Abstract
Nitric oxide (NO) is an intercellular messenger involved in multiple bodily functions. Prolonged NO exposure irreversibly inhibits respiration by covalent modification of mitochondrial cytochrome oxidase, a phenomenon of pathological relevance. However, the speed and potency of NO's metabolic effects at physiological concentrations are incompletely characterized. To this end, we set out to investigate the metabolic effects of NO in cultured astrocytes from mice by taking advantage of the high spatiotemporal resolution afforded by genetically encoded Förster resonance energy transfer (FRET) nanosensors. NO exposure resulted in immediate and reversible intracellular glucose depletion and lactate accumulation. Consistent with cytochrome oxidase involvement, the glycolytic effect was enhanced at a low oxygen level and became irreversible at a high NO concentration or after prolonged exposure. Measurements of both glycolytic rate and mitochondrial pyruvate consumption revealed significant effects even at nanomolar NO concentrations. We conclude that NO can modulate astrocytic energy metabolism in the short term, reversibly, and at concentrations known to be released by endothelial cells under physiological conditions. These findings suggest that NO modulates the size of the astrocytic lactate reservoir involved in neuronal fueling and signaling.
Keywords: astrocyte, cytochrome c oxidase (complex IV), glucose metabolism, nitric oxide, pyruvate, AR-C155858, MCT, cytochalasin B, Glut1, lactate
Introduction
In brain tissue and retina, NO contributes to both vasodilation and vasoconstriction (1, 2). The vasoactive effects of NO are mediated by the enzymes guanylate cyclase, cytochrome P450 epoxygenase, and ω-hydroxylase, which sense different NO concentrations. Guanylate cyclase is stimulated in the picomolar to low nanomolar range, whereas cytochrome P450 enzymes are inhibited at tens to hundreds of nanomolar NO (1, 3). However, there is more to NO than vasodilation. Endothelial cells express both endothelial NO synthase (eNOS)3 and inducible NO synthase (iNOS) (4) resulting in NO production strong enough to sustain up to 70 nm extracellular NO in response to shear stress (5), to reach cells millimeters away in bicameral cultures (6), or to permeate across layers of astrocytes and myelin toward axons in vivo (7). A fourth well characterized molecular target of NO is mitochondrial cytochrome oxidase (EC 1.9.3.1), whose sensitivity to NO is similar to that of cytochrome P450 (8, 9). As inhibition of cytochrome oxidase reduces local oxygen consumption, endothelial NO has been proposed to extend the effective zone of oxygenation away from the vessel (10). A role for NO-dependent mitochondrial inhibition in brain tissue was suggested upon the observation that NO inhibits astrocytic respiration, resulting in stimulated glycolysis and lactate production (11, 12). Conceivably, inhibition of cytochrome oxidase may not only play a tonic distributive role but may also participate in activity-dependent neurometabolic coupling (13). However, there are aspects that need further exploring, notably the speed and potency of the metabolic effects in the face of actual NO concentrations found under physiological conditions. Also important is to know whether the modulation is reversible, as prolonged or high concentration exposures to NO are known to cause irreversible, non-physiological nitrosylation of cytochrome oxidase and other targets (8, 14).
In recent years, genetically encoded optical sensors have been introduced that afford sensitive real-time monitoring of energy metabolites and metabolic fluxes in individual cells. A distinctive advantage of these tools over standard isotope-based methodologies is that they can detect functional changes before they are obscured by desensitization or compensatory adaptations. Using these tools we were able to show that K+ and NH4+, which are released by active neurons, trigger acute changes in astrocytic glucose consumption and lactate production (15–18). The aim of this study was to investigate whether NO is capable of modulating energy metabolism in astrocytes with the speed, reversibility, and potency expected for a physiological signal.
Results
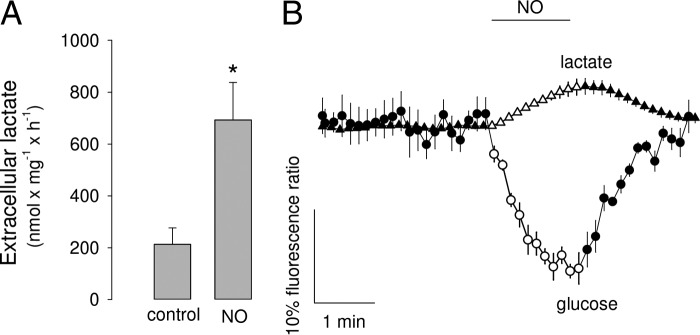
As reported previously for rat astrocytes, prolonged exposure of mouse astrocytes in culture to the slow nitric oxide donor DETANO (half-time 20 h; 0.5 mm equivalent to 1.4 μm steady-state NO (11)) led to increased extracellular lactate (Fig. 1A), consistent with cytochrome oxidase inhibition and secondary glycolysis stimulation. To investigate the speed of the phenomenon, cytosolic glucose and lactate were measured using the genetically encoded FRET sensors FLII12Pglu700μΔ6 (19) and Laconic (20), respectively. Astrocytes were studied in the presence of neurons, which foster their functional and metabolic differentiation (21). For real-time experiments, NO was generated by dissolving NOC-9 (half-time 2.7 min) 1 min before the onset of culture superperfusion. Each NOC-9 molecule releases two NO molecules, but there is some loss along the perfusion line so that the actual concentration of NO measured in the imaging chamber was found to be similar to that of NOC-9 added (NO (μm) of 8 ± 1, 21 ± 1, and 120 ± 1 at 10, 20, and 100 μm NOC-9, respectively). Monitored by FRET imaging, astrocytes showed immediate intracellular glucose depletion and lactate accumulation (Fig. 1B), indicative of fast glycolysis stimulation. A NOC-9-treated buffer that had been depleted of NO by 1 h of gassing with air/CO2 was without apparent effect on the intracellular glucose level (data not shown). The small change in Laconic signal is consistent with the high concentration of lactate found in resting astrocytes (1.4 mm (17)) and with the dose-response curve of this sensor (20).
