Abstract
Helping kin or nonkin can provide direct fitness benefits, but helping kin also benefits indirect fitness. Why then should organisms invest in cooperative partnerships with nonkin, if kin relationships are available and more beneficial? One explanation is that a kin-limited support network is too small and risky. Even if additional weaker partnerships reduce immediate net cooperative returns, individuals extending cooperation to nonkin can maintain a larger social network which reduces the potential costs associated with losing a primary cooperation partner. Just as financial or evolutionary bet-hedging strategies can reduce risk, investing in quantity of social relationships at the expense of relationship quality (‘social bet-hedging’) can reduce the risks posed by unpredictable social environments. Here, we provide evidence for social bet-hedging in food-sharing vampire bats. When we experimentally removed a key food-sharing partner, females that previously fed a greater number of unrelated females suffered a smaller reduction in food received. Females that invested in more nonkin bonds did not do better under normal conditions, but they coped better with partner loss. Hence, loss of a key partner revealed the importance of weaker nonkin bonds. Social bet-hedging can have important implications for social network structure by influencing how individuals form relationships.
Keywords: biological markets, centrality, cooperation, bet-hedging, reciprocity, social networks
1. Introduction
When cooperative relationships require an investment of time or energy, individuals should invest preferentially in the partner yielding the greatest cooperative returns [1–3]. However, if cooperative relationships take time to develop and partners are not always available, then a strategy that focuses investments in the single most-profitable partnership is risky. When partner availability is unpredictable, a better strategy would diversify cooperative investments across more partners to reduce the potential costs of losing a key partnership. We call this strategy social bet-hedging. Like other forms of bet-hedging [4], this strategy can be advantageous even if it reduces average short-term returns.
Bet-hedging strategies avoid risk. Social bet-hedging is analogous to evolutionary bet-hedging, where phenotypes with less temporally variable reproductive success outbreed phenotypes yielding reproductive success that is higher on average but more temporally variable [4]. This occurs because optimizing growth rates (or returns on investment) requires increasing the geometric, rather than arithmetic, mean. An evolutionary bet-hedging strategy can maximize geometric mean fitness, even at the expense of a lower arithmetic mean fitness, by coping better with rare stressful conditions [4].
By spreading cooperative investments to more partners, social bet-hedging strategies can reduce the temporal variance in cooperative returns caused by changes in partner availability. Investing in new relationships can be beneficial even if this requires diverting time and energy away from the most-profitable cooperative relationship which yields the greatest inclusive fitness return rate (e.g. the strongest reciprocator or closest kin).
Social bet-hedging might explain why female common vampire bats (Desmodus rotundus) that have strong reciprocal food-sharing relationships with close kin still regurgitate food to other nonkin [5–7]. Vampire bats are susceptible to starvation and depend on a network of food-sharing partners to feed them after unsuccessful foraging nights. The strongest, most reliable, and most balanced food-sharing bonds develop between mothers and daughters, but even for these close kin, the direct fitness benefits of food sharing might exceed the indirect fitness benefits [5–10]. The best known predictor of sharing rates within familiar pairs is not kinship, but the reciprocal rate of sharing [5,8]. If feeding close kin yields reciprocal sharing benefits that are equal or greater to feeding nonkin, why invest in nonkin bonds?
Sharing only with kin could be risky because relatives can be lost for various reasons. A starved female with only one or a few close maternal kin in her food-sharing network might not find her primary close kin donor, for example, if this partner also failed to feed or switched to a different roost on that night—which happens frequently [10]. To compensate for this risk, a social bet-hedging female would foster new bonds by diverting some of her social time and energy away from mothers and daughters and towards other females. Even if each of these additional partners is less related and reciprocates less, this strategy could dramatically increase long-term survival by reducing the risk of failing to find a primary donor when in dire need.
