Abstract
The aim of this study was to determine the resistance phenotypes of selected enteric bacteria isolated from non-human primates at a wildlife-human interface. Bacterial isolates from faecal samples of non-human primates at two wildlife rehabilitation centres in South Africa were screened for the presence of Escherichia coli. The biochemical characterisation of E. coli and E. coli-like bacteria revealed both adonitol positive and sorbitol negative strains – a unique characteristic of Escherichia fergusonii and Escherichia coli K99. Further tests were carried out to identify the isolates, namely growth on Simmons citrate agar supplemented with 2% adonitol and biochemical tests based on their ability to ferment cellobiose and d-arabitol. Antimicrobial sensitivity was determined with microbroth dilution tests employing microtitre plates with 21 different antimicrobial drugs. Molecular characterisation was done with a duplex polymerase chain reaction (PCR) assay that targeted the yliE and EFER_1569 genes. E. fergusonii strains were confirmed by the presence of a 233 bp segment of the yliE gene and a 432 bp segment of the EFER_1569 gene.
Twenty-three E. coli-like bacteria were confirmed as E. fergusonii based on the confirmatory tests and they were in 100% agreement. Approximately 87% of them were resistant to polymyxins B and E (colistin) as well as the carbapenem group with occasional resistance to amikacin.
This is the first reported isolation and identification of E. fergusonii strains in non-human primates. The findings point to E. fergusonii as a possible emerging pathogen of zoonotic importance.
Keywords: Escherichia fergusonii, Antimicrobial resistance, Non-human primates, Wildlife
1. Introduction
In the last four decades, descriptions of Escherichia blattae [5], E. hermanii [3], E. vulneris [4], E. fergusonii [7] and E. albertii [15] have been published as additions to the genus Escherichia. The recent identification of other species in this genus and genomic clades of Escherichia coli has been attributed to the improvement in diagnostic techniques [12]. As a result of the availability of an increasing variety of growth media and the introduction of PCR-based assays and whole genome sequencing, phenotypic similarities are not limiting factors anymore in differentiating between species of the genus Escherichia.
Escherichia fergusonii was proposed as a new species within the genus Escherichia and family Enterobacteriaceae in 1985 with a 64% similarity to Escherichia coli when analysed by means of DNA hybridisation [7]. This bacterial strain was formerly known as Enteric Group 10, due to its biochemically distinct nature compared to other species and bio-groups of the genus Enterobacteriaceae. DNA-DNA hybridisation to determine the relatedness of E. fergusonii strains to other species within the genus Enterobacteriaceae using 32P-labeled DNA in 60 °C hydroxyapatite, showed 90–97% relatedness to the type strain (holotype) E. fergusonii ATCC 35469. In relation to other species, the closest was Escherichia coli with up to 64% similarity [7].
E. fergusonii was initially isolated from human clinical samples collected from the blood, urine, abdominal wounds and faeces of patients [7]. It has been isolated from gall bladder fluids of patients [10] and from food products such as montasio cheese [17]. It has also been isolated from faecal samples of goats [13], sheep [1], horses [25], turkeys, ostriches [14], chickens [20], cattle and pigs [7].
Aside the recent report of E. fergusonii isolations in fish in Egypt [11]; no other reports of E. fergusonii isolations have been documented from Africa either in humans or in animals. Since the majority of reported E. fergusonii isolations in other parts of the world were case reports [12], data on host species dynamics, reservoirs and transmission are currently unknown.
2. Literature review
In common with other Escherichia species, E. fergusonii is a rod-shaped, Gram-negative member of the family Enterobacteriaceae. It is a non-spore-forming, predominantly motile, peritrichous flagellated bacterium. It has a diameter of 0.8–1.5 μm and lengths from 2 to 5 μm. E. fergusonii grows optimally on growth media at 37–40 °C under aerobic conditions with variations between 21 and 45 °C [12], [23].
Citrate adonitol agar has been reported to be an effective selective growth medium for isolating E. fergusonii from faecal samples [26]. This medium was first described in 1984 as a selective medium for the isolation of certain K99 E. coli strains based on their ability to ferment adonitol. Recent studies also employed the use of CHROMAgar Orientation media in isolating Escherichia species. Escherichia coli colonies appear pink on the growth medium whilst E. fergusonii colonies appear pink with brown discoloration surrounding the colonies [16].
