Abstract
Background
Many echocardiographic parameters have been proposed to evaluate right ventricular (RV) systolic function. We comprehensively assessed a wide range of quantitative echocardiographic parameters in a single cohort compared with same-day cardiovascular magnetic resonance (CMR).
Methods and results
92 subjects were examined prospectively: Group 1 consisted of 46 healthy controls (21 males, 33.4 ± 11.4 years), Group 2 consisted of 46 patients (20 males, 38.5 ± 18.9 years) undergoing RV functional assessment by CMR (1.5 T). Echocardiography was performed on the same day as CMR; fractional area change (RVFAC), myocardial performance index via spectral Doppler (RVMPI), RVMPI via Doppler tissue imaging (RVMPI-DTI), peak systolic myocardial velocity by DTI (RVSm), tricuspid annular plane systolic excursion (TAPSE), speckle tracking strain, and three dimensional right ventricular ejection fraction (3DE-RV). Linear regression, Bland–Altman and receiver-operator-characteristic (ROC) analyses were performed. At ROC analysis, the most predictive echocardiographic methods were; RVFAC (AUC = 0.892), RVMPI (AUC 0.785), TAPSE (AUC 0.849) and 3DE-RV (AUC 0.909). 3DE-RV appeared the most accurate compared to CMR, although underestimated true RV volumes.
Conclusion
As compared to CMR; 3DE-RV, RVFAC, TAPSE and RVMPI were the most reliable predictors of RV function. These parameters can be recommended for clinical use.
Abbreviation: 3DE-RV, three-dimensional echo right ventricular ejection fraction; CMR, cardiovascular magnetic resonance; DTI, Doppler tissue imaging; ε, strain; EF, ejection fraction; IVCT, isovolumic contraction time; IVRT, isovolumic relaxation time; LV, left ventricle; MPI, myocardial performance index; RVSm, peak systolic myocardial velocity; 3DE, three dimensional echocardiography; RV, right ventricular; RVOT, right ventricular outflow tract; RVSm, s prime: right ventricular peak systolic myocardial velocity; SR, strain rate; TAPSE, tricuspid annular peak systolic excursion; TOF, tetralogy of Fallot; TR, tricuspid regurgitation
Keywords: Echocardiography, Right ventricle, Right ventricular function, Magnetic resonance imaging
1. Introduction
Right ventricular (RV) systolic function is prognostically significant in the management of various cardiac conditions including many congenital abnormalities, cardiomyopathy, heart failure, valvular pathologies, and pulmonary hypertension [1], [2], [3], [4]. Accurate quantitative assessment of this chamber is crucial for informing clinical decisions [5].
Several echocardiographic parameters for the quantitative assessment of RV systolic function have been studied (Table 1). Each method, however, has limitations. Echocardiographic methods are challenged by the uniquely crescentic and highly trabeculated anatomy of the RV. The RV is also positioned retro-sternally and anterior to the LV which can result in a differing size and functional appearance depending on the axis in which it is viewed [6].
Table 1.
Echocardiographic methods & limitations for the assessment of RV function.
| Echocardiographic method | Limitations |
|---|---|
| Qualitative assessment | Inter-observer variability, poorly defined endocardium (8). |
| RV Ejection Fraction | Poorly defined endocardium, requirement of 2 orthogonal views with a common long axis and failure to include the infundibulum (9). Relies on geometric assumptions (8). |
| Doppler Tissue Imaging (DTI) | Does not take segmental function into account, is affected by load and heart rate (9). Is sensitive to Doppler cursor alignment. |
| Three-dimensional echocardiography (3DE) (8) | Requires high quality images of the right ventricle, poorly defined endocardium produces inaccurate results (10). |
| Myocardial Performance Index (MPI) (11) | Does not take segmental function into account, is affected by load and heart rate (9). |
| Tricuspid annular peak systolic excursion (TAPSE) (12) | Is sensitive to Doppler curser alignment, does not take segmental function into account, is affected by load and heart rate (9). |
| Doppler Strain (ε) and Strain Rate Imaging (6) | Angle dependent, poor signal to noise ratio and is load sensitive (13). Is sensitive to Doppler curser alignment. |
| 2D Strain (ε) and Strain Rate Imaging (6) | Motion of myocardium perpendicular to the ultrasound beam has a higher degree of error than DTI strain, through plane motion (affecting the arrangement of speckles between frames) could result in errors, lower temporal resolution (17) |
At the present time, transthoracic echocardiography (TTE) is the most commonly used method for assessing RV function as it is non-invasive, inexpensive and widely available throughout hospitals and private institutions.
We comprehensively assessed a wide range of quantitative echocardiographic parameters of RV function in a single, large cohort compared with the reference standard of cardiovascular magnetic resonance (CMR). We aimed to identify the most accurate parameters for predicting quantitative RV systolic function, and thence to derive normal cut-off values which could be incorporated into routine TTE examination.
