Abstract
In November 2014, New South Wales Health was notified of a cluster of respiratory illness in a veterinary school. Active case finding identified another case at a local equine stud. All cases had exposure to the equine fetal membranes of Mare A. This tissue subsequently tested positive for Chlamydia psittaci using quantitative real-time polymerase chain reaction. We conducted a cohort study of the university and stud farm staff to determine risk factors for disease. Nine people were exposed to the fetal membranes of Mare A. Of these, five cases of psittacosis were identified. Two required hospital admission. Contact with birds was not associated with illness (RR = 0.5, 95% CI = 0.09–2.73). People who had direct contact with the abnormal fetal membranes were more likely to develop disease (RR = 11.77, 95% CI = 1.02–∞). The emergence of an association between horse exposure and C. psittaci infection has important implications for the prevention and control of psittacosis.
Article summary line: Investigation of an outbreak of psittacosis in a rural veterinary school demonstrates novel source of infection for psittacosis through exposure to abnormal equine fetal membranes.
1. Introduction
Psittacosis is a systemic infectious disease caused by Chlamydia psittaci. It is characterised by fever, malaise, myalgias and atypical pneumonia [1]. Complications include myocarditis, endocarditis, hepatitis, reactive arthritis, and neurological abnormalities [2]. Birds are the major zoonotic reservoir [3]. However, outbreaks of psittacosis have occurred in the absence of direct bird exposure. Epidemiological studies have demonstrated associations time spent outdoors and the suggested mechanism of infection is through aerosolisation of infectious particles shed by birds through the process of lawn mowing [5], [6]. There is also limited evidence of person to person spread [7].
Previous studies have demonstrated the presence of C. psittaci infection in horses [8], [9], however, to our knowledge transmission to humans has not previously been demonstrated [4]. Limited studies suggest horses are only occasional hosts of C. psittaci, and infections in horses can cause respiratory disease and fetal abortion [8], [9], [10]. We describe an outbreak of probable psittacosis within a veterinary school linked to exposure to infected equine fetal membranes, in which we aimed to establish the likely route of exposure.
2. Background
On 21 November 2014, the Department of Primary Industries, the government department overseeing agriculture in New South Wales (NSW), reported four cases of respiratory illness among staff and students at a veterinary school to Health Protection New South Wales (NSW). The veterinary school is located in Wagga Wagga, a city with a population of approximately 63,000 persons, located in rural Australia. The affected staff worked in the veterinary reproduction unit where students were undertaking a rotation at the time. Active case finding identified a further case of atypical pneumonia in a human at a local equine stud, where one of the students worked. Through this outlier, we established a common exposure to the equine fetal membranes of Mare A among all the cases, generating the hypothesis that the membranes of Mare A were the source of the outbreak. The fetal membranes subsequently tested positive for C. psittaci using quantitative real-time polymerase chain reaction (qPCR). An outbreak investigation team, coordinated by Health Protection NSW, was set up. Health Protection NSW has statutory responsibility for disease control and surveillance in NSW.
3. Methods
3.1. Case definition and identification
We defined a suspected case as a person who reported exposure to the fetal membranes of Mare A and who had clinical features of community acquired pneumonia OR at least two symptoms of fever, headache, myalgia, dry cough or dyspnoea in Wagga Wagga, between 1 November and 14 December 2014. In addition to the features above, we required a probable cause to have a single high IgG titer (> 32) to C. psittaci demonstrated by micro-immunofluorescence (MIF) and a confirmed case to have a fourfold rise in antibody titer to C. psittaci by MIF or detection of C. psittaci by nucleic acid testing.
Active case finding was conducted at the veterinary school through an online communication system for students and an email and newsletter for staff. Additional cases were also identified by interviewing cases.
3.2. Epidemiological investigation
We conducted a site visit to the equine stud and veterinary school on 5 March 2015. We interviewed all persons exposed to the equine fetal membranes of Mare A using a standard questionnaire designed for the outbreak. We collected information on demographics, clinical history, laboratory investigations, and potential exposures. Degree of exposure to the membranes was further delineated into the following non-exclusive categories: 1) attendance at the examination of the membranes, 2) scrubbing of contaminated floors, 3) direct contact with bagged membranes, 4) direct contact with exposed membranes, and 5) manipulation of membranes (i.e. inverting the membranes for examination). The use of personal protective equipment (PPE) was also examined.
