Abstract
Subsidies from adjacent ecosystems can alter recipient food webs and ecosystem functions, such as herbivory. Emerging aquatic insects from streams can be an important prey in the riparian zone. Such aquatic subsidies can enhance predator abundances or cause predators to switch prey, depending on the herbivores. This can lead to an increase or decrease of in situ herbivores and herbivory. We examined the effects of aquatic subsidies on a simplified terrestrial food web consisting of two types of herbivores, plants and predators (spiders). In our six-week experiment, we focused on the prey choice of the spiders by excluding predator immigration and reproduction. In accordance with predator switching, survival of leafhoppers increased in the presence of aquatic subsidies. By contrast, the presence of aquatic subsidies indirectly reduced weevils and herbivory. Our study shows that effects of aquatic subsidies on terrestrial predators can propagate through the food web in contrasting ways. Thereby, the outcome of the trophic cascade is determined by the prey choice of predators.
Keywords: aquatic-terrestrial linkages, indirect effects, prey choice, spiders, aquatic subsidy, trophic cascades
1. Introduction
Consumers can benefit from the dispersal of organisms across ecosystem boundaries, which can in turn affect food webs [1]. Such subsidies can cause different responses in the recipient food web. First, an increase of predator abundance can affect the in situ prey (i.e. apparent competition) [2]. Second, a switch from in situ to subsidy prey can reduce predation pressure on the in situ prey [3]. Streams and their riparian zones represent ecosystems that are linked via fluxes of material and organisms [4]. Aquatic subsidies like aquatic insects typically complete their life cycle in the terrestrial ecosystem [5] and can be an important prey for terrestrial predators such as riparian spiders [6]. Consequently, the abundance of terrestrial spiders can be enhanced along streams, reducing terrestrial insects and herbivory [7]. In the field, this apparent competition between aquatic insects and terrestrial herbivores is mediated by predator numbers, but it remains unknown if differences in predator density in riparian areas are a result of aquatic subsidies or differences in habitat conditions [8]. By contrast, indirect positive effects of aquatic subsidies on terrestrial herbivores were observed, presumably caused by a switch of a riparian predator from leafhoppers to the subsidies [9]. Such indirect positive effects of aquatic subsidies on terrestrial herbivores can dominate in riparian food webs [9,10]. However, indirect effects of such subsides depend on differences in the quality and mobility of prey species [11]. Studies on the role of aquatic subsidies for plants [7,12] and for trophic cascades including more than one prey type in an experiment [9] are scarce. We examined how aquatic subsidies affect a terrestrial model food web consisting of two types of herbivores with differing predator avoidance strategies, plus plants and spiders. We focus on short-term effects by keeping predator numbers constant, thus excluding predator immigration and reproduction.
We hypothesized: (H1) different survival of the two terrestrial herbivores in the presence of aquatic subsidy, and (H2) increased herbivory in the presence of aquatic subsidy due to predator switching.
2. Material and methods
(a). Study species
We used species that are common on riparian nettle stands of the study region in realistic field densities. Spiders, Tetragnatha sp. and Pisaura mirablis, were collected from late April until early May. Tetragnatha was chosen because it is common in riparian areas and feeds on aquatic subsidies [13] and Pisaura because it appears in high numbers on nettle plants and uses them as a hunting ground [14]. Tetragnatha relies on orb webs for prey capture, while Pisaura is a non-web-building sit-and-wait predator that can also patrol vegetation for prey [15]. Leafhoppers and weevils (Phyllobius sp.) were used as terrestrial herbivores and are common on riparian nettle stands. While leafhoppers feed on plant sap, weevils feed on the leaf lamina and are thus responsible for the leaf damage in previous field experiments [14]. Leafhoppers avoid predation mostly through their effective sensing of approaching predators in combination with escape movements. Weevils, rather, rely on their mechanical defence plus thanatosis to avoid predation [16]. Nettles, Urtica dioica, were used as a model plant to determine herbivory, because they are widely distributed along streams and offer a habitat for a high diversity of arthropods [17]. Nettles were collected four weeks before introducing them into the mesocosms from the riparian zone of a stream and were planted in fertilized soil (nitrogen 230 mg l−1) in 6 × 6 × 9 cm pots. The plants were vacuumed for 5 s to remove herbivores before the experiment; slugs and snails were removed by hand.