Simmons et al. [24] described a novel PCR assay for the detection of E. fergusonii directly from caecal and cloacal samples of poultry without pre-enrichment. For that study, primers targeting specific genes, including yliE (encoding a conserved hypothetical protein of the cellulose synthase and regulator of cellulose synthase island), EFER_1569 (encoding a hypothetical protein, putative transcriptional activator for multiple antibiotic resistance), and EFER_3126 (encoding a putative triphosphoribosyl-dephospho-coenzyme A [CoA]), were designed for the detection of E. fergusonii by conventional and real-time PCR methods.
A fluorescent dye-labeled probe for the enumeration of E. fergusonii cells was tested by in situ hybridisation and epifluorescence microscopy and found to be able to stain cells of E. coli, Shigella spp. and E. fergusonii [22]. The specificity of the probe is limited to the aforementioned species; having being tested on 169 other species.
These molecular techniques improved the sensitivity and speed of diagnosis of E. fergusonii infections. As an opportunistic pathogen, usually isolated from infections in which the causative pathogen is initially unknown, molecular detection speeds up the detection/confirmation processes considerably [23].
Earlier experiments on E. fergusonii strains recorded resistance to ampicillin, tetracycline and co-trimoxazole and susceptibility to cephalosporin and netilmicin [6].
Lagacé-Wiens et al. [16] also reported the presence of large quantities (> 108) colony-forming units per litre of urine (CFU/l) of extended-spectrum β-lactamase (ESBL)-producing E. fergusonii in a 76-year-old Caucasian woman presented to the emergency department of a community hospital. This report was the first case of a clinical isolate of multidrug-resistant Escherichia fergusonii expressing an extended-spectrum-β-lactamase (ESBL).
Ampicillin-resistant E. fergusonii isolates from farm animals were tested for extended-spectrum β-lactamase (ESBL) phenotypes by double disc diffusion tests using three indicator cephalosporins: cefotaxime, ceftazidime and cefoxitin, both alone and in combination with amoxicillin-clavulanic acid (AMC). PCR and sequencing of SHV, CTX-M, and TEM β-lactamase genes as described by Mulvey et al. [19] revealed the presence of TEM-1 and SHV-12 genes – the presence of the latter conferring the ESBL phenotype [16].
In another study on day-old-chick models [8], it was observed after genome analysis of an isolated E. fergusonii strain that the existence of several resistance genes to multiple classes of antibiotics was present in the strain, thus making the treatment of infection caused by such E. fergusonii strains difficult when using currently available antimicrobials [8].
The presence of multiple resistance genes in their genomes gives rise to multi-drug resistant E. fergusonii strains. Most E. fergusonii strains that are isolated from both humans and animals – in cases where general first-line drug treatments for bacterial infections fail – are multi-drug resistant [23], [2].
3. Materials and methods
3.1. Study location and animals
Two wildlife rehabilitation centres located within 10 km of each other and approximately 30 km from the Kruger National Park served as study sites for this project. These centres were established to provide temporary sanctuary for injured and orphaned wildlife, rehabilitate them to a point of self-support and their ultimate release and introduction into nature, where they naturally belong. They rehabilitate and provide sanctuary to over 500 orphaned, injured, abused, ex-laboratory baboon and vervet monkey populations. In both centres, the non-human primates were classified into three groups and this influenced the sampling strategies.
The first group had regular human contact and were mostly orphaned juveniles that were fed, bathed and cuddled regularly. The second group of non-human primates had been weaned off frequent contact with humans with occasional contact when it was time to feed them. There was no cuddling or bathing of the animals in this group and they only came in contact with staff/volunteers during feeding lasting a maximum of 10 min, 3 times daily. The third group had previous human contact and lived in enclosures similar to their natural habitats with almost no human contact. They fed off natural shoots in their enclosures and food was only supplemented in harsh weather conditions when plants they feed on were not available.
3.2. Sample collection and processing
There were three major groups defined per rehabilitation centre for sampling purposes in line with the groups within the centres. Three hundred fresh faecal samples were obtained from vervets and baboons in sampling groups using sterile swabs immersed in Stuart's transport medium. The animals were anally swabbed early mornings between 6 and 7 am, for three days by staff at the centres. One-hundred-and-fifty (150) baboon faecal samples and fifty faecal samples from vervets were collected at one wildlife rehabilitation centre whilst another 100 faecal samples from vervets were collected at the second wildlife rehabilitation centre, in triplicates.