2. Methods
This study was approved by the local human research ethics committee, and all participants gave informed consent.
We examined a total of 92 subjects in a prospective manner: Group 1 consisted of 46 healthy age-matched control subjects investigated under the study protocol; Group 2 consisted of 46 patients undergoing routine clinical CMR for quantitative evaluation of RV function. All subject's age, gender and body surface area (BSA), were recorded at the time of the study. Table 2 lists the demographics and clinical characteristics of subjects within each group. The majority of the subjects in group 2 were being evaluated by CMR for congenital heart disease, including tetralogy of Fallot, arrythmogenic right ventricular dysplasia, Ebstein's anomaly, pulmonary hypertension and septal defects (Table 3). Of the subjects in group 2, 13 subjects (29%) were found to have pulmonary hypertension by echo (RVSP > 40 mmHg) and 17 subjects (38%) were found to have grade ≥ 2/4 tricuspid regurgitation (TR). The controls were assessed by a consultant cardiologist for normal cardiac status via a standard 12-lead ECG, blood pressure assessment, clinical examination and a questionnaire. One test patient was excluded due to limitations of the CMR equipment (non-compatible implant). The population group was indicative of a ‘real life’ data set in that no patients were excluded due to poor image quality and all measurements were able to be obtained.
Table 2.
Demographic and clinical characteristics of study groups.
| Parameter | Control (group1) | Test (group2) |
|---|---|---|
| Gender (M:F) | 21:25 | 20:25 |
| Age (yrs) | 33.4 ± 11.4 | 38.5 ± 18.9 |
| BSA (m2) | 1.84 ± 0.20 | 1.79 ± 0.24 |
Age and BSA values are expressed as mean ± standard deviation.
Table 3.
Mean and standard deviations for all RV parameters (function and volume).*
| Group | N | Mean | SD | Std. error mean | Sig. (2-tailed) | |
|---|---|---|---|---|---|---|
| CMR data Right ventricular ejection fraction (%) |
Control | 46 | 55.72 | 4.810 | .709 | 0.000 |
| Test | 45 | 46.98 | 8.220 | 1.225 | ||
| CMR Indexed Diastolic Volume/BSA (ml/m2) |
Control | 46 | 95.500 | 15.8909 | 2.3430 | 0.000 |
| Test | 45 | 122.044 | 33.5003 | 4.9939 | ||
| 3DE Indexed Diastolic Volume/BSA (ml/m2) |
Control | 46 | 51.122 | 13.0717 | 1.9273 | 0.000 |
| Test | 45 | 90.158 | 27.5984 | 4.1141 | ||
| 2-dimensional echo parameters: | ||||||
| 3DE-RV(%) | Control | 46 | 55.09 | 4.834 | .713 | 0.000 |
| Test | 46 | 45.39 | 10.786 | 1.590 | ||
| RVFAC (%) | Control | 46 | 44.95 | 4.644 | .685 | 0.000 |
| Test | 46 | 34.36 | 11.945 | 1.761 | ||
| RVMPI (unitless) | Control | 46 | .243 | .1064 | .0157 | 0.007 |
| Test | 46 | .327 | .1763 | .0260 | ||
| RVMPI (DTI) (unitless) | Control | 46 | .4620 | .09280 | .01368 | 0.946* |
| Test | 46 | .4639 | .17091 | .02520 | ||
| RVSm (cm/s) | Control | 46 | 12.986 | 1.6661 | .2457 | 0.000 |
| Test | 46 | 9.811 | 2.3013 | .3393 | ||
| TAPSE (cm) | Control | 46 | 2.2093 | .41809 | .06164 | 0.000 |
| Test | 46 | 1.6200 | .54577 | .08047 | ||
| DTI ε Systolic_Base (%) | Control | 46 | − 20.009 | 13.5976 | 2.0049 | 0.017* |
| Test | 46 | − 13.585 | 11.7582 | 1.7336 | ||
| DTI ε Systolic_Mid (%) | Control | 46 | − 25.026 | 11.6670 | 1.7202 | 0.005 |
| Test | 46 | − 18.152 | 11.2605 | 1.6603 | ||
| DTI ε Systolic_Apical (%) | Control | 46 | − 26.174 | 14.2436 | 2.1001 | 0.001 |
| Test | 46 | − 16.039 | 12.6333 | 1.8627 | ||
| Speckle tracking ε base (%) | Control | 46 | − 21.528 | 14.6809 | 2.1646 | 0.008 |
| Test | 46 | − 14.672 | 9.0193 | 1.3298 | ||
| Speckle tracking ε mid (%) | Control | 46 | − 29.535 | 10.7568 | 1.5860 | 0.000 |
| Test | 46 | − 19.141 | 10.6114 | 1.5646 | ||
| Speckle tracking ε apex (%) | Control | 46 | − 28.335 | 10.4242 | 1.5370 | 0.003 |
| Test | 46 | − 21.222 | 11.5258 | 1.6994 |
No significant difference between control and test subject means at p ≥ 0.01 for all parameters.
