Abstract
Purpose
Abnormal brain perfusion is a critical mechanism in neonatal brain injury. The aim of the present study was to compare Cerebral Blood Flow (CBF) evaluated with ASL MRI in three groups of neonates: preterms without brain lesions on MRI (PN), preterms with periventricular white matter lesions (PNp) and term neonates with normal MRI (TN). The correlation between CBF and clinical outcome was explored.
Materials and methods
The institutional review board approved this prospective study and waived informed consent. The perfusion ASL data from 49 consecutive preterm neonates (PN) studied at term-equivalent age and 15 TN were evaluated. Statistically significant differences in gray matter CBF were evaluated by using a linear mixed-model analysis and Mann-Whitney U test. Logistic regression analysis was used to assess the relation between CBF and neuromotor outcome at 12 months.
Results
Comparison of means indicated that the CBF of the whole brain were significantly higher in PN compared to TN (P = 0.011). This difference remained significant when considering the frontal (P = 0.038), parietal (P = 0.002), temporal (P = 0.030), occipital (P = 0.041) and cerebellar (P = 0.010) gray matter. In the PN group, lower CBF in basal ganglia was associated with a worse neuromotor outcome (P = 0.012).
Conclusions
ASL MRI demonstrated differences in brain perfusion of the basal ganglia between PN and TN. In PN, a positive correlation between CBF and neuromotor outcome was demonstrated in this area.
Abbreviations: ASL, arterial spin labeling; CBF, cerebral blood flow; HIE, hypoxic-ischemic encephalopathy; PN, preterm neonate with normal MRI; PNp, preterm neonate periventricular white matter lesions; PVL, periventricular leukoencephalopathy; TN, term neonate with normal MRI
Keywords: Brain perfusion, Neonate, Prematurity
Highlights
-
•
Different ASL cerebral perfusion between preterm and term neonates
-
•
Global reduction of CBF values in preterm neonates with white matter lesions
-
•
ASL identifies preterm neonates at higher risk for sub-optimal neuromotor development.
-
•
Worst 12-months neuromotor outcome in preterm neonates with lower CBF of basal ganglia
1. Introduction
Approximately 10–12% of all live births in developed countries are preterm. They are a population at high risk for brain damage and neurodevelopmental disabilities (Blencowe et al., 2012). Improvements in health care for preterm infants have vastly improved survival rates, although comorbid medical and neurodevelopmental difficulties, including cerebral palsy and intellectual disabilities, persist (Saigal and Doyle, 2008). Even in the absence of overt disabilities, a significantly lower percentage of preterms subsequently achieve higher educational and social levels compared to full-terms (Ment et al., 2009). As such, the consequences of preterm birth are considered a serious public health issue, especially for very preterm births (gestational age < 32 weeks).
Establishing an association between neurodevelopmental outcome and brain imaging performed around birth would favor earlier treatment strategies to improve long-term outcomes. Currently, there is no accurate method of identifying all preterm neonates at risk of abnormal developmental outcome. Conventional Magnetic Resonance Imaging (MRI) is considered the most sensitive neuroimaging modality for assessing brain myelination and perinatal brain injuries (i.e., cerebral hemorrhage and periventricular leukomalacia). These factors are highly predictive for subsequent motor abnormalities. However, cognitive and behavioral disorders may occur in the absence of MRI abnormalities (and vice versa) thereby limiting the application of conventional MRI as a predictor of neuro-psychological outcome (Tortora et al., 2015).
Previous studies have focused on the potential role of the advanced diffusion (Arzoumanian et al., 2003) and functional (Smyser et al., 2010, Navarra et al., 2016) MRI for predicting neurodevelopmental outcome of preterm neonates. Cerebral blood flow (CBF) is tightly linked to brain metabolism, and provides another window into neonatal brain development (Greisen, 2005). Further, impaired autoregulation of the CBF is thought to contribute to the development of brain damage in preterm neonates (Greisen, 2005). Immaturity of both the vascular network and vasoactive signaling in preterm neonates may alter cerebral perfusion pressure, partial pressure of oxygen and carbon dioxide, and neuronal metabolism, thus affecting brain development (Brew et al., 2014). Accordingly, perfusion-MRI techniques could reveal subtle abnormalities in an apparently normal brain.
Arterial Spin Labeling (ASL) MR imaging non-invasively assesses brain perfusion and allows a direct quantitative measurement of CBF without administrating contrast material or exposure to ionizing radiation (Detre and Alsop, 1999). The primary aim of this study was to compare CBF as measured with ASL MRI in three different groups of neonates (preterm neonates without brain lesions at MRI (PN), preterm neonates with periventricular white matter lesions at MRI (PNp) and term neonates with normal MRI (TN). A secondary aim was to evaluate the correlation between global and regional CBF and clinical outcomes in PN.