(b). Experimental design
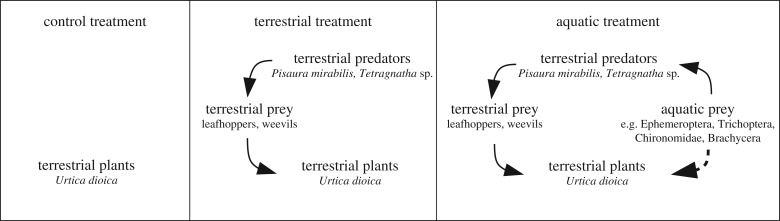
We conducted a six-week mesocosm experiment beside a stream in the research facility Eußerthal (49°15′17.86″ N 7°57′41.69″ E) in southwest Germany. The experiment consisted of three treatments: control (plants only, six replicates), terrestrial food web (plants, terrestrial herbivores and spiders, 13 replicates) and terrestrial food web with aquatic subsidies (as previous plus aquatic subsidies, 13 replicates) (figure 1). Organisms were introduced in 25 × 25 × 90 cm mesocosms (aerarium by Matthäus Hahn e.K., Altdorf, Germany). One specimen of Tetragnatha and two female and one male Pisaura per mesocosm were introduced in the food web treatments. If either Tetragnatha or Pisaura disappeared completely from a mesocosm, this was compensated by adding a new individual of the respective species. Five leafhoppers and two or three individuals of weevils were introduced fortnightly and weekly, respectively. To quantify herbivory, three leaves per plant in each mesocosm were marked with a coloured string at study initiation. The marked leaves were photographed twice per week. Herbivory was quantified by subtracting the areas of individual leaves from the first and last picture using the software ImageJ v. 1.48v [18]. Owing to leaf fall, one additional leaf was marked in the third and in the fifth week. The length of each nettle shoot was measured on the first and last day of the experiment. After the experiment, dry weight of nettles was determined after drying for 24 h at 60°C. Aquatic insects were collected from the stream using emergence traps with a basal area of 0.25 m2 each; thus the input mimicked the quantitative and temporal pattern of field emergence (see [19]). The living insects from one randomly chosen emergence trap were transferred to each mesocosm twice a week. At the end of the experiment, organisms were collected by vacuuming the mesocosms and plants for at least 5 s with a leaf blower (modified STIHL SH86 blower; Stihl, Waiblingen, Germany) to quantify the number of survived individuals.
Figure 1.
Design of the three treatments (control: only plants; terrestrial: terrestrial plants, prey and predators; aquatic: terrestrial treatment including aquatic subsidies). Solid lines indicate direct effects between organisms and dashed lines potential indirect effects of aquatic subsidies.
(c). Data analysis
Treatment effects on spider survival and size were tested with a generalized linear model (GLM) with Poisson distribution, followed by an ANOVA with a χ2-test. To test the effects on Pisaura biomass a linear model was used. Effects of aquatic subsidies on herbivores were tested with a GLM with Poisson distribution, followed by an ANOVA with a χ2-test. To test whether the two herbivores respond differently to the treatments we conducted a GLM with binomial error distribution. To evaluate the effects of aquatic subsidies on herbivory, we calculated the weighted mean of consumed leaf area per plant and day, given the different time spans of leaf observation. A generalized linear mixed model (GLMM) with gamma distribution, log link and the individual plant as random effect was used to account for between-plant variation using penalized quasi-likelihood (PQL) [20]. Again GLMMs, but combined with a PQL and a Tukey test, were performed to test for significant differences of the plant metrics between the treatments [21]. Statistics and graphics were done in R v. 3.3.1 [22].
