Abstract
Wesselsbron disease is a neglected mosquito transmitted Flavivirus infection that causes abortions and has teratogenic effects on sheep and cattle in Africa. Human can also be infected. The detection of human or animal cases is complicated by the non-specific symptoms close to Rift Valley Fever (RVF) in domestic livestock species or Dengue like syndrome in humans. Then, these detections are usually made during RVF investigations in sheep. These domestic animals should take a role in the life cycle of the virus but some evidences of Wesselsbron virus (WSLV) presence in wild animals suggest that the latter may be involved in the virus maintenance in nature. However, the reservoir status of wild vertebrate in general and rodents particularly for WSLV is only based on an isolation from a Cape short-eared gerbil in southern Africa. Most of WSLV isolations are from southern parts of Africa even if it has been found in western and central Africa or Madagascar. In Senegal, there are serological evidences of WSLV circulation in human since the 1970s and some isolations, the last one of which dates back in 1992. Despite the detection of the virus on mosquitoes until the 2000s in different parts of the country, no new human case has been noted. In this paper, we report the WSLV re-emergence in eastern Senegal in 2013 with 2 human cases and its first isolation from a black rat Rattus rattus. Sequencing analyses show the circulation of the same strain between these humans and the commensal rodent. The putative impact on WSLV transmission to human populations could be more important if the reservoir status of the black rat is confirmed. Focused survey in human populations, specific entomological and mammalogical investigations would permit a better understanding of the life cycle of the virus and its impact on public health.
Keywords: Wesselsbron virus, Rodents, Black rat, Eastern Senegal
Highlights
-
•
Wesselsbron virus (WSLV) re-emerged in eastern Senegal in 2013 with 2 human cases and a black rat Rattus rattus.
-
•
Sequencing data showed circulation of the same strain among humans and black rat.
-
•
First reported isolation of WSLV from rodent since a Cape short-eared gerbil collected in 1968 in South Africa.
-
•
The potential impact on WSLV transmission to human could be more important should R. rattus be confirmed as reservoir.
-
•
Surveillance and field investigation on WSLV life cycle should be carried out to assess associated risk and impact of WSL on human health.
1. Introduction
Wesselsbron disease (WSL) is a mosquito borne infection of sheep and cattle in Africa which can also infect human in whom fever and myalgia are the most common symptoms. It is caused by Wesselsbron virus (WSLV) which belongs to the Flavivirus genus. WSLV was first isolated in blood of a febrile man and dead lamb during an outbreak in 1955 in Wesselsbron in the Free State Province, South Africa [17], [34].
The disease in sheep is clinically similar to Rift Valley fever (RVF) with abortions and 20% mortality in pregnant ewes. Hydrops amnii and teratogenic effects such as arthrogryposis, hydrocephaly or neurogenic muscular atrophy are also observed in lambs [4] whereas infection causes less severe fever in goat, cattle, and pig [5]. WSL has been described as a cause of neurological disease in two horses of South Africa (Venter et al., 2008) [38]. WSL outbreaks can be unnoticed as they are often concomitant with RVF epidemics in South Africa [35] and its incidence can be underestimated. In humans, the infection causes arthralgia, myalgia and fever during a short and mild acute phase [33].
WSLV has a wide geographic distribution in Africa [34] and viral isolations from mosquitoes have been reported in South Africa, Botswana, Zimbabwe, Uganda, Mozambique, Uganda, Cameroon, Central African Republic, Mauritania, Senegal, Nigeria, DR Congo and Madagascar [36]. Aedes mosquitoes are generally associated with virus detection [18].
WSLV was also isolated from several domestic livestock species including camels, cattle, pigs, donkeys and horses, and serological evidence of its circulation was found in wild animals including South African zebras [2] and wild ruminants from Chad [2], [34] which raised the question of its reservoir. The reservoir status of wild vertebrate for WSLV is also suspected since an isolation from Cape short-eared gerbil Desmodillus auricularis in southern Africa (Kokernot et al., 1960) [39].
Between 1955 and 2011, 35 WSLV isolates were obtained from humans, mostly from Central African Republic (CAR) and South Africa [33], [37], Jupp and Kemp, 1998 [40], [13], [36]. In western Africa, most human isolates are from Senegal (4 strains in 1965, 1974, 1982 and 1992).
In Senegal, WSLV was serologically detected in most of the children from Upper Casamance and Eastern Senegal between 1972 and 1975 [29]. Swanepoel [34] reports a laboratory-acquired infection in 1965 and another one in 1974. A human infected isolation was also obtained in 1992 in the capital city Dakar (Annual report of Institut Pasteur de Dakar (IPD), 1992) after a previous one 10 years before.
