Abstract
Maternally inherited Wolbachia endosymbionts manipulate arthropod reproduction in various ways. In the butterfly Eurema mandarina, a cytoplasmic incompatibility-inducing Wolbachia strain wCI and the associated mtDNA haplotypes are known to originate from the sister species Eurema hecabe, which offered a good case study for microbe-mediated hybrid introgression. Besides wCI, some females with the Z0 karyotype harbour a distinct Wolbachia strain wFem, which causes all-female production by meiotic drive and feminization. We report that a considerable proportion of E. mandarina females (65.7%) were infected with both wCI and wFem (CF) on Tanegashima Island. While females singly infected with wCI (C) produced offspring at a 1 : 1 sex ratio, CF females produced only females. Although Z-linked sequence polymorphism showed no signs of divergence between C and CF females, mtDNA split into two discrete clades; one consisted of C females and the other CF females, both of which formed a clade with E. hecabe but not with uninfected E. mandarina. This suggests that CF matrilines also, but independently, experienced a selective sweep after hybrid introgression from E. hecabe. Distinct evolutionary forces were suggested to have caused C and CF matrilines to diverge, which would be irreversible because of the particular phenotype of wFem.
Keywords: Eurema, feminization, hybrid introgression, meiotic drive, mitochondrial DNA, Wolbachia
1. Introduction
Wolbachia, a maternally inherited, ubiquitous endosymbiont of arthropod and nematodes, is well known for its ability to manipulate host reproduction in order to facilitate its transmission in various ways, which can be categorized into two groups: cytoplasmic incompatibility (CI) and female-biased sex-ratio distortion [1]. CI is a lethal phenotype in embryos produced by mating between infected males and uninfected females, thus providing a reproductive advantage for infected females compared with uninfected ones. Besides CI, Wolbachia can bias its host sex ratio towards females by inducing thelytokous parthenogenesis, male-killing or feminization [1]. It was recently demonstrated that Wolbachia can also cause meiotic drive (MD), leading to all-female offspring [2].
Because of its reproductive manipulations, Wolbachia infection spreads and persists in host populations, together with maternally inherited mitochondria. This hitchhiking effect of mitochondria through Wolbachia selective sweep was first identified in Drosophila simulans [3], followed by similar findings in other insects [4]. Importantly, Wolbachia and its associated mitochondria can cross species boundaries; cytoplasmic elements that move into sibling species via hybridization may persist and spread in the new host due to the effect of Wolbachia-induced reproductive manipulations (such as CI), which would otherwise disappear easily due to the genetic drift [4]. Importantly, the hybrid introgression followed by Wolbachia selective sweep can confound molecular phylogenetic analyses based on mitochondrial DNA (mtDNA) sequences [4].
The common grass yellow, Eurema mandarina (Lepidoptera: Pieridae), offered a good model case wherein hybrid introgression and selective sweep caused by CI-inducing Wolbachia (strain wCI) resulted in the fixation of mtDNA haplotypes derived from Eurema hecabe (i.e. a sister species of E. mandarina) in most populations, except those on the northernmost part of the main island of Japan, where uninfected individuals remain [5].
Independently, some individuals in particular populations of E. mandarina and E. hecabe were found to harbour a sex-ratio-distorting Wolbachia, the strain wFem, in addition to wCI [6,7]. In both species, females singly infected with wCI (C females) produce offspring at a 1 : 1 sex ratio, whereas females doubly infected with wCI and wFem (CF females) produce all-female offspring. On subtropical Okinawa-jima Island, two out of ten E. mandarina females were considered CF females and produced only females [6,8]. Exclusive production of females by CF females was also reported from temperate Tanegashima Island [9,10], but systematic analyses on their Wolbachia infection status and offspring sex ratio have yet to be performed.
Recent studies revealed that E. mandarina C females have the WZ sex chromosome system, whereas CF females have the Z0 sex chromosome system [2,11]. Wolbachia was found to affect female meiosis and prevent the production of Z-bearing oocytes, thereby producing exclusively Z0 embryos that carry a paternal Z. Moreover, Wolbachia is responsible for female sex determination in Z0 individuals, which would otherwise be determined as males owing to the absence of the female-determining W chromosome [2]. Therefore, the Wolbachia-induced coordinated dual effects (i.e. MD and feminization) are considered responsible for the all-female production in E. mandarina.
We speculated that the distinct phenotypes exerted in C and CF matrilines are subjected to different selective forces. We aimed to establish (i) how common Wolbachia-induced MD is and (ii) whether the two distinct Wolbachia infection statuses (i.e. C and CF) affect mtDNA diversity in E. mandarina.