Figure 1.
Fast modulation of astrocytic metabolism by NO. A, concentration of lactate in the extracellular medium of pure astrocytic cultures treated for 60 min with or without 0.5 mm DETANO, equivalent to 1.4 μm free NO (11) (n = three experiments). B, effect of 2 μm NO on astrocytic glucose and lactate (n = 5 cells, representative of three experiments).
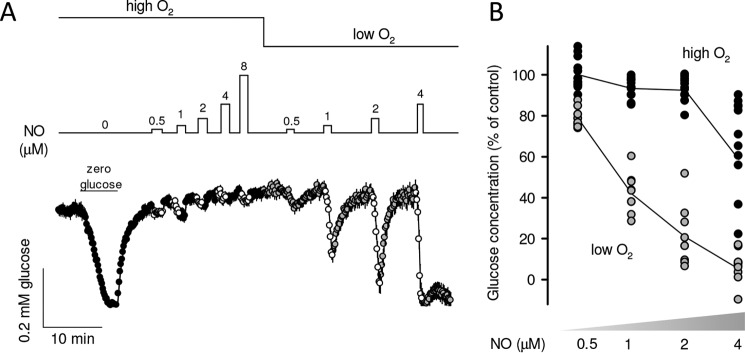
NO competes for the oxygen-binding site at cytochrome oxidase, and therefore NO functions mediated by this enzyme are characteristically sensitive to oxygen (22), which is not the case for guanylate cyclase and cytochrome P450. To test for possible cytochrome oxidase involvement, the response of intracellular glucose to NO was first compared at high and low oxygen concentrations (467 ± 9 and 62 ± 3 μm) that were achieved by superfusing cultures with buffers equilibrated with 95% air, 5% CO2 or 95% N2, 5% CO2, respectively. Fig. 2, A and B, shows that glucose depletion was more intense at lower oxygen levels, consistent with cytochrome oxidase involvement. Transient NO exposures were followed by quick recovery of glucose to pre-stimulation levels, but there was no recovery of glucose homeostasis at high NO (Fig. 2A), as expected from irreversible nitrosylation of cytochrome oxidase (14). Additional evidence for cytochrome oxidase participation was provided by delayed or absent glucose recovery after more prolonged NO exposure (data not shown). Considering the sensitivity to oxygen, the measurements described below were performed in low oxygen.
Figure 2.
Oxygen sensitivity of the metabolic response to NO. A, astrocytes expressing the glucose sensor were first transiently depleted of glucose for calibration purposes and then exposed to increasing NO concentrations in the presence of 467 μm (high O2) and 62 μm (low O2; n = 10 cells from a single experiment). B, magnitude of the glucose depletion in three similar experiments, with 3–5 cells each. Line plot shows the average glucose depletion at each NO concentration.
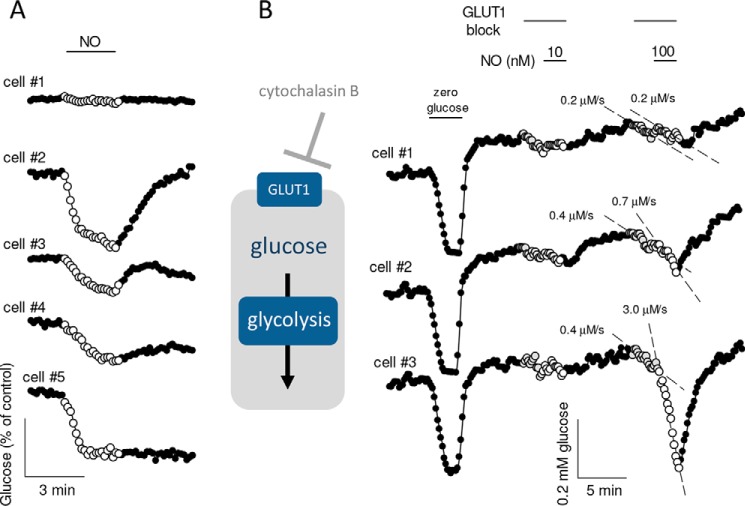
Intracellular glucose depletion is an indirect readout of glycolytic modulation, which may be obscured by parallel changes in glucose transporter activity (23). Indeed, when astrocytes were tested in the presence of a glucose transport blocker to probe glucose consumption directly (24), it became evident that NO stimulates glycolysis at lower concentrations, at least in a subpopulation of cells. Whereas 10 nm was without effect on the rate of glucose consumption, a significant response was observed at 100 nm in 16 out of 22 cells (p < 0.05; n = 3 experiments). Fig. 3B shows data from a single experiment in which one cell did not respond, a second cell showed a small response, and the third showed a very robust stimulation at 100 nm. Still, glucose consumption may not reflect the true sensitivity of mitochondria to NO.