We compared proportions by case status using the Wilcoxon rank-sum and Fisher's exact tests. We calculated relative risks (RR) and 95% confidence intervals (95% CI) to quantify the association between exposure variables and illness. We used exact logistic regression to calculate an odds ratio (OR) approximating the RR, when RR was unable to be calculated. Data analysis was performed using Stata IC 13.
3.3. Laboratory investigation
Respective treating clinicians obtained acute and convalescent serological samples from three of the five cases. Sera were tested for Chlamydia sp. antibodies. Using enzyme immunoassay (EIA) and further differentiated by species (Chlamydia psittaci, Chlamydia pneumoniae and Chlamydia trachomatis) using microimmunofluorescence (MIF). For the two hospitalised cases, serology for Q fever, leptospirosis, brucellosis, mycoplasma, toxoplasma, Ross River virus, and urinary legionella and pneumococcal antigens were collected to exclude other likely diagnoses. A serology sample was also obtained from the mare and tested for Chlamydia sp. EIA. The equine fetal membranes were stored at − 20 °C in a freezer at the veterinary school (for teaching purposes). We investigated the membranes with qPCR and examined histologically. To address the potential of environmental contamination, we swabbed the internal aspect of the membranes – this method has been validated in subsequent investigations of aborted fetuses, showing concordance of internally sampled membranes with sampling from the fetus (Gabor M, personal communication, 17 October 2016). qPCR was performed targeting genes specific for C. psittaci, C. trachomatis, C. pneumoniae and C. abortus, as well as Coxiella burnetii, Rickettsia spp. and Hendra virus. DNA was extracted from 10 to 20 mg of tissue using the Wizard® Genomic DNA Purification Kit (Promega, WI USA) according to the manufacturer’s instructions. Chlamydial qPCR was performed using a Corbett real-time platform with genus-specific [11], [12] and species-specific primers for C. psittaci [13], [14], [15], [16]. qPCR tests for C. pneumoniae and C. trachomatis DNA were performed in parallel, using primers based on published species-specific sequences to exclude inadvertent species cross-reactions [14], [15]. For histology, formalin fixed tissues were embedded in paraffin, sectioned (5 μm) and stained with hematoxylin and eosin.
4. Results
4.1. Descriptive epidemiology
A total of nine people were exposed to the fetal membranes of Mare A. Mare A, normally kept at a farm in Goulburn, NSW was transferred to a stud farm in Wagga Wagga three weeks before foaling. She foaled on 5 November 2014 with two stud staff in attendance. A third stud staff member examined the membranes and stored them in a porous plastic feed bag later in the morning. The membranes were grossly abnormal: the chorionic surface of the fetal membranes displayed a diffuse dark red to black discolouration. (See Fig. 1). The foal died one week later – the cause of death was not further investigated by stud staff.
Fig. 1.
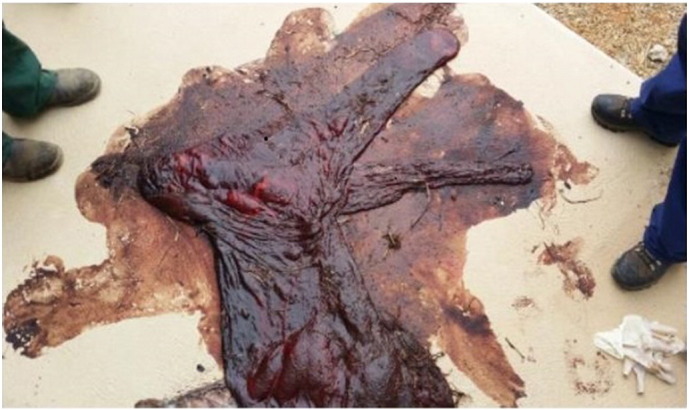
Infected equine fetal membranes from Mare A,Wagga Wagga, 5 November 2014.