Even if Monlun et al. [26] found that WSLV did not seem to represent a major public health concern in southeastern Senegal, entomological investigations between 1998 and 1999 in some parts of Senegal and Mauritania, spanning the Senegal River basin permitted to detect 51 WSLV strains from Aedes vexans [11]. Already, > 40 strains were found from 1974 to 1999 in Kedougou, Barkedji and Yonofere, a village in Saint Louis region (Annual report of IPD, 1999).
Moreover, recent emergences of viruses within populations initially naive showed the necessity of anticipation of risk factors. In this purpose, in the Kedougou region (southeastern Senegal), a surveillance of arboviruses has been undertaken on mosquitoes and human populations.
In this paper, we report on the re-emergence WSLV in Eastern Senegal in 2013 with 2 human cases and its first isolation from the rodent Rattus rattus (the black rat).
2. Material and methods
2.1. Study sites
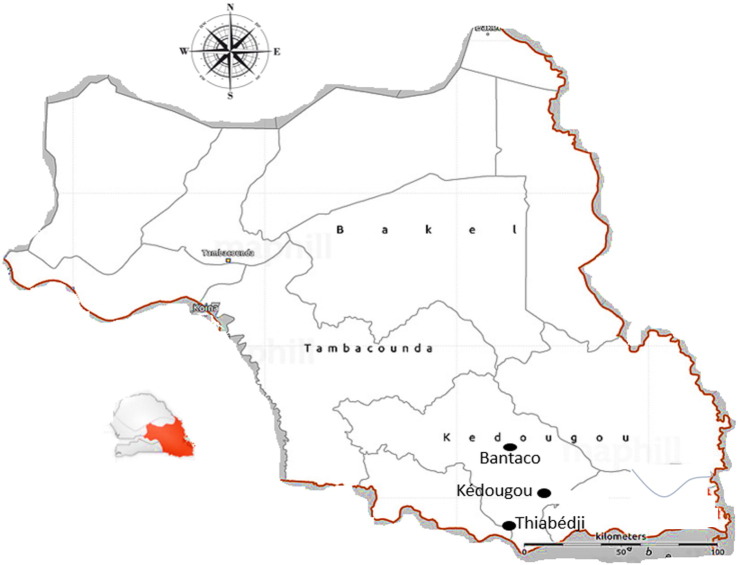
In the Kedougou region (Fig. 1), a surveillance of acute febrile illness (AFI) on human populations has been led since 2009 [31]. Patients presenting AFI were recruited from seven healthcare facilities of the Kedougou region, including Ninefesha rural hospital, Kedougou and Saraya health centres, Bandafassi and Khossanto health posts, the Kedougou military health post, and the Catholic Mission mobile team, which targets populations in remote areas.
Fig. 1.
Map of Kedougou region with the different localities of origin of the different isolates of this study.
2.2. Samples collection
Samples were collected from human presenting acute febrile illnesses and from rodent trapped in Kedougou. Serum or blood specimens of patients with AFI, as well as brain, serum and blood collected from rodents, are then submitted to the Arboviruses and Hemorrhagic Fever viruses Unit of IPD for a diagnostic of arboviruses.
Trapping sessions targeting rodents and shrews have been undertaken in a number of localities of Eastern Senegal to study the putative circulation of arboviruses in these small mammal populations. Briefly, traps (wire-mesh locally made and folding aluminium Sherman© traps) were set inside buildings for trapping sessions of one to six consecutive days with peanut butter as bait [6]. One of each type of traps was set per room and inspected for captures each morning. Each trapped specimen was identified to the species level based on morphological or geographical knowledge [14], [16] or, in case of ambiguity, by further molecular or chromosomal analyses (see details in [12], [15], [21]). Trapped individuals were euthanatized by cervical dislocation as recommended by Mills et al. [25]. The brain, the tissues and the serum of each individual were separated and put in dry ice for transportation to IPD. Permission to work within the different villages was obtained from appropriate authorities, and animals were correctly treated following Sikes et al. [32].
2.3. Viral isolation and identification
Each sample was inoculated into 2- to 3-days-old suckling mice for isolation of live virus (Shope and Sather, 1979) [41] according to a protocol approved by the National Health Laboratory Service Animal Ethics Committee (reference number, 107/06).
Suckling mice derived viruses were identified by reverse transcription PCR targeting a segment at the partial NS5 gene using the generic Flavivirus primers pair FD3/FU1 [20] and Sanger sequencing performed out-of-house by GENEWIZ, Inc. (Essex, UK).
