Abstract
Emerging and re-emerging pathogens represent a substantial threat to public health, as demonstrated with numerous outbreaks over the past years, including the 2013–2016 outbreak of Ebola virus in western Africa. Coronaviruses are also a threat for humans, as evidenced in 2002/2003 with infection by the severe acute respiratory syndrome coronavirus (SARS-CoV), which caused more than 8000 human infections with 10% fatality rate in 37 countries. Ten years later, a novel human coronavirus (Middle East respiratory syndrome coronavirus, MERS-CoV), associated with severe pneumonia, arose in the Kingdom of Saudi Arabia. Until December 2016, MERS has accounted for more than 1800 cases and 35% fatality rate. Finding an animal model of disease is key to develop vaccines or antivirals against such emerging pathogens and to understand its pathogenesis. Knowledge of the potential role of domestic livestock and other animal species in the transmission of pathogens is of importance to understand the epidemiology of the disease. Little is known about MERS-CoV animal host range. In this paper, experimental data on potential hosts for MERS-CoV is reviewed. Advantages and limitations of different animal models are evaluated in relation to viral pathogenesis and transmission studies. Finally, the relevance of potential new target species is discussed.
Keywords: Animal model, Coronavirus (CoV), Emerging pathogen, Middle East respiratory syndrome (MERS), Reservoir
Abbreviations: BSL, biosafety level; DPP4, dipeptidyl peptidase-4; FDA, Food and Drug Administration; HCoV, human coronaviruses; hDPP4, human dipeptidyl peptidase-4; MERS-CoV, Middle East respiratory syndrome coronavirus; NHP, Nonhuman primates; PI, post-inoculation; RDB, receptor binding domain; SARS-CoV, severe acute respiratory syndrome coronavirus; URT, upper respiratory tract; WHO, World Health Organization
1. Introduction
Over the past years, outbreaks of zoonotic diseases and growing resistance against antibiotics have emphasized the need for interdisciplinary collaboration between human health, veterinary medicine and environmental sciences, a concept commonly known as “One health” [1]. Most of emerging diseases are zoonotic [2]. For instance, the human flu pandemics have originated in domestic animals and wildlife, and have been driven by ecological, behavioral, or socioeconomic changes [3]. In these cases, the reaction time between detection of a new outbreak and application of medical countermeasures are critical in terms of epidemic control. To understand the potential role that animal sources could play in virus dissemination and the epidemiology of the disease, surveillance studies, as well as experimental infections in potential target species, are required. Furthermore, after having identified the novel or re-emerged virus responsible of the outbreak, it is important to rapidly provide an accurate diagnosis as a basis for quarantine measures. It is also imperative to focus on the search for new vaccines and treatments for highly pathogenic viruses, especially for those that represent a threat to human and animal health, particularly livestock.
Until the beginning of the last decade, human coronaviruses (HCoV) infections were considered to be restricted to the upper respiratory tract (URT), with low mortality rate, and recognized as the second ranked cause of the common cold after rhinoviruses [4]. However, in the late-2002, the severe acute respiratory syndrome coronavirus (SARS-CoV) emerged in China. It rapidly spread worldwide with more than 8000 causalities and a lethality rate of 10% [5]. Ten years later, a novel HCoV associated with severe pneumonia emerged in the Kingdom of Saudi Arabia [6]. The new CoV was named Middle East respiratory syndrome coronavirus (MERS-CoV), and by March 2014, a total of 207 cases and 45% fatalities were recorded. One month later, only in April 2014, an increase in human cases was registered with at least 217 more infected people and 38 fatalities. More recently, as of December 2016, 1842 cases of MERS-CoV have been reported to the World Health Organization (WHO), including at least 652 deaths [7].
Besides coronaviruses, highly pathogenic viruses belonging to other families represent a threat to either human or animal health, or both. One of the most recent examples is the outbreak of Ebola virus (Filoviridae) in West Africa, which started in December of 2013 in Guinea and evolved as the largest Ebola outbreak recorded with more than 28,600 cases [8]. Furthermore, during recent years, outbreaks caused by other emerging viral pathogens from Arenaviridae, Bunyaviridae, and Flaviviridae families among others, disturbed public and private health, social networks and the economies of the affected countries [9], [10]. Prevention and control of emerging and reemerging viral diseases is efficient when several actions are combined: i.e. creating diagnostic networks and surveillance programs, training medical and veterinary staff, informing the population about sanitary measures, and also promoting research on prophylaxis, treatments and on the causative agent pathogenesis. Regarding the last point, animal models are crucial to study the viral and host factors contributing to the disease as well as transmission outcomes of virus infection and to allow pre-clinical testing of antiviral drugs and vaccines. Non-human primates (NHP) are the preferred models for pathogenesis studies, and potential vaccine and treatment testing, as they better translate to humans [11]. However, working with NHP is costly, with limited availability, and raises ethical problems. Therefore small-animal models are usually the first choice for drug screening. The United States Food and Drug Administration's (FDA) Animal Rule provides guidelines concerning the appropriateness of animal models for licensing purposes [12]. Additionally, by controlling the disease in animal reservoirs and/or in intermediate hosts, virus transmission to humans can be significantly reduced [13], [14]. This is particularly true for domestic or feral animals for which efficient vaccines and vaccination strategies can be implemented [15]. Therefore, in cases of new pathogenic virus outbreaks, the search for natural hosts or potential target animals (as opposed to laboratory animals) seems to be relevant not only to implement prophylactic solutions but also to improve the preparedness for an eventual global extension of diseases. Nowadays, this task is rendered possible by the availability of improved biosafety levels 3 and 4 (BSL3 and 4) animal facilities, which can accommodate large animal experimentation with such highly virulent pathogens [16].