3. Results
Although we observed Pisaura and Tetragnatha consuming aquatic subsidies, no effect of the treatment on the survival (Pisaura: 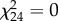 , p = 1; Tetragnatha:
, p = 1; Tetragnatha: 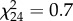 , p = 0.405), biomass (F22 = 0, p = 0.901) or size (
, p = 0.405), biomass (F22 = 0, p = 0.901) or size (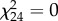 , p = 0.922) of the predators was detected. The indirect effect of aquatic subsidies differed significantly between the two types of herbivores (t24 = 2.9, p = 0.009). The addition of aquatic subsidies caused an increased survival of leafhoppers (figure 2a;
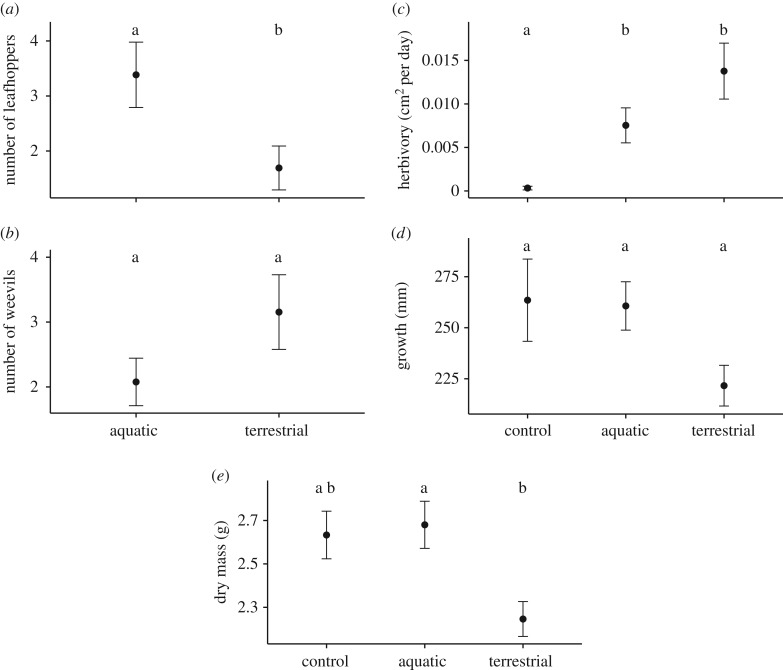
, p = 0.922) of the predators was detected. The indirect effect of aquatic subsidies differed significantly between the two types of herbivores (t24 = 2.9, p = 0.009). The addition of aquatic subsidies caused an increased survival of leafhoppers (figure 2a; 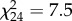 , p = 0.006), and there was a non-significant trend for weevil numbers to decrease in the presence of aquatic subsidies (figure 2b;
, p = 0.006), and there was a non-significant trend for weevil numbers to decrease in the presence of aquatic subsidies (figure 2b; 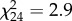 , p = 0.088), in accordance with leaf area loss. Herbivory was reduced by 55% in the aquatic compared with the terrestrial food web treatment (figure 2c; z28,128 = 2.2, p = 0.064). Plant dry mass of the aquatic treatment was 1.19 times higher than without the addition of aquatic subsidies (figure 2e; z28,126 = −2.3, p = 0.046), with a similar trend for growth (figure 2d; z28,126 = −2.2, p = 0.063).
, p = 0.088), in accordance with leaf area loss. Herbivory was reduced by 55% in the aquatic compared with the terrestrial food web treatment (figure 2c; z28,128 = 2.2, p = 0.064). Plant dry mass of the aquatic treatment was 1.19 times higher than without the addition of aquatic subsidies (figure 2e; z28,126 = −2.3, p = 0.046), with a similar trend for growth (figure 2d; z28,126 = −2.2, p = 0.063).
Figure 2.
Effects of aquatic subsidies on (a) number of leafhoppers, (b) number of weevils, (c) herbivory (cm² per day), (d) plant growth (mm), (e) plant dry mass (g). Control: only plants; terrestrial: control + herbivores + spiders; aquatic: terrestrial + aquatic subsidies. Letters indicate means that are significantly different (p < 0.05). Data are presented as mean (filled circle) ± s.e.
4. Discussion
As hypothesized, the effect of aquatic subsidies on weevils was different from that on leafhoppers. Leafhopper survival increased in the presence of aquatic subsidies. Similarly, a previous experiment found enhanced leafhopper survival in the presence of aquatic subsidies in an arctic ecosystem with wolf spiders as predators [9]. Opposite to leafhoppers, predation of weevils increased in the presence of aquatic subsidies. Weevils use thanatosis and mechanical defence [16] to avoid predation. When the mobility of hungry predators is enhanced in the presence of aquatic subsidies, this could lead to higher encounter rates and predation on the relatively stationary weevils. In addition, spiders might invest relatively more time and energy for capturing highly active and relatively small-bodied prey such as chironomids. Under this situation, spiders might prefer the larger and higher-quality weevils to the plant sap feeding leafhoppers. As an alternative explanation for the decrease of weevils under aquatic subsidies, a higher leafhopper herbivory in this treatment could have induced plant defence compounds that may have caused higher movement in weevils, enhancing their encounter rate with and mortality due to Pisaura [23,24]. Future research should test these hypotheses to uncover the underlying mechanisms.
