Abstract
Establishing how Ediacaran organisms moved and fed is critical to deciphering their ecological and evolutionary significance, but has long been confounded by their non-analogue body plans. Here, we use computational fluid dynamics to quantitatively analyse water flow around the Ediacaran taxon Parvancorina, thereby testing between competing models for feeding mode and mobility. The results show that flow was not distributed evenly across the organism, but was directed towards localized areas; this allows us to reject osmotrophy, and instead supports either suspension feeding or detritivory. Moreover, the patterns of recirculating flow differ substantially with orientation to the current, suggesting that if Parvancorina was a suspension feeder, it would have been most efficient if it was able to re-orient itself with respect to current direction, and thus ensure flow was directed towards feeding structures. Our simulations also demonstrate that the amount of drag varied with orientation, indicating that Parvancorina would have greatly benefited from adjusting its position to minimize drag. Inference of facultative mobility in Parvancorina suggests that Ediacaran benthic ecosystems might have possessed a higher proportion of mobile taxa than currently appreciated from trace fossil studies. Furthermore, this inference of movement suggests the presence of musculature or appendages that are not preserved in fossils, but which would noneltheless support a bilaterian affinity for Parvancorina.
Keywords: Parvancorina, Ediacaran, paleobiology, computational fluid dynamics
1. Background
The Ediacaran Period (approx. 635–541 Ma) marks a key interval in Earth's history, which culminated in the evolution and diversification of animals. This Period documents the first appearance of large, complex multicellular lifeforms, referred to as the ‘Ediacara biota' [1]. The phylogenetic affinities of many of the Ediacara biota remain contentious; this is largely due to their body plans, which have no counterparts in the present day. Consequently, progress in deciphering their ecology and biology has been limited, hampering our understanding of the early evolution of complex eukaryotes.
The application of computer modelling approaches has recently shed light on key characteristics of long-extinct organisms, including feeding mode and mobility in Ediacaran taxa [2], which has greatly advanced understanding of both their ecology and phylogenetic affinities [2,3]. Here, we use computational fluid dynamics (CFD) to evaluate competing hypotheses for feeding and mobility in the enigmatic Ediacaran taxon Parvancorina. Parvancorina is a small, bilaterally symmetrical fossil with a shield-like base and a dorsal T-shaped ridge known exclusively from Ediacaran (approx. 555–550 Ma) deposits in Australia and Russia [4–6]. This taxon occurs in shallow-marine sediments between storm and fair-weather wave base, which preserve evidence for variable currents [7,8]. Debate surrounds the phylogenetic affinities and mode of life of Parvancorina: Glaessner [4] interpreted Parvancorina as a mobile detritivore that fed at the sediment–water interface; Laflamme et al. [9] binned virtually all the White Sea organisms (including Parvancorina) as sessile osmotrophs; and Rahman et al. [2] interpreted the coeval taxon Tribrachidium as a sessile suspension feeder and suggested that many other enigmatic Ediacaran taxa may have led similar lifestyles. Distinguishing between these competing models is critical to understanding the paleobiology and affinity of Parvancorina, and also sheds light on the complexity of Ediacaran ecosystems.
2. Material and methods
We created three-dimensional digital models of three Parvancorina morphotypes: (i) Parvancorina minchami from South Australia [5]; (ii) Parvancorina minchami from Russia [6]; and (iii) Parvancorina sagitta from Russia [10]. Because these specimens might have been subjected to post-mortem vertical compaction, we produced additional models with the relief increased by 15% and 30% (see electronic supplementary material, S1). To evaluate the functional significance of the raised T-shaped ridge, we constructed null models that consisted of only the shield-shaped base. Although our three-dimensional models are accurate representations of the specimens figured in previous studies, we note that there is still some ambiguity regarding the morphology of Parvancorina (especially on the ventral side).
CFD simulations of water flow were performed in COMSOL. For all simulations, the Parvancorina model was fixed to the lower surface of a half-cylinder (electronic supplementary material, S2). Water flow was simulated with a velocity inlet at the upstream end of the half-cylinder and a zero-pressure outlet at the downstream end. Slip boundary conditions were assigned to the top and sides of the half-cylinder, and no-slip boundary conditions were assigned to the Parvancorina model and the lower surface of the half-cylinder (electronic supplementary material, S2; figure S3). The domain was meshed using free tetrahedral elements and the shear stress transport turbulence model was used to solve the Reynolds-averaged Navier–Stokes equations (electronic supplementary material, S3 and S4). A stationary solver was used to compute the steady-state flow patterns.