In this article, the current situation of comprehension on potential hosts for MERS-CoV is reviewed. Based on the coronaviruses experience, benefits and limitations of these species as animal models and transmission studies are discussed.
2. Animal models for MERS research
Several review articles have described and discussed animal models for MERS-CoV infection [17], [18], [19], [20]. In this section, the current status of animal models for MERS disease reproduction is briefly summarized.
After the identification of MERS-CoV in 2012 [6], the efforts were directed to develop an animal model to study pathogenesis and to test the efficacy of vaccines and/or treatments in vivo. Similar to SARS-CoV, rhesus macaques have demonstrated susceptibility to MERS-CoV [21], [22], [23]. A work led by Munster demonstrated that the common marmoset is also suitable as a MERS-CoV model [24]. They showed that this model recapitulates the disease observed in humans; therefore, findings in the evaluation of potential therapeutic strategies might be implemented in humans. However, small animals are required for controlled, large and comprehensive studies. While, at first, experiences with SARS-CoV turned out to be very helpful for the research on MERS-CoV, the development of a small animal model for MERS was a more difficult task [18], [19]. Raj and collaborators rapidly identified dipeptidyl peptidase-4 (DPP4) as the functional receptor for MERS-CoV [25], and DPP4 is present in lung cells of many rodents. Thus, rodents were expected to be susceptible for MERS-CoV. However, and as predicted by the crystal structure analysis of the MERS-CoV receptor binding domain (RBD) with the human DPP4 (hDDP4) extracellular domain [26], so far, no rodent model is naturally permissive for MERS-CoV infection. In Syrian hamster, the DPP4 receptor was shown to be expressed on bronchiolar epithelium, but inoculation of MERS-CoV via aerosols or intratracheal routes with different doses did not lead to productive infection [27]. Wild type and immune-deficient mice were also tested for MERS-CoV infection without success [28]. Since then, several groups have been focused on new strategies to develop a small animal model susceptible to MERS-CoV infection. It was found that mouse cells could be made permissive for MERS-CoV when expressing hDPP4. Consequently, the hDPP4 was transduced into mouse lungs using an adenovirus vector, which resulted in animals susceptible to MERS-CoV infection. These mice exhibited pneumonia and extensive inflammatory-cell infiltration with the presence of virus in the lungs [29]. Recently, a transgenic mice model expressing hDPP4, highly susceptible to MERS-CoV infection and able to display systemic lesions, has been developed [30]. As demonstrated for several diseases, transgenic animal models have become an important tool to improve medical research [31]. On the other hand, glycosylation of the murine DPP4 is a major factor impacting the receptor function by blocking the binding to MERS-CoV [32]. Therefore, the modification of the mouse genome to match the sequence in the hDPP4 made this species susceptible to MERS-CoV infection [33]. Accordingly, these newly established mice models are useful to evaluate the efficacy of vaccines and therapeutic agents against MERS-CoV infection [30], [34], [35], [36]. VelocImmune and VelociGene technologies have been used to develop a humanized mouse model for MERS-CoV infection [36]; these methodologies can be also applied for other pathogens in future emerging epidemics.
3. MERS-CoV animal reservoir and the role of domestic animals
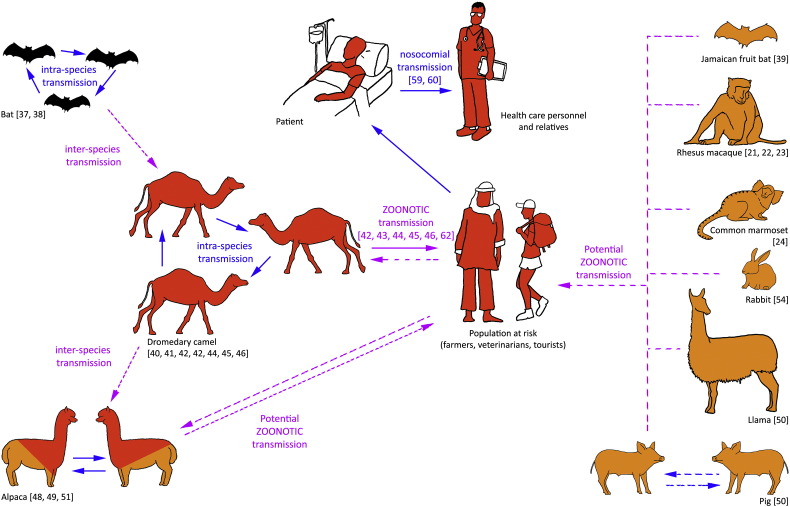
Researchers worldwide have identified several animal species which could have a role in the transmission of MERS-CoV to humans (summarized in Fig. 1). Bats have been suggested to be the reservoir for MERS-CoV, but RNA of MERS-like CoVs (and no MERS-CoV) has been found in several bat families (Vespertillionidae, Molosidae, Nycteridae and Emballonuridae) in Africa, America, Asia and Europe [37], [38]. Recently, an experimental infection with Jamaican fruit bats (Artibeus jamaicensis) confirmed that MERS-CoV can replicate in bats [39]. However, MERS-CoV strains causing disease in humans have not been so far identified in bats. The presence of MERS-CoV neutralizing antibodies has been reported in dromedary camels (Camelus dromedaries) [40], [41] and, more recently, a link between MERS-CoV infection in camels and a human infection in Qatar has been suggested [42], [43], [44], [45]. Most importantly, the MERS-CoV strain that caused the 2015 human outbreak was found in dromedary camels; in fact, phylogenetic analyses indicate that MERS-CoV was generated in this species by recombination [46]. In addition, Adney and collaborators have provided experimental evidence to support the role of dromedary camels as a MERS-CoV reservoir [47]. Recently, evidence was provided that other members of the Camelidae family such as alpaca (Vicugna pacos) and llama (Lama glama), are also susceptible to MERS-CoV infection [48], [49], [50]. Field studies with alpacas performed in Qatar confirmed this finding [51]. A recently published experimental study has demonstrated that domestic pigs are also susceptible to MERS-CoV infection, suggesting the possibility of MERS-CoV circulation in other unsuspected animal species such suidae [50]. While DPP4 in silico predictions and in vitro studies suggested that other livestock species such as goats, sheep, or horses could be susceptible to MERS-CoV infection, experimental data suggested the opposite [50]. Each natural and potential host for MERS-CoV infection is discussed below in detail and summarized in Table 1, Table 2.