2. Materials and methods
2.1. Patients
The institutional review board approved this prospective study. The brain MR imaging acquisitions of 49 consecutive PNs acquired from January 2011 to December 2013 within five days of term-corrected age as part of an ongoing screening of preterm neonates (study approved by the Ethics Committee of our University and Local Health Authority) were included in this study. The parents or legal guardians of the neonates provided written informed consent prior to acquisitions. A control group of 15 consecutive TN, who showed periventricular hyperechogenicity at routine early cranial ultrasound without asphyxia at birth, underwent an MRI exam within five days of birth. Eleven of the 15 TN who did not show brain lesions at MRI exam and presented a normal neurological status at the twelve-month follow-up visit were enrolled in the study.
2.2. MR imaging
MR imaging was performed with a 3 T whole-body system (Achieva 3.0 T X-Series; Philips Healthcare, Best, Netherlands) using an 8-channel head receiver array. Neonates were fed and sedated with 0.05 mg oral Midazolam per kilogram of body weight immediately prior to initiating acquisitions. During the scan, neonates were laid in a supine position and swaddled in blankets. Molded foam was placed around the body of the neonate to minimize head movement. Ear protection was always used and consisted of commercially available neonatal earmuffs (MiniMuffs; Natus Medical, San Carlos, California) and adapted ear-canal plugs. Heart rate and oxygen saturation were monitored during the MR imaging session by an intensive care neonatologist with eight years of experience (RS). All neonates underwent the same standard clinical MR imaging protocol (Table 1).
Table 1.
MRI sequences parameters.
| Sequence | Slice thickness (mm) | Matrix | FOV (mm) | Interslice (mm) | TR (ms) | TE (ms) | FA (°) | SAR (W/kg) | dB |
|---|---|---|---|---|---|---|---|---|---|
| 3D T1-FFE | 1 | 220 × 151 | 180 | 0 | 9.2 | 4.3 | 10 | 0.0 | 5.4 |
| Axial T2-TSE | 3 | 256 × 256 | 220 | 0.5 | 3000 | 80 | – | < 0.2 | 6.5 |
| Axial T2-FFE | 3 | 256 × 256 | 220 | 0.5 | 974 | 16 | 18 | < 50% | 2.9 |
| Axial DWI | 4 | 176 × 176 | 220 | 1 | 2380 | 65 | – | < 0.1 | 9.5 |
ASL was implemented using signal targeting and alternating radiofrequency (EPISTAR) with pulsed arterial spin labeling and a multi-slice single-shot echo planar imaging (EPI) readout with parallel imaging (SENSE factor = 2.3) and the following settings: TR/TE, 400/20 ms; flip angle, 40°; matrix size, 80 × 77; FOV, 240 × 240 mm; slice thickness, 6 mm; 14 axial sections with a 0 mm gap; 30 label/control pairs; total scan time, 4.08 min; 100 mm labeling slab thickness of with a gap of 20 mm; and 1250 ms label delay.
2.3. MR data analysis
2.3.1. T1-weighted images evaluation
A neuroradiologist (MC) with 10 years of experience in neonatal neuroimaging blinded to grouping reviewed the MR imaging studies of all neonates using a workstation equipped with a professional DICOM viewer (OsiriX Imaging Software; http://www. osirix-viewer.com). The T1 and T2-weighted axial images were evaluated in order to establish the presence and location of punctate or cystic white matter lesions typical of PN (Back et al., 2007).
Punctate white matter lesions were defined as T1 hyperintense regions in the periventricular white matter with a diameter of < 5 mm that did not necessarily present a corresponding decreased signal intensity on T2-weighted images. Cystic white matter lesions were described as T2 hyperintense and T1 hypointense periventricular roundish areas. Since dead cystic tissue is generally not perfused, neonates with multiple white matter lesions and > 5 mm of axial diameter were not enrolled.
2.4. ASL data analysis
A preliminary image-quality assessment of the acquired ASL sequences was performed evaluating the general image quality, noise (quantitatively), and the presence of artifacts.