Sample size was random and representative of at least 25% of the total population.
3.3. Bacterial isolation
To isolate Gram-negative bacteria, the 300 faecal samples were inoculated on MacConkey agar under sterile conditions and incubated for 24 h at 37 °C. Suspect E. coli and E. coli-like isolates were then inoculated on Eosin Methylene Blue (EMB) agar.
3.4. Biochemical tests
Biochemical characterisation of E. coli and E. coli-like isolates were carried out using the Remel® RapID One panel strips (Remel, UK). Oxidase tests were done prior to the panel strip tests to ensure only oxidase-negative isolates were tested - as recommended by the manufacturer. Samples were transferred to inoculation fluids and then into wells imbibed with one reactive ingredient each and incubated at 37 °C for 4 h. The biochemical profiles were then determined using the Remel Eric system (Remel, UK) after incubation, based on colour changes in the inoculated wells - as indicated by the manufacturer.
Adonitol positive and sorbitol negative E. coli-like isolates were classified as suspected E. fergusonii strains and picked out for further testing. Two tests were carried out for further confirmation of E. coli and E. coli-like colonies, namely selective isolations on Simmons citrate agar supplemented with 2% adonitol [9] and biochemical tests based on their ability to ferment cellobiose and d-arabitol as described by Huys et al. [15].
3.5. Antimicrobial susceptibility tests
Antimicrobial susceptibility tests were carried out using the Sensititre GNX2F MIC microdilution plates (Trekds, UK) imbibed with 21 different antimicrobial drugs in serial dilutions in order to determine the minimum inhibitory concentrations. To determine the susceptibility of an isolate to the selected antimicrobials, 3–5 colonies of an isolate grown on fresh nutrient agar plates were picked, emulsified in sterile water and adjusted to a 0.5 McFarland Standard visually. 10 μl of the suspension was then transferred into a tube of cation-adjusted Mueller-Hinton broth (Trekds, UK) with TES buffer to give an inoculum of 1 × 105 cfu/ml. The procedure was repeated for 23 suspect E. fergusonii isolates.
All the plates were then incubated at 34–36 °C for 18–24 h in a non-CO2 incubator. The plates were read using the UV lamp readers commonly used for reading PCR products on agarose gels instead of the conventional MIC plate readers, for easier reading. Results were interpreted based on the CLSI MIC Interpretive guidelines except for the drug Colistin, which was unavailable on the CLSI guidelines and was interpreted based on the EUCAST MIC breakpoints. The drugs used in the test were particularly selected for this research because they are antimicrobial drugs often used in treating bacterial infections in humans in South Africa [18].
3.6. Duplex PCR
A duplex PCR for molecular confirmation of E. fergusonii targeting two genes, namely the yliE gene and the EFER_1569, were carried out on 23 isolates - as previously described by Simmons et al. [24].
The PCR conditions we used in our study were slightly modified to suit the Taq polymerase used in the reaction as well as to match the concentrations of DNA used. The reported [24] annealing temperature of 56.5 °C was adjusted to 60 °C and the number of cycles per reaction kept at 30. The 25 μl final volume of the PCR mixture contained 12.5 μl of Kapa 2G Fast Multiplex PCR kit (Kapa Biosystems, Cape Town, South Africa), 0.5 μM of each primer, 8.5 μl of molecular grade water, and 2 μl of bacterial DNA. The cycling conditions were 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 56.5 °C for 30 s, 72 °C for 30 s, and then a hold at 4 °C. The PCR products were separated on a 2% Tris–acetate-EDTA buffer agarose electrophoresis gel stained with ethidium bromide (1 μl/10 ml) and the bands were referenced to a GeneRuler 100 bp DNA ladder (Fermentas, Ottawa, Ontario) to size the amplicons [24]. Positive samples were confirmed by the presence of 233 bp of the yliE gene and 432 bp of the EFER_1569 gene.
4. Results
4.1. Biochemical tests
On the Remel RapID ONE plates, the E. coli-like bacteria were adonitol positive and sorbitol negative with test code number 4100271, which was on the further analysed on the Remel ERIC electronic compendium. The E. coli-like bacteria “passed” all the confirmatory biochemical tests and were adonitol positive, sorbitol negative and able to ferment cellobiose and d-arabitol. They produced yellow growths on Simmons citrate agar supplemented with 2% adonitol.