Fig. 1.
Illustration of the known and potential host range of Middle East respiratory syndrome coronavirus (MERS-CoV). Dromedary camel is a demonstrated reservoir of MERS-CoV, but other species might act as hosts. In red: reported MERS-CoV-seropositive species and/or species in which virus has been naturally detected. In orange: other animal species that might be considered potential hosts since they are experimentally susceptible to MERS-CoV infection. Alpacas fit into both scenarios (red and orange). In black: animals (bats, to date) in which RNA of different sequences of MERS-CoV-like viruses have been found. Continuous arrows represent already described intra- and inter-species transmission events. Discontinuous arrows represent potential inter-species transmission.
Table 1.
Summary of MERS-CoV shedding and presence of virus in different tissues in the potential animal reservoirs for MERS-CoV after experimental inoculation.
| Species | Route and dose of inoculation | MERS-CoV shedding | MERS-CoV RNA in tissues MERS-CoV antigen in tissues |
Infectious MERS-CoV in tissues | References | |
|---|---|---|---|---|---|---|
| Camelids | Dromedary camels (Camelus dromedarius) | IT, IN, conjunctival or IN only; 107 TCID50 | Viral RNA in NS (1 to 13 dpi) Infectious virus in NS (1 to 6 dpi) |
URT, LRT, tracheal LN, pulmonary LN, cervical LN, tonsil, PSG, intestine, liver, spleen, kidney, heart, adrenal Nasal respiratory epithelial cells |
URT, trachea, large bronchus, tracheal LN |
[47], [53] |
| Alpacas (Vicugna pacos) | IN; 107 PFU in 3 mL saline solution | Infectious virus in NS (1 to 5 dpi) | URT, trachea Nasal respiratory epithelial cells |
ND | [48], [49] | |
| Llamas (Lama glama) | IN; 107 TCID50 in 3 mL saline solution | Viral RNA in NS (1 to 15 dpi) Infectious virus in NS (1 to 7 dpi) |
URT, trachea and bronchus Nasal respiratory epithelial cells |
ND | [50] | |
| Non-camelid domestic species | Domestic pig (Sus scrofa domesticus) | IN; 107 TCID50 in 3 mL saline solution | Viral RNA in NS (1 to 10 dpi) Infectious virus in NS (1 to 4 dpi) |
URT, trachea and bronchus Nasal respiratory epithelial cells |
ND | [50] |
| Rabbit (Oryctolagus cuniculus) | IN (1 × 106 TCID50), IT (4 × 106 TCID50) | Viral RNA in NS (1 to 10 dpi) Infectious virus in NS (1 to 7 dpi) |
Mainly in URT, LRT Nasal respiratory and bronchiolar epithelial cells |
ND | [54] | |
| NHP | Rhesus macaques (Macaca mulatta) | IT, OC, oral, IN; 7 × 106 TCID50/IT, 6,5 × 107 TCID50 | Viral RNA in NS, BAL samples, and few OS | URT, lung, mediastinal LN Type I and II pneumocytes, alveolar MΦ |
Lung | [21], [22], [23] |
| Common marmoset (Callithrix jacchus) | OC, oral, IT, IN; 5 × 106/IT, 5 × 107 TCID50 | Viral RNA in NS and OS | URT, lung, mediastinal LN, blood Type I pneumocytes, alveolar MΦ |
Nasal mucosa, trachea, lung | [24], [55] |
Abbreviations: BAL, bronchoalveolar lavages; dpi, days post inoculation; IN, intranasal; IT, intratracheal; LN, lymph node; LRT, lower respiratory tract; MΦ, macrophages; ND, non-determined; NHP, non-human primates; NS, nasal swabs; OC, ocular; OS, oropharyngeal swabs; PFU, plaque-forming units; PSG, parotid salivary gland; RNA, ribonucleic acid; TCID50, 50% tissue culture infectious dose; URT, upper respiratory tract.
Table 2.