3. CMR assessment
CMR imaging was performed on a 1.5 Tesla GE Signa Twinspeed system (GE Medical Systems, Milwaukee, WI, USA) with an 8-element cardiac phased array coil. Cine images were acquired using a steady state free precession (SSFP) acquisition (TE 1.3 ms, TR 3.0 ms, flip angle 45, bandwidth +/− 125 kHz, FOV 35 cm, slice thickness 8 mm, gap 2 mm, matrix 224 × 224, number of averages 1). Twenty cardiac phases per slice location were reconstructed. All images were acquired at end expiration using respiratory bellows. Quantitative analysis of the right ventricle (including end diastolic volume, end systolic volume and ejection fraction) was performed using the modified RV short axis series, which has been shown to have increased accuracy and reproducibility [7].
4. Echocardiographic assessment
Echocardiography was performed on the same day as CMR using a commercially available ultrasound platform (iE33, Philips Medical Systems, Andover, Massachusetts) with an S5–1 transducer and an X3–1 matrix-array transducer. Two-dimensional (2D), motion-mode (M-mode), Pulsed-wave (PW) Doppler, Continuous-wave (CW) Doppler, DTI and three-dimensional (3DE) imaging was performed primarily from standard apical 4-chamber view. Atypical views were not utilized in this study to ensure reproducibility. RV quantitative parameters were consistent with current American Society of Echocardiography (ASE) guidelines [8] and acquired as follows; fractional area change (RVFAC), myocardial performance index via spectral Doppler (RVMPI), RVMPI via Doppler tissue imaging (RVMPI-DTI), peak systolic myocardial velocity by DTI (RVSm), tricuspid annular plane systolic excursion (TAPSE), strain (ε) and strain rate (SR) rate via DTI, ε and SR via speckle tracking, and three dimensional right ventricular ejection fraction (3DE-RV). Images were stored digitally on the Prosolv database (Prosolv Cardiovascular Analyser, Indianapolis). 3D images were obtained from the apical 4-chamber view with the patient in the left lateral decubitus position. Full volume loops were acquired over four cardiac cycles with held respiration and analysis of RV function was performed offline. Strain and SR images were obtained from the apical 4-chamber view, and a clip of three consecutive cycles obtained for off line analysis. Using QLAB software (Philips), 3DE-RV and peak ε/SR systolic velocities were taken at the basal, mid and apical segments. The RV systolic period was defined as the time from pulmonary valve opening (PVO) to pulmonary valve closure (PVC).
5. Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) unless otherwise stated. Demographic and clinical characteristics of the subject groups were assessed for significant differences using a two-tailed t-test assuming equal variances.
To compare test modalities (CMR and echo parameters) for significant similarity based on the raw data, Kendall's tau non-parametric correlation analysis was performed. Linear regression analyses were performed to assess the relationship between CMR and echo parameters. The CMR RVEF parameter was identified as the dependent variable while the echo parameters of RV function assessment were identified as the independent variables. For 3D RVEF, CMR REVF and volume comparisons, Bland–Altman analysis was performed to assess the level of agreement. The results of all testing parameters including CMR and echo parameters were placed into the categories of normal or abnormal RV function based on published values. McNemar Chi-squared analyses were conducted to evaluate for normal and abnormal RV function as referenced by CMR. Correlation coefficients were computed for the seven RV function analysis methods, being 3DE-RV, RVFAC, RVMPI, RVMPI (DTI), RVSm, TAPSE and ε and SR (18 parameters as identified in Table 3). Using the modified Bonferroni approach to control for Type 1 errors across the 18 correlations, a p value of less than 0.01 was required for significance. Receiver operating characteristic curves were then applied to evaluate the predictive ability of echo parameters compared to CMR RVEF.
6. Results
There was no significant difference noted in ages between group 1 and group 2 (p = 0.10). The mean and standard deviations for RV function parameters between the two groups are demonstrated in Table 3. The results of the correlation analyses (Kendall's tau) show that 4 echo parameters were statistically similar when compared to CMR RVEF (p < 0.01). CMR RVEF had a significant correlation with 3DE-RV, RVFAC, RVSm and TAPSE (p < 0.01). The other fourteen echo parameters demonstrated no significant similarity to CMR RVEF (p ≥ 0.01).