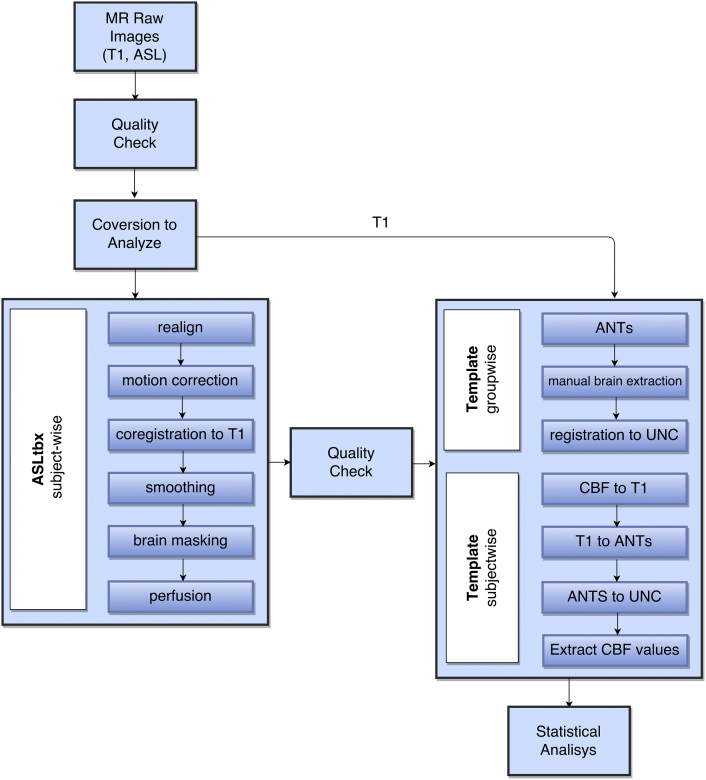
The ASL data processing toolbox ASLtbx was used (Wang, 2012). The toolbox included preprocessing steps of tag and control ASL series realignment, motion correction, coregistration with T1-anatomic sequence, smoothing and brain masking for excluding out-of-brain voxel (Wang, 2012) (Fig. 1). The standard toolbox was modified to incorporate the standardized hematocrit values for each individual subject (Jopling et al., 2009, Varela et al., 2011). Quantitative estimates of the regional CBF were performed using the cerebral blood flow maps and the formula described by Wang et al. (2008).
Fig. 1.
Flowchart of the pipeline used for ASL data analysis.
The resulting perfusion weighted images were inspected visually (blinded to group) for residual motion artifacts and subjects with severe artifacts were excluded. A manual segmentation process was used to delineate the boundaries of ventricles and main arterial vessels (anterior, middle and posterior cerebral arteries) in order to exclude these regions from subsequent analysis.
The segmentation of the brain in gray, white matter and lobes was performed in different steps: an anatomical template that includes T1-weighted sequence datasets of all neonates was built using the Anatomical Normalization Tools (ANTs) Template construction pipeline (Avants et al., 2013), then the MR-ASL sequence of each subject was recorded to the template through a nonlinear registration process using the FMRIBs Linear Image Registration Tool (FLIRT) of FSL (Jenkinson et al., 2012). Subsequently, the extracted brain with removal of the scalp was registered to the 0-atlas (neonates) of the UNC Infant 0-1-2 Atlases developed by the Biomedical Research Imaging Group of the University of North Carolina School of Medicine. The segmentations provided with this atlas were used to segment the registered perfusion maps for group analysis (Shi et al., 2001).
Probabilistic segmentations from International Consortium for Brain Mapping (known as the ICBM database) (Fonov et al., 2011) linearly recorded in UNC 0 atlas space, were used to determine CBF of the whole brain and of the gray matter of each lobe (frontal, parietal, temporal, occipital), cerebellum and basal ganglia. The highest segmentation threshold was applied (99%).
2.5. Neuromotor outcome
Neurologic examinations of the neonates were routinely performed at 12 months of age by a pediatric neurologist who was blinded to the MRI results. Neonates were classified according to the World Health Organization development scale into three groups: 1) “normal” (normal neurologic examination findings); 2) “mildly abnormal” (mild hypertonia, hypotonia, and/or asymmetry); and 3) “definitely abnormal” (severe hypertonia, cerebral palsy) (Rosenbaum and Stewart, 2004).
2.6. Statistical analysis
Statistical analysis was performed with SPSS Statistics for Mac, Version 21.0 (IBM, Armonk, New York). The level of significance was set at P < 0.05. A false-discovery-rate (FDR) correction for multiple comparisons was applied.
Significant group differences in gray and white matter CBF in each brain lobe were evaluated with a linear mixed-model analysis. In order to evaluate the influence of gestational age, a non-parametric correlation test between gestational age and perfusion data was performed. The mean CBF values in each brain region of neonates of different groups were compared with the Mann-Whitney U test.
The presence of a relationship between the neuromotor outcome at 12 months and the CBF values was tested in the preterm group using logistic regression with a backward stepwise conditional method.
3. Results
The ASL acquisitions of 37/49 (76%) PNs did not show residual motion artifacts at the final image-quality analysis and were included in the study. 8/37 (22%) PNs (2 females; 7 “early” preterms (gestational age < 32 weeks); average postmenstrual age at MRI, 40.1 ± 0.9 weeks; range 39–41 weeks) presented periventricular white matter lesions at MRI (PNp group). 6/8 PNp presented T1-hyperintense punctate white matter lesions (four in both frontal and parietal lobes and two in parietal lobe) and 2/8 PNp showed cystic white matter lesions (major axial diameter < 2 mm) in the parietal lobes.
The remaining 29 PNs (16 females; 16 “early” preterms; average postmenstrual age at MRI, 39.8 ± 1.2 weeks; range 38–41 weeks) showed no alterations at MRI (PN group). 11 TN without brain lesions at MRI (six females; average postmenstrual age at MRI, 40.8 ± 0.5 weeks; range 40–42 weeks) were included in the analysis as a control group (TN group).