2.4. RNA extraction and RT-PCR amplification
RNA was extracted from suckling mice derived viruses stocks using the QIAamp RNA Viral Kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. RNA was eluted in 50 μl of AVE buffer and stored at − 80 °C until use. For cDNA synthesis, 10 μl of viral RNA was mixed with 1 μl of the reverse primer (2 pmol) and the mixture was heated at 95 °C for 2 min. Reverse transcription was performed in 20 μl mixture containing mixed of 2.5 U RNasin (Promega, Madison, USA), 1 μl of deoxynucleotide triphosphate (dNTP) (10 mM each DNTP), 5 U of AMV reverse transcriptase (Promega, Madison, USA) and incubated at 42 °C for 60 min. PCR products were generated using the primers FD3/FU1 to amplify partial NS5 region. Five microliters of cDNA were mixed with 10 × buffer, 5 μl of each primer, 5 μl of dNTPs 10 mM, 3 μl of MgCl2, and 0.5 μl of Taq polymerase (Promega, Madison, USA).
2.5. Genome sequencing and analysis
The genomic polyprotein of each confirmed WSLV strain was obtained using overlapping sets of primers (Table 1) designed with the complete genome of the WSLV reference strain from South Africa (GenBank accession number: gi | 389595538 | gb | JN226796.1 |; https://www.ncbi.nlm.nih.gov/nuccore/389595538/, consulted on January 07th 2017). Sequences obtained were merged using EMBOSS Merger software and final results were analyzed using the Basic Local Alignment Tool (BLAST, www.ncbi.nlm.nih.gov/).
Table 1.
Designed primers used for genome sequencing.
| Primers pair | Primers name | Orientation | Primers position | Sequence (5′ → 3′) |
|---|---|---|---|---|
| 1 | WSL1F | F | 4nt–23nt | ATATTCTGCGTGCTAATCGT |
| WSL1R | R | 1467nt–1486nt | CCATAGCCTGTAAAAGCAAC | |
| 2 | E-FW-WSL | F | 1387nt–1406nt | CCACTCAGGAGCAAAGAAGG |
| E-REV-WSL | R | 2362nt–2381nt | TGAAGCCCATTGACATTGAA | |
| 3 | WSL3F | F | 2392nt–2411nt | GAGCCTTACTGCTAGTGCTG |
| WSL3R | R | 3829nt–3848nt | GATCTCCTAATGCAAGTTGG | |
| 4 | WSL4F | F | 3559nt–3578nt | GATGAAGAGGTTCTCCATGA |
| WSL4R | R | 4996nt–5015nt | CATAAAGGCCAATGACATCT | |
| 5 | WSL5F | F | 4741nt–4760nt | TGCAGGAAAAAGAATGACTC |
| WSL5R | R | 6180nt–6199nt | AACTGGCATGTCAAGTCTCT | |
| 6 | WSL6F | F | 6107nt–6126nt | ACCAAGACAACAACAAGTCC |
| WSL6R | R | 7571nt–7590nt | CCATTGTAAGCAAGTCCAAT | |
| 7 | WSL7F | F | 7303nt–7322nt | TCCAGTAGTTGATGGGAATC |
| WSL7R | R | 8368nt–8387nt | ACACGTCTCCTTCTATGACG | |
| 8 | WSL8F | F | 8297nt–8316nt | AACATCACTCACATGGTCAA |
| WSL8R | R | 9332nt–9351nt | ACCACCTTGTTTTTGTATGC | |
| 9 | NS5-FW-WSL | F | 9084nt–9104nt | TGGGATTCYTAAATGAAGACC |
| NS5-REV-WSL | R | 10,081nt–10,101nt | GTCTGATGTGGATTGTCTTCT | |
| 10 | WSL10F | F | 9556nt–9575nt | GAGATGTTGGCTTGACAGAT |
| WSL10R | R | 10,790nt–10,811nt | CACTAGTTGGTTCTCAACTTCC | |
| 11 | WSL Rat Gap F1 | F | 1041nt–1060nt | GCAGCTGCGTGACTTTGATA |
| WSL Rat Gap R1 | R | 1575nt–1594nt | GTCGTGAACCCATTGCTTGT | |
| 12 | WSL Rat Gap F2 | F | 2156nt–2175nt | ACAATGAAAGGAGCCCAACG |
| WSL Rat Gap R2 | R | 2873nt–2892nt | ACCCGTATTGAGTTCCACAC | |
| 13 | WSL Rat Gap F3 | F | 3356nt–3373nt | CCAGAATGGTGCTGTCGC |
| WSL Rat Gap R3 | R | 4262nt–4281nt | AGTAGCACTCCACCAACAGC | |
| 14 | WSL Rat Gap F4 | F | 4669nt–4688nt | AGTCGGAGTGGTGAAGGATG |
| WSL Rat Gap R4 | R | 5372nt–5391nt | GTCGGCTCTAACATGCGATG | |
| 15 | WSL Rat Gap F5 | F | 5508nt–5527nt | TATTCATGTCAGCCACCCCT |
| WSL Rat Gap R5 | R | 6360nt–6379nt | ACTGCATACCCTGGTGTCAA | |
| 16 | WSL Rat Gap F6 | F | 6780nt–6799nt | TAATACCAGAACCGGGCACA |
| WSL Rat Gap R6 | R | 7479nt–7499nt | TGCCTTCAATAAGTGGTCCCA | |
| 17 | WSL human Gap F1 | F | 5790nt–5809nt | TGGCCACTGACATAGCTGAA |
| WSL human Gap R1 | R | 6588nt–6607nt | AGCCAGCATCACCAGTAAAA |
Nucleotide sequences alignment were generated using the MUSCLE algorithm implemented in Mega version 6 (Tamura, Stecher, Peterson, Filipski, and Kumar 2013) [42].