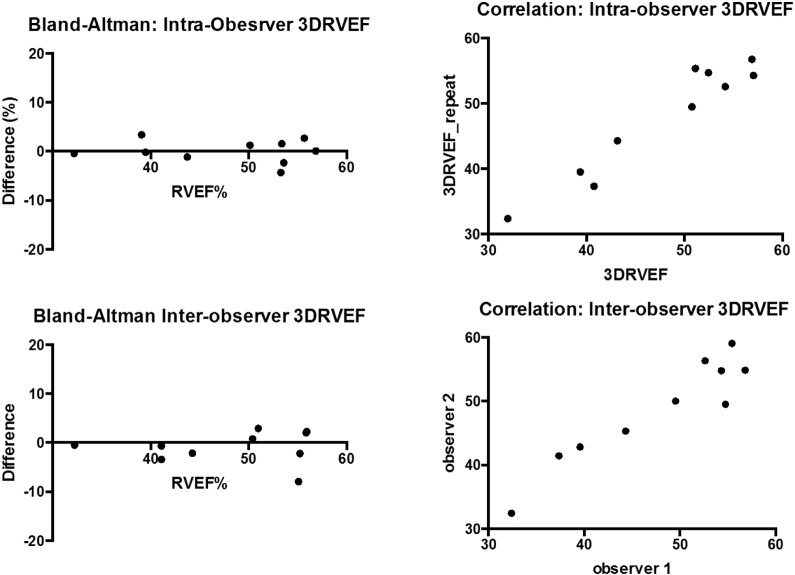
Intra-observer and inter-observer variation with regard to RV measurement using echo parameters was minimal. Measurement of variation was analysed using Pearson and Bland Altman techniques and showed no significant difference between observers or with re-measurement with a single observer (Fig. 4).
Fig. 4.
Bland–Altman analysis of inter-observer reproducibility of 3D-RVEF.
The currently used normal RVEF as estimated via echocardiography is 67 +/− 8% [9]; while the CMR normal RVEF is 49–72% [10]. In the assessment of both groups, 4 echo parameters produced significantly similar results to CMR RVEF (p > 0.05) while 15 parameters were not significantly similar. The significantly similar parameters were RVFAC, RVMPI, TAPSE and 3DE-RVusing CMR reference ranges (Table 4).
Table 4.
McNemar Chi-Square tests demonstrating statistical similarity to published reference ranges.
| N | Exact Sig (2-tailed) | |
|---|---|---|
| CMR EF & RVFAC | 91 | 0.388⁎ |
| CMR EF & RVMPI | 91 | 0.629⁎ |
| CMR EF & TAPSE | 91 | 0.791⁎ |
| CMR EF & 3DE-RV(CMR ref. ranges) | 91 | 0.607⁎ |
Significant similarity at p > 0.05.
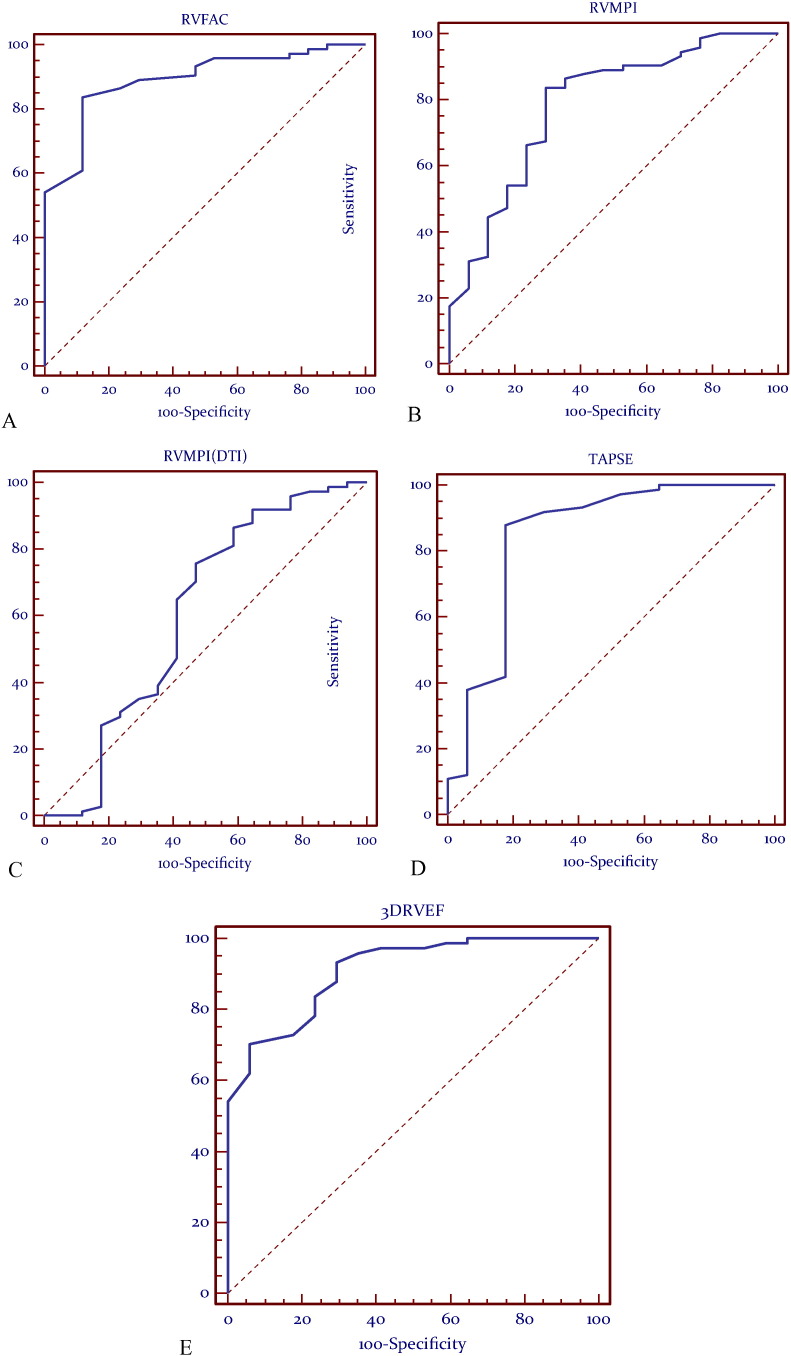
At receiver operating characteristic (ROC) analysis the most predictive models were identified as RVFAC, RVMPI, TAPSE and 3DE-RV (Fig. 1) (p = 0.0001). RVMPI using Doppler tissue imaging had an AUC of 0.606 and a p value of 0.18 which does not suggest a high level of validity or predictive ability (Table 5). Cut-off values to separate normal from abnormal RV function were determined for each parameter, based on these data (Table 4).