The outcome of the trophic cascade of aquatic subsidy on the terrestrial food web contrasted with our hypothesis. Rather than reducing plant dry mass through the release of terrestrial herbivores from predation, plant dry mass increased along with a non-significant reduction in herbivory and higher growth in the treatment receiving aquatic subsidies. This can be explained by the reduction of leaf chewing weevils in the presence of aquatic subsidies. Plant performance was more strongly affected by the reduction of the main herbivore (weevils) than by enhanced plant sucking leafhopper densities.
Even leafhopper densities can respond to aquatic subsidies in contrasting ways. The enhancement of leafhoppers by aquatic subsidies in our study and in a previous experiment [9] contrasts with reduced leafhopper densities on nettle plants next to a river in a field survey [7]. The difference is probably due to predator accumulation in these prey-rich areas, which was excluded from both experimental studies. Accordingly, short-term release of leafhoppers from predation in the presence of alternative prey would turn into enhanced leafhopper predation after predator densities have responded to the availability of aquatic food. Thus, indirect effects of aquatic subsidies on terrestrial food webs represent a case of transient dynamics caused by resource pulses [25], whereby short-term release of (at least some) in situ prey from predation contrasts with a long-term increase in predation pressure. Our observation that even short-term effects of subsidies affect in situ prey in contrasting ways further highlights the complexity of effects of aquatic subsidies on riparian food webs.
Acknowledgements
We thank two anonymous referees for valuable suggestions on a previous version of the manuscript, and Johanna Dupré, Katharina Frisch and Maya Nakajima for assistance.
Data accessibility
Datasets for the whole experiment are publicly accessible in the Dryad data repository at (http://dx.doi.org/10.5061/dryad.24r3m) [19].
Authors' contributions
All authors designed the study. N.G. carried out the field work, conducted the statistical analysis and drafted the manuscript. R.B., R.B.S. and M.H.E. provided feedback on the analysis, discussion of results and writing of the manuscript. All authors gave final approval for publication and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the Ministry for Education, Science and Culture (MBWWK) of Rhineland-Palatinate (Project AufLand).
References
- 1.Polis GA, Strong DR. 1996. Food web complexity and community dynamics. Am. Nat. 147, 813–846. ( 10.1086/285880) [DOI] [Google Scholar]
- 2.Holt RD, Lawton JH. 1994. The ecological consequences of shared natural enemies. Annu. Rev. Ecol. Syst. 25, 495–520. ( 10.1146/annurev.es.25.110194.002431) [DOI] [Google Scholar]
- 3.Abrams PA, Matsuda H. 1996. Positive indirect effects between prey species that share predators. Ecology 77, 610–616. ( 10.2307/2265634) [DOI] [Google Scholar]
- 4.Schulz R, et al. 2015. Review on environmental alterations propagating from aquatic to terrestrial ecosystems. Sci. Total Environ. 538, 246–261. ( 10.1016/j.scitotenv.2015.08.038) [DOI] [PubMed] [Google Scholar]
- 5.Post DM, Doyle MW, Sabo JL, Finlay JC. 2007. The problem of boundaries in defining ecosystems: a potential landmine for uniting geomorphology and ecology. Geomorphology 89, 111–126. ( 10.1016/j.geomorph.2006.07.014) [DOI] [Google Scholar]
- 6.Krell B, Röder N, Link M, Gergs R, Entling MH, Schäfer RB. 2015. Aquatic prey subsidies to riparian spiders in a stream with different land use types. Limnol. Ecol. Manage. Inland Waters 51, 1–7. ( 10.1016/j.limno.2014.10.001) [DOI] [Google Scholar]
- 7.Henschel JR, Mahsberg D, Stumpf H. 2001. Allochthonous aquatic insects increase predation and decrease herbivory in river shore food webs. Oikos 93, 429–438. ( 10.1034/j.1600-0706.2001.930308.x) [DOI] [Google Scholar]