4.2. Antimicrobial susceptibility tests and resistance profiles
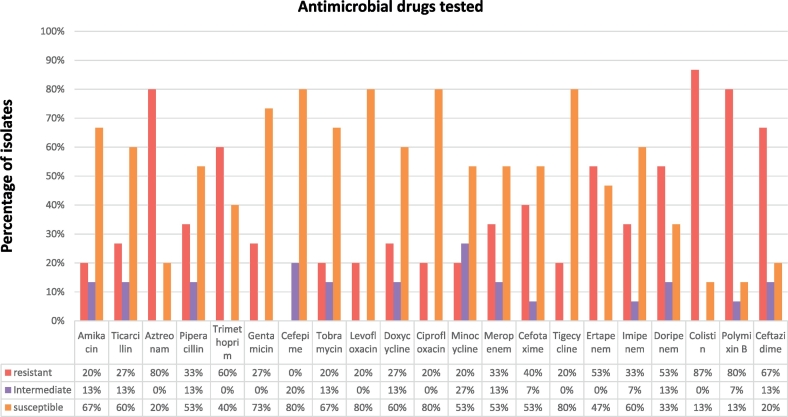
The drugs most bacteria were resistant to included polymyxin B and colistin. Seventy percent (i.e. 16 isolates) of the E. coli-like isolates were resistant to polymyxin B and colistin as well as to the carbapenems with occasional resistance recorded against aminoglycosides (Fig. 1). As compared to confirmed E. coli isolates isolated in the same research, the resistance profiles bore over 80% similarity with higher levels of resistance recorded in latter for all its isolates.
Fig. 1.
Antimicrobial sensitivity of 15 (randomly selected) out of the 23 Escherichia fergusonii isolates.
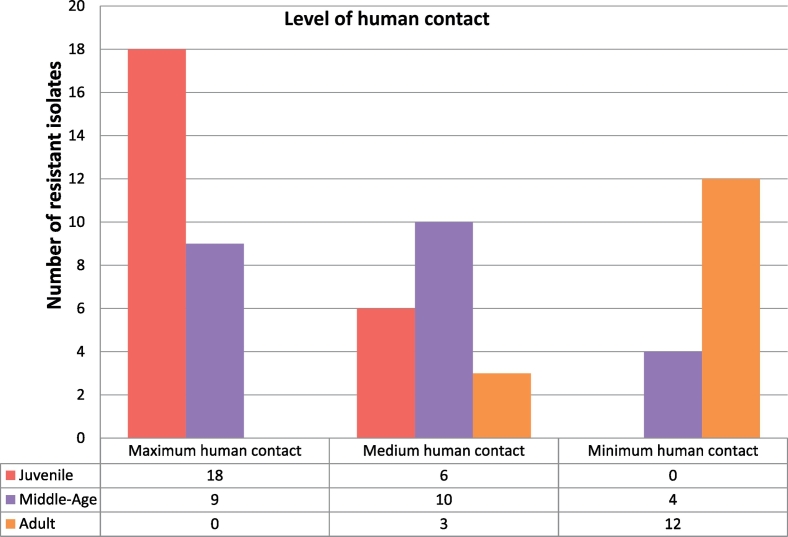
The highest level of resistance was observed in group 1 primates. Comparatively, group 1 had 20% and 23% (Table 1) higher numbers of resistant strains than group 2 and group 3 respectively. This was observed across all the antimicrobials tested, especially polymyxin B and colistin (Fig. 2).
Table 1.
The number of multi-drug resistant E. fergusonii strains isolated and the source of the isolates.
| E. fergusonii isolates from vervets | E. fergusonii isolates from baboons | ||
|---|---|---|---|
| Number of antimicrobial drugs E. fergusonii isolates were resistant to | 1 | 1 | 0 |
| 2 | 0 | 2 | |
| 3 | 2 | 1 | |
| 4 | 1 | 0 | |
| 5 | 0 | 2 | |
| 6 | 0 | 0 | |
| 7 | 1 | 0 | |
| 8 | 0 | 1 | |
| 9 | 1 | 1 | |
| 10 | 0 | 0 | |
| ≥ 10 | 2 | 0 | |
| Total number | 8 | 7 | |
| % Multi resistant (≥ 3) | 87.5 | 71.4 | |
Fig. 2.
A representation of the number of multi-drug resistant E. coli-like isolates in the sampling group.