2. Material and methods
(a). Insects
In May 2015, adults of E. mandarina (33 females and 29 males) were collected on Tanegashima Island, Kagoshima Prefecture, Japan (figure 1). Offspring obtained from each female were reared in the laboratory (electronic supplementary material, S1).
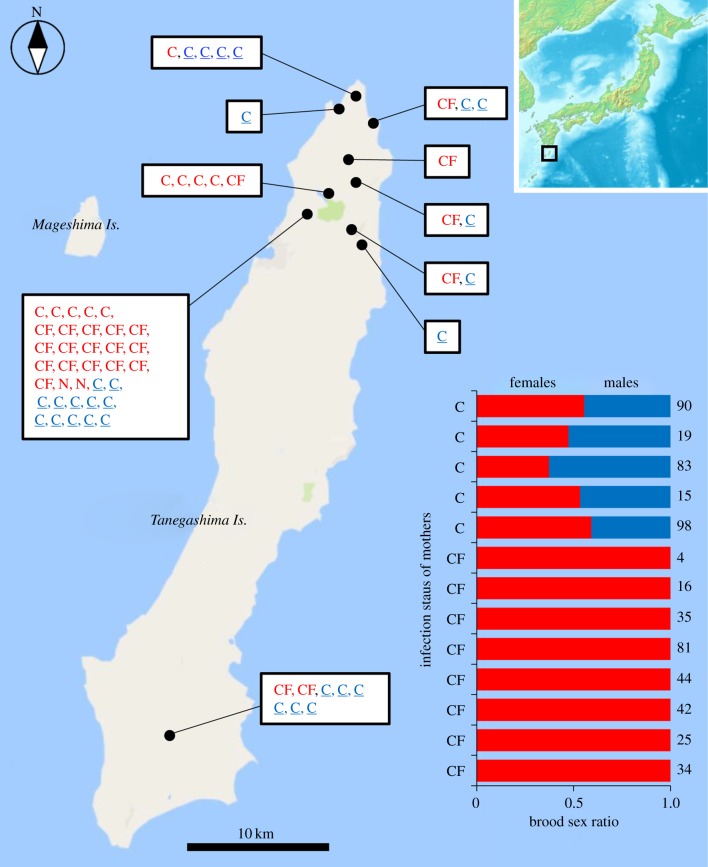
Figure 1.
Ten sampling sites of E. mandarina on Tanegashima Island. Red, females; blue and underlined, males; CF, doubly infected with wCI and wFem; C, singly infected with wCI. The bar graph represents the brood sex ratio produced from 13 females (five C and eight CF females) collected in this study. Brood size is given on the right. Original map images were from Google Maps (Google Inc.).
(b). Diagnostic PCR and sequencing of Wolbachia wsp gene
Infection with either wCI or wFem strain of Wolbachia was examined by specific PCR. The PCR products were then sequenced to confirm the identity of Wolbachia strains (electronic supplementary material, S1).
(c). Phylogenetic analysis of host nuclear and mtDNA genes
An intron region of the Z-linked triose phosphate isomerase (Tpi), mitochondrial cytochrome c oxidase subunit I (COI) and mitochondrial cytochrome c oxidase subunit III (COIII) and adjacent tRNA (referred to as COIII hereafter) were amplified by PCR and directly sequenced. For phylogenetic analyses, we also included previously published sequences of COI and COIII of E. hecabe collected from Southeast Asia [12] (electronic supplementary material, S1 and S2).
3. Results
(a). CF females are widespread in Eurema mandarina on Tanegashima Island
PCR and sequencing showed that all of the 33 wild-caught E. mandarina females were infected with wCI [6,7]. Among them, 23 females were also infected with wFem [6,7] (table 1). Offspring obtained from eight CF females were all females (281 females in total), while five C females produced both males and females (156 and 149 in total; figure 1).
Table 1.
Number of E. mandarina adults collected in Tanegashima Island in 2015. C, singly infected with wCI; CF, doubly infected with wCI and wFem; n.a., unknown (samples lost).