3. Results
3.1. Isolation and identification of WSLV in black rat sample
Between May 2012 and December 2013, 6 trapping sessions yielded 1313 small mammal captures. After inoculation in suckling mice, WSLV was isolated from the brain of a male black rat trapped in November 2013 in Kedougou. Day 4-brain derived virus identity was done by RT-PCR targeting the Flavivirus partial NS5. The RT-PCR amplicon obtained showed a sequence which was highly conserved after comparison to the reference strain (GenBank accession number: gi | 389595538 | gb | JN226796.1 |), with 99% of homology.
3.2. Isolation and identification of WSLV in human sera
Two human WSLV cases from the region of Kedougou were confirmed from patients showing AFI. The first case was a 4-year-old girl from Thiabedji (Fig. 1), a village located about 35 km from Kedougou, presenting in local healthcare facility in July 2013 with a fever over 39 °C, headache and diffuse pains associated with malaria. The second one was a 30-year-old woman from Bantaco (Fig. 1) presenting jaundice and fever in February 2014 in Kedougou and who was found Hepatitis E positive. Both samples were tested by RT-PCR. Amplification of polymerase region (NS5) showed a similitude of 100% and 99% respectively for the first and second case compared to the strain found in black rats. An additional test of complement fixation test targeting WSLV was performed as described by Casey [3] on the suckling mice derived virus obtained from the Thiabedji case sample to check the consistency with sequencing results (data not shown).
These two co-infected WSLV human cases recovered after few days without sequelae.
3.3. Phylogeny of WSLV isolates
Using 17 designed overlapping primers pairs (Table 1), sequencing of the coding regions of the virus genome permitted to obtain 10,643 bp and 10,648 bp for the human cases from Thiabedji (isolate WSLV-IP248525/SEN/2013; access number: KY056256) and Bantaco (isolate IP262451/SEN/2014; access number: KY056257), respectively, and 10,645 bp for the black rats' one (isolate IP259570/SEN/2013; access number: KY056258).
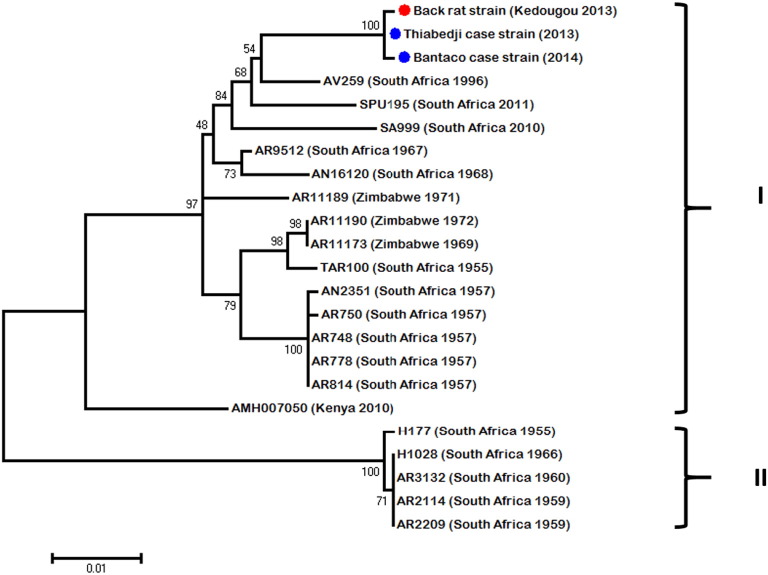
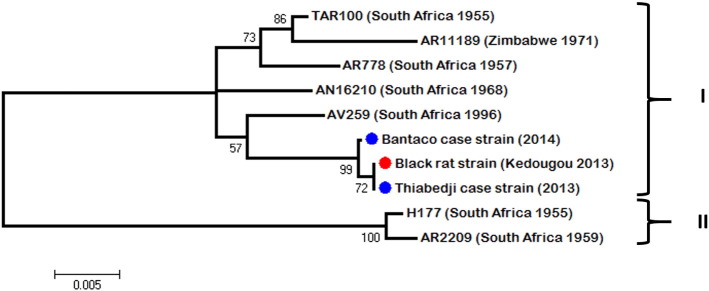
Pairwise nucleotide comparison of the three genomes yielded an average of 1% nucleotide diversity across the black rat strain (ref. in GenBank) and the two human cases from Thiabedji (ref. in GenBank) and Bantaco (ref. in GenBank). The sequences of partial polymerase (NS5) and envelope (E) genes of the WSLV strains isolated in this study were aligned with the other available sequences obtained from isolates of South Africa, Kenya and Zimbabwe (from 1955 to 2011). Analysis of both regions showed two clades. The Senegalese strains isolated from eastern Senegal were included in a first clade (I) containing the largest number of sequences from South Africa, Zimbabwe and Kenya (Fig. 2). The other one (II) (Fig. 3) contained some South African strains isolated before 1970s. Intra-clade diversity was at most 1%. Inter-clade diversity was of 6% in sequenced region of polymerase (NS5) and 8% in envelope (E). Despite this diversity, no geographical or spatial correlation could be observed as previously argued by Weyer et al. [36].