Summary of clinical signs, pathological findings and target cells in tissues of natural and potential reservoir hosts for MERS-CoV infection as experimental animal models.
| Species | Clinical signs | Gross findings | Histopathological lesions | Target cells in tissues | References | |
|---|---|---|---|---|---|---|
| Camelids | Dromedary camels (Camelus dromedarius) | Mild respiratory disease, nasal discharge | Not present | Multifocal moderate rhinitis, tracheitis and bronchitis with epithelial necrosis. Hyperplasia of lymph nodes and tonsil | Respiratory epithelial cells in the URT | [47], [53] |
| Alpacas (Vicugna pacos) | Not observable | Not present | Squamous metaplasia of the epithelium of the turbinate. Hypertrophy and hyperplasia of lymph nodes | Respiratory epithelial cells in the URT | [48], [49] | |
| Llamas (Lama glama) | Mild mucus secretion in one nostril | Not present | Mild to severe rhinitis | Respiratory epithelial cells in the URT | [50] | |
| Non-camelid domestic species | Domestic pig (Sus scrofa domesticus) | Mild excretion of mucus in the nose | Not present | Mild to severe rhinitis | Respiratory epithelial cells in the URT | [50] |
| Rabbit (Oryctolagus cuniculus) | Not observable | Not present | Focal mild to moderate rhinitis with necrosis | Respiratory epithelial cells in the URT | [54] | |
| NHP | Rhesus macaquesa(Macaca mulatta) | Fever, mild to moderate respiratory disease | Lung congestion and nodules in lung No extra pulmonary lesions |
Multifocal mild-to-moderate interstitial pneumonia | Type I and II pneumocytes and alveolar macrophages | [21], [22], [23] |
| Common marmoseta(Callithrix jacchus) | Mild to severe respiratory disease | Congestion of bronchioles | Diffuse interstitial infiltration in lower lung lobes, bronchointerstitial pneumonia | Type I pneumocytes and alveolar macrophages | [24] |
Abbreviations: NHP, non-human primates; URT, upper respiratory tract.
Also animal models of disease (translation to human).
3.1. Camelids
Dromedary camels are the main source of MERS-CoV zoonotic transmission (reviewed in [52]). In experimental intranasal inoculations with MERS-CoV, only mild clinical signs (i.e. nasal discharge) with URT infection were observed. Viral RNA was detected in nasal swabs, in upper and lower respiratory tracts, and also in extra-pulmonary tissues (i.e. lymph nodes, tonsil, intestine, liver, adrenal gland, etc.). In contrast, infectious virus was only detected in the URT, trachea, large bronchus and tracheobronchial lymph node. Gross lesions were not observed in dromedary camels, but inflammation in the nasal cavity, trachea and bronchus was present. The virus replication in dromedaries was only detected in epithelial cells in the URT [47], [53].
Llamas and alpacas, also known as domestic new world camelids, developed a similar clinical-pathological picture to that of dromedaries after experimental MERS-CoV infection. In both species the virus was inoculated via intranasal route, and either no clinical signs (alpacas) or mild mucus secretion (llamas) was observed. MERS-CoV was detected in nasal swabs, and in the URT and trachea of both llamas and alpacas. None of the species showed lesions macroscopically, but microscopically mild to severe rhinitis was detected in alpacas as well as metaplasia of the epithelium of the turbinate in alpacas. Similar to dromedaries, the epithelial cells in the URT were the main target cells for virus replication. Concomitant to an antibody response, the virus was cleared from the URT 7 to 10 days after experimental infection [48], [49], [50].
3.2. Non-camelid domestic species
After intranasal inoculation of MERS-CoV, only mild excretion of mucus was observed in 6 to 8-week old domestic pigs [50]. Viral RNA was detected in nasal swabs, in the URT, trachea and bronchus. Although gross lesions were not present in pigs, they showed mild to moderate rhinitis, with virus replication observed in the epithelial cells in the URT. Shedding of MERS-CoV was detected in nasal swabs from days 1 to 10 PI, but infectious virus was only detected until day 4 PI. Viral RNA was also detected in the URT, trachea and bronchus [50].
New Zealand white rabbits did not exhibit clinical signs or significant gross lesions at necropsy after experimental MERS-CoV inoculation; thus, they were considered an animal model of asymptomatic infection [54]. Similar to pigs, mild to moderate rhinitis with necrosis was observed, and respiratory epithelial cells in the URT were identified as the target cells for MERS-CoV replication. Viral RNA was present in nasal swabs, and upper and lower respiratory tracts. Infectious virus was also detected in nasal swabs up to 7 days PI [54].
3.3. Non-human primates
The rhesus macaque was used as the first animal model developed for MERS-CoV infection, showing mild to moderate respiratory disease from day 1 to 4 PI after intratracheal inoculation [21]. Gross lesions were present only in the lung, consisting in congestion and presence of nodules, and the main observed microscopical lesion was interstitial pneumonia. Although MERS-CoV RNA was detected in nasal swabs, bronchoalveolar lavage samples, oropharyngeal swabs, and also in some upper and lower respiratory tract tissue samples, infectious virus was only isolated from the lungs. MERS-CoV replication occurred in type I and II pneumocytes, and viral antigen co-localized with sites of pneumonia [21]. Macaques represent a useful model to study mild MERS-CoV infection because they develop a transient respiratory disease similar to humans.