For each model, simulations were performed with an inlet velocity of 0.1, 0.2 and 0.5 m s−1 (Reynolds numbers 400–12 000), reflecting typical current velocities for shallow-marine settings on the continental shelf, in which Parvancorina evidently thrived [7,8]. To explore the sensitivity of the results to mesh size, we carried out simulations for the models of the original morphotypes using three additional mesh sizes (electronic supplementary material, S4; figures S5 and S6). Given the variability of current directions in shallow off-shore settings, it was important to test whether hydrodynamic performance varied with orientation to the current. As such, simulations were conducted with all models at three different orientations. CFD results were visualized as two-dimensional cross-sections of flow velocity magnitude, and drag forces and their coefficients were calculated to quantify flow around the Parvancorina models (electronic supplementary material, S3).
3. Results
Sensitivity analyses established that a moderate mesh represents the best balance between accuracy and computational duration (electronic supplementary material, S4), and was used in all subsequent analyses.
In all CFD simulations, fluid velocity decreases rapidly where the flow encounters the model, with a steep velocity gradient developing as the flow approaches the lower margin of the flow domain. A region of low-velocity recirculation develops in the wake downstream of the Parvancorina models, but not in the null models (figure 1; electronic supplementary material, S5). The magnitude of recirculation varies depending on the morphotype, and is also governed by the orientation, inlet velocity and modelled height. The flow is directed to different parts of the organism depending on the orientation to the current.
Figure 1.
Computer simulations of water flow around Parvancorina models (all original height). From left to right: P. minchami [S. Australia], P. minchami [White Sea] and P. saggita. (a) Digital models (scale bars, 1 cm). (b–e) Results of CFD simulations visualized as two-dimensional plots (horizontal and vertical cross-sections) of flow velocity magnitude with flow vectors (arrows). (b) Original models at 0° to the current. (c) Original models at 90° to the current. (d) Original models at 180° to the current. (e) Null models at 0° to the current. Ambient flow from left to right (uniform inlet velocity of 0.2 m s−1).
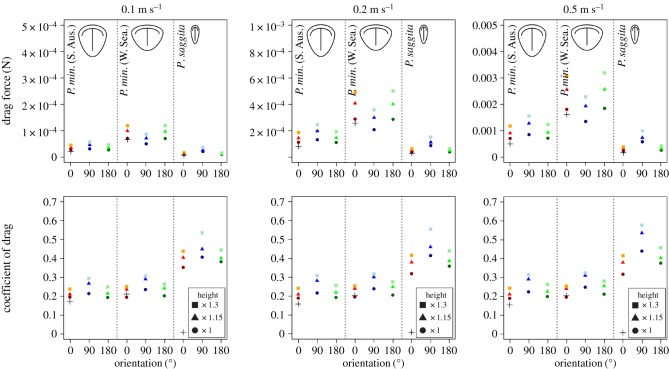
Drag forces increase as the size of the model, inlet velocity and relief increase (figure 2). For all morphotypes, drag forces are similar regardless of whether the model is oriented at 0 or 180° to the current. However, P. minchami [South Australia] and P. sagitta produce the most drag when oriented at 90°, whereas P. minchami [White Sea] produces the least drag in this position. The coefficients of drag increase with relief. In all morphotypes, the drag coefficients are greatest when the models are oriented at 90° (figure 2). The null models produce substantially lower drag forces and drag coefficients than the equivalent models of the original morphotypes.
Figure 2.
Drag forces (top) and drag coefficients (bottom) for Parvancorina models in CFD simulations; crosses indicate results for null models.
4. Discussion
The results demonstrate that, regardless of orientation to the flow, current velocity or morphotype, Parvancorina did not distribute flow evenly across the surface of the organism, and instead directed recirculated flow towards localized areas (figure 1). Similar flow patterns were seen at all modelled heights, indicating that these results are robust to post-mortem vertical compaction. This allows us to reject the possibility that Parvancorina was an osmotroph; in Ediacaran taxa that are inferred to have fed osmotrophically, flow patterns diffuse uniformly across the entire external surface in a way that facilitates direct absorption of nutrients across membranes [11] (although also see [12]). Instead, flow patterns associated with Parvancorina are much more consistent with either suspension feeding or a more active method of nutrient acquisition such as detritivory.
If Parvancorina was a suspension feeder, we would expect the external morphology of the organism to direct flow to specific parts of the organism with specialized food-capturing structures. In agreement with this, our CFD simulations recover patterns of recirculating flow around the body of Parvancorina; however, the regions to which the flow was directed varied depending on the orientation to the current (figure 1). Thus, in order to sustain a suspension feeding lifestyle, Parvancorina would need to have a particular alignment to the prevailing current direction to ensure flow was directed towards putative feeding structures. Controlling its position on the seafloor would therefore be highly preferable in order to feed effectively in shallow-water environments characterized by shifting currents.