The membranes were taken to the university by one of the staff members at the stud, who was also a student at the university, and examined by two academic staff and three students for teaching purposes. The examination involved inverting the membranes. The next day, a technical staff member from the university transferred the bagged membranes into a watertight bag and stored it in the freezer.
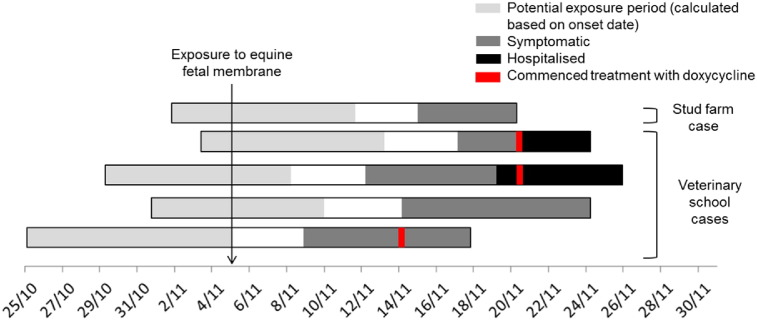
From this cohort of nine, a total of five cases of psittacosis (three probable, two suspected) were identified; an attack rate of 56%. The clinical features of the five cases are summarised in Table 1. All cases reported fever, fatigue, headache and clinical signs of pneumonia as identified by medical practitioners. Four of the five cases received chest X-rays and all had evidence of lobar consolidation. The onset dates of illness ranged from 9 November – 17 November 2014. (Fig. 2) Overlapping periods of exposure, calculated from onset dates based on an incubation period between 5 and 14 days, indicated a likely point-source exposure between 3 and 5 November, consistent with the known date of exposure to the fetal membrane of Mare A on 5 November. Active case-finding at the university did not identify any cases of atypical pneumonia not linked to the membranes over the same period.
Table 1.
Clinical presentations of patients with probable and suspected psittacosis, Wagga Wagga, November – December 2014.
| Signs and symptoms | No. (%) cases, n = 5 |
|---|---|
| Fever | 5 (100) |
| Cough | 2 (40) |
| Myalgia | 4 (80) |
| Clinical or radiological signs of pneumonia | 5 (100) |
| Fatigue | 5 (100) |
| Rash | 0 (0) |
| Headache | 5 (100) |
| Nausea | 2 (40) |
| Chest pain | 1 (20) |
Fig. 2.
Onset dates, potential exposure period, and duration of symptoms for cases of probable or suspected psittacosis, Wagga Wagga, November – December 2014.
Two cases were hospitalised and three cases were treated by primary care physicians. The case descriptions of the hospitalised cases below:
University staff member A was a 25 year old female admitted on 19 November 2015 with fever (40.1 °C), headache, back pain, myalgia and a mild cough. Her initial investigations revealed normal white cell count (WCC) (5.4 × 109/L) but an elevated C reactive protein (CRP) (182 mg/L). A chest radiograph demonstrated right middle lobe consolidation. She was commenced on oral doxycycline 100 mg bd for two weeks. On day 2 she developed inspiratory chest pain, and dyspnoea. Electrocardiogram (ECG) initially showed normal sinus rhythm with minor ST elevation in the inferolateral leads and minor PR segment depression in lead II and later showed normalisation of the ST segments with T wave inversion in the lateral leads, typical of evolutionary changes of acute pericarditis. Serum high-sensitivity cardiac troponin T level was elevated (987 ng/ml, upper limit of normal 50 ng/ml) in keeping with myocardial injury. Echocardiogram showed a structurally normal heart with no pericardial effusion. Cardiac magnetic resonance imaging (cMR) showed minor gadolinium enhancement in the inferoseptal wall consistent with a small region of myocarditis. The patient's chest pain resolved by day 7 after treatment with naproxen 500 mg twice daily. Echocardiogram remained normal on follow-up 2 months later.