3.1. Brain perfusion
The linear mixed-model analysis showed a statistically significant difference in mean CBF measured in the gray matter of each group of neonates (P = 0.004). No statistically significant hemispheric differences in CBF were observed in any group (P = 0.855) or between the “early” and “late” PN in the PNp and PN groups (P = 0.090).
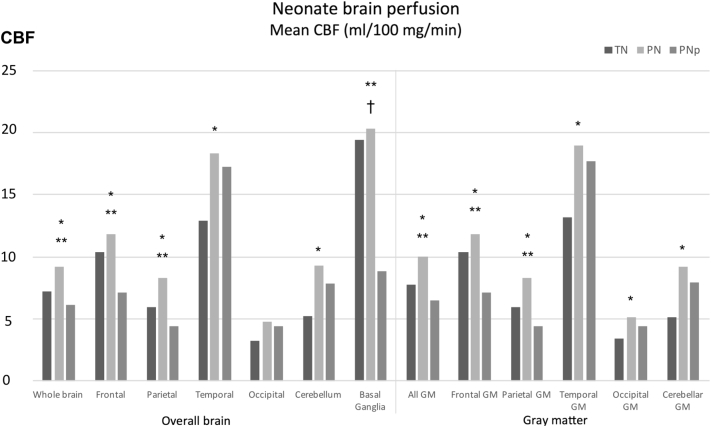
Comparison of means indicated that the CBF of the whole brain of PN was significantly higher than TN (P = 0.011). This difference remained significant when considering the frontal (P = 0.038), parietal (P = 0.002), temporal (P = 0.030), occipital (P = 0.041) and cerebellar gray matter (P = 0.010), separately (Fig. 2).
Fig. 2.
Regional mean CBF (ml of blood/100 mg/min) in the three groups of neonates (dark gray: TN, lite gray: PN, gray: PNp). *PN vs TN; †PNp vs TN; **PN vs PNp P < 0.05.
Comparison of means indicated that the CBF of the whole brain of PNp was significantly lower than PN (P = 0.013). The difference also remained significant when considering the frontal (P = 0.006), and parietal (P < 0.001) gray matter and the basal ganglia (P < 0.001) separately (Fig. 1). Finally, PNp showed significantly lower CBF in the basal ganglia when compared with TN (P = 0.004) (Table 2) (Fig. 3).
Table 2.
Regional median and range of CBF (ml/100 g/min) in the three group of neonates. (MLM: linear mixed model analysis; M-W: Mann-Whitney U test; FDR-corrected).
| Brain regions | Neonate group |
p |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TN |
PN |
PNp |
MLM | M-W | M-W | M-W | |||||||
| Median | Min | Max | Median | Min | Max | Median | Min | Max | PN vs TN | PNp vs TN | PN vs PNp | ||
| Whole brain | 7.18 | 4.04 | 11.55 | 9.20 | 5.72 | 12.61 | 6.13 | 2.13 | 10.32 | 0.004 | 0.011 | 0.492 | 0.013 |
| All GM | 7.72 | 4.41 | 12.83 | 9.98 | 6.36 | 13.86 | 6.53 | 2.30 | 11.10 | 0.002 | 0.011 | 0.492 | 0.008 |
| Frontal | 10.36 | 5.88 | 17.18 | 11.81 | 7.38 | 18.27 | 7.15 | 2.72 | 15.43 | 0.003 | 0.041 | 0.310 | 0.006 |
| Frontal GM | 10.38 | 5.88 | 17.20 | 11.82 | 7.38 | 18.28 | 7.16 | 2.72 | 15.40 | 0.003 | 0.038 | 0.310 | 0.006 |
| Parietal | 5.96 | 2.04 | 9.30 | 8.33 | 3.89 | 13.27 | 4.41 | 2.45 | 8.19 | < 0.001 | 0.002 | 0.351 | < 0.001 |
| Parietal GM | 5.96 | 2.03 | 9.31 | 8.33 | 3.89 | 13.28 | 4.41 | 2.45 | 8.19 | < 0.001 | 0.002 | 0.351 | < 0.001 |
| Temporal | 12.88 | 7.37 | 27.03 | 18.37 | 5.49 | 30.43 | 17.24 | 2.15 | 26.14 | 0.266 | 0.033 | 0.492 | 0.414 |
| Temporal GM | 13.21 | 7.65 | 27.80 | 19.01 | 5.57 | 31.82 | 17.68 | 2.19 | 26.75 | 0.243 | 0.030 | 0.492 | 0.373 |
| Occipital | 3.27 | 1.32 | 8.31 | 4.73 | 2.21 | 12.70 | 4.43 | 0.90 | 6.61 | 0.107 | 0.079 | 0.952 | 0.221 |