4.3. Screening for yliE and EFER_1569 genes - molecular identification of E. fergusonii
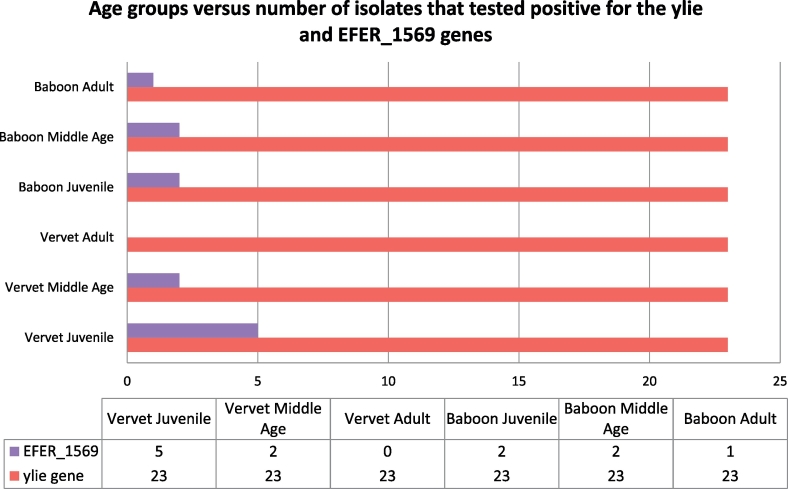
All 23 E. coli – like isolates screened were positive for yliE genes whilst 12 were positive for EFER_1569 (Fig. 3).
Fig. 3.
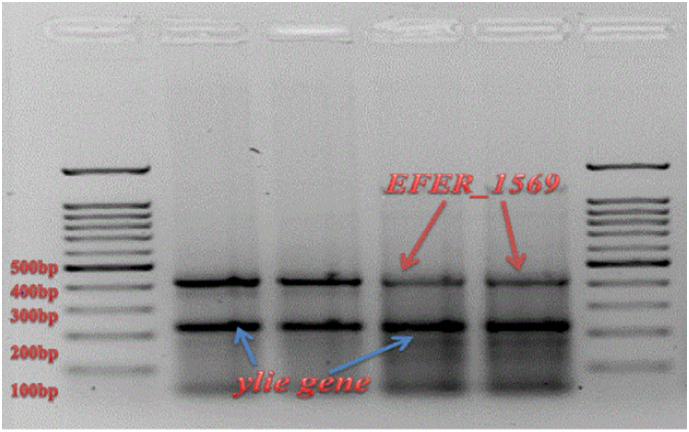
PCR assay product (gel image) depicting the presence of yliE (233 bp) and EFER_1569 (432 bp) genes in selected E. fergusonii isolates.
5. Discussion
All E. fergusonii bacteria isolated from juvenile vervet monkeys in both facilities yielded positive results with the molecular detection of EFER_1569 (multiple antimicrobial resistance) whilst in the other groups, variable amplification of EFER_1569 was observed (Fig. 4). The protein encoded by the yliE gene synthesises biofilms for colonisation resistance in commensal Escherichia spp. (233 bp) whilst the EFER_1569 gene encodes a transcriptional activator for multiple antibiotic resistances (432 bp) solely in E. fergusonii [24].
Fig. 4.
The results of the PCR assay to detect the presence of yliE and EFER_1569 genes in the E. fergusonii isolates.
The highest level of resistance was observed in group 1. Comparatively, group 1 had 20% and 23% higher levels of resistance than group 2 and group 3 respectively. This was observed for all the antimicrobials tested, and especially polymyxin B and colistin. Decreasing levels of antimicrobial resistance were observed with increasing age, with isolates from older animals being less resistant. This resistance profile was similar in both camps. An 87.5% multi-drug resistance was recorded for isolates from vervets tested against antimicrobial drugs whilst those from baboons totalled 71.4%.
This is the first documented isolation and confirmation of E. fergusonii in South Africa. It is also the first report of successful isolation from non-human primates globally. The presence of EFER_1569 genes in all the isolates from young vervets that had consistent human contact is suggestive of the possible routes of transmission into the non-human primate population. However, this cannot be confirmed without molecular fingerprinting analyses, which would infer insights into the transmission.