| sexual phenotype | C | CF | n.a. | total |
|---|---|---|---|---|
| females | 10 | 23 | 2 | 35 |
| males | 29 | 0 | 0 | 29 |
(b). Divergence of mtDNA between C and CF females in E. mandarina
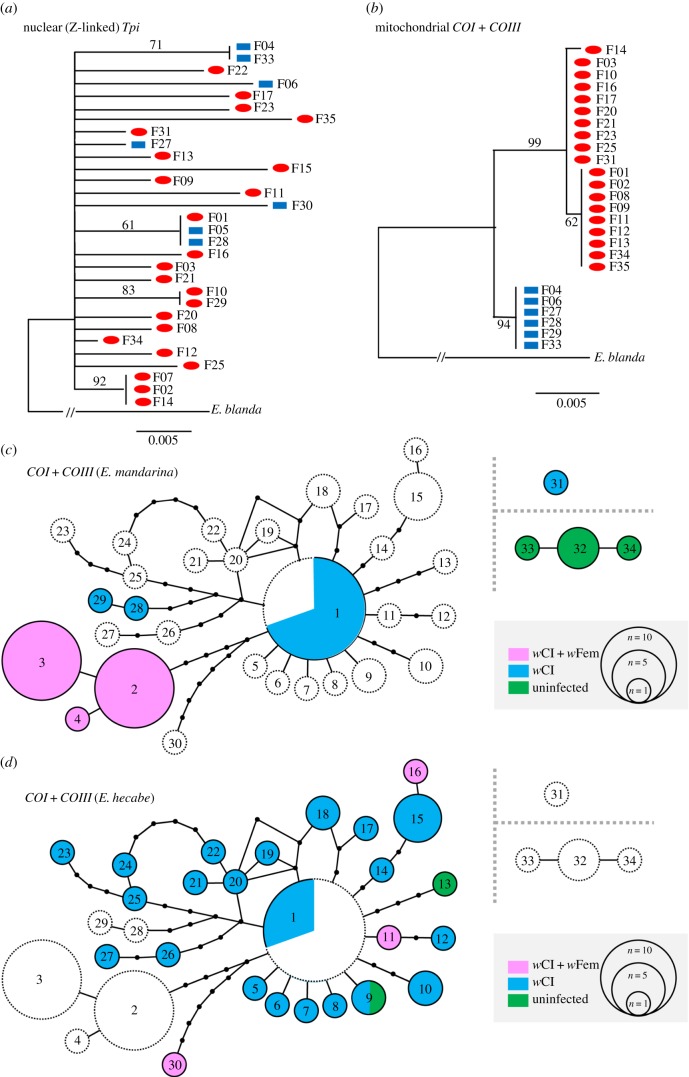
Among 30 wild-caught females of E. mandarina (22 CF and eight C females), Z-linked Tpi (430 bp) was polymorphic in 31 nucleotide sites, constituting 24 haplotypes. Because the Z is driven out of Z0 females [2], Tpi is always a marker of paternal origin in CF females. The maximum-likelihood (ML) tree based on Tpi did not show any signs of divergence between CF and C lineages (figure 2a), indicating that CF and C lineages share a common gene pool through males.
Figure 2.
Molecular phylogenetic analyses of Eurema butterflies. ML trees of E. mandarina collected on Tanegashima Island based on nuclear Tpi (a) and concatenated sequences of mitochondrial COI and COIII (b). Red ovals, CF females; blue rectangles, C females. A haplotype spanning network of E. mandarina and E. hecabe based on concatenated sequences of COI and COIII (c,d). Haplotypes of E. mandarina (c) and E. hecabe (d) are highlighted. Each circle represents a single haplotype with an area proportional to the sample size. Dots: missing haplotypes. Circles and dots are connected by single-nucleotide substitutions. Pink, CF females; blue, C females; green, uninfected females. Dotted circles and dotted parts of circles represent haplotypes that are absent in this particular species but are present in the sister species.
By contrast, ML trees based on mtDNA exhibited a clear distinction between CF and C (figure 2b). Among 25 E. mandarina females (19 CF and six C females), concatenated sequence of COI and COIII (2032 bp) was polymorphic in 16 nucleotide sites, constituting four haplotypes; one haplotype (corresponding to haplotype 1 in electronic supplementary material, S2) was possessed only by C females, while three haplotypes (corresponding to haplotypes 2, 3 and 4 in electronic supplementary material, S2) were possessed only by CF females. It is apparent that COI and COIII did not undergo recombination (electronic supplementary material, S3).
(c). Phylogenetic analysis of Eurema mandarina and Eurema hecabe based on mtDNA
Mitochondrial haplotypes of E. mandarina collected on Tanegashima Island were compared with the previously published mitochondrial haplotypes of E. mandarina and E. hecabe, which were collected in other populations in Japan and Southeast Asia [12] (figure 2c,d).
On the haplotype spanning network based on the concatenated sequences of COI and COIII (1777 bp in total), haplotypes of E. hecabe, irrespective of the infection status, were indistinguishable from the haplotypes of E. mandarina C females, except for an enigmatic Hong Kong-derived sample (CN2-a; haplotype 31 in figure 2c) [12], but were different from CF females of E. mandarina (figure 2c,d). Unique haplotypes of E. mandarina CF females compared with others may suggest a different evolutionary history of E. mandarina CF matrilines.