Fig. 2.
Unrooted maximum likelihood tree of 948-bp of the NS5 region of WSLV strains isolated in this study (blue circle for human strain and red circle for black rat strain) and in previous ones from southern Africa countries.
Fig. 3.
Unrooted maximum likelihood tree of 845-bp of the E region of WSLV strains isolated in this study (blue circle for human strain and red circle for black rat strain) and in previous ones from southern Africa countries.
4. Discussion
The Kedougou region is an area prone to infectious diseases [7], [9], [10]. The surveillance of AFI on human populations is then very important for the anticipation of possible emergence. In this study, the first human case of WSL disease was a co-infection of a malaria positive young girl from Bantaco. In Kedougou, malaria remains highly prevalent with a circulation of at least two Plasmodium species [28]. Sow et al. [32] showed that concurrent infection malaria/arboviruses occurred among 48.7% of patients infected with arboviruses in southeastern Senegal from July 2009 to March 2013. According to this previous study where co-infected patients were significantly younger than non-co-infected ones, the WSL case from Bantaco is a 4-year-old girl. If mosquito biting infection seems obvious, this co-infection could be the consequence of consecutive bites from two different species. In fact, only 10 strains of WSLV have been isolated in Anopheles vectors from Senegal, Central African Republic and Cameroon from 1962 to 1999 (Annual report of Arboviruses and Hemorrhagic Fever Viruses Unit of the Institute Pasteur, Dakar, 1999). However we should note the possibility of an asymptomatic carriage of Plasmodium, which often occurs in Senegal as showed by Zwetyenga et al. [37] or Males et al. [22] for examples.
The second case from Thiabedji is another co-infection during a concomitant hepatitis E virus (HEV) outbreak (personal communication). Thiabedji is a village located near to some little mining areas where breeding sites have proliferated promoting vector/human contacts in unhealthy environment.
An interesting fact in this study is the WSLV isolation in a black rat specimen trapped in Kedougou. To our knowledge, it is the second isolation of the virus from a rodent after D. auricularis in South Africa (Kokernot et al., 1960) [39]. The analysis showed that the same WSLV strain has circulated among the patients and the black rat. From this, it appears that this commensal rodent may have been involved in WSLV circulation in dwelling areas.
Previous studies on WSLV transmissibility on mice model showed various results. If Muspratt et al. [27] showed that about 90% of artificially infected Ae. circumluteolus mosquitoes were able to transmit WSLV to mice while successful transmission rate was very low for most of other studies including Ae. caballus s.l. [18], Culex theileri [24], Aedes aegypti and Cx. quinquefasciatus [30]. Therefore, inoculation of WSLV by mosquitoes seems to be exceptional in rodents and it can explain the small number of isolates obtained from them. The recent environmental changes in Kedougou with the substantial increase of mosquito breeding sites near of human habitats and gold mining areas could foster the transmission on a black rat specimen. However, entomological studies in this area at this same time did not permit any WSLV detection in the mosquito pools trapped (data not shown). As for many arboviruses, vertical transmission appears to not be sufficient for explaining virus maintenance in nature and secondary cycles involving vertebrates is another track study [8]. Except the Namaqua gerbil D. auricularis in which viremia induced by WSLV (7.2 LD50/ml; 1–3 days) could induce infection in mosquito [1], [24], rodents appeared to be refractory to WSLV as showed by McIntosh [23] with Mastomys natalensis or Aethomys chrysophylus for example. However, the black rat was not involved in these studies. The Namaqua gerbil is a desert species most often meet in the dry Kalahari area, between Botswana, Namibia and western South Africa. The black rat is an urban species close to human habitats and also one of the most invasive in the world. The putative impact on human populations concerning WSLV transmission could be then more important if the reservoir status of this rat is confirmed. It would certainly be too anticipate to link these two last human cases with the recent introduction of black rat specimens in southeastern Senegal at the beginning of the 21st century [19] but investigations on the implication of this species in the life cycle of WSLV should be undertaken.