University staff member B was a 44 year old male admitted on 20 November 2014 with fever (measured at 39.3 °C) headache, myalgia and arthralgia of the small joints. Initial investigations revealed a normal WCC 6.8 × 109/L and CRP of 26 mg/L. Clinical signs of pneumonia were confirmed with a chest radiograph demonstrating left lower lobe consolidation. He was commenced on oral doxycycline 100 mg bd and amoxicillin with clavulanic acid 875 + 125 mg on day 1 and discharged on day 4.
4.2. Laboratory findings
Three cases underwent serological testing, all of whom received antibiotic treatment 2–4 days after onset of symptoms (Fig. 2). All three cases were positive for Chlaymdia sp. on EIA. However, none had a four-fold rise in titer to C. psittaci or any other Chlamydial sp. on species specific testing using MIF. One case demonstrated a single high titer (median titer 128), two demonstrated falling titres to C. psittaci on MIF. (Table 2).
Table 2.
Psittacosis serology for probable or suspected cases of psittacosis, Wagga Wagga, November – December 2014.
|
Chlamydia psittaci microimmunofluorescence |
Case status | ||||||
|---|---|---|---|---|---|---|---|
| Onset to first sample (days) | First titer | First to second sample (days) | Second titer | First to final sample (days) | Final titer | ||
| University staff A | 8 | < 128 | 26 | 128 | 74 | < 128 | Probable |
| University staff B | 3 | 128 | 25 | < 128 | 99 | 128 | Probable |
| Student A | 12 | 128 | 125 | 128 | Not taken | Not taken | Probable |
| Student B | Not taken | Not taken | Not taken | Not taken | Not taken | Not taken | Suspected |
| Stud staff | Not taken | Not taken | Not taken | Not taken | Not taken | Not taken | Suspected |
The mare's serology was also positive for Chlamydial sp. using EIA. The equine fetal membranes were qPCR positive for Chlamydia genus (and C. psittaci-species. It should be noted that a weak positive signal could also be detected in the C. abortus qPCR, however, the fetal membranes were negative for C. trachomatis, C. pneumoniae, C. burnetii, Rickettsia spp., Kingella spp., and Hendra virus. Aerobic and anaerobic cultures were also negative. The histology results from 3 December 2014 showed moderate to marked congestion and oedema with focal perivascular to diffuse mild infiltration of the interstitium with mixed inflammatory cells mainly neutrophils and macrophages. Moderate autolysis interfered with histological interpretation. The diagnosis was of mild, diffuse interstitial placentitis.
4.3. Risk factors for infection
Risk factors for infection were evaluated using the cohort of nine people exposed to the fetal membranes. There were no significant differences in age or sex between those who developed illness and those who did not (Table 3). There was no association between exposure to known risk factors: e.g. birds, lawn-mowing (with or without a grass-catcher) and developing disease. The relative risk for specific exposure to the fetal membranes of Mare A was not calculated as it was part of the case definition (Table 4).
Table 3.
Demographic characteristics of cohort exposed to the fetal membranes of Mare A by case status, Wagga Wagga, November – December 2014.
| Demographic characteristics | Ill, n = 5 | Not ill, n = 4 | p-Values |
|---|---|---|---|
| Median age (range) | 36 (25–63) | 25 (23–63) | 0.6547a |
| Sex – no. male (%) | 3 (60) | 1 (25) | 0.524b |
No significant difference by Wilcoxon rank-sum.
Fisher's exact tests.
Table 4.
Exposure characteristics of cohort exposed to the fetal membranes of Mare A by case status, Wagga Wagga, November – December 2014.
| Exposures | No. (%) ill, n = 5 | No. (%) not ill, n = 4 | Relative risk | 95% CI |
|---|---|---|---|---|
| Travel outside of Wagga Wagga | 3 (60) | 1 (25) | 1.88 | 0.56–6.31 |
| Birds | 1 (20) | 2 (50) | 0.5 | 0.09–2.73 |
| Contact with psittacosis case | 0 (0) | 0 (0) | 0 | – |
| Regular occupational exposure to equine fetal membranes | 3 (60) | 3 (75) | 0.75 | 0.24–2.32 |
| Mowing lawns without grass-catcher | 1 (20) | 1 (25) | 0.88 | 0.19–4.03 |
Direct contact with the fetal membranes (RR = 11.77, 95% CI 1.02–∞) and manipulation of the fetal membranes of Mare A (RR = 2.33, 95% CI 0.99–5.49) were associated with an increased risk of disease, however only direct contact was statistically significant. There was no association between the hosing or scrubbing of floors and developing disease (Table 5). Both cases who manipulated the membranes were subsequently hospitalised, suggesting that a higher degree of exposure was associated with more severe disease. None of the cohort wore either masks or protective eyewear, although gloves were worn.