Consistent with published literature, primate groups with frequent human contact had higher levels of bacteria resistant to antimicrobial drugs than those with less contact [27], [28], [29], [30], [31]. Resistant bacteria were also observed to diminish with the age of the animals.
Polymyxin B sulphate and colistin had not been used for many years (from the 1970′s to early 2000) for the treatment of bacterial infection in humans because less toxic antimicrobials were available [32], [33]. However, increasing resistance to the carbapenems (such as imipenem and meropenem) – which are often considered as the last resort treatment for nosocomial infections caused by multi-resistant Gram-negative organisms – has resulted in a return of the polymyxins and colistin [34]. It's re-use started early in the 21st century [33], [35]. Thus, the high rates of resistance shown by all the Gram-negative strains isolated in this project are not unexpected (since they are not new drugs) but of concern because of the dependence of current antimicrobial treatment programmes of humans on these drugs.
The presence of multi-drug resistant E. fergusonii strains in the vervet monkeys and baboons may be attributed to two factors:
Firstly, E. fergusonii strains may have been present in Africa long enough to acquire drug resistance but have not been identified. Since reported isolations outside of Africa have mostly been coincidental, it can be hypothesised that it is not a novel strain in Africa but has eluded detection. Its similarity to E. coli, Shigella and even Salmonella strains phenotypically have probably contributed to its lack of detection until recently.
Alternatively, international volunteers may be responsible for the introduction of the strains into the non-human primate populations. The rehabilitation centres are both run by international volunteers who travel to South Africa to work with these primates for a few weeks before returning to their countries. It is possible that these strains were transferred from some volunteers to these animals during handling. E. fergusonii has been identified as an emerging pathogen on every other continent except Africa. Thus, it may have been transferred from international visitors to local primates. Since the human handlers were not sampled, these assertions remain speculative.
The mcr-1 colistin resistance gene has been recently reported in Avian-Pathogenic Escherichia coli in South Africa [21]. In that same report, the presence of the gene is associated with travellers and the trade of food animals, supporting the earlier assertions. At this point, however, in the primates, this is conjecture and could not be verified for the purpose of this article.
6. Conclusion
Evidence is accumulating that suggests that E. fergusonii is an emerging multi-drug resistant pathogen that requires further investigation. Its zoonotic potential must be substantiated in order to implement control measures for possible future outbreaks. The absence of data from Africa must also be addressed and further research must be undertaken to investigate E. fergusonii populations both in humans and in animals. As an opportunistic pathogen, its role in infections of immunocompromised patients must also be studied especially in regions with high tuberculosis and HIV infection rates. Further research on the relatedness of E. coli K99 and E. fergusonii.
Funding
None.
Competing interests
None declared.
Ethical approval
Animal Ethics Committee, University of Pretoria. Reference number: V076-13.
References
- 1.Bain M., Green C. Isolation of Escherichia fergusonii in cases clinically suggestive of salmonellosis. Vet. Rec. 1999;144:511. [PubMed] [Google Scholar]