Basic phylogenetic analysis of the genome of the three isolates showed that the same WSLV strain has probably circulated among humans and the black rat specimen in Kedougou region between 2013 and 2014. The current results let envisage the circulation of two lineages, a first one (clade I) found in some countries of southern Africa, in Kenya and Senegal and another one (clade II) detected in South Africa between 1955 and 1960. More isolates and sequences from other parts of Africa could help for a better understanding of the phylogenetic situation of WSLV at least in Africa.
WSLV impact on public health, although no fatal human case has been identified, may be underestimated given the lack of monitoring of the virus. In fact, most of the cases have been noted in a context of other investigations. The described WSLV human cases were found to be co-infection.
Further investigations are needed in human populations, insects and mammals to understand the life cycle and the impact on public health of the virus. Conferring to the hypothesis of rodents' implication in general, the most abundant order among mammals, in the life cycle of the virus, combined with the Aedini populations' extension worldwide, WSLV could have a larger distribution than expected.
Financial support
This study is a part supported by the French National Research Agency (ANR) CEP&S 2011 under the references ANR-11-CEPL-0010 and ANR-11-JSV7-0006 and by the Institut Pasteur (IPD24_01) de Dakar funds.
Disclosures regarding real or perceived conflicts of interest
No competing interests in this scientific work.
Acknowledgements
We thank the CBGP teams of IRD (Institut de Recherche pour le Développement) of Montpellier, France and Dakar, Senegal for their outstanding work for the rodent trappings.
References
- 1.Arbovirus Research Unit, National Institute for Virology, Sandringham, unpublished laboratory records, 1953 to 1985.
- 2.Barnard B.J. Antibodies against some viruses of domestic animals in southern African wild animals. Onderstepoort J. Vet. Res. 1997 Jun;64(2):95–110. [PubMed] [Google Scholar]
- 3.Casey H.L. Standardized Diagnostic Complement Fixation and Adaptation to Microtest. U.S. Public Health Monograph No. 74. U.S. Public Health Service; Washington, D.C: 1965. II. Adaptation of LCBF-method microtechnique; pp. 31–34. [PubMed] [Google Scholar]
- 4.Coetzer J.A.W., Barnard B.J.H. Hydrops amnii in sheep associated with hydranencephaly and arthrogryposis with Wesselsbron disease and Rift Valley fever viruses as ethological agents. Onderstepoort J. Vet. Res. 1977;44:119–126. [PubMed] [Google Scholar]
- 5.Coetzer J.A., Theodoridis A. Clinical and pathological studies in adult sheep and goats experimentally infected with Wesselsbron disease virus. Onderstepoort J Vet Res. 1982 March;49(1):19–22. [PubMed] [Google Scholar]
- 6.Dalecky A., Ba K., Piry S., Lippens C., Diagne C.A., Kane M., Sow A., Diallo M., Niang Y., Konecny A., Sarr N., Artige E., Charbonnel N., Granjon L., Duplantier J.M., Brouat C. Range expansion of the invasive house mouse Mus musculus domesticus in Senegal, West Africa: a synthesis of trapping data over three decades, 1983–2014. Mammal Rev. 2015;45(3):176–190. [Google Scholar]
- 7.Diallo Diawo, Sall Amadou A., Buenemann Michaela, Chen Rubing, Faye Oumar, Diagne Cheikh T., Faye Ousmane, Ba Yamar, Dia Ibrahima, Watts Douglas, Weaver Scott C., Hanley Kathryn A., Diallo Mawlouth. Landscape ecology of sylvatic chikungunya virus and mosquito vectors in Southeastern Senegal. PLoS Negl. Trop. Dis. June 2012;6(6) doi: 10.1371/journal.pntd.0001649. 10.1371/journa.pntd.0001649 Published online 2012 June 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diallo D., Chen R., Diagne C.T., Ba Y., Dia I., Sall A.A., Weaver S.C., Diallo M. Bloodfeeding patterns of sylvatic arbovirus vectors in southeastern Senegal. Trans. R. Soc. Trop. Med. Hyg. 2013 Mar;107(3):200–203. doi: 10.1093/trstmh/trs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diallo Diawo, Sall Amadou A., Diagne Cheikh T., Faye Oumar, Hanley Kathryn A., Buenemann Michaela, Ba Yamar, Faye Ousmane, Weaver Scott C., Diallo Mawlouth. Patterns of a sylvatic yellow fever virus amplification in Southeastern Senegal, 2010. Am.J.Trop. Med. Hyg. 2014;90(6):1003–1013. doi: 10.4269/ajtmh.13-0404. 