On the other hand, common marmosets exhibited moderate to severe respiratory disease from 1 to 13 days after inoculation of MERS-CoV through multiple routes (ocular, oral, intratracheal and intranasal) [24]. Similar to macaques, gross findings were present only in the lung and correlated with moderate to severe bronchointerstitial pneumonia. MERS-CoV antigen was detected by immunohistochemistry in both type I and II pneumocytes and alveolar macrophages, but the virus replicated only in type I pneumocytes and macrophages. Similar results were observed in marmosets after inoculation of MERS-CoV only via intratracheal route [55]. However, the outcome of MERS-CoV infection in marmosets has been controversial after the publication of a recent study by Johnson and collaborators, which demonstrated no lethality after intratracheal inoculation [56]. The outcome of experimental infections in both species is reviewed in recent publications [17], [18], [19], [20].
4. Advantages and disadvantages of animal hosts used for MERS-CoV experimental infection
As described in the previous section, a number of animal species have been described as either natural reservoir (dromedary camel) or potential intermediate hosts of MERS-CoV, each one with its benefits and limitations (Table 3) when used as experimental infection models. Camelids (dromedary camels, alpacas and llamas), non-camelid domestic species (pigs and rabbits), and NHP (rhesus macaques and common marmosets) have been experimentally demonstrated to be susceptible to MERS-CoV infection, but with differences among them [21], [22], [23], [24], [47], [48], [49], [50], [53], [54]. Experiments with dromedary camels, the natural MERS-CoV host, and probably the first target for controlling MERS through vaccination [53], are costly and represent a high security risk for animal caretakers because of the difficulty in handling these animals under appropriate biosafety conditions. The main advantages of using the llama or alpaca models are that both belong to the family Camelidae, have smaller size, more gentle behavior, and are more available at a commercial level than dromedary camels; importantly, specific reagents for immune monitoring have been developed for new world camelids [57]. Therefore, they may be useful surrogates for dromedaries under experimental conditions. However, both models are also quite expensive and require large and complex BSL3 facilities. In contrast to camelids, other domestic species such as pigs and rabbits are readily available, with lower cost and easier handling. Additionally, an extensive panel of specific-immunological reagents is available for these species. When compared to camelids, however, lower MERS-CoV titers were detected in nasal cavities and tissue samples of pigs and rabbits during the infection. Furthermore their usefulness as animal models for transmission studies has not yet been addressed.
Table 3.
Advantages and limitations of natural and potential intermediate hosts for MERS-CoV infection as experimental animal models as well as for transmission studies.
| Species | Advantages | Limitations | |
|---|---|---|---|
| Camelids | Dromedary camels (Camelus dromedarius) |
|
|
| Alpacas (Vicugna pacos) |
|
|
|
| Llamas (Lama glama) |
|
|
|
| Non-camelid domestic species | Domestic pig (Sus scrofa domesticus) |
|
|
| Rabbit (Oryctolagus cuniculus) |
|
|
|
| NHP | Rhesus macaques (Macaca mulatta) |
|
|
| Common marmoset (Callithrix jacchus) |
|
|
Abbreviations: BSL3, biosafety level 3; MERS-CoV, Middle East respiratory syndrome; NHP, non-human primates; NS, nasal swabs.
Contrary to the mentioned species, both macaques and common marmosets develop clinical disease relatively similar to humans. In that respect, phylogenetically-related species as baboons [58], which live in Africa and Arabic Peninsula, might also play a role in the transmission of the virus. However, little attention has been paid to these species since no sero-epidemiology has been documented. There are important limitations when working with NHP models, namely the complex husbandry requirements that lead to substantially increased costs, some controversy results among different groups. Besides practical considerations, human-specific immunological reagents cross-react with NHP species and are widely available.
5. Conclusion and future steps
As summarized in this review, several species of animals are susceptible to experimental MERS-CoV infection; thus, they might act as potential intermediate hosts of the disease. However, the presence of viral RNA and/or specific antibodies against the virus has been only demonstrated in the field in dromedaries and alpacas [41], [51]. At the light of recent experimental studies, it seems that the list of potential host targets for MERS-CoV is not closed. MERS surveillance programs should be implemented in endemic areas in animal species for which experimental evidence of susceptibility has been provided and species closely related to them.
SARS and MERS outbreaks taught us many lessons, and one of the most important is that, even in the absence of an overt threat, there is the possibility of the re-emergence of a virus or other similar viruses. On the other hand, and since the first case of MERS, continuous new cases have been described in different countries around the world [59], [60], [61], [62]. This underlines the importance of the development of animal models closer to the natural host targets. The key role of domestic animals and wildlife in the transmission of MERS-CoV should be further elucidated; meanwhile, countermeasures against deadly coronaviruses must be further explored since the risk of a global outbreak is not negligible. Noteworthy, after more than a decade of SARS and five years of MERS epidemics, there are still no licensed preventive or therapeutic drugs available that could be used in case of an eventual re-emergence of SARS or MERS. This scenario is not the outcome of technical issues, since effective vaccine prototypes against those pathogens are already available [53], [63], [64]. In case of MERS-CoV, vaccination of dromedary camels, the main source of zoonotic transmission, might be useful to control the spread of MERS [53]. However, when developing a vaccine, besides testing the protection efficacy, researchers need to think about social problems such as the reticence of camel owners to vaccinate their animals. Thus, the development of a dual vaccine able to protect against both, MERS-CoV and camelpox virus (an endemic disease in the Middle East, Africa and Asia) might be an ideal solution [53]. Recently, another dual-vaccine for humans and animals against MERS-CoV and rabies virus has been designed [65]. Political aspects have also a key role in the release of a vaccine into the market. Unless the requirements and timings for vaccine licensing procedures are facilitated, pharmaceutical companies will unlikely invest in their development taking into account the current market demand. Moreover, fragmentation of intellectual property rights may also adversely affect the development of vaccines to combat those infections [66].