Table 5.
Type of exposure to equine fetal membranes of Mare A for the nine people exposed to the fetal membranes of Mare A by case status.
| No. (%) ill, n = 5 | No. (%) not ill, n = 4 | Relative risk | 95% CI | |
|---|---|---|---|---|
| – | – | |||
| Observation of membranes | 5 (100) | 3 (75) | 2.85a | 0.20–∞a |
| Hosing/scrubbing of floors | 1 (20) | 3 (75) | 0.31 | 0.05–1.80 |
| Direct contact with bagged membranes | 1 (20) | 2 (50) | 0.5 | 0.09–2.73 |
| Direct contact with membranes | 5 (100) | 1 (25) | 11.77a | 1.02–∞a |
| Manipulation of membranes | 2 (40) | 0 (0) | 2.33 | 0.99–5.49 |
∞: Positive infinity.
Exact logistic regression was used to calculate odds ratios and 95% confidence intervals (CI).
5. Discussion
The clinical features of fever, cough, myalgia, and nausea were consistent with other reported cases of psittacosis in the published literature [1], [17]. In this cohort, all five cases had either abnormal chest findings on clinical examination or chest radiograph findings demonstrating lobar consolidation. Both chest radiographs were consistent with the typical pattern of consolidation affecting a single lobe [1]. One of the probable cases (university staff A) was complicated by myopericarditis, which has been described in the literature infrequently [3].
This study demonstrates an association between direct exposure to the infected equine fetal membrane of Mare A and developing psittacosis. The temporality of the cluster was consistent, with the exposure falling within the calculated exposure period for all cases. All cases had direct contact with the membranes demonstrating a high level of specificity. A biological gradient was demonstrated with the two most severe cases, requiring hospitalisation, having the greatest degree of contact with the membranes.
Human infection with C. abortus related to parturient goats and sheep have previously been described, and providing biological plausibility for this mode of transmission [20], [21], [22], [23], [24]. C. abortus is highly related and previously indistinguishable from C. psittaci until innovations in testing led to a reclassification [25]. Indeed, in this current report, a weak C. abortus signal was detected in the fetal membranes, likely due to cross-reactivity of the qPCR primers.
Despite having minimal information about the mare's clinical presentation, the presence of a serological response and histological findings suggestive of placentitis strongly suggest infection rather than an incidental finding. Limited studies suggest horses are only occasional hosts of C. psittaci, and can cause respiratory disease and fetal abortion [8], [9], [10]. As the main natural reservoir of C. psittaci, we consider birds as the most likely source of equine infection [2]. It is feasible that Mare A could have also contracted psittacosis from wild bird populations in the local area. Previous outbreaks of psittacosis linked to wild birds have demonstrated the presence of epizootic transmission among bird populations in rural areas at higher altitudes [5], [6]
The likely modes of transmission from infected fetal membranes to humans are either via the airborne route or direct inoculation of the eyes or nose [26], [27]. There is limited evidence that respiratory protection and eye protection reduce the risk of disease [28]. In this cohort we were unable to determine whether these measures provided any protection as none of the cohort were wearing masks or protective eyewear while handing the fetal membrane.
There are a number of limitations in this study. While the low number of cases in the outbreak limits the representativeness of these findings, the consistent epidemiological and microbiological features of this outbreak point strongly towards the equine fetal membranes as the source of the outbreak. The small numbers in the outbreak also limited the statistical analysis that could be appropriately applied. Exact methods were used as samples sizes were small [29]. The odds ratios calculated, using exact logistic regression, approximate the relative risk when the outcomes are rare, therefore the use of exact logistic regression may have exaggerated the risk association demonstrated in this study [30].