10.4269/ajtmh.13-0404 June 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diallo Diawo, Sall Amadou A., Diagne Cheikh T., Faye Oumar, Faye Ousmane, Ba Yamar, Hanley Kathryn A., Buenemann Michaela, Weaver Scott C., Diallo Mawlouth. Zika Virus Emergence in Mosquitoes in Southeastern Senegal, 2011. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109442. 10.1371/journal.pone.0109442 Published online 2014 October 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diallo M., Nabeth P., Ba K., Sall A.A., Ba Y., Mondo M., Girault L., Abdalahi M.O., Mathiot C. Mosquito vectors of the 1998–1999 outbreak of Rift Valley Fever and other arboviruses (Bagaza, Sanar, Wesselsbron and West Nile) in Mauritania and Senegal. Med. Vet. Entomol. Jun 2005;19(2):119–126. doi: 10.1111/j.0269-283X.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- 12.Dobigny G., Tatard C., Kane M., Gauthier P., Brouat C., Bâ K., Duplantier J.-M. A cytotaxonomic and DNA-based survey of rodents from Northern Cameroon and Western Chad. Mamm. Biol. 2011;76:417–427. [Google Scholar]
- 13.Durand J.-P., de Camaret X., Zeller H., Sall A., Tock F., Czarnecki E., Régis R., Guesdon S., Tolou H. First isolation of Wesselsbron virus in Chad. Virologie. 2001;5(4):305–306. [Google Scholar]
- 14.Granjon L., Duplantier J.M. IRD Editions/Publications scientifiques du Muséum; Marseille, France: 2009. Les Rongeurs de l'AfriqueSahélo-soudanienne. [Google Scholar]
- 15.Granjon L., Dobigny G. The importance of cytotaxonomy in understanding the biogeography of African rodents: Lake Chad murids as an example. Mammal Rev. 2003;33:77–91. [Google Scholar]
- 16.Happold M., Happold D.C.D. Bloomsbury Publishing; London, UK: 2013. Mammals of Africa, Vol. IV, Hedgehogs, Shrews and Bats. [Google Scholar]
- 17.Karabatsos N., editor. International Catalogue of Arboviruses, Including Certain Other Viruses of Vertebrates. third ed. CDC Div. Vector-Borne Infect. Dis.; Ft. Collins: 1985. (San Antonio, Texas: Am. Soc. Trop. Med. Hyg, The 1986–1995 Supplements to the International Catalogue). [DOI] [PubMed] [Google Scholar]
- 18.Kokernot R.H., Paterson H.E., De Meillon B. Studies on the transmission of Wesselsbron virus by Aedes. (Ochlerotatus) caballus (Theo.) S. Afr. Med. J. 1958;32:546–548. [PubMed] [Google Scholar]
- 19.Konečnỷ A. Université de Montpellier 2; 2009. Consequences of Anthropogenic Changes on Rodent Communities and Populations: Study Cases on Native and Introduced Species in Eastern Senegal. [Google Scholar]
- 20.Kuno G., Chang G.J.J., Tsuchiya K.R., Karabatsos N., Cropp C.B. Phylogeny of the genus flavivirus. J. Virol. 1998;10(1):73–83. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecompte E., Brouat C., Duplantier J.M., Galan M., Granjon L., Loiseau A., Mouline K., Cosson J.F. Molecular identification of four cryptic species of Mastomys (Rodentia, Murinae) Biochem. Syst. Ecol. 2005;33:681–689. [Google Scholar]
- 22.Males S., Gaye O., Garcia A. Long-term asymptomatic carriage of Plasmodium falciparum protects from malaria attacks: a prospective study among Senegalese children. Clin. Infect. Dis. Feb 15 2008;46(4):516–522. doi: 10.1086/526529. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh B.M. Susceptibility of some African wild rodents to infection with various arthropod borne viruses. Trans. R. Soc. Trop. Med. Hyg. 1961;55:63. doi: 10.1016/0035-9203(61)90041-4. [DOI] [PubMed] [Google Scholar]
- 24.McIntosh B.M. University of Pretoria; Pretoria: 1980. The Epidemiology of Arthropod-borne Viruses in Southern Africa. (D.Sc.thesis) [Google Scholar]
- 25.Mills J.N., Childs J.E., Ksiazek T.G., Peters C.J., Velleca W.M. U.S. Department of Health & Human Services, CDC; Atlanta, Georgia, USA: 1995. Methods for Trapping and Sampling Small Mammals for Virologic Testing. [Google Scholar]