| Occipital GM | 3.41 | 1.31 | 8.42 | 5.10 | 2.39 | 11.68 | 4.41 | 0.97 | 6.42 | 0.058 | 0.041 | 0.904 | 0.158 |
| Cerebellum | 5.18 | 2.85 | 15.12 | 9.30 | 2.65 | 14.33 | 7.85 | 2.21 | 12.27 | 0.168 | 0.010 | 0.442 | 0.148 |
| Cerebellar GM | 5.15 | 2.75 | 14.81 | 9.18 | 2.78 | 14.27 | 7.95 | 2.22 | 12.02 | 0.161 | 0.010 | 0.441 | 0.148 |
| Basal ganglia | 19.40 | 13.42 | 35.88 | 20.33 | 11.85 | 29.10 | 8.83 | 1.39 | 20.25 | 0.001 | 0.952 | 0.004 | < 0.001 |
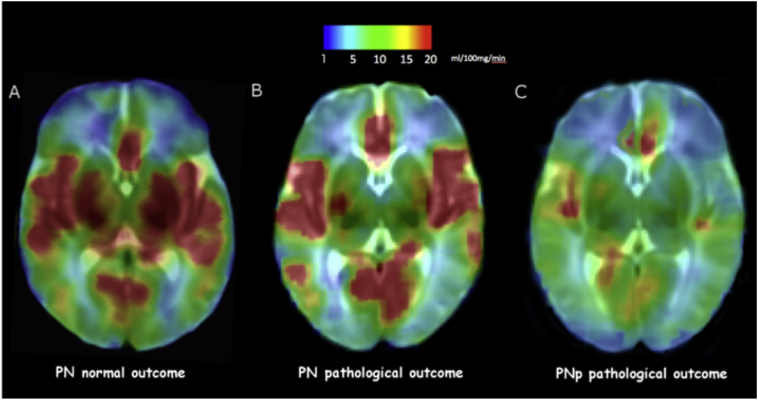
Fig. 3.
ASL maps of three preterm neonates. A) and B) CBF maps of two PN who presented normal and abnormal outcome respectively, showing reduced perfusion of basal ganglia in B. C) CBF map of a PNp neonate showing a global reduction of brain perfusion. Color scale indicates CBF (ml/100 mg/min).
3.2. Relationship between CBF and neuromotor outcome
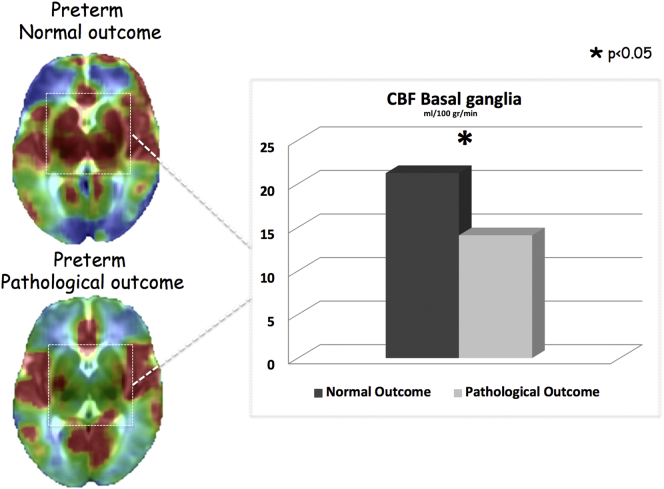
Neuromotor outcomes at 12 months are reported in Table 3. In PNp the presence of white matter lesions was highly predictive of an abnormal neuromotor outcome at 12 months (100% of neonates). Therefore, we focused our attention on the relationship between CBF and neuromotor outcome of PN, which was the only group that presented a highly heterogeneous neuromotor outcome. PN with an adverse outcome (“definitively abnormal” and “mildly abnormal” outcome) showed significantly lower CBF in the basal ganglia compared to PN with “normal” outcome (17.5 ± 4.3 vs 22.2 ± 4.8 ml/100 mg/min) (P = 0.012) (Fig. 4).
Table 3.
Clinical outcome results.
| Definitively abnormal | Mildly abnormal | Normal | Lost to follow-up | |
|---|---|---|---|---|
| Severe hypertonia, cerebral palsy | Mild hypertonia | Healthy | ||
| TN | 0/11 (0%) | 0/11 (0%) | 11/11 (100%) | 0/11 (0%) |
| PN | 0/29 (0%) | 10/29 (34.5%) | 15/29 (51.7%) | 4/29 (13.8%) |
| PNp | 7/8 (87.5%) | 1/8 (12.5%) | 0/8 (0%) | 0/8 (0%) |
Fig. 4.
PN presenting pathological outcome at 12 months showed lower CBF values in the basal ganglia region. The CBF of the basal ganglia resulted to be an independent predictive factor for neuromotor outcome at 12 months in the PN group.
To evaluate whether CBF of PN was predictive of the neuromotor outcome, a logistic regression analysis was performed and the CBF of all lobes except basal ganglia were removed in a backward stepwise analysis. Mean basal ganglia CBF was an independent predictive factor for neuromotor outcome in PN at 12 months (odds ratio = 0.494 95% CI, 0.315–0.785; P = 0.002). The correlation analysis excluded a confounding effect of the gestational age of PN on the regression analysis results (P > 0.05).