Figure 3.
Stimulation of the rate of glucose consumption by nanomolar NO. A, astrocytes expressing the glucose sensor were exposed for 3 min to 2 μm NO (n = 5 cells, representative of four experiments). B, astrocytes expressing the glucose sensor were first transiently depleted of glucose for calibration purposes, and then the rate of glucose consumption was estimated in the presence of the GLUT1 blocker cytochalasin B (20 μm) before and after addition of 10 nm NO to the superfusate. After washout, the same protocol was repeated at 100 nm NO. Data are shown for three cells, which responded to 100 nm NO with varying degrees of glucose consumption stimulation.
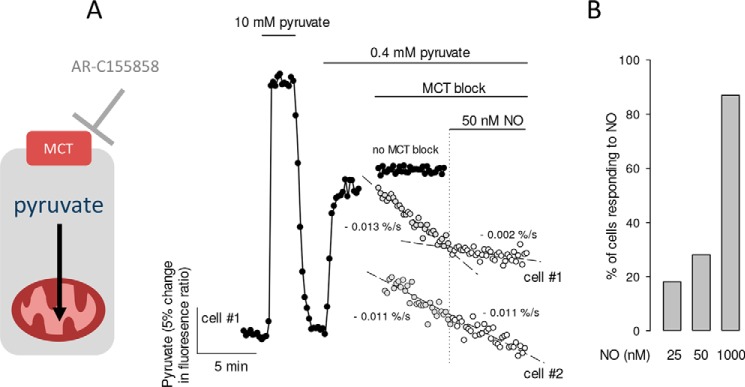
For a more direct and sensitive assessment of the metabolic response to low nanomolar NO, the consumption of pyruvate by mitochondria was monitored with the genetically encoded FRET nanosensor Pyronic. The rationale of this method, which has been described in detail elsewhere (25), is that in the presence of extracellular pyruvate and absence of glucose, cells become net pyruvate consumers, and therefore, when pyruvate influx into the cell is acutely blocked with an inhibitor of the monocarboxylate transporter, the cytosolic concentration of pyruvate goes down as fast as pyruvate is consumed by mitochondria. Fig. 4A shows a representative experiment, which involved a transient exposure to a high concentration of pyruvate (10 mm) to illustrate the range of the FRET sensor, followed by establishment of a steady-state at 0.4 mm extracellular pyruvate, in which pyruvate influx and mitochondrial pyruvate consumption are matched. Measurement in the presence of the MCT1–2 blocker AR-C155858 showed that some cells responded to 25–50 nm NO with a quick reduction of mitochondrial pyruvate consumption (e.g. cell 2 in Fig. 4, A and B).
Figure 4.
Inhibition of the rate of mitochondrial pyruvate consumption by nanomolar NO. A, astrocytes expressing the pyruvate sensor were first transiently exposed to 10 mm pyruvate to demonstrate the range of the sensor and then stabilized in 0.4 mm extracellular pyruvate. The trace in closed symbols illustrates a cell left in the steady-state condition. For the representative cells shown as gray symbols, mitochondrial pyruvate consumption was measured in the presence of the MCT blocker AR-C155858 (1 μm) in the absence and then in the presence of 50 nm NO. In cell #1 pyruvate consumption was strongly inhibited by NO, and in cell #2 pyruvate consumption remained constant (trace displaced for clarity). B, summary of three to five similar experiments for each concentration, with at least five cells each.
Discussion
The main observations here are that the inhibitory effects of NO on astrocytic mitochondria and glycolysis are fast and reversible and that they can be detected in the low nanomolar range, providing fresh support to the hypothesis that NO modulation of astrocytic cytochrome oxidase plays a physiological role in the brain.
After reports of the reversible inhibitory effect of NO on respiration appeared in 1994 (22, 26, 27), it was hypothesized that NO may be a physiological regulator of oxygen consumption in mammalian tissues (8). Studies in cultured astrocytes showed that NO, used at 1.4 μm over a period of minutes, stimulated glycolysis and lactate production via inhibition of cytochrome oxidase (11, 12). By monitoring the effect of NO in real time, we were able to observe that the stimulation of glucose consumption is immediate, leading to intracellular glucose depletion and lactate accumulation. The immediate translation of mitochondrial inhibition into glycolysis stimulation is not trivial, as it was not observed after acute mitochondrial matrix acidification by glutamate (28, 29) or by NH4+ (18). The effect was dose-dependent and could be detected at 100 nm NO. Consistent with cytochrome oxidase involvement, the response was enhanced at low oxygen, and it became irreversible at a high NO (9, 30). A more direct investigation revealed inhibition of mitochondrial pyruvate consumption that could be detected in some cells even at 25 nm. We do not have an obvious explanation for the differential sensitivity of individual astrocytes to NO. Oxygen consumption is markedly more sensitive to NO in state 3 than in state 4 (8), whereas in situ mitochondria are in neither state (31), so perhaps the sensitivity of astrocytes to NO relates to their metabolic status. Unfortunately, the lowest oxygen concentration we could reach by bubbling the superfusate with N2 was 62 μm, which is still over the physiological brain tissue oxygen of 30 μm (32). Given that the KD value of cytochrome oxidase for oxygen is in the low micromolar range (9), it seems likely that NO will inhibit astrocytic mitochondria with even more effectiveness in vivo. For example, mitochondria isolated from brown adipose tissue showed a 6-fold increase in the potency of NO to inhibit respiration when oxygen was lowered from 72 to 32 μm (33).