- 2.Fricke W.F., McDermott P.F., Mammel M.K., Zhao S., Johnson T.J., Rasko D.A., Fedorka-Cray P.J., Pedroso A., Whichard J.M., Leclerc J.E., White D.G., Cebula T.A., Ravel J. Antimicrobial resistance-conferring plasmids with similarity to virulence plasmids from avian pathogenic Escherichia coli strains in Salmonella enterica serovar Kentucky isolates from poultry. Appl. Environ. Microbiol. 2009;75:5963–5971. doi: 10.1128/AEM.00786-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner D.J., Davis B.R., Steigerwalt A.G., Riddle C.F., McWorther A.C., Allen S.D. Atypical biogroups of Escherichia coli found in clinical specimens and description of Escherichia hermanii sp. nov. J. Clin. Microbiol. 1982;15:703–713. doi: 10.1128/jcm.15.4.703-713.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner D.J., McWorther A.C., Knutson J.K., Steigerwalt A.G. Escherichia vulneris: a new species of Enterobacteriaceae associated with human wounds. J. Clin. Microbiol. 1982;15:1133–1140. doi: 10.1128/jcm.15.6.1133-1140.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess N.R., McDermott S.N., Whiting J. Aerobic bacteria occurring in the hind-gut of the cockroach, Blatta orientalis. J. Hyg. 1973;71:1–7. doi: 10.1017/s0022172400046155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhury A., Nath G., Tikoo A., Sanyal S.C. Enteropathogenicity and antimicrobial susceptibility of new Escherichia spp. J. Diarrhoeal Dis. Res. 1999;17:85–87. [PubMed] [Google Scholar]
- 7.Farmer J.J., III, Fanning G.R., Davis B.R., O'Hara C., Riddle C., Hickman-Brenner F.W. Escherichia fergusonii and Enterobacter taylorae, two new species of Enterobacteriaceae from clinical specimens. J. Clin. Microbiol. 1985;21:77–81. doi: 10.1128/jcm.21.1.77-81.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forgetta V., Rempel H., Malouin F., Vaillancourt R., Jr., Topp E., Dewar K. Pathogenic and multidrug-resistant Escherichia fergusonii from broiler chicken. Poult. Sci. 2012;91:512–525. doi: 10.3382/ps.2011-01738. [DOI] [PubMed] [Google Scholar]
- 9.Foster G., Evans J., Tryland M., Hollamby S., MacArthur I., Gordon E. Use of citrate adonitol agar as a selective medium for the isolation of Escherichia fergusonii from a captive reindeer herd. Vet. Microbiol. 2010;144:484–486. doi: 10.1016/j.vetmic.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Funke G., Hany A., Altwegg M. Isolation of Escherichia fergusonii from four different sites in a patient with pancreatic carcinoma and cholangiosepsis. J. Clin. Microbiol. 1993;31:2201–2203. doi: 10.1128/jcm.31.8.2201-2203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaafar A.Y., Younes A.M., Kenawy A.M., Soliman W.S., Mohamed L.A. Escherichia fergusonii: a new emerging bacterial disease of farmed Nile Tilapia (Oreochromis niloticus) Glob. Vet. 2015;14(2):268–273. [Google Scholar]
- 12.Gaastra W., Kusters J.G., van Duijkeren E., Lipman L.J. Escherichia fergusonii. Vet. Microbiol. 2014;172(1–2):7–12. doi: 10.1016/j.vetmic.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Hariharan H., Lopez A., Conboy G., Coles M., Muirhead T. Isolation of Escherichia fergusonii from the feces and internal organs of a goat with diarrhoea. Can. Vet. J. 2007;48:630–631. [PMC free article] [PubMed] [Google Scholar]
- 14.Herráez P., Rodriguez F., Espinosa de los Monteros A., Acosta B., Jaber J., Castellano J., Castro A. Fibrino-necrotic typhilitis caused by Escherichia fergusonii in ostriches. Avian Dis. 2005;49:167–169. doi: 10.1637/7221-061104r. [DOI] [PubMed] [Google Scholar]
- 15.Huys G., Cnockaert M., Janda J.M., Swings J. Escherichia albertii sp. nov., a diarrhoeagenic species isolated from stool specimen of Bangladeshi children. Int. J. Syst. Evol. Microbiol. 2003;53:807–810. doi: 10.1099/ijs.0.02475-0. [DOI] [PubMed] [Google Scholar]
- 16.Lagacé-Wiens P.R.S., Baudry P.J., Pang P., Hammond G. First description of an extended-spectrum-b-lactamase producing multi-drug resistant Escherichia fergusonii strain in a patient with cystitis. J. Clin. Microbiol. 2010;48:2301–2302. doi: 10.1128/JCM.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maifreni M., Frigo F., Bartolomeoli I., Innocente N., Biasutti M., Marino M. Identification of the Enterobacteriaceae in Montasio cheese and assessment of their amino acid decarboxylase activity. J. Dairy Res. 2013;80:122–127. doi: 10.1017/S002202991200074X. [DOI] [PubMed] [Google Scholar]
- 18.Mendelson M., Whitelaw A., Nicol M., Brink A. Wake up South Africa! The antibiotic ‘horse’ has bolted. S. Afr. Med. J. 2012;102(7):607–608. doi: 10.7196/samj.5759. [DOI] [PubMed] [Google Scholar]