To test this idea, we quantify the impact, in terms of total food received, of removing a past key food donor for individual bats in need. Previously, Carter & Wilkinson [6] demonstrated that females that fed more nonkin females in previous years subsequently received more food in the absence of this key donor (see Methods), but this finding could simply mean that better-connected bats always receive more food. Here, we extend our analysis of this experiment to show that, as predicted by social bet-hedging, helping more nonkin did not increase food received when key donors were available, but it reduced the negative impact on food received when a key donor was removed.
2. Methods
We used data from a previous experiment [6], where a female subject was isolated and fasted for 24 h, then reintroduced to a captive colony of 27–34 individually marked conspecifics to measure food donated by each groupmate. Mean dyadic donation rates were calculated from 1337 dyadic regurgitation observations among 14 captive females using 91 fasting trials over a 4-year period (see electronic supplementary material, [6]). Relatedness was estimated using maternal pedigree and 19 microsatellite markers (see [6]). For each female, a unique key donor with a strong history of food sharing was selected for temporary removal; key donors were either the subject's highest-ranking donor (nine cases), second-highest ranking donor (four cases), or a lower-ranking donor but the highest-ranking recipient (one case) (see electronic supplementary material, [6]). During two control trials, a female that had never fed the subject was excluded by either removing it or fasting it on the same night. During three subsequent test trials, the subject's key donor was similarly excluded [6]. A previous analysis showed that bats that fed more nonkin females in past years received more food during test trials [6], but the social bet-hedging hypothesis predicts that this relationship should be most important when key donors are removed, not when they are present.
Here, we fitted linear models to predict the amount of food received with and without key donors present as well as the change in total food received (difference in food received per trial) when key donors were removed. We included the number of nonkin females fed in the past 4 years to represent investment in the size of a social support network. We did not include the number of kin fed because this depended on the number of kin available. We also did not include the number of males fed because stable bonds in the wild are female–female. To control for sampling bias, we included the control variable opportunity to donate, which is the number of trials where the subject could have fed another bat (see electronic supplementary material). The distribution of residuals did not deviate from normal (Shapiro Wilk's test: W = 0.98, p = 0.95). To visualize results, we plotted mean food received against residual past sharing to nonkin females—the residuals from a regression of the number of unrelated females fed on number of opportunities to donate (to control for the latter).
3. Results
Under typical conditions, when key donors were present, the number of female nonkin fed in previous years did not predict the amount of food received; instead the trend was negative (figure 1a; R2 = 0.43, β = −52.4, t = −1.81, p = 0.155). However, feeding more female nonkin did predict receiving more food later when a female's key donor was absent (figure 1b; R2 = 0.56, β = 56.0, t = 3.68, p = 0.004). A bat's proclivity to invest in female nonkin therefore predicted the change in total food she received when key donors were removed (R2 = 0.58, F2,11 = 7.54; β = 108.4, t = 3.48, p = 0.005; figure 1c). Females that fed more female nonkin coped better with partner removal. This result was robust to several variations in the analysis (see electronic supplementary material).
Figure 1.
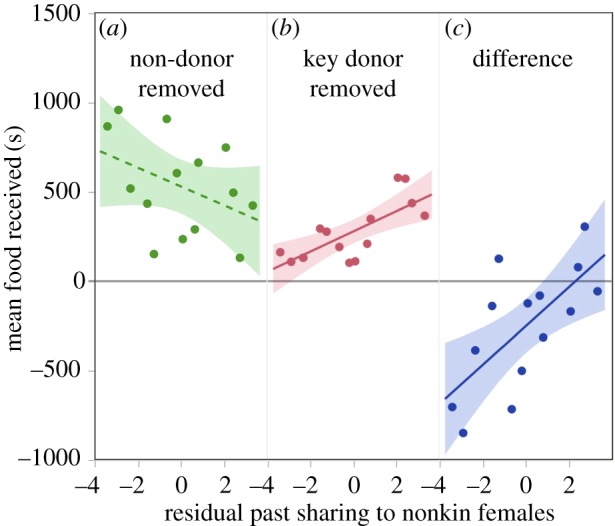
Bats with a higher propensity to help unrelated females suffered smaller losses in total food received when a key donor was removed as a potential donor. Proclivity to feed more nonkin females (x-axis) did not positively correlate with food received when a non-donor was absent (a) but it did when the key donor was absent (b). Feeding more unrelated females predicted smaller reductions in food received when the key donor was removed (c). Shading shows 95% CI of the slope.