- 26.Monlun E., Zeller H., Le Guenno B., Traoré-Lamizana M., Hervy J.P., Adam F., Ferrara L., Fontenille D., Sylla R., Mondo M. Surveillance of the circulation of arbovirus of medical interest in the region of eastern Senegal. Bull. Soc. Pathol. Exot. 1993;86(1):21–28. [PubMed] [Google Scholar]
- 27.Muspratt J., Smithburn K.C., Paterson H.E., Kokernot R.H. Studies on arthropod-borne viruses of Tongaland. X. The laboratory transmission of Wesselsbron virus by the bite of Aedes (Banksinella) circumluteolus Theo. S. Afr. J. Med. Sci. Oct 1957;22(2–3):121–126. [PubMed] [Google Scholar]
- 28.Niang M., Thiam L.G., Sow A., Loucoubar C., Bob N.S., Diop F., Diouf B., Niass O., Mansourou A., Varela M.L., Perraut R., Sall A.A., Toure-Balde A. A molecular survey of acute febrile illnesses reveals Plasmodium vivax infections in Kedougou, southeastern Senegal. Malar. J. Jul 19 2015;14:281. doi: 10.1186/s12936-015-0808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renaudet J., Jan C., Ridet J., Adam C., Robin Y. A serological survey of arboviruses in the human population of Senegal. Bull. Soc. Pathol. Exot. Filiales. Mar-Apr 1978;71(2):131–140. [PubMed] [Google Scholar]
- 30.Simasathien P., Olson L.C. Factors influencing the vector potential of Aedes aegypti and Culex quinquefasciatus for Wesselsbron virus. J. Med. Entomol. Dec 30 1973;10(6):587–590. doi: 10.1093/jmedent/10.6.587. [DOI] [PubMed] [Google Scholar]
- 31.Sow A., Loucoubar C., Diallo D., Faye O., Ndiaye Y., Senghor C.S., Dia A.T., Faye O., Weaver S.C., Diallo M., Malvy D., Sall A.A. Concurrent malaria and arbovirus infections in Kedougou, southeastern Senegal. Malar. J. 2016;15:47. doi: 10.1186/s12936-016-1100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sikes RS, Gannon WI, the Animal Care and Use Committee of the American Society of Mammalogists (2011).
- 33.Swanepoel R. Wesselsbron virus disease. In: Monath T.P., editor. The Arboviruses Epidemiology and Ecology. vol. 5. CRC Press, Inc.; Boca Raton, FL: 1989. [Google Scholar]
- 34.Weiss K.E., Haig D.A., Alexander R.A. Wesselsbron virus—a virus not previously described, associated with abortion in domestic animals. Onderstepoort Vet. J. 1956;27:183–195. [Google Scholar]
- 35.Weiss K.E. Rift Valley fever—a review. Bull. Epizoot. Dis. Afr. 1957;5:431–458. [Google Scholar]
- 36.Weyer J., Thomas J., Leman P.A., Grobbelaar A.A., Kemp A., Paweska J.T. Human cases of Wesselsbron disease, South Africa 2010–2011. Vector Borne Zoonotic Dis. 2013 May;13(5):330–336. doi: 10.1089/vbz.2012.1181. (Epub 2013 Mar 8) [DOI] [PubMed] [Google Scholar]
- 37.Zwetyenga J., Rogier C., Spiegel A., Fontenille D., Trape J.F., Mercereau-Puijalon O. A cohort study of Plasmodium falciparum diversity during the dry season in Ndiop, a Senegalese village with seasonal, mesoendemic malaria. Trans. R. Soc. Trop. Med. Hyg. Jul–Aug 1999;93(4):375–380. doi: 10.1016/s0035-9203(99)90122-0. [DOI] [PubMed] [Google Scholar]
- 38.Venter M., Human S., Gerdes G.H., Williams J., Leman P.A., Kemp A. Proceedings of the South African Society for Veterinary Epidemiology and Preventive Medicine 8th Annual Conference, 20–22 August. Gauteng, South Africa; 2008. Investigation of the pathogenesis of West Nile virus and other zoonotic flavi and alpha viruses in humans and horses in South Africa. [Google Scholar]
- 39.Kokernot R.H., Smithburn K.C., Paterson H.E., de Meillon B. Further isolations of Wesselsbron virus from mosquitos. S. Afr. Med. J. 1960;34:871–874. [PubMed] [Google Scholar]
- 40.Jupp P.G., Kemp A. Studies on an outbreak of Wesselsbron virus in the Free State Province. South Africa. J. Am. Mosquito. Contr. 1998;14:40–45. [PubMed] [Google Scholar]
- 41.Shope E., Sather G.E. Arboviruses. In: Lenette E.H., Schmidt N.J., editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. American Public Health Association; Washington DC: 1979. pp. 767–814. [Google Scholar]
- 42.Tamura Koichiro, Stecher Glen, Peterson Daniel, Filipski Alan, Kumar Sudhir. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]