Sources and concentration of NO in brain tissue
The brain contains the highest activity of nitric-oxide synthase of any tissue (34). The accepted view of exclusive expression of nNOS in neurons and eNOS in endothelium of capillaries and large vessels (7, 35–37) was recently confirmed by quantitative RNA sequencing (38), a technique that in addition revealed substantial endothelial mRNA expression of iNOS, the isoform with the highest capacity for NO production and previously thought to be expressed only under pathological conditions. In contrast, almost no transcripts for nNOS, eNOS, or iNOS were detected in astrocytes, oligodendrocytes, oligodendrocyte precursor cells, or microglia. Thus, astrocytes appear as NO targets that may in principle be reached by endothelial or by neuronal NO. Neuronal NOS is stimulated by synaptic activity, whereas eNOS responds to flow-induced shear stress and also to synaptic activity via endothelial NMDA receptors (34, 39, 40).
The physiological concentration of NO is contentious. Mathematical modeling of NO distribution within tissue is hampered by insufficient information about actual rates of production, diffusion, and scavenging (41). According to one study, distribution of NO at neuronal nNOS is restricted to the immediate vicinity of the synaptic cleft so that even at the production site, NO may not reach astrocytes (42). Moreover, perisynaptic astrocytic processes possess very few mitochondria compared with larger, more distant, astrocytic processes (43). These considerations speak against inhibition of astrocytic metabolism by neuronal NO, but neurons may still modulate the metabolism of astrocytic mitochondria via other signals such as glutamate and NH4+ (18, 44, 45).
In contrast, there are multiple lines of evidence pointing to endothelial NO. One is geometrical. A study of the optic nerve, a tract of white matter, showed that endothelial NO can diffuse far enough to modify axonal excitability (7). Before reaching axons, endothelial NO must traverse astrocytic end-feet and then partition into the membranous myelin sheath. NO is four times more soluble and diffuses 10 times slower in lipid membranes than in water (46), so its concentration at astrocytic end-feet will be much higher than that within axons. Of note, end-feet mitochondria have been located within 100 nm of the endothelium (47). Electrode measurements of NO in brain tissue span from picomolar to micromolar (48), a perplexing range that has been ascribed to experimental conditions and to unstirred layer artifacts at the tip of NO-consuming electrodes (49). According to the latter, electrode measurements underestimate physiological NO, which would reach well into the nanomolar range (49), a conclusion consistent with the wealth of evidence for cytochrome P450 epoxygenase and ω-hydroxylase involvement in brain blood flow regulation. NO inhibits cytochrome P450 and cytochrome c oxidase with similar potency (1–3, 33, 40, 50). It stands to reason that if there is enough NO for one, there must be enough for the other. Further supporting the idea that the endothelium generates enough NO to modulate astrocytic cytochrome oxidase is the observation that NO production by brain vascular endothelial cells stabilizes HIF1-α in astrocytes lying millimeters away and in a guanylate cyclase-independent manner (6, 51).
Possible roles of cytochrome oxidase inhibition by NO, lactate, and oxygen gradient
Endothelial cells appear to be immune to their own NO production, as they have few mitochondria and rely almost exclusively on glycolysis for ATP production (52–54). It is not known to what extent their low mitochondrial ATP production is explained by NO inhibition of oxidative phosphorylation, either reversible or by nitrosylation. The reversibility of the inhibitory effect of NO on the respiration of parenchymal cells led to the speculation that endothelial NO may inhibit oxygen consumption at perivascular regions, thereby improving its delivery to the rest of the tissue (10, 55). Along with previous observations in astrocytes and synaptosomes (6, 11, 12, 22, 51), the present findings are consistent with such a mechanism being at work in brain tissue (Fig. 5), acting together with activity-dependent vasodilation (32, 56) to promote an adequate oxygen supply to cellular regions away from vessels. At the same time, inhibition of pyruvate oxidation by NO is expected to support astrocytic tricarboxylic acid cycle anaplerosis for the generation of building blocks for biosynthesis.
Figure 5.