4. Discussion
Our results support the social bet-hedging hypothesis. By helping nonkin, individuals appear to maintain a wider support network than would be possible through only helping close kin. This suggests that female vampire bats can reduce the costs of losing a key donor by ‘not putting all their eggs in one basket’.
The social bet-hedging hypothesis makes three key assumptions. First, it assumes that individuals shift cooperative investments to and from individuals based on their relative cooperative returns, as predicted by reciprocity and biological market theory (e.g. models of partner control and partner choice) [1–3].
Second, it assumes not only that there are fitness benefits to having both more cooperative partners and stronger relationships [11–17], but also that individuals often face a trade-off between investing in relationship quantity versus quality (strength). If cooperative relationships require continuous investment, then merely increasing the number of weak connections can reduce overall cooperative returns, just as increasing offspring production at the expense of offspring quality does not reliably increase fitness [18]. On the other hand, strengthening each relationship can come at the expense of relationship quantity, so individuals might therefore divert investments towards partners that yield lower indirect fitness or reciprocal returns simply to create more relationships.
Third, social bet-hedging only makes sense if lost cooperative partnerships cannot be replaced instantly and effortlessly (as evidenced by figure 1a). Backup partners must already be in place. Social bet-hedging therefore assumes that new relationships require time and energy to develop. This seems true for food-sharing vampire bats [5–10].
Social bet-hedging may also exist for other cooperative behaviours. For example, female baboons increase their social grooming rates and groom more partners after the death of a close female relative [19], suggesting that investments in more relationships can help to compensate for the loss of a key social partner. In humans, although relationship quality is better than relationship quantity at predicting received social support [20], people appear to benefit from a greater number of weaker friendships in environments where friends are more likely to leave [21].
Many models of cooperation focus on pairwise interactions (e.g. [2]), but cooperative ‘exchange rates’ are determined by the supply and demand of cooperative services and partners—properties of the larger social network [22]. Many cooperative species might allocate cooperative investments across several partners and compare the varying return rates from each [3,22]. It remains unclear, however, if or how different social animals balance the quality and quantity of social ties. By influencing the number and strength of connections in a social network, strategies like social bet-hedging can both shape, and be shaped by, social network structure.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Organization for Bat Conservation for their extraordinary support. Ronald Noë, Rachel Crisp, Julia Vrtilek and two anonymous reviewers provided comments that improved the manuscript.
Ethics
All procedures were approved by the University of Maryland Institutional Animal Care and Use Committee (Protocol R-10-63).
Data accessibility
The data supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
G.G.C. and D.R.F. conceived the analysis, G.G.C. carried out the analysis, and D.R.F. and G.S.W. advised the analysis. G.G.C. drafted the manuscript; D.R.F. and G.S.W. revised it critically for important intellectual content. All authors gave final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
We have no competing interests.
Funding
Work by G.G.C. was supported by a Ford Predoctoral Fellowship, a Dissertation Improvement Grant from the National Science Foundation (IOS-1311336), and grants from the American Society of Mammalogists and Animal Behavior Society.