In order to translate these results to a clinical setting, two neuro-radiologists jointly positioned a circular VOI with a 3 mm radius for each lenticular nucleus (right and left) using axial-reformatted 3D T1-wieghted FFE weighted passing through the genu of the internal capsule. Perfusion was then measured on co-registered ASL sequences and the mean of the two VOIs for each patient was used in a ROC analysis. The results were statistically significant (P = 0.037) with a lower threshold (12.2 ml of blood/100 ml of tissue per minute) but with similar sensitivity (88.9%) and specificity (50.0%) as those obtained for the more time consuming group analysis based on automated segmentation.
4. Discussion
In this study, brain perfusion measured non-invasively using ASL MRI was assessed in three groups of neonates: preterms without brain lesions, preterms with periventricular white matter injury and term neonates with normal conventional MRI. The results showed that PN with a lower ASL-measured CBF in basal ganglia had a worse neuromotor outcome at one year of age.
Our results indicated that the CBF of the whole brain of PN was significantly higher than in TN. The higher CBF of PN is likely related to their greater postnatal age compared to TN (42 ± 22 and 5 ± 2 days, respectively). De Vis et al. demonstrated that CBF progressively increases during the first period of life as synaptogenesis, myelination, and brain functional activity progress (De Vis et al., 2013). Miranda et al. observed that MR-ASL cerebral perfusion was significantly higher in preterm neonates studied at term corrected age than in the term neonates, indicating that brain perfusion may be influenced by developmental and postnatal age (Miranda et al., 2006). In addition, our results were concordant with the findings of previous PET and Xe-CT studies that reported an association between higher brain perfusion and metabolism with increasing postnatal age (Chiron et al., 1992).
Higher CBF was observed in the parietal, temporal and occipital lobes and in the cerebellum of PN as compared to TN. This likely reflects the intense cellular maturation and synaptogenesis associated with the development of sensory (for example, auditory) systems and motor function. This concept is reinforced by other studies demonstrating that the volume of these lobes increase exponentially during the first months of life (Tzarouchi et al., 2009). On the other hand, CBF in the basal ganglia did not differ between PN and TN. This region also had the highest CBF, suggesting earlier prenatal maturation compared to the cortex (Chiron et al., 1992, Chugani et al., 1987). The lack of a significantly different CBF of the basal ganglia between PN and TN supports the hypothesis that at term-equivalent age the perfusion of these regions has already reached a more advanced stage of maturation, regardless of the gestational age at birth.
PVL are an MRI-detectable white matter lesions typical of PN and the principal pathogenic factors that induce PVL development include hypoperfusion of deep white matter end zones and maternal infection/inflammation (Volpe, 2008). In this study PNp showed significantly lower values of CBF compared to PN, especially in the frontal and parietal lobes and in basal ganglia. This finding could be the consequence of the previously demonstrated neuronal/axonal loss in preterm neonates (Volpe, 2009, Massaro et al., 2013). A neuropathological study performed on 41 preterm infants indicated that neuronal loss and gliosis were most common in the thalamus, caudate and putamen of infants with white matter lesions (Pierson et al., 2007). In this study white matter perfusion was not evaluated due to the limits of using ASL to accurately measure this compartment which have been amply reported in literature (van Gelderen et al., 2008).
The presence of MRI lesions indicative of PVL in PN studied at term represents an important predictor of both poor neuromotor outcome and cognitive delay during the preschool and early school years (Tortora et al., 2015, Skiöld et al., 2012). However, even PN without PVL may develop neuropsychological deficits (Skiöld et al., 2012). In the present study, all PNp presented an adverse neuromotor outcome at 12 months but more than a third of the PN also developed neuromotor deficits. These findings demonstrate that in PN the absence of white matter abnormality on MRI is not fully predictive of a normal outcome (low specificity). However, a positive correlation between CBF in the basal ganglia and neuromotor outcome was found in PN.
Our findings differ from the results of recent studies (De Vis et al., 2015, Wintermark et al., 2011) that demonstrated hyperperfusion in the brain of term neonates with hypoxic-ischemic encephalopathy (HIE). The differences were probably the consequence of the different time that elapsed following insult. That is, hyperperfusion reported after HIE is considered an acute hemodynamic compensation attempt induced by perinatal hypoxia-ischemia, whereas the hypoperfusion in PNp indicates a chronic phase of brain injury.
The evolution of regional brain perfusion and metabolism during the period of postnatal growth may correlate with the development of neuroanatomical structures and functional maturation (Chugani and Phelps, 1986). Therefore, the higher CBF in the basal ganglia of PN with normal outcome could reflect a more advanced stage of brain development. But, the lower CBF values of basal ganglia of PN with abnormal outcome support the hypotheses that prematurity influences the autoregulation of brain perfusion, contributing to the delayed maturation of brain (du Plessis, 2009). This observed selective effect on the basal ganglia is in accordance with their advanced development around birth and the greater involvement following perinatal asphyxia (Barkovich et al., 1995).