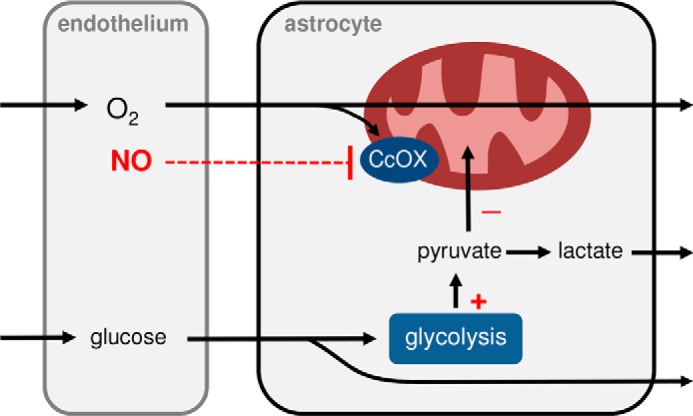
Possible modulatory roles of endothelial NO on astrocytic energy metabolism. Oxygen, NO, and glucose reach astrocytes from the endothelium. By inhibiting mitochondrial cytochrome oxidase (CcOX) and stimulating glucose consumption in astrocytes, NO contributes to refilling the astrocytic lactate reservoir while increasing the availability of oxygen for cells situated deeper in the brain parenchyma.
Acting in synergy with NH4+, which also inhibits mitochondrial pyruvate consumption (18), endothelial NO is expected to contribute to the standing astrocytic lactate reservoir (17, 57, 58) that is acutely released to the interstitium “on demand” via a K+-activated lactate channel (17). Lactate is a metabolic fuel and also an intercellular signal that modulates multiple functions in brain tissue, from vascular tone to memory processing (59–61), and therefore the effect of NO on astrocytic mitochondria may be understood as a modulator of these functions.
Experimental procedures
Standard reagents and inhibitors were acquired from Sigma or Merck. Plasmids encoding the FRET sensors FLII12Pglu700μΔ6 (19), Laconic (20), and Pyronic (25) are available from Addgene. Viral vectors Ad FLII12Pglu700μΔ6, Ad Laconic, and Ad Pyronic (all serotype 5) were custom made by Vector Biolabs.
Animals and cultures
Procedures involving animals were carried out according to the Guide for the Care and Use of Laboratory Animals, National Research Council. Procedures were approved by the Centro de Estudios Científicos Animal Care and Use Committee. The reports of the procedures comply with the ARRIVE guidelines. Mixed neuronal glial primary cultures (day 8–16) were prepared from mixed F1 1–3-day-old mice (C57BL/6J × CBA/J), as described previously (24). The animals were maintained on a 12-h day/night cycle at constant room temperature with free access to water and standard mouse fodder in the animal facility of Centro de Estudios Científicos. Lactate release from pure astrocytic subcultures was estimated enzymatically using 4 mm hydrazine as a pyruvate sink (62). NO in superfusates was measured using a colorimetric kit according to the manufacturer's instructions (Abcam). Oxygen in superfusates was measured using the oxygen probe WTW multi 340i (Wissenschaftlich-Technische-Werkstätten, Germany).
Fluorescence imaging
Detailed protocols for the use of the fluorescent sensors for glucose, lactate, and pyruvate are available (63–65). Cultured cells were imaged with an upright Olympus FV1000 confocal microscope and a 440-nm solid-state laser or with Olympus IX70 or BX51 microscopes equipped with Cairn Research monochromators and Optosplits, and either a Hamamatsu Orca or Rollera camera. Cells were superfused at room temperature with a 95% air, 5% CO2- or a 95% N2, 5% CO2-gassed solution of the following composition (mm): 112 NaCl, 3 KCl, 1.25 CaCl2, 1.25 MgCl2, 2 glucose, 0.1 to 1 sodium lactate, 10 HEPES, and 24 NaHCO3, pH 7.4. Data are presented as means ± S.E. Differences between experimental groups were assessed with the Student's t test. p values < 0.05 were considered significant and are indicated with an asterisk.
Author contributions
A. S. M. designed and conducted most of the experiments, analyzed the results, and wrote the paper with L. F. B. R. A. M. conducted experiments with the pyruvate sensor. A. G. conducted experiments on nitric oxide reversibility. G. P. G. conducted extracellular lactate determinations. L. F. B. conceived the idea for the project, analyzed the results, and wrote the paper with A. S. M.
Acknowledgments
We thank Karin Alegría for expert assistance with cell culture and Planta Valdivia of Arauco S.A., particularly Manager Sergio Carreño Moscoso and Production sub-manager Manuel González Saldivia, for kindly lending the oxygen electrode. We also thank Karen Everett for critical reading of the manuscript. The Centro de Estudios Científicos is funded by the Chilean Government through the Centers of Excellence Base Financing Program of Conicyt.
This work was supported in part by Fondecyt Grants 1130095 and 1160317. The authors declare that they have no conflicts of interest with the contents of this article.
This article was selected as one of our Editors' Picks.
- eNOS
- endothelial NO synthase
- iNOS
- inducible NO synthase
- nNOS
- neuronal nitric-oxide synthase.