Conflicts of interest
None.
Acknowledgements
This review work was performed as part of the Zoonotic Anticipation and Preparedness Initiative (ZAPI project) [Innovative Medicines Initiative (IMI) grant 115760] with assistance and financial support from IMI and the European Commission and contributions from EFPIA partners. The funding from CERCA Programme/Generalitat de Catalunya to IRTA is also acknowledged.
References
- 1.Rubin C., Myers T., Stokes W., Dunham B., Harris S., Lautner B. Review of institute of medicine and national research council recommendations for one health initiative. Emerg. Infect. Dis. 2013;19(12):1913–1917. doi: 10.3201/eid1912.121659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morse S.S., Mazet J.A.K., Woolhouse M., Parrish C.R., Carroll D., Karesh W.B. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380(9857):1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mäkelä M.J., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpimäki M. Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 1998;36(2):539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization, WHO 2014. http://www.who.int/csr/don/2016_ (Accessed on December 15, 2016)
- 6.Bermingham A., Chand M.A., Brown C.S., Aarons E., Tong C., Langrish C. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to United Kingdom from the Middle East, September 2012. Euro Surveill. 2012;17(40):20290. [PubMed] [Google Scholar]
- 7.World Health Organization, WHO 2016. http://www.who.int/emergencies/mers-cov/en/ (Accessed on December 15, 2016)
- 8.World Health Organization, WHO 2014. https://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/ (Accessed on December 15, 2016)
- 9.Paweska J.T., Sewlall N.H., Ksiazek T.G., Blumberg L.H., Hale M.J., Lipkin W.I. Nosocomial outbreak of novel arenavirus infection, southern Africa. Emerg. Infect. Dis. 2009;15(10):1598–1602. doi: 10.3201/eid1510.090211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X.J., Liang M.F., Zhang S.Y., Liu Y., Li J.D., Sun Y.L. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011;364(16):1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safronetz D., Geisbert T.W., Feldmann H. Animal models for highly pathogenic emerging viruses. Curr. Opin. Virol. 2013;3(2):205–209. doi: 10.1016/j.coviro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration, FDA . US Department of Health and Human Services. May 2014 ed. FDA; Silver Spring, MD: 2014. Guidance for industry product development under the animal rule. [Google Scholar]
- 13.Plumb G., Babiuk L., Mazet J., Olsen S., Rupprecht C., Pastoret P.P. Vaccination in conservation medicine. Rev. Sci. Tech. 2007;26(1):229–241. [PubMed] [Google Scholar]
- 14.Adams L.G., Khare S., Lawhon S.D., Rossetti C.A., Lewin H.A., Lipton M.S. Enhancing the role of veterinary vaccines reducing zoonotic diseases of humans: linking systems biology with vaccine development. Vaccine. 2011;29(41):7197–7206. doi: 10.1016/j.vaccine.2011.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alharbi N.K. Vaccines against Middle East respiratory syndrome coronavirus for humans and camels. Rev. Med. Virol. 2016 Oct 27 doi: 10.1002/rmv.1917. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowenthal J. Overview of the CSIRO Australian Animal Health Laboratory. J. Infect. Public Health. 2016 May–Jun;9(3):236–239. doi: 10.1016/j.jiph.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baseler L.J., Falzarano D., Scott D.P., Rosenke R., Thomas T., Munster V.J. An acute immune response to Middle East respiratory syndrome coronavirus replication contributes to viral pathogenicity. Am. J. Pathol. 2016;186(3):630–638. doi: 10.1016/j.ajpath.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Doremalen N., Munster V.J. Animal models of Middle East respiratory syndrome coronavirus infection. Antivir. Res. 2015;122:28–38. doi: 10.1016/j.antiviral.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton T.C., Subbarao K. Development of animal models against emerging coronaviruses: from SARS to MERS coronavirus. Virology. 2015;479–480:247–258. doi: 10.1016/j.virol.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baseler L., de Wit E., Feldmann H. A comparative review of animal models of Middle East respiratory syndrome coronavirus infection. Vet. Pathol. 2016;53(3):521–531. doi: 10.1177/0300985815620845. [DOI] [PubMed] [Google Scholar]
- 21.de Wit E., Rasmussen A.L., Falzarano D., Bushmaker T., Feldmann F., Brining D.L. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc. Natl. Acad. Sci. U. S. A. 2013;110(41):16598–16603. doi: 10.1073/pnas.1310744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munster V.J., de Wit E., Feldmann H. Pneumonia from human coronavirus in a macaque model. N. Engl. J. Med. 2013;368(16):1560–1562. doi: 10.1056/NEJMc1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao Y., Bao L., Deng W., Xu L., Li F., Lv Q. An animal model of MERS produced by infection of rhesus macaques with MERS coronavirus. J. Infect. Dis. 2014;209(2):236–242. doi: 10.1093/infdis/jit590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falzarano D., de Wit E., Feldmann F., Rasmussen A.L., Okumura A., Peng X. Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PLoS Pathog. 2014;10(8):e1004250. doi: 10.1371/journal.ppat.1004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Müller M.A., Dijkman R. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang N., Shi X., Jiang L., Zhang S., Wang D., Tong P. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23(8):986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Wit E., Prescott J., Baseler L., Bushmaker T., Thomas T., Lackemeyer M.G. The Middle East respiratory syndrome coronavirus (MERS-CoV) does not replicate in Syrian hamsters. PLoS One. 2013;8(7):e69127. doi: 10.1371/journal.pone.0069127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman C.M., Matthews K.L., Goicochea L., Frieman M.M. Wild-type and innate immune-deficient mice are not susceptible to the Middle East respiratory syndrome coronavirus. J. Gen. Virol. 2014;95(Pt 2):408–412. doi: 10.1099/vir.0.060640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J., Li K., Wohlford-Lenane C., Agnihothramn S.S., Fett C., Zhao J. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc. Natl. Acad. Sci. U. S. A. 2014;111(13):4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agrawal A.S., Garron T., Tao X., Peng B.H., Wakamiya M., Chan T.S. Generation of a transgenic mouse model of Middle East respiratory syndrome coronavirus infection and disease. J. Virol. 2015;89(7):3659–3670. doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houdebine L.M. Transgenic animal models in biomedical research. Methods Mol. Biol. 2007;360:163–202. doi: 10.1385/1-59745-165-7:163. [DOI] [PubMed] [Google Scholar]
- 32.Peck K.M., Cockrell A.S., Yount B.L., Scobey T., Baric R.S., Heise M.T. Glycosylation of mouse DPP4 plays a role in inhibiting Middle East respiratory syndrome coronavirus infection. J. Virol. 2015;89(8):4696–4699. doi: 10.1128/JVI.03445-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cockrell A.S., Yount B.L., Scobey T., Jensen K., Douglas M., Beall A. A mouse model for MERS coronavirus-induced acute respiratory distress syndrome. Nat Microbiol. 2016;2:16226. doi: 10.1038/nmicrobiol.2016.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tai W., Zhao G., Sun S., Guo Y., Wang Y., Tao X. A recombinant receptor-binding domain of MERS-CoV in trimeric form protects human dipeptidyl peptidase 4 (hDPP4) transgenic mice from MERS-CoV infection. Virology. 2016;499:375–382. doi: 10.1016/j.virol.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu H., Sun S., Xiao H., Feng J., Guo Y., Tai W. Single-dose treatment with a humanized neutralizing antibody affords full protection of a human transgenic mouse model from lethal Middle East respiratory syndrome (MERS)-coronavirus infection. Antivir. Res. 2016;132:141–148. doi: 10.1016/j.antiviral.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pascal K.E., Coleman C.M., Mujica A.O., Kamat V., Badithe A., Fairhurst J. Pre- and postexposure efficacy of fully human antibodies against Spike protein in a novel humanized mouse model of MERS-CoV infection. Proc. Natl. Acad. Sci. U. S. A. 2015;112(28):8738–8743. doi: 10.1073/pnas.1510830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Benedictis P., Marciano S., Scaravelli D., Priori P., Zecchin B., Capua I. Alpha and lineage C beta CoV infections in Italian bats. Virus Genes. 2014;48(2):366–371. doi: 10.1007/s11262-013-1008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Memish Z.A., Mishra N., Olival K.J., Fagbo S.F., Kapoor V., Epstein J.H. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg. Infect. Dis. 2013;19(11):1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munster V.J., Adney D.R., van Doremalen N., Brown V.R., Miazgowicz K.L., Milne-Price S. Replication and shedding of MERS-CoV in Jamaican fruit bats (Artibeus jamaicensis) Sci. Rep. 2016;6:21878. doi: 10.1038/srep21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corman V.M., Jores J., Meyer B., Younan M., Liljander A., Said M.Y. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992–2013. Emerg. Infect. Dis. 2014;20(8):1319–1322. doi: 10.3201/eid2008.140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reusken C.B., Haagmans B.L., Müller M.A., Gutierrez C., Godeke G.J., Meyer B. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect. Dis. 2013;13(10):859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haagmans B.L., Al Dhahiry S.H., Reusken C.B., Raj V.S., Galiano M., Myers R. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect. Dis. 2014;14(2):140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Müller M.A., Meyer B., Corman V.M., Al-Masri M., Turkestani A., Ritz D. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect. Dis. 2015;15(5):559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farag E.A., Reusken C.B., Haagmans B.L., Mohran K.A., Stalin Raj V., Pas S.D. High proportion of MERS-CoV shedding dromedaries at slaughterhouse with a potential epidemiological link to human cases, Qatar 2014. Infect. Ecol. Epidemiol. 2015 Jul 15;5:28305. doi: 10.3402/iee.v5.28305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raj V.S., Farag E.A., Reusken C.B., Lamers M.M., Pas S.D., Voermans J. Isolation of MERS coronavirus from a dromedary camel, Qatar, 2014. Emerg. Infect. Dis. 2014 Aug;20(8):1339–1342. doi: 10.3201/eid2008.140663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabir J.S., Lam T.T., Ahmed M.M., Li L., Shen Y., Abo-Aba S.E. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science. 2016;351(6268):81–84. doi: 10.1126/science.aac8608. [DOI] [PubMed] [Google Scholar]