- 19.Mulvey M.R., Bryce E., Boyd D., Ofner-Agostini M., Christianson S., Simor A.E. Ambler class A extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp. in Canadian hospitals. Antimicrob. Agents Chemother. 2004;48:1204–1214. doi: 10.1128/AAC.48.4.1204-1214.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh J.-Y., Kang M.-S., An B.-K., Shin E.-G., Kim M.-J., Kwon J.-H. Isolation and epidemiological characterisation of heat labile enterotoxin producing Escherichia fergusonii from healthy chickens. Vet. Microbiol. 2012;160:170–175. doi: 10.1016/j.vetmic.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Perreten V., Strauss C., Collaud A., Gerber D. Colistin resistance gene mcr-1 in avian-pathogenic Escherichia coli in South Africa. Antimicrob. Agents Chemother. 2016;60:4414–4415. doi: 10.1128/AAC.00548-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regnault B., Martin-Delautre S., Lejay-Collin M., Lefèvre M., Grimont P.A. Oligonucleotide probe for the visualisation of Escherichia coli/Escherichia fergusonii cells by in situ hybridisation: specificity and potential applications. Res. Microbiol. 2000;151(7):521–533. doi: 10.1016/s0923-2508(00)00222-9. [DOI] [PubMed] [Google Scholar]
- 23.Savini V., Catavitello C., Talia M., Manna A., Pompetti F., Favaro M. Multidrug-resistant Escherichia fergusonii: a case of acute cystitis. J. Clin. Microbiol. 2008;46:1551–1552. doi: 10.1128/JCM.01210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons K., Rempel H., Block G., Forgetta V., Vaillancourt R., Jr., Mal-ouin F. Duplex PCR methods for the molecular detection of Escherichia fergusonii isolates from broiler chickens. Appl. Environ. Microbiol. 2014;80:1941–1948. doi: 10.1128/AEM.04169-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss A.T.A., Lubke-Becker A., Krentz M., van der Grinten E. Enteritis and septicemia in a horse associated with infection by Escherichia fergusonii. J. Equine Vet. Sci. 2011;31:361–364. [Google Scholar]
- 26.Wragg P., La Ragione R.M., Best A., Reichel R., Anjum M.F., Mafura M. Characterisation of Escherichia fergusonii isolates from farm animals using an Escherichia coli virulence gene array and tissue culture adherence assays. Res. Vet. Sci. 2009;86:27–35. doi: 10.1016/j.rvsc.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Blanco G., Lemus J.A., Grande J. Microbial pollution in wildlife: linking agricultural manuring and bacterial antimicrobial resistance in red-billed choughs. Environ. Resist. 2009;109:405–412. doi: 10.1016/j.envres.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Kozak G.K., Boerlin P., Janecko N., Reid-Smith R.J., Jardine C. Antimicrobial resistance in Escherichia coli isolates from swine and wild mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl. Environ. Microbiol. 2009;75:559–566. doi: 10.1128/AEM.01821-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Literak I. Highly variable patterns of antimicrobial resistance in commensal Escherichia coli isolates from pigs, sympatric rodents, and flies. Microb. Drug Resist. 2009;15:229–237. doi: 10.1089/mdr.2009.0913. [DOI] [PubMed] [Google Scholar]
- 30.Cole D. Free-living Canada geese and antimicrobial resistance. Emerg. Infect. Dis. 2005;11:935–938. doi: 10.3201/eid1106.040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rolland R.M., Hausfater G., Marshall B., Levy S.B. Antibiotic resistant bacteria in wild primates: increased prevalence in baboons feeding on human refuse. Appl. Environ. Microbiol. 1985;49:791–794. doi: 10.1128/aem.49.4.791-794.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans M.E., Feola D.J., Rapp R.P. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant Gram-negative bacteria. Ann. Pharmacother. 1999;33(9):960–967. doi: 10.1345/aph.18426. [DOI] [PubMed] [Google Scholar]
- 33.Biswas S., Brunel J.M., Dubus J.C., Reynaud-Gaubert M., Rolain J.M. Colistin: an update on the antibiotic of the 21st century. Expert Rev. Anti-Infect. Ther. 2012;10:917–934. doi: 10.1586/eri.12.78. [DOI] [PubMed] [Google Scholar]
- 34.Michalopoulos A.S., Karatza D.C. Multidrug-resistant Gram-negative infections: the use of colistin. Expert Rev. Anti-Infect. Ther. 2010;8:1009–1017. doi: 10.1586/eri.10.88. [DOI] [PubMed] [Google Scholar]
- 35.Dhariwal A.K., Tullu M.S. Colistin: Re-emergence of the 'forgotten' antimicrobial agent. J. Postgrad. Med. 2013;59:208–215. doi: 10.4103/0022-3859.118040. [DOI] [PubMed] [Google Scholar]