References
- 1. Metea M. R., and Newman E. A. (2006) Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J. Neurosci. 26, 2862–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Attwell D., Buchan A. M., Charpak S., Lauritzen M., Macvicar B. A., and Newman E. A. (2010) Glial and neuronal control of brain blood flow. Nature 468, 232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellamy T. C., Griffiths C., and Garthwaite J. (2002) Differential sensitivity of guanylyl cyclase and mitochondrial respiration to nitric oxide measured using clamped concentrations. J. Biol. Chem. 277, 31801–31807 [DOI] [PubMed] [Google Scholar]
- 4. Yamakawa H., Jezova M., Ando H., and Saavedra J. M. (2003) Normalization of endothelial and inducible nitric-oxide synthase expression in brain microvessels of spontaneously hypertensive rats by angiotensin II AT1 receptor inhibition. J. Cereb. Blood Flow Metab. 23, 371–380 [DOI] [PubMed] [Google Scholar]
- 5. Andrews A. M., Jaron D., Buerk D. G., Kirby P. L., and Barbee K. A. (2010) Direct, real-time measurement of shear stress-induced nitric oxide produced from endothelial cells in vitro. Nitric Oxide 23, 335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marcillac F., Brix B., Repond C., Jöhren O., and Pellerin L. (2011) Nitric oxide induces the expression of the monocarboxylate transporter MCT4 in cultured astrocytes by a cGMP-independent transcriptional activation. Glia 59, 1987–1995 [DOI] [PubMed] [Google Scholar]
- 7. Garthwaite G., Bartus K., Malcolm D., Goodwin D., Kollb-Sielecka M., Kollb-Sielecka M., Dooldeniya C., and Garthwaite J. (2006) Signaling from blood vessels to CNS axons through nitric oxide. J. Neurosci. 26, 7730–7740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown G. C. (2001) Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim. Biophys. Acta 1504, 46–57 [DOI] [PubMed] [Google Scholar]
- 9. Antunes F., Boveris A., and Cadenas E. (2004) On the mechanism and biology of cytochrome oxidase inhibition by nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 101, 16774–16779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas D. D., Liu X., Kantrow S. P., and Lancaster J. R. Jr. (2001) The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. U.S.A. 98, 355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Almeida A., Almeida J., Bolaños J. P., and Moncada S. (2001) Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc. Natl. Acad. Sci. U.S.A. 98, 15294–15299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Almeida A., Moncada S., and Bolaños J. P. (2004) Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 6, 45–51 [DOI] [PubMed] [Google Scholar]
- 13. Bolaños J. P. (2016) Bioenergetics and redox adaptations of astrocytes to neuronal activity. J. Neurochem. 139, 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clementi E., Brown G. C., Feelisch M., and Moncada S. (1998) Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. U.S.A. 95, 7631–7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bittner C. X., Valdebenito R., Ruminot I., Loaiza A., Larenas V., Sotelo-Hitschfeld T., Moldenhauer H., San Martín A., Gutiérrez R., Zambrano M., and Barros L. F. (2011) Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J. Neurosci. 31, 4709–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruminot I., Gutiérrez R., Peña-Münzenmayer G., Añazco C., Sotelo-Hitschfeld T., Lerchundi R., Niemeyer M. I., Shull G. E., and Barros L. F. (2011) NBCe1 mediates the acute stimulation of astrocytic glycolysis by extracellular K+. J. Neurosci. 31, 14264–14271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sotelo-Hitschfeld T., Niemeyer M. I., Mächler P., Ruminot I., Lerchundi R., Wyss M. T., Stobart J., Fernández-Moncada I., Valdebenito R., Garrido-Gerter P., Contreras-Baeza Y., Schneider B. L., Aebischer P., Lengacher S., San Martín A., et al. (2015) Channel-mediated lactate release by K+-stimulated astrocytes. J. Neurosci. 35, 4168–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lerchundi R., Fernández-Moncada I., Contreras-Baeza Y., Sotelo-Hitschfeld T., Mächler P., Wyss M. T., Stobart J., Baeza-Lehnert F., Alegría K., Weber B., and Barros L. F. (2015) NH4+ triggers the release of astrocytic lactate via mitochondrial pyruvate shunting. Proc. Natl. Acad. Sci. U.S.A. 112, 11090–11095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takanaga H., Chaudhuri B., and Frommer W. B. (2008) GLUT1 and GLUT9 as major contributors to glucose influx in HepG2 cells identified by a high sensitivity intramolecular FRET glucose sensor. Biochim. Biophys. Acta 1778, 1091–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. San Martín A., Ceballo S., Ruminot I., Lerchundi R., Frommer W. B., and Barros L. F. (2013) A genetically encoded FRET lactate sensor and its use to detect the Warburg effect in single cancer cells. PLoS ONE 8, e57712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mamczur P., Borsuk B., Paszko J., Sas Z., Mozrzymas J., Wiśniewski J. R., Gizak A., and Rakus D. (2015) Astrocyte-neuron crosstalk regulates the expression and subcellular localization of carbohydrate metabolism enzymes. Glia 63, 328–340 [DOI] [PubMed] [Google Scholar]