- 8.Paetzold A, Smith M, Warren PH, Maltby L. 2011. Environmental impact propagated by cross-system subsidy: chronic stream pollution controls riparian spider populations. Ecology 92, 1711–1716. ( 10.1890/10-2184.1) [DOI] [PubMed] [Google Scholar]
- 9.Dreyer J, Hoekman D, Gratton C. 2016. Positive indirect effect of aquatic insects on terrestrial prey is not offset by increased predator density: aquatic insect positive indirect effect. Ecol. Entomol. 41, 61–71. ( 10.1111/een.12272) [DOI] [Google Scholar]
- 10.Sabo JL, Power ME. 2002. Numerical response of lizards to aquatic insects and short-term consequences for terrestrial prey. Ecology 83, 3023–3036. ( 10.1890/0012-9658(2002)083[3023:NROLTA]2.0.CO;2) [DOI] [Google Scholar]
- 11.Eubanks MD, Denno RF. 2000. Health food versus fast food: the effects of prey quality and mobility on prey selection by a generalist predator and indirect interactions among prey species. Ecol. Entomol. 25, 140–146. ( 10.1046/j.1365-2311.2000.00243.x) [DOI] [Google Scholar]
- 12.Bultman H, Hoekman D, Dreyer J, Gratton C. 2014. Terrestrial deposition of aquatic insects increases plant quality for insect herbivores and herbivore density: terrestrial effects of midge deposition. Ecol. Entomol. 39, 419–426. ( 10.1111/een.12118) [DOI] [Google Scholar]
- 13.Kato C, Iwata T, Wada E. 2004. Prey use by web-building spiders: stable isotope analyses of trophic flow at a forest-stream ecotone. Ecol. Res. 19, 633–643. ( 10.1111/j.1440-1703.2004.00678.x) [DOI] [Google Scholar]
- 14.Bucher R, Menzel F, Entling MH. 2015. Risk of spider predation alters food web structure and reduces local herbivory in the field. Oecologia 178, 571–577. ( 10.1007/s00442-015-3226-5) [DOI] [PubMed] [Google Scholar]
- 15.Roberts MJ. 1996. Collins field guide. Spiders of Britain and northern Europe. London, UK: HarperCollins Publishers. [Google Scholar]
- 16.Reitze M, Nentwig W. 1991. Comparative investigations into the feeding ecology of six Mantodea species. Oecologia 86, 568–574. ( 10.1007/BF00318324) [DOI] [PubMed] [Google Scholar]
- 17.Davis BNK. 1989. The European distribution of insects on stinging nettles, Urtica dioica L.: a field survey. Bolletino Zool. 56, 321–326. ( 10.1080/11250008909355658) [DOI] [Google Scholar]
- 18.Rasband W. 2011. ImageJ. (http://imagej.nih.gov/ij)
- 19.Graf N, Entling MH, Schäfer RB, Bucher R. 2017. Data from: Contrasting effects of aquatic subsidies on a terrestrial trophic cascade. Dryad Digital Repository. ( 10.5061/dryad.24r3m) [DOI] [PMC free article] [PubMed]
- 20.Venables WN, Ripley BD, Venables WN. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer. [Google Scholar]
- 21.Bretz F, Hothorn T, Westfall PH. 2011. Multiple comparisons using R. Boca Raton, FL: CRC Press. [Google Scholar]
- 22.R Development Core Team. 2011. R, a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 23.Kaplan I, Lynch ME, Dively GP, Denno RF. 2007. Leafhopper-induced plant resistance enhances predation risk in a phytophagous beetle. Oecologia 152, 665–675. ( 10.1007/s00442-007-0692-4) [DOI] [PubMed] [Google Scholar]
- 24.Bálint J, Zytynska SE, Salamon RV, Mehrparvar M, Weisser WW, Schmitz OJ, Benedek K, Balog A. 2016. Intraspecific differences in plant chemotype determine the structure of arthropod food webs. Oecologia 180, 797–807. ( 10.1007/s00442-015-3508-y) [DOI] [PubMed] [Google Scholar]
- 25.Holt RD. 2008. Theoretical perspectives on resource pulses. Ecology 89, 671–681. ( 10.1890/07-0348.1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Graf N, Entling MH, Schäfer RB, Bucher R. 2017. Data from: Contrasting effects of aquatic subsidies on a terrestrial trophic cascade. Dryad Digital Repository. ( 10.5061/dryad.24r3m) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Datasets for the whole experiment are publicly accessible in the Dryad data repository at (http://dx.doi.org/10.5061/dryad.24r3m) [19].