References
- 1.Trivers RL. 1971. The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57. ( 10.1086/406755) [DOI] [Google Scholar]
- 2.Axelrod R, Hamilton WD. 1981. The evolution of cooperation. Science 211, 1390–1396. ( 10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
- 3.Noë R, Hammerstein P. 1994. Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 35, 1–11. ( 10.1007/BF00167053) [DOI] [Google Scholar]
- 4.Philippi T, Seger J. 1989. Hedging one's evolutionary bets, revisited. Trends Ecol. Evol. 4, 41–44. ( 10.1016/0169-5347(89)90138-9) [DOI] [PubMed] [Google Scholar]
- 5.Carter GG, Wilkinson GS. 2013. Food sharing in vampire bats: reciprocal help predicts donations more than relatedness or harassment. Proc. R. Soc. B 280, 20122573 ( 10.1098/rspb.2012.2573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter GG, Wilkinson GS. 2015. Social benefits of non-kin food sharing by female vampire bats. Proc. R. Soc. B 282, 20152524 ( 10.1098/rspb.2015.2524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilkinson GS. 1984. Reciprocal food sharing in the vampire bat. Nature 308, 181–184. ( 10.1038/308181a0) [DOI] [Google Scholar]
- 8.Carter GG, Wilkinson G. 2013. Does food sharing in vampire bats demonstrate reciprocity? Commun. Integr. Biol. 6, e25783 ( 10.4161/cib.25783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson GS. 1988. Reciprocal altruism in bats and other mammals. Ethol. Sociobiol. 9, 85–100. ( 10.1016/0162-3095(88)90015-5) [DOI] [Google Scholar]
- 10.Wilkinson GS. 1985. The social organization of the common vampire bat: I. Pattern and cause of association. Behav. Ecol. Sociobiol. 17, 111–121. [Google Scholar]
- 11.Kokko H, Johnstone RA, Clutton-Brock TH. 2001. The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187–196. ( 10.1098/rspb.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seyfarth RM, Cheney DL. 1984. Grooming, alliances and reciprocal altruism in vervet monkeys. Nature 308, 541–543. ( 10.1038/308541a0) [DOI] [PubMed] [Google Scholar]
- 13.Seyfarth RM, Cheney DL. 2012. The evolutionary origins of friendship. Annu. Rev. Psychol. 63, 153–177. ( 10.1146/annurev-psych-120710-100337) [DOI] [PubMed] [Google Scholar]
- 14.Seyfarth RM, Silk JB, Cheney DL. 2014. Social bonds in female baboons: the interaction between personality, kinship and rank. Anim. Behav. 87, 23–29. ( 10.1016/j.anbehav.2013.10.008) [DOI] [Google Scholar]
- 15.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2009. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B 276, 3099–3104. ( 10.1098/rspb.2009.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2010. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361. ( 10.1016/j.cub.2010.05.067) [DOI] [PubMed] [Google Scholar]
- 17.Wittig RM, Crockford C, Lehmann J, Whitten PL, Seyfarth RM, Cheney DL. 2008. Focused grooming networks and stress alleviation in wild female baboons. Horm. Behav. 54, 170–177. ( 10.1016/j.yhbeh.2008.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith CC, Fretwell SD. 1974. The optimal balance between size and number of offspring. Am. Nat. 108, 499–506. ( 10.1086/282929) [DOI] [Google Scholar]
- 19.Engh AL, Beehner JC, Bergman TJ, Whitten PL, Hoffmeier RR, Seyfarth RM, Cheney DL. 2006. Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus). Proc. R. Soc. B 273, 707–712. ( 10.1098/rspb.2005.3378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franks HM, Cronan TA, Oliver K. 2004. Social support in women with fibromyalgia: is quality more important than quantity? J. Community Psychol. 32, 425–438. ( 10.1002/jcop.20011) [DOI] [Google Scholar]
- 21.Oishi S, Kesebir S. 2012. Optimal social-networking strategy is a function of socioeconomic conditions. Psychol. Sci. 23, 1542–1548. ( 10.1177/0956797612446708) [DOI] [PubMed] [Google Scholar]
- 22.Fruteau C, Voelkl B, van Damme E, Noë R. 2009. Supply and demand determine the market value of food providers in wild vervet monkeys. Proc. Natl Acad. Sci. USA 106, 12 007–12 012. ( 10.1073/pnas.0812280106) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been uploaded as part of the electronic supplementary material.