- 22. Brown G. C., and Cooper C. E. (1994) Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 356, 295–298 [DOI] [PubMed] [Google Scholar]
- 23. Barros L. F., San Martín A., Sotelo-Hitschfeld T., Lerchundi R., Fernández-Moncada I., Ruminot I., Gutiérrez R., Valdebenito R., Ceballo S., Alegría K., Baeza-Lehnert F., and Espinoza D. (2013) Small is fast: astrocytic glucose and lactate metabolism at cellular resolution. Front. Cell. Neurosci. 7, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bittner C. X., Loaiza A., Ruminot I., Larenas V., Sotelo-Hitschfeld T., Gutiérrez R., Córdova A., Valdebenito R., Frommer W. B., and Barros L. F. (2010) High resolution measurement of the glycolytic rate. Front. Neuroenergetics 2, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. San Martín A., Ceballo S., Baeza-Lehnert F., Lerchundi R., Valdebenito R., Contreras-Baeza Y., Alegría K., and Barros L. F. (2014) Imaging mitochondrial flux in single cells with a FRET sensor for pyruvate. PLoS ONE 9, e85780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., and Schapira A. H. (1994) Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 345, 50–54 [DOI] [PubMed] [Google Scholar]
- 27. Schweizer M., and Richter C. (1994) Nitric oxide potently and reversibly deenergizes mitochondria at low oxygen tension. Biochem. Biophys. Res. Commun. 204, 169–175 [DOI] [PubMed] [Google Scholar]
- 28. Loaiza A., Porras O. H., and Barros L. F. (2003) Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J. Neurosci. 23, 7337–7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Azarias G., Perreten H., Lengacher S., Poburko D., Demaurex N., Magistretti P. J., and Chatton J. Y. (2011) Glutamate transport decreases mitochondrial pH and modulates oxidative metabolism in astrocytes. J. Neurosci. 31, 3550–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martínez-Ruiz A., Cadenas S., and Lamas S. (2011) Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free Radic. Biol. Med. 51, 17–29 [DOI] [PubMed] [Google Scholar]
- 31. Brand M. D., and Nicholls D. G. (2011) Assessing mitochondrial dysfunction in cells. Biochem. J. 435, 297–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Devor A., Sakadzic S., Saisan P. A., Yaseen M. A., Roussakis E., Srinivasan V. J., Vinogradov S. A., Rosen B. R., Buxton R. B., Dale A. M., and Boas D. A. (2011) “Overshoot” of O(2) is required to maintain baseline tissue oxygenation at locations distal to blood vessels. J. Neurosci. 31, 13676–13681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koivisto A., Matthias A., Bronnikov G., and Nedergaard J. (1997) Kinetics of the inhibition of mitochondrial respiration by NO. FEBS Lett. 417, 75–80 [DOI] [PubMed] [Google Scholar]
- 34. Garthwaite J., and Boulton C. L. (1995) Nitric oxide signaling in the central nervous system. Annu. Rev. Physiol 57, 683–706 [DOI] [PubMed] [Google Scholar]
- 35. Stanarius A., Töpel I., Schulz S., Noack H., and Wolf G. (1997) Immunocytochemistry of endothelial nitric-oxide synthase in the rat brain: a light and electron microscopical study using the tyramide signal amplification technique. Acta Histochem. 99, 411–429 [DOI] [PubMed] [Google Scholar]
- 36. Blackshaw S., Eliasson M. J., Sawa A., Watkins C. C., Krug D., Gupta A., Arai T., Ferrante R. J., and Snyder S. H. (2003) Species, strain and developmental variations in hippocampal neuronal and endothelial nitric-oxide synthase clarify discrepancies in nitric oxide-dependent synaptic plasticity. Neuroscience 119, 979–990 [DOI] [PubMed] [Google Scholar]
- 37. Chan Y., Fish J. E., D'Abreo C., Lin S., Robb G. B., Teichert A. M., Karantzoulis-Fegaras F., Keightley A., Steer B. M., and Marsden P. A. (2004) The cell-specific expression of endothelial nitric-oxide synthase: a role for DNA methylation. J. Biol. Chem. 279, 35087–35100 [DOI] [PubMed] [Google Scholar]
- 38. Zhang Y., Chen K., Sloan S. A., Bennett M. L., Scholze A. R., O'Keeffe S., Phatnani H. P., Guarnieri P., Caneda C., Ruderisch N., Deng S., Liddelow S. A., Zhang C., Daneman R., Maniatis T., et al. (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. LeMaistre J. L., Sanders S. A., Stobart M. J., Lu L., Knox J. D., Anderson H. D., and Anderson C. M. (2012) Coactivation of NMDA receptors by glutamate and d-serine induces dilation of isolated middle cerebral arteries. J. Cereb. Blood Flow Metab. 32, 537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stobart J. L., Lu L., Anderson H. D., Mori H., and Anderson C. M. (2013) Astrocyte-induced cortical vasodilation is mediated by d-serine and endothelial nitric-oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 110, 3149–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen K., Pittman R. N., and Popel A. S. (2008) Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid. Redox Signal. 10, 1185–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garthwaite J. (2016) From synaptically localized to volume transmission by nitric oxide. J. Physiol 594, 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Derouiche A., Haseleu J., and Korf H. W. (2015) Fine astrocyte processes contain very small mitochondria: glial oxidative capability may fuel transmitter metabolism. Neurochem. Res. 40, 2402–2413 [DOI] [PubMed] [Google Scholar]