This finding needs to be replicated in a larger series but, if verified, it has the possibility of identifying PN at high risk for sub-optimal neuropsychological development using ASL perfusion at term-corrected age could significantly impact their management by identifying the infants who require medical/rehabilitation therapy. Post-hoc evaluation of results obtainable by manually positioning VOIs indicated that basing a prognosis on the perfusion of the basal ganglia in a clinical setting is valid. Therefore, the results of this study can be easily translated into a clinical setting allowing patient evaluation in a sufficiently short time period; i.e., before the patient is removed from the scanner.
The main limitation of this study was that the only clinical finding that was consistently available from patient records was the neuromotor outcome at 12 months. In addition, the number of PN enrolled precluded a more detailed statistical analysis; specifically, the definition of a CBF threshold able to distinguish PN with normal and adverse neuromotor outcome with high diagnostic accuracy.
Another limitation of this study regards the use of a PASL sequence has been reported to have a lower image quality when compared with other ASL perfusion sequences (i.e., pCASL) for the study of neonates. However, the recommended quality assurance steps (Alsop et al., 2015) were applied while determining the best delay time for our scanner/study population/sequence characteristics using a multiphase sequence. The validated PASL settings for quantitatively evaluating neonatal brain perfusion provided adequate gray matter SNR. Furthermore, a significant percentage of ASL acquisitions of our population was excluded from the analysis due to the motion artifacts. In this study, tag-acquisition delay time was also not corrected for individual hematocrit but a standardized hematocrit correction was applied.
Since ASL perfusion is highly susceptible to head movements, a preliminary quality check of the images should be performed immediately after the acquisition of the sequence and studies affected by motion artifacts could be repeated during the same examination.
Multicenter studies with larger sample sizes will be required to further refine the cut-off but the identification of the area of interest for modifications of perfusion is an important first-step.
5. Conclusion
This study demonstrated differences in cerebral perfusion between PN and TN. PN showed higher CBF compared to TN and PNp. PNp showed a global reduction of brain perfusion. Also, CBF in the basal ganglia of PN was positively associated with neuromotor outcome at 12 months and could represent a non-invasive method for identifying PN with a high risk for sub-optimal development. The ability to identify PN at risk of sub-optimal neuromotor development could greatly impact the management of rehabilitative procedures reducing sequela.
Institution from which the work originated
ITAB — Institute of Advanced Biomedical Technologies, University “G. d'Annunzio” Via Luigi Polacchi 11, 66100 Chieti, Italy.
Funding sources
The contribution of John A. Detre was supported by the National Institutes of Health (grant numbers MH080729 and EB015893).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.05.023.
Contributor Information
Domenico Tortora, Email: domenicotortora@gaslini.org.
Peter Angelo Mattei, Email: pamattei@unich.it.
Riccardo Navarra, Email: riccardo.navarra@unich.it.
Valentina Panara, Email: v.panara@rad.unich.it.
Rita Salomone, Email: ritasalomone@tiscali.it.
Andrea Rossi, Email: andrearossi@gaslini.org.
John A. Detre, Email: detre@mail.med.upenn.edu.
Massimo Caulo, Email: caulo@unich.it.
Appendix A. Supplementary data
The following is the supplementary data to this article.
A. Unregistered masked tagged-untagged subtraction of a single representative subject. B. Relative perfusion map.
References
- Alsop D.C., Detre J.A., Golay X. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: aconsensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia. Magn. Reson. Med. 2015;73:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzoumanian Y., Mirmiran M., Barnes P.D. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am. J. Neuroradiol. 2003;24:1646–1653. [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Stauffer M., Song G., Wu B., Gee J.C. The Insight ToolKit image registration framework. Front. Neuroinform. 2013;8(44) doi: 10.3389/fninf.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.A., Riddle A., McClure M.M. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38:724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- Barkovich A.J., Westmark K., Partridge C. Perinatal asphyxia: MR findings in the first 10 days. AJNR Am. J. Neuroradiol. 1995;16:427–438. [PMC free article] [PubMed] [Google Scholar]
- Blencowe H., Cousens S., Oestergaard M.Z. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- Brew N., Walker D., Wong F.Y. Cerebral vascular regulation and brain injury in preterm infants. Am. J. Phys. Regul. Integr. Comp. Phys. 2014;306:773–786. doi: 10.1152/ajpregu.00487.2013. [DOI] [PubMed] [Google Scholar]
- Chiron C., Raynaud C., Mazière B. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J. Nucl. Med. 1992;33:696–703. [PubMed] [Google Scholar]
- Chugani H.T., Phelps M.E. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science. 1986;231:840–843. doi: 10.1126/science.3945811. [DOI] [PubMed] [Google Scholar]