- 47.Adney D.R., van Doremalen N., Brown V.R., Bushmaker T., Scott D., de Wit E. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg. Infect. Dis. 2014;20(12):1999–2005. doi: 10.3201/eid2012.141280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adney D.R., Bielefeldt-Ohmann H., Hartwig A.E., Bowen R.A. Infection, replication, and transmission of Middle East respiratory syndrome coronavirus in alpacas. Emerg. Infect. Dis. 2016;22(6):1031–1037. doi: 10.3201/eid2206.160192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crameri G., Durr P.A., Klein R., Foord A., Yu M., Riddell S. Experimental infection and response to rechallenge of alpacas with Middle East respiratory syndrome coronavirus. Emerg. Infect. Dis. 2016;22(6):1071–1074. doi: 10.3201/eid2206.160007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vergara-Alert J., van den Brand J.M., Widagdo W., Muñoz M., Raj S. Livestock susceptibility to infection with Middle East respiratory syndrome coronavirus. Emerg. Infect. Dis. 2017 Feb;15:23(2). doi: 10.3201/eid2302.161239. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reusken C.B., Schilp C., Raj V.S., De Bruin E., Kohl R.H., Farag E.A. MERS-CoV infection of alpaca in a region where MERS-CoV is endemic. Emerg. Infect. Dis. 2016;22(6):1129–1131. doi: 10.3201/eid2206.152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohd H.A., Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus (MERS-CoV) origin and animal reservoir. Virol. J. 2016;13:87. doi: 10.1186/s12985-016-0544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haagmans B.L., van den Brand J.M., Raj V.S., Volz A., Wohlsein P., Smits S.L. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science. 2016;351(6268):77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- 54.Haagmans B.L., van den Brand J.M., Provacia L.B., Raj V.S., Stittelaar K.J., Getu S. Asymptomatic Middle East respiratory syndrome coronavirus infection in rabbits. J. Virol. 2015;89(11):6131–6135. doi: 10.1128/JVI.00661-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan J.F., Yao Y., Yeung M.L., Deng W., Bao L., Jia L. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson R.F., Via L.E., Kumar M.R., Cornish J.P., Yellayi S., Huzella L. Intratracheal exposure of common marmosets to MERS-CoV Jordan-n3/2012 or MERS-CoV EMC/2012 isolates does not result in lethal disease. Virology. 2015;485:422–430. doi: 10.1016/j.virol.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis W.C., Heirman L.R., Hamilton M.J., Parish S.M., Barrington G.M., Loftis A. Flow cytometric analysis of an immunodeficiency disorder affecting juvenile llamas. Vet. Immunol. Immunopathol. 2000;74(1–2):103–120. doi: 10.1016/s0165-2427(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 58.Eichler E.E., DeJong P.J. Biomedical applications and studies of molecular evolution: a proposal for a primate genomic library resource. Genome Res. 2002;12(5):673–678. doi: 10.1101/gr.250102. [DOI] [PubMed] [Google Scholar]
- 59.Cowling B.J., Park M., Fang V.J., Wu P., Leung G.M., Wu J.T. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill. 2015;20(25):7–13. doi: 10.2807/1560-7917.es2015.20.25.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu J., Yi L., Zou L., Zhong H., Liang L., Song T. Imported case of MERS-CoV infection identified in China, May 2015: detection and lesson learned. Euro Surveill. 2015;20(24) doi: 10.2807/1560-7917.es2015.20.24.21158. (pii: 21158) [DOI] [PubMed] [Google Scholar]
- 61.Korean Society of Infectious Diseases, Korean Society for Healthcare-associated Infection Control and Prevention The Same Middle East Respiratory Syndrome-Coronavirus (MERS-CoV) yet Different Outbreak Patterns and Public Health Impacts on the Far East Expert Opinion from the Rapid Response Team of the Republic of Korea. Infect. Chemother. 2015;47(4):247–251. doi: 10.3947/ic.2015.47.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Tawfiq J.A., Zumla A., Memish Z.A. Coronaviruses: severe acute respiratory syndrome coronavirus and Middle East respiratorysyndrome coronavirus in travelers. Curr. Opin. Infect. Dis. 2014;27(5):411–417. doi: 10.1097/QCO.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 63.Roper R.L., Rehm K.E. SARS vaccines: where are we? Expert Rev. Vaccines. 2009;8(7):887–898. doi: 10.1586/erv.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Honda-Okubo Y., Barnard D., Ong C.H., Peng B.H., Tseng C.T., Petrovsky N. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J. Virol. 2015;89(6):2995–3007. doi: 10.1128/JVI.02980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wirblich C., Coleman C.M., Kurup D., Abraham T.S., Bernbaum J.G., Jahrling P.B. One-health: a safe, efficient, dual-use vaccine for humans and animals against Middle East respiratory syndrome coronavirus and rabies virus. J. Virol. 2017 Jan 3;91(2) doi: 10.1128/JVI.02040-16. (pii: e02040-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simon J.H., Claassen E., Correa C.E., Osterhaus A.D. Managing severe acute respiratory syndrome (SARS) intellectual property rights: the possible role of patent pooling. Bull. World Health Organ. 2005;83(9):707–710. [PMC free article] [PubMed] [Google Scholar]