During this investigation we were unable to confirm any cases of C. psittaci disease in humans using serology despite repeated testing. Previous studies have demonstrated that the rises in antibody titer may take between 2 weeks and 3 months after onset and the lack of an antibody response may be due to the effect of antibiotic treatment [6]. In this study, clinical features characteristic of psittacosis, a rise in C. psittaci specific titer, albeit non-diagnostic, a clear epidemiological link, as well as the absence of an alternative diagnosis was considered sufficient evidence to avoid misclassification. We recommend that if psittacosis is considered, nucleic acid amplification testing should be used to give a rapid and specific diagnosis. DNA can be detected in sputum, throat specimens, whole blood and urine [13].
The difficulties in confirming a diagnosis of psittacosis using serology in this cohort suggests that psittacosis is likely to be under-recognised in the community. National passive surveillance, reporting a total of 31 cases in the past year, is likely to be an under-representation since mild cases may not seek medical attention, patients may not have timely or appropriate samples taken, particularly where the index of suspicion of such disease is low, and early treatment may affect the sensitivity of serology results [31].
Alongside previous studies, this outbreak emphasises the need for medical practitioners to consider psittacosis as a diagnosis in patients without direct exposure to birds [5], [6]. A recent study in Germany using qPCR identified C. psittaci as the most prevalent chlamydial species causing community acquired pneumonia (2.1%). The study was unable to identify any association between psittacosis and bird contact [32]. Similar results were found in an Australian study in which 29 cases (60%) did not have direct bird contact [17]. Further research using qPCR technology is required to characterise the prevalence of C. psittaci disease attributable to equine and other non-avian sources.
The emergence of an association between equine fetal membranes and psittacosis in humans has important implications for disease prevention and control. To prevent infection in at risk groups, the US Compendium of Measures to Control Chlamydophila psittaci Infection recommends the donning of PPE when handling potentially infected birds, and using a biological safety cabinet and other strategies to prevent aerosolisation of infectious particles, when performing necropsies of potentially infected birds [4]. In veterinary medicine, equine fetal membranes are routinely examined for completeness and for pathology. We recommend similar measures should be implemented for the examination of abnormal equine fetal membranes.
This outbreak highlights the inter-relationships between animal and human health and demonstrates the public health knowledge that can be gained from collaboration between veterinary and human medicine. Collaboration with professionals in public health and infectious diseases facilitated the establishment of a likely causative pathogen and the gathering of evidence towards establishing a causative relationship. Whilst this paper focuses on human health, the presence of C. psittaci infection in Australian horse populations also has implications for veterinary medicine.
To conclude, this report of an outbreak of psittacosis associated with exposure to equine fetal membranes highlights the emergence of an unusual source of psittacosis infection. While previous studies have demonstrated the presence of C. psittaci infection in horses, horses have not previously been considered an important source of infection. We recommend the use of PPE for the examination of abnormal equine fetal membranes to protect veterinarians and other staff involved in the handling of horses. Further research is required to characterise the prevalence of C. psittaci disease attributable to equine and other non-avian sources.
Funding
This work was supported by the Health Protection NSW. While undertaking this work Jocelyn Chan was completing a Masters of Philosophy (Applied Epidemiology) at Australian National University, funded by the Western Sydney Local Health District.
Declaration of interest
None.
Acknowledgements
We would like to thank Seonaid Grimmer and Shane Raidal from Charles Sturt University for facilitating the investigation; Sheena Adamson and Kirsty Hope at Communicable Diseases Branch and staff at the Department of Primary Industry for their contributions. We would also like to thank Cate D'Este for her biostatistics advice. We acknowledge laboratory staff at the Institute of Clinical Pathology and Medical Research, Westmead and the Elizabeth Macarthur Agricultural Institute who performed the technical work on the samples. Thanks also to Adam Polkinghorne for reviewing manuscript. We also thank the staff and students who participated in the outbreak investigation.