The inference that Parvancorina could adjust its position on the sediment surface receives strong support from the calculated drag forces, which varied depending on orientation (figure 2). P. minchami [South Australia] and P. sagitta produced the least drag when oriented at 0° or 180° to the current, whereas P. minchami [White Sea] produced the least drag when oriented at 90° to the current. Organisms living in environments characterized by changing current directions typically evolve low-relief profiles to minimize drag [13], which is an important force acting upon organisms attempting to remain in place upon the substrate. Among these, organisms with a sessile life habit frequently adopt approximately symmetrical (and hemispherical) body shapes that present the same cross-sectional profile to water flow regardless of current direction (e.g. barnacles). In contrast, mobile organisms adopt body shapes that minimize drag in one direction to the current (e.g. caddisfly larvae and water penny beetles [13]). Our simulations show that all morphotypes produced different amounts of drag in different orientations, supporting the idea that Parvancorina was facultatively mobile and thus able to adjust its position so that drag was minimized.
Inference of suspension feeding also raises questions surrounding the function of the T-shaped ridge. The characteristic flow patterns seen around Parvancorina largely disappear in the null models (figure 1), indicating that the ridge is key for generating recirculation. In addition, all three null models produce significantly less drag than the corresponding geometry with ridges, suggesting the ridge did not reduce drag. Given the absence of macroscopic raptorial predators in Ediacaran ecosystems, it is also unlikely that this structure performed a protective function. Among extant marine invertebrates, structures in which a concave side faces upstream most commonly occur in connection with passive suspension feeding [13], which hints that the ridge may have played some role in influencing fluid flow and the supply of suspended food particles.
Paterson et al. [14] described fossil beds preserving multiple Parvancorina in a common orientation; taphonomic and morphological evidence allowed them to reject post-mortem hydrodynamic sorting, and instead supports the notion that Parvancorina was able to alter its position on the substrate at some stage of its life cycle. Our results support this idea by illustrating that if Parvancorina was a suspension feeder, flow patterns would only have supported feeding in a specific direction to the current, and thus Parvancorina would need to have been facultatively mobile to feed effectively. This is particularly true if the direction of bottom currents were variable, as is common in most nearshore settings, as well as for Ediacaran localities in South Australia [15] (although if currents were constant throughout the life of the organism, it is conceivable that Parvancorina was only mobile early in development [14]). However, although our data are consistent with the interpretation of Parvancorina as a mobile suspension feeder, they do not reject a deposit feeding strategy sensu Glaessner [4]. Nevertheless, deposit feeding would also imply a mobile lifestyle. The results of CFD therefore provide indirect evidence for facultative mobility in Parvancorina, despite the lack of preserved appendages or associated trace fossils.
Finally, we suggest that computer modelling approaches may help elucidate the phylogenetic affinities of many Ediacaran taxa. The inference of facultative mobility supports the interpretation of Parvancorina as a total-group bilaterian (along with the presence of bilateral symmetry and differentiated anterior–posterior and dorsal–ventral regions; see discussion of Kimberella in [16]), broadly in agreement with [4]. More generally, the inference of mobility in Parvancorina in the absence of preserved appendages or traces raises the possibility that other Ediacaran organisms might also have been capable of movement, and suggests a much more dynamic picture of Ediacaran benthic ecosystems.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
All authors thank Mary Droser for discussions and encouragement, John Patterson for sending proofs of their manuscript and acknowledge detailed reviews from both the editor and two anonymous reviewers.
Ethics statement
Ethical approval was not required.
Data accessibility
3-D models and CFD simulation files are available at: http://dx.doi.org/10.5061/dryad.2601h (see also [17]). Supplementary files include supplementary text and figures.
Authors' contributions
All authors conceived the study. I.A.R. and B.G. produced 3-D models of Parvancorina. I.A.R. performed CFD simulations. S.A.F.D. and I.A.R. wrote the first draft of the manuscript. All authors contributed to subsequent versions, are accountable for the content and gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
All authors acknowledge generous support from a Vanderbilt University International Travel Grant awarded to S.A.F.D. I.A.R. was funded by an 1851 Royal Commission Fellowship and the Oxford University Museum of Natural History. R.A.R. was funded by NSF grant nos. DEB 1331980 and PLR 134175 to N. Smith and the Natural History Museum of LA County. M.L. thanks the National Science and Engineering Research Council of Canada (NSERC 2013 Discovery Grant RGPIN 435402).