- Chugani H.T., Phelps M.E., Mazziotta J.C. Positron emission tomography study of human brain functional development. Ann. Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- De Vis J.B., Petersen E.T., de Vries L.S. Regional changes in brain perfusion during brain maturation measured non-invasively with arterial spin labeling MRI in neonates. Eur. J. Radiol. 2013;82(3):538–543. doi: 10.1016/j.ejrad.2012.10.013. [DOI] [PubMed] [Google Scholar]
- De Vis J.B., Hendrikse J., Petersen E.T. Arterial spin-labelling perfusion MRI and outcome in neonates with hypoxic-ischemic encephalopathy. Eur. Radiol. 2015;25:113–121. doi: 10.1007/s00330-014-3352-1. [DOI] [PubMed] [Google Scholar]
- Detre J.A., Alsop D.C. Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. Eur. J. Radiol. 1999;30:115–124. doi: 10.1016/s0720-048x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:1053–8119. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelderen P., de Zwart J.A., Duyn J.H. Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magn. Reson. Med. 2008;59:788–795. doi: 10.1002/mrm.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greisen G. Autoregulation of cerebral blood flow in newborn babies. Early Hum. Dev. 2005;81:423–428. doi: 10.1016/j.earlhumdev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jopling J., Henry E., Wiedmeier S.E., Christensen R.D. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009;123 doi: 10.1542/peds.2008-2654. [DOI] [PubMed] [Google Scholar]
- Massaro A.N., Bouyssi-Kobar M., Chang T., Vezina L.G., du Plessis A.J., Limperopoulos C. Brain perfusion in encephalopathic newborns after therapeutic hypothermia. AJNR Am. J. Neuroradiol. 2013;34(8):1649–1655. doi: 10.3174/ajnr.A3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment L.R., Hirtz D., Hüppi P.S. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8(11):1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- Miranda M.J., Olofsson K., Sidaros K. Noninvasive measurements of regional cerebral perfusion in preterm and term neonates by magnetic resonance arterial spin labeling. Pediatr. Res. 2006;60(3):359–363. doi: 10.1203/01.pdr.0000232785.00965.b3. [DOI] [PubMed] [Google Scholar]
- Navarra R., Sestieri C., Conte E. Perinatal MRI diffusivity is related to early assessment of motor performance in preterm neonates. Neuroradiol. J. 2016 Apr;29(2):137–145. doi: 10.1177/1971400915628019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson C.R., Folkerth R.D., Billiards S.S. Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol. 2007;114:619–631. doi: 10.1007/s00401-007-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis A.J. The role of systemic hemodynamic disturbances in prematurity-related brain injury. J. Child Neurol. 2009;24:1127–1140. doi: 10.1177/0883073809339361. September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum P., Stewart D. The World Health Organization international classification of functioning, disability and health: a model to guide clinical thinking, practice and research in the field of cerebral palsy. Semin. Pediatr. Neurol. 2004;11:5–10. doi: 10.1016/j.spen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Saigal S., Doyle L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Shi F., Yap P.T., Wu G. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One. 2001;6(4) doi: 10.1371/journal.pone.0018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiöld B., Vollmer B., Böhm B. Neonatal magnetic resonance imaging and outcome at age 30 months in extremely preterm infants. J. Pediatr. 2012;160:559–566. doi: 10.1016/j.jpeds.2011.09.053. (e551) [DOI] [PubMed] [Google Scholar]
- Smyser C.D., Inder T.E., Shimony J.S. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora D., Panara V., Mattei P.A. Comparing 3 T T1-weighted sequences in identifying hyperintense punctate lesions in preterm neonates. Am. J. Neuroradiol. 2015;36:581–586. doi: 10.3174/ajnr.A4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzarouchi L.C., Astrakas L.G., Xydis V. Age-related grey matter changes in preterm infants: an MRI study. NeuroImage. 2009;47:1148–1153. doi: 10.1016/j.neuroimage.2009.03.072. [DOI] [PubMed] [Google Scholar]
- Varela M., Hajnal J.V., Petersen E.T., Golay X., Merchant N., Larkman D.J. A method for rapid in vivo measurement of blood T1. NMR Biomed. 2011;24:80–88. doi: 10.1002/nbm.1559. [DOI] [PubMed] [Google Scholar]
- Volpe J.J. fifth ed. Elsevier; Philadelphia: 2008. Neurology of the Newborn. [Google Scholar]
- Volpe J.J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Ze. Improving cerebral blood flow quantification for arterial spin labeled perfusion MRI by removing residual motion artifacts and global signal fluctuations. Magn. Reson. Imaging. 2012;30(10):1409–1415. doi: 10.1016/j.mri.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Aguirre G.K., Rao H. Empirical ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn. Reson. Imaging. 2008;26(2):261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermark P., Hansen A., Gregas M.C. Brain perfusion in asphyxiated newborns treated with therapeutic hypothermia. AJNR Am. J. Neuroradiol. 2011;32:2023–2029. doi: 10.3174/ajnr.A2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Unregistered masked tagged-untagged subtraction of a single representative subject. B. Relative perfusion map.