Fig. 1.
Receiver-operator characteristic (ROC) curves demonstrating validity of (A) RVFAC, (B) RVMPI, (C) RVMPI-DTI, (D)TAPSE, (E) 3DE as compared to CMR RVEF.
Table 5.
Combined groups: ROC curves.
| Echo method | Sensitivity (%) | Specificity (%) | Area under curve (95% CI) | Determined cut off | Significance level (p) |
|---|---|---|---|---|---|
| RVFAC (%) | 83.78 | 88.24 | 0.892 | ≥ 36% | 0.0001 |
| RVMPI | 83.78 | 70.59 | 0.785 | < 0.37 | 0.0001 |
| RVMPI-DTI | 75.68 | 52.94 | 0.606 | < 0.51 | 0.1800 |
| TAPSE (cm) | 87.84 | 82.35 | 0.849 | ≥ 1.50 | 0.0001 |
| 3DE-RV(%) | 70.27 | 94.12 | 0.909 | ≥ 50% | 0.0001 |
| Comparison of RV echo parameters | |||
|---|---|---|---|
| CMR EF ≥ 45% (n = 74) |
< 45% (n = 17) |
P value | |
| RV strain (%) | |||
| a) 3 segments (mid, apical, apex) | − 25.1 ± 7.7 | − 15.6 ± 4.9 | < 0.0001 |
| b) 4 segments (basal, mid, apical, apex) | − 24.6 ± 6.1 | − 16.5 ± 4.6 | < 0.0001 |
| c) 7 segments | − 22.4 ± 4.5 | − 15.3 ± 4.2 | < 0.0001 |
| RVFAC (%) | 42.6 ± 8.3 | 29.2 ± 10.3 | < 0.0001 |
| RVMPI | 0.26 ± 0.13 | 0.39 ± 0.19 | 0.0009 |
| TAPSE (cm) | 2.0 ± 0.5 | 1.5 ± 0.6 | < 0.0001 |
| 3D RVEF (%) | 53.4 ± 5.7 | 39.4 ± 9.7 | < 0.0001 |
| Logistic regression analysis using RV echo parameters | ||
|---|---|---|
| Odds ratio (95%CI) | P value | |
| RV strain (3 segments: mid, apical, apex) | 1.06 (0.93–1.24) | 0.40 |
| RV FAC | 1.08 (0.99–1.18) | 0.09 |
| 3D RVEF | 1.26 (1.08–1.53) | 0.0009 |
| RV strain (4 segments) | 1.09 (0.93–1.31) | 0.30 |
| RV FAC | 1.08 (0.99–1.18) | 0.10 |
| 3D RVEF | 1.24 (1.08–1.52) | 0.01 |
| RV strain (7 segments) | 1.22 (0.98–1.57) | 0.09 |
| RV FAC | 1.08 (0.99–1.19) | 0.09 |
| 3D RVEF | 1.21 (1.05–1.47) | 0.03 |
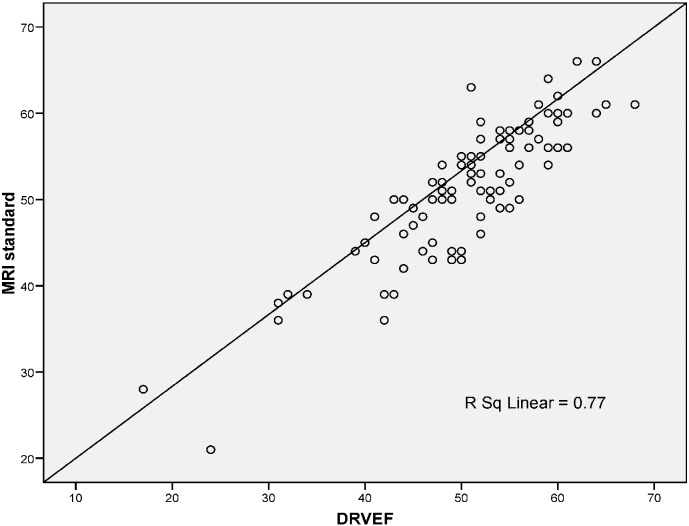
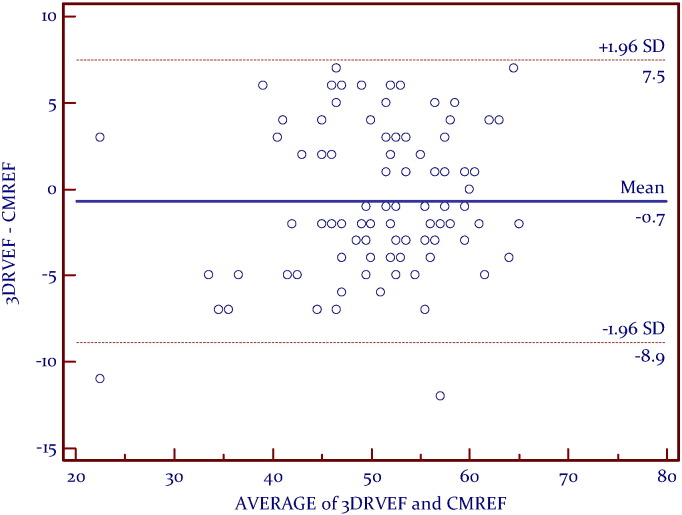
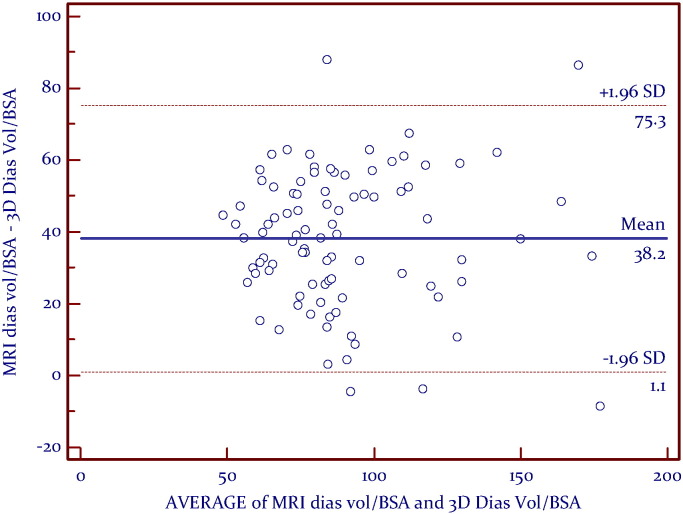