References
- 1.Narbonne GM. 2005. The Ediacara biota: Neoproterozoic origin of animals and their ecosystems. Annu. Rev. Earth Planet. Sci. 33, 421–442. ( 10.1146/annurev.earth.33.092203.122519) [DOI] [Google Scholar]
- 2.Rahman IA, Darroch SAF, Racicot RA, Laflamme M. 2015. Suspension feeding in the enigmatic Ediacaran organism Tribrachidium demonstrates complexity of Neoproterozoic ecosystems. Sci. Adv. 1, e1500800 ( 10.1126/sciadv.1500800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghisalberti M., Gold DA, Laflamme M., Clapham ME, Narbonne GM, Summons RE, Johnston DT, Jacobs DK. 2014. Canopy flow analysis reveals the advantage of size in the oldest communities of multicellular eukaryotes. Curr. Biol. 24, 305–309. ( 10.1016/j.cub.2013.12.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glaessner MF. 1980. Parvancorina—an arthropod from the Late Precambrian (Ediacarian) of South Australia. Ann. Nat. Hist. Mus. Wien 1, 83–90. [Google Scholar]
- 5.Glaessner MF. 1958. New fossils from the base of the Cambrian in South Australia. T. Roy. Soc. South Aust. 81, 185–188. [Google Scholar]
- 6.Naimark EB, Ivantsov AY. 2009. Growth variability in the late Vendian problematics Parvancorina Glaessner. Paleontol. J. 43, 12–18. ( 10.1134/S003103010901002X) [DOI] [Google Scholar]
- 7.Gehling JG, Droser ML. 2013. How well do fossil assemblages of the Ediacara Biota tell time? Geology 41, 447–450. ( 10.1130/G33881.1) [DOI] [Google Scholar]
- 8.Droser ML, Gehling JG. 2015. The advent of animals: the view from the Ediacaran. Proc. Natl. Acad. Sci. U.S.A. 112, 4865–4870. ( 10.1073/pnas.1403669112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laflamme M, Darroch SAF, Tweedt SM, Peterson KJ, Erwin DH. 2013. The end of the Ediacara biota: extinction, biotic replacement, or Cheshire Cat? Gondwana Res. 23, 558–573. ( 10.1016/j.gr.2012.11.004) [DOI] [Google Scholar]
- 10.Ivantsov AY, Malakhovskaya YE, Serezhnikova EA. 2004. Some problematic fossils from the Vendian of the southeastern White Sea region. Paleontol. J. 38, 3–9. [Google Scholar]
- 11.Singer A, Plotnick R, Laflamme M. 2012. Experimental fluid mechanics of an Ediacaran frond. Palaeontol. Electron. 15, 1–14. [Google Scholar]
- 12.Liu AG, Kenchington CG, Mitchell EG. 2015. Remarkable insights into the paleoecology of the Avalonian Ediacaran macrobiota. Gondwana Res. 27, 1355–1380. ( 10.1016/j.gr.2014.11.002) [DOI] [Google Scholar]
- 13.Vogel S. 1994. Life in Moving fluids: the Physical Biology of Flow. Princeton, NJ: Princeton University Press. [Google Scholar]
- 14.Paterson JR, Gehling JG, Droser ML, Bicknell RDC. 2017. Rheotaxis in the Ediacaran epibenthic organism Parvancorina from South Australia. Sci. Reports 7, 45539 ( 10.1038/srep45539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gehling JG. 2000. Environmental interpretation and a sequence stratigraphic framework for the terminal Proterozoic Ediacara Member within the Rawnsley Quartzite, South Australia. Precambrian Res. 100, 65–95. ( 10.1016/S0301-9268(99)00069-8) [DOI] [Google Scholar]
- 16.Cunningham JA, Liu AG, Bengtson S, Donoghue PCJ. 2017. The origin of animals: can molecular clocks and the fossil record be reconciled? BioEssays 39, 1–12. ( 10.1002/bies.201600120) [DOI] [PubMed] [Google Scholar]
- 17.Darroch SAF, Rahman IA, Gibson B, Racicot RA, Laflamme M. 2017. Data from: Inference of facultative mobility in the enigmatic Ediacaran organism Parvancorina. Dryad Digital Repository. ( 10.5061/dryad.2601h) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Darroch SAF, Rahman IA, Gibson B, Racicot RA, Laflamme M. 2017. Data from: Inference of facultative mobility in the enigmatic Ediacaran organism Parvancorina. Dryad Digital Repository. ( 10.5061/dryad.2601h) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
3-D models and CFD simulation files are available at: http://dx.doi.org/10.5061/dryad.2601h (see also [17]). Supplementary files include supplementary text and figures.