References
- 1.Yung A.P., Grayson M.L. Psittacosis–a review of 135 cases. Med. J. Aust. 1988;148(5):228–233. doi: 10.5694/j.1326-5377.1988.tb99430.x. [DOI] [PubMed] [Google Scholar]
- 2.Bennett J., Dolin R., Mandell Mandell G. fifth ed. Churchill Livingstone; Philadelphia; London: 2000. Douglas and Bennett's Principles and Practice of Infectious Diseases. [Google Scholar]
- 3.Stewardson A.J., Grayson M.L. Psittacosis. Infect. Dis. Clin. N. Am. 2010;24(1):7–25. doi: 10.1016/j.idc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Compendium of measures to control Chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis) vol. 49 (Rr-8) Centers for Disease Control and Prevention; 2000. pp. 3–17. (MMWR Recommendations and Reports: Morbidity and Mortality Weekly Report). [PubMed] [Google Scholar]
- 5.Telfer B.L., Moberley S.A., Hort K.P., Branley J.M., Dwyer D.E., Muscatello D.J. Probable psittacosis outbreak linked to wild birds. Emerg. Infect. Dis. 2005;11(3):391–397. doi: 10.3201/eid1103.040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams J., Tallis G., Dalton C., Ng S., Beaton S., Catton M. Community outbreak of psittacosis in a rural Australian town. Lancet. 1998;351(9117):1697–1699. doi: 10.1016/S0140-6736(97)10444-5. [DOI] [PubMed] [Google Scholar]
- 7.Wallensten A., Fredlund H., Runehagen A. Multiple human-to-human transmission from a severe case of psittacosis, Sweden, January–February 2013. Euro Surveillance. 2014;19(42) doi: 10.2807/1560-7917.es2014.19.42.20937. [DOI] [PubMed] [Google Scholar]
- 8.Szeredi L., Hotzel H., Sachse K. High prevalence of chlamydial (Chlamydophila psittaci) infection in fetal membranes of aborted equine fetuses. Vet. Res. Commun. 2005;29(Suppl. 1):37–49. doi: 10.1007/s11259-005-0835-1. [DOI] [PubMed] [Google Scholar]
- 9.Theegarten D., Sachse K., Mentrup B., Fey K., Hotzel H., Anhenn O. Chlamydophila spp. infection in horses with recurrent airway obstruction: similarities to human chronic obstructive disease. Respir. Res. 2008;9(1):14. doi: 10.1186/1465-9921-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodolakis A., Yousef Mohamad K. Zoonotic potential of Chlamydophila. Vet. Microbiol. 2010;140(3–4):382–391. doi: 10.1016/j.vetmic.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 11.DeGraves F.J., Gao D., Kaltenboeck B. High-sensitivity quantitative PCR platform. BioTechniques. 2003;34(1):106–110. doi: 10.2144/03341rr01. (12-5) [DOI] [PubMed] [Google Scholar]
- 12.DeGraves F.J., Gao D., Hehnen H.R., Schlapp T., Kaltenboeck B. Quantitative detection of Chlamydia psittaci and C. pecorum by high-sensitivity real-time PCR reveals high prevalence of vaginal infection in cattle. J. Clin. Microbiol. 2003;41(4):1726–1729. doi: 10.1128/JCM.41.4.1726-1729.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branley J.M., Roy B., Dwyer D.E., Sorrell T.C. Real-time PCR detection and quantitation of Chlamydophila psittaci in human and avian specimens from a veterinary clinic cluster. Eur. J. Clin. Microbiol. Infect. Dis. 2008;27(4):269–273. doi: 10.1007/s10096-007-0431-0. [DOI] [PubMed] [Google Scholar]