A linear relationship was found between CMR RVEF and 3DE (Fig. 2). Bland–Altman analysis demonstrated very good agreement, with a mean difference of − 0.7% and a narrow limits of agreement with discrepancies of 8.2% (Fig. 3). Correlation analyses (Kendall's tau) showed that RV volumes calculated by CMR were statistically larger than those calculated by 3DE (p < 0.05) (Table 3). Bland–Altman analysis demonstrated a clinically important disagreement in RV volumes with the mean difference 38.2 ml and wide limits of agreement, with discrepancies of 37.1 ml (Fig. 5).
Fig. 2.
Linear regression analysis demonstrating the linear relationship between MRI standard (CMR RVEF) and 3DE-RV.
Fig. 3.
Bland–Altman analysis between CMREF and 3DE-RV, demonstrating very good agreement.
Fig. 5.
Combined Groups: Bland Altman analysis between indexed CMR Diastolic volume and indexed 3DE-RV Diastolic volume/BSA.
Multiple logistic regression showed that RV strain, RVFAC, RVMPI, TAPSE and 3DRVEF performed well at discrimination patients with RV dysfunction by CMR (RVEF < 45%), table x. Strain analysis using 3, 4, and 7 segments all performed similarly for differentiation of patients with RV dysfunction.
7. Discussion
We evaluated multiple quantitative echocardiographic parameters for assessment of RV function in a single, prospectively-enrolled cohort compared to the reference standard of CMR. Although previous studies have evaluated various echocardiographic methods of assessing RV function [3], [11], [12], [13], this is the first known attempt to comprehensively assess a wide range of echocardiographic parameters in a single study cohort. We also compared RV volume as measured using 3DE compared to volumes produced by CMR [14], [15], [16]. Inter and intra-observer measurements of these parameters were excellent.