- 44. Jackson J. G., O'Donnell J. C., Takano H., Coulter D. A., and Robinson M. B. (2014) Neuronal activity and glutamate uptake decrease mitochondrial mobility in astrocytes and position mitochondria near glutamate transporters. J. Neurosci. 34, 1613–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jackson J. G., and Robinson M. B. (2015) Reciprocal regulation of mitochondrial dynamics and calcium signaling in astrocyte processes. J. Neurosci. 35, 15199–15213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Möller M., Botti H., Batthyany C., Rubbo H., Radi R., and Denicola A. (2005) Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J. Biol. Chem. 280, 8850–8854 [DOI] [PubMed] [Google Scholar]
- 47. Mathiisen T. M., Lehre K. P., Danbolt N. C., and Ottersen O. P. (2010) The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58, 1094–1103 [DOI] [PubMed] [Google Scholar]
- 48. Hall C. N., and Garthwaite J. (2009) What is the real physiological NO concentration in vivo? Nitric Oxide 21, 92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bohlen H. G. (2013) Is the real in vivo nitric oxide concentration pico- or nanomolar? Influence of electrode size on unstirred layers and NO consumption. Microcirculation 20, 30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rodríguez-Juárez F., Aguirre E., and Cadenas S. (2007) Relative sensitivity of soluble guanylate cyclase and mitochondrial respiration to endogenous nitric oxide at physiological oxygen concentration. Biochem. J. 405, 223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brix B., Mesters J. R., Pellerin L., and Jöhren O. (2012) Endothelial cell-derived nitric oxide enhances aerobic glycolysis in astrocytes via HIF-1α-mediated target gene activation. J. Neurosci. 32, 9727–9735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Culic O., Gruwel M. L., and Schrader J. (1997) Energy turnover of vascular endothelial cells. Am. J. Physiol. 273, C205–C213 [DOI] [PubMed] [Google Scholar]
- 53. Quintero M., Colombo S. L., Godfrey A., and Moncada S. (2006) Mitochondria as signaling organelles in the vascular endothelium. Proc. Natl. Acad. Sci. U.S.A. 103, 5379–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Davidson S. M., and Duchen M. R. (2007) Endothelial mitochondria: contributing to vascular function and disease. Circ. Res. 100, 1128–1141 [DOI] [PubMed] [Google Scholar]
- 55. Poderoso J. J., Carreras M. C., Lisdero C., Riobó N., Schöpfer F., and Boveris A. (1996) Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch. Biochem. Biophys. 328, 85–92 [DOI] [PubMed] [Google Scholar]
- 56. Buxton R. B., and Frank L. R. (1997) A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J. Cereb. Blood Flow Metab. 17, 64–72 [DOI] [PubMed] [Google Scholar]
- 57. Mächler P., Wyss M. T., Elsayed M., Stobart J., Gutierrez R., von Faber-Castell A., Kaelin V., Zuend M., San Martín A., Romero-Gómez I., Baeza-Lehnert F., Lengacher S., Schneider B. L., Aebischer P., Magistretti P. J., et al. (2016) In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab. 23, 94–102 [DOI] [PubMed] [Google Scholar]
- 58. Kasparov S. (2016) Are astrocytes the pressure-reservoirs of lactate in the brain? Cell Metab. 23, 1–2 [DOI] [PubMed] [Google Scholar]
- 59. Barros L. F. (2013) Metabolic signaling by lactate in the brain. Trends Neurosci. 36, 396–404 [DOI] [PubMed] [Google Scholar]
- 60. Tang F., Lane S., Korsak A., Paton J. F., Gourine A. V., Kasparov S., and Teschemacher A. G. (2014) Lactate-mediated glia-neuronal signalling in the mammalian brain. Nat. Commun. 5, 3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang J., Ruchti E., Petit J. M., Jourdain P., Grenningloh G., Allaman I., and Magistretti P. J. (2014) Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. U.S.A. 111, 12228–12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rosenberg J. C., and Rush B. F. (1966) An enzymatic-spectrophotometric determination of pyruvic and lactic acid in blood. Methodologic aspects. Clin. Chem. 12, 299–307 [PubMed] [Google Scholar]
- 63. Hou B. H., Takanaga H., Grossmann G., Chen L. Q., Qu X. Q., Jones A. M., Lalonde S., Schweissgut O., Wiechert W., and Frommer W. B. (2011) Optical sensors for monitoring dynamic changes of intracellular metabolite levels in mammalian cells. Nat. Protoc. 6, 1818–1833 [DOI] [PubMed] [Google Scholar]
- 64. Barros L. F., Baeza-Lehnert F., Valdebenito R., Ceballo S., and Alegría K. (2014) in Springer Protocols: Brain Energy Metabolism (Waagepetersen H. S., and Hirrlinger J., eds) pp. 145–159, Springer, Berlin [Google Scholar]
- 65. San Martín A., Sotelo-Hitschfeld T., Lerchundi R., Fernández-Moncada I., Ceballo S., Valdebenito R., Baeza-Lehnert F., Alegría K., Contreras-Baeza Y., Garrido-Gerter P., Romero-Gómez I., and Barros L. F. (2014) Single-cell imaging tools for brain energy metabolism: a review. Neurophotonics 1, 011004 [DOI] [PMC free article] [PubMed] [Google Scholar]