- 14.Madico G., Quinn T.C., Boman J., Gaydos C.A. Touchdown enzyme time release-PCR for detection and identification of Chlamydia trachomatis, C. pneumoniae, and C. psittaci using the 16S and 16S-23S spacer rRNA genes. J. Clin. Microbiol. 2000;38(3):1085–1093. doi: 10.1128/jcm.38.3.1085-1093.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messmer T.O., Skelton S.K., Moroney J.F., Daugharty H., Fields B.S. Application of a nested, multiplex PCR to psittacosis outbreaks. J. Clin. Microbiol. 1997;35(8):2043–2046. doi: 10.1128/jcm.35.8.2043-2046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menard A., Clerc M., Subtil A., Megraud F., Bebear C., de Barbeyrac B. Development of a real-time PCR for the detection of Chlamydia psittaci. J. Med. Microbiol. 2006;55(Pt 4):471–473. doi: 10.1099/jmm.0.46335-0. [DOI] [PubMed] [Google Scholar]
- 17.Branley J.M., Weston K.M., England J., Dwyer D.E., Sorrell T.C. Clinical features of endemic community-acquired psittacosis. New Microbes New Infect. 2014;2(1):7–12. doi: 10.1002/2052-2975.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgensen D.M. Gestational psittacosis in a Montana sheep rancher. Emerg. Infect. Dis. 1997;3(2):191–194. doi: 10.3201/eid0302.970214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janssen M.J., van de Wetering K., Arabin B. Sepsis due to gestational psittacosis: a multidisciplinary approach within a perinatological center–review of reported cases. Int. J. Fertil. Wom. Med. 2006;51(1):17–20. [PubMed] [Google Scholar]
- 22.Hyde S.R., Benirschke K. Gestational psittacosis: case report and literature review. Mod. Pathol. 1997;10(6):602–607. [PubMed] [Google Scholar]
- 23.Walder G., Hotzel H., Brezinka C., Gritsch W., Tauber R., Wurzner R. An unusual cause of sepsis during pregnancy: recognizing infection with chlamydophila abortus. Obstet. Gynecol. 2005;106(5 Pt 2):1215–1217. doi: 10.1097/01.AOG.0000161060.69470.9c. [DOI] [PubMed] [Google Scholar]
- 24.Meijer A., Brandenburg A., de Vries J., Beentjes J., Roholl P., Dercksen D. Chlamydophila abortus infection in a pregnant woman associated with indirect contact with infected goats. Eur. J. Clin. Microbiol. Infect. Dis. 2004;23(6):487–490. doi: 10.1007/s10096-004-1139-z. [DOI] [PubMed] [Google Scholar]
- 25.Everett K.D., Bush R.M., Andersen A.A. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 1999;49(Pt 2):415–440. doi: 10.1099/00207713-49-2-415. [DOI] [PubMed] [Google Scholar]
- 26.Anderson D.C., Stoesz P.A., Kaufmann A.F. Psittacosis outbreak in employees of a turkey-processing plant. Am. J. Epidemiol. 1978;107(2):140–148. doi: 10.1093/oxfordjournals.aje.a112516. [DOI] [PubMed] [Google Scholar]
- 27.Psittacosis at a turkey processing plant–North Carolina, 1989. vol. 39 (27) 1990. pp. 460-1–467-9. (MMWR Morbidity and Mortality Weekly Report). [PubMed] [Google Scholar]
- 28.Williams C.J., Sillis M., Fearne V., Pezzoli L., Beasley G., Bracebridge S. Risk exposures for human ornithosis in a poultry processing plant modified by use of personal protective equipment: an analytical outbreak study. Epidemiol. Infect. 2013;141(9):1965–1974. doi: 10.1017/S0950268812002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clayton D., Hills M. Oxford, GBR; OUP Oxford: 1993. Statistical Models in Epidemiology. [Google Scholar]
- 30.Zhang J., Yu K.F. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 31.Communicable Diseases Network Australia . Department of Health; 2015. National Notifiable Diseases Surveillance System - Current CDNA fortnightly Report. [Google Scholar]
- 32.Dumke R., Schnee C., Pletz M.W., Rupp J., Jacobs E., Sachse K. Mycoplasma pneumoniae and Chlamydia spp. infection in community-acquired pneumonia, Germany, 2011–2012. Emerg. Infect. Dis. 2015;21(3):426–434. doi: 10.3201/eid2103.140927. [DOI] [PMC free article] [PubMed] [Google Scholar]