At ROC analysis, the most predictive echocardiographic methods were; RVFAC, RVMPI, TAPSE, RV strain and 3DE-RV. All other echo parameters did not significantly correlate with CMR RVEF. These parameters can be used to reliably separate normal from abnormal RV function based on a comparison between these parameters with CMR RVEF. No discrepancy between geometric and longitudinal measurements was identified, as has been previously reported [17].
7.1. RVFAC
In the current study, an RVFAC cut-off value of 36% separated a normal from an abnormal RVEF. This is similar to previous works [2].
7.2. TAPSE
TAPSE measurement of 1.37 cm to 2.96 cm indicates normal RV function [18]. The current study identified a similar normal cut-off value for normal RV function as ≥ 1.5 cm.
7.3. MPI
The assessment of RVMPI is unaffected by heart rate, is not limited by the non-geometric shape of the ventricle and is a measure of the overall systolic and diastolic function of the ventricle [19], [20], [21], [22]. The normal value for RVMPI (spectral Doppler) is reported as 0.28 +/− 0.04 (11) while the normal value of RVMPI-DTI is reported as 0.41 +/− 0.06 (22). In the current study, a normal RVMPI was < 0.37 while a normal RVMPI-DTI was < 0.51. RVMPI and RVMPI (DTI) were both found able to reliably differentiate between a normal and abnormal RVEF in group 2, but only RVMPI (spectral Doppler) was able to reliably differentiate between a normal and abnormal RVEF in the combined analysis.
7.4. RVSm
Right ventricular peak systolic myocardial velocity (RVSm) by tissue Doppler imaging (RVSm) of < 11.5 cm/s predicts RVEF < 45% [23]. This cut-off value was applied to the current study, where it was found that RVSm did not significantly relate to RVEF by CMR. RVSm measures localized velocities within one segment of the RV (indicated by the sample volume) and assumes that this velocity reflects the systolic function of the entire RV. It is also influenced by myocardial tethering and cardiac translational motion; that is, DTI is not able to distinguish between actively contracting myocardium and passive myocardial motion [24].
7.5. Strain imaging
RV Strain (ε) is an evolving technique for quantitation of RV function, and is not influenced by passive motion of the myocardium [25]. Strain analysis using 3, 4, and 7 segments all performed similarly for differentiation of patients with RV dysfunction, but at odds ratio analysis strain did not perform as well as RVFAC and 3DRVEF.
7.6. 3DE
We found that the RVEF estimated via 3DE was highly statistically similar to the RVEF via CMR and that 3DE was predictive and valid (as determined using ROC curves). [Fig. 4, Fig. 5]. 3DE-RV was statistically similar to CMR RVEF in all groups. The normal 3DE-RVcut-off was found to be ≥ 50% which similar to previously published values. However, the volumes calculated by CMR were statistically greater than those calculated by 3DE which correlates with previous studies [14], [15], [16].
7.7. Limitations
Strain imaging was performed using commercially available speckle tracking software, but not on a dedicated right ventricular package. The study patients with right ventricular dysfunction were a heterogeneous cohort, largely consisting of adult congenital heart disease. However, this is the cohort in which RV quantitation by echo is clinically relevant.
8. Conclusion
We demonstrate that several echocardiographic measurements for RV function and RV volume produce results consistent with those obtained via CMR. Our data suggest that 3DE, RVFAC, TAPSE, RVMPI would be the most appropriate parameters to incorporate into routine echocardiographic examination for the quantitative evaluation of RV function. In particular, the present data identify that cutoff values for 3DE-RVEF of ≥ 50%, RVFAC of ≥ 36%, TAPSE of ≥ 1.5 cm and RVMPI of < 0.37 are indicative of normal RV systolic function.
Financial disclosure
Dr. Hamilton-Craig was supported by the Smart Futures Fellowship (DSITIA). The authors report no conflicts of interest.
Acknowledgements
Robyn Riley, Andrew Trotter and the staff of the Centre of Excellence in Cardiovascular MRI, Prince Charles Hospital.
References
- 1.D'Andrea A. Right ventricular myocardial dysfunction in adult patients late after repair of tetralogy of fallot. Int. J. Cardiol. 2004;94(2–3):213–220. doi: 10.1016/j.ijcard.2003.04.033. [DOI] [PubMed] [Google Scholar]
- 2.Haddad F. Right ventricular myocardial performance index predicts perioperative mortality or circulatory failure in high-risk valvular surgery. J. Am. Soc. Echocardiogr. 2007;20(9):1065–1072. doi: 10.1016/j.echo.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Kjaergaard J., Sogaard P., Hassager C. Quantitative echocardiographic analysis of the right ventricle in healthy individuals. J. Am. Soc. Echocardiogr. 2006;19(11):1365–1372. doi: 10.1016/j.echo.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Morner S. Right ventricular dysfunction in hypertrophic cardiomyopathy as evidenced by the myocardial performance index. Int. J. Cardiol. 2008;124(1):57–63. doi: 10.1016/j.ijcard.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Schwerzmann M. Comparison of echocardiographic and cardiac magnetic resonance imaging for assessing right ventricular function in adults with repaired tetralogy of fallot. Am. J. Cardiol. 2007;99(11):1593–1597. doi: 10.1016/j.amjcard.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 6.Schenk P. Accuracy of echocardiographic right ventricular parameters in patients with different end-stage lung diseases prior to lung transplantation. J. Heart Lung Transplant. 2000;19(2):145–154. doi: 10.1016/s1053-2498(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 7.Strugnell W.E. Modified RV short axis series–a new method for cardiac MRI measurement of right ventricular volumes. J. Cardiovasc. Magn. Reson. 2005;7(5):769–774. doi: 10.1080/10976640500295433. [DOI] [PubMed] [Google Scholar]
- 8.Rudski L.G. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. (quiz 786-8) [DOI] [PubMed] [Google Scholar]
- 9.Tamborini G. Reference values for right ventricular volumes and ejection fraction with real-time three-dimensional echocardiography: evaluation in a large series of normal subjects. J. Am. Soc. Echocardiogr. 2010;23(2):109–115. doi: 10.1016/j.echo.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Kjaer A. Right-sided cardiac function in healthy volunteers measured by first-pass radionuclide ventriculography and gated blood-pool SPECT: comparison with cine MRI. Clin. Physiol. Funct. Imaging. 2005;25(6):344–349. doi: 10.1111/j.1475-097X.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 11.Miller D. The relation between quantitative right ventricular ejection fraction and indices of tricuspid annular motion and myocardial performance. J. Am. Soc. Echocardiogr. 2004;17(5):443–447. doi: 10.1016/j.echo.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Ramani G.V., Edelman K., Lopez-Candales A. Standard measures of right ventricular function assessment in adult patients with acute sickle cell crises. Int. J. Cardiol. 2009;132(3):448–450. doi: 10.1016/j.ijcard.2007.08.099. [DOI] [PubMed] [Google Scholar]
- 13.Kjaergaard J. Evaluation of right ventricular volume and function by 2D and 3D echocardiography compared to MRI. Eur. J. Echocardiogr. 2006;7(6):430–438. doi: 10.1016/j.euje.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Crean A.M. 3D Echo systematically underestimates right ventricular volumes compared to cardiovascular magnetic resonance in adult congenital heart disease patients with moderate or severe RV dilatation. J. Cardiovasc. Magn. Reson. 2011;13:78. doi: 10.1186/1532-429X-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoo N.S. Assessments of right ventricular volume and function using three-dimensional echocardiography in older children and adults with congenital heart disease: comparison with cardiac magnetic resonance imaging. J. Am. Soc. Echocardiogr. 2009;22(11):1279–1288. doi: 10.1016/j.echo.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins C. Reproducibility of right ventricular volumes and ejection fraction using real-time three-dimensional echocardiography: comparison with cardiac MRI. Chest. 2007;131(6):1844–1851. doi: 10.1378/chest.06-2143. [DOI] [PubMed] [Google Scholar]
- 17.Tamborini G. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur. J. Echocardiogr. 2009;10(5):630–634. doi: 10.1093/ejechocard/jep015. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Candales A. Right ventricular systolic function is not the sole determinant of tricuspid annular motion. Am. J. Cardiol. 2006;98(7):973–977. doi: 10.1016/j.amjcard.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 19.Bleeker G.B. Assessing right ventricular function: the role of echocardiography and complementary technologies. Heart. 2006;92(Suppl. 1):i19–i26. doi: 10.1136/hrt.2005.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eidem B.W. Usefulness of the myocardial performance index for assessing right ventricular function in congenital heart disease. Am. J. Cardiol. 2000;86(6):654–658. doi: 10.1016/s0002-9149(00)01047-x. [DOI] [PubMed] [Google Scholar]
- 21.Tei C. Doppler echocardiographic index for assessment of global right ventricular function. J. Am. Soc. Echocardiogr. 1996;9(6):838–847. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 22.Rojo E.C. Disagreement between tissue Doppler imaging and conventional pulsed wave Doppler in the measurement of myocardial performance index. Eur. J. Echocardiogr. 2006;7(5):356–364. doi: 10.1016/j.euje.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Pellerin D. Tissue Doppler, strain, and strain rate echocardiography for the assessment of left and right systolic ventricular function. Heart. 2003;(89 Suppl. 3) doi: 10.1136/heart.89.suppl_3.iii9. (p. iii9-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson B. second ed. MGA Graphics; Manly, Australia: 2007. Echocardiography — The Normal Examination and Echocardiographic Measurements; pp. 266–271. [Google Scholar]
- 25.Friedberg M.K., Rosenthal D.N. New developments in echocardiographic methods to assess right ventricular function in congenital heart disease. Curr. Opin. Cardiol. 2005;20(2):84–88. doi: 10.1097/01.hco.0000154446.65835.08. [DOI] [PubMed] [Google Scholar]