4. Discussion
This study suggested that Wolbachia-induced MD is widespread among E. mandarina on Tanegashima Island. Considering the fact that the sex ratios of collected butterflies are usually male-biased (probably due to the behavioural differences between males and females) [13], the nearly 1 : 1 sex ratio (33 females and 29 males) of the collected butterflies probably reflects a female-biased primary sex ratio. Assuming that the ratio of CF and C females in this population is 23/10 and that equal numbers of offspring are produced by these females, the sex ratio of the subsequent generation would be 5.6 females per male. Because the coexistence of all-female-producing CF females and normal C females is unstable under simple model formulations, some unknown factors might be involved in selection for or against CF females in a frequency-dependent manner. Alternatively, this polymorphism may be transient, leading to the extinction or fixation of CF matrilines (the latter would lead to a population collapse). Either way, our study highlights that Wolbachia-induced MD has a significant impact on the ecology and evolution of E. mandarina.
This study also reveals distinct nucleotide divergence in mitochondrial lineages in a population of E. mandarina according to Wolbachia infection status (i.e. C versus CF). This can be explained by distinct selective forces acting on the two types of matrilines, one showing a 1 : 1 sex ratio and the other all females. The reason for the clear distinction of mtDNA despite the potentially incomplete vertical transmission of wFem [10] can be considered as follows. Failure in Wolbachia vertical transmission in a CF matriline would result in all-male production [2,6,8], and hence the extinction of the particular mtDNA haplotype of the CF matriline. In typical cases of all-female production caused by male-killing Wolbachia, however, removal of Wolbachia would result in the production of females that produce 1 : 1 sex-ratio offspring. Therefore, divergence in mtDNA may be purged, to some extent, by the matrilines that lost Wolbachia. Contrastingly, C and CF females of E. mandarina would lead to an irreversible divergence of mtDNA. Although empirical evidence is lacking, this would also be the case in Ostrinia moths, wherein Wolbachia causes male-killing, but the elimination of Wolbachia results in the production of all-male offspring [14,15].
By comparison with the previously published sequences of E. hecabe and E. mandarina, we found that the mtDNA haplotypes of CF females formed a unique clade (figure 2d). Although these haplotypes apparently originated from E. hecabe, the distinctiveness from those of E. mandarina C females suggests that the C and CF females may have independently experienced hybrid introgression from E. hecabe, possibly representing consecutive mitochondrial sweep, firstly by wCI and then by wFem. In the cherry fruit fly, Rhagoletis cerasi, consecutive mtDNA sweeps were suggested due to multiple invasions of host populations by CI-inducing Wolbachia strains [16]. The fact that the mtDNA haplotypes possessed by E. mandarina CF females were not found in E. hecabe may imply that the introgression of CF mtDNA occurred a long time ago, so that considerable nucleotide substitution in mtDNA may have made these haplotypes extremely rare or extinct in E. hecabe. However, alternative possibilities still remain such as mitochondrial introgression together with wFem from another yet unknown Eurema species or horizontal transmission of Wolbachia multiple times in E. hecabe but only once in E. mandarina. Unlike the male-killing cases, the appearance of a suppressor against MD in the host would result in all males [2,8], leading to the elimination of Wolbachia as well as the accompanying mtDNA from the population.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Ethics
Collection of E. mandarina, a non-endangered butterfly, made in prefectural roadsides on Tanegashima Island, does not violate any laws.
Data accessibility
Sequences of Tpi, COI and COIII were submitted to DDBJ/EMBL/GenBank (electronic supplementary material, S2). Information for butterfly samples was deposited in Dryad (http://dx.doi.org/10.5061/dryad.7qf5f) [17].
Authors' contributions
M.M., M.N. and D.K. designed the study. M.M., T.K., K.Y. and D.K. collected and analysed the data. M.M., M.N., K.Y. and D.K. wrote the manuscript with input from T.K. All authors approved the final manuscript and are accountable for its content.
Competing interests
The authors have no competing interests.
Funding
This work was supported by a JSPS KAKENHI grant no. (16K08106) to D.K.
References
- 1.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6, 741–751. ( 10.1038/nrmicro1969) [DOI] [PubMed] [Google Scholar]
- 2.Kageyama D. et al 2017. Master manipulation continued: feminizing Wolbachia endosymbiont distorts sex chromosome inheritance. bioRχiv beta. ( 10.1101/115386) [DOI] [Google Scholar]
- 3.Hale LR, Hoffmann AA. 1990. Mitochondrial DNA polymorphism and cytoplasmic incompatibility in natural populations of Drosophila simulans. Evolution 44, 1383–1386. ( 10.2307/2409298) [DOI] [PubMed] [Google Scholar]
- 4.Hurst GD, Jiggins FM. 2005. Problems with mitochondrial DNA as a marker in population, phylogeographic and phylogenetic studies: the effects of inherited symbionts. Proc. R. Soc. B 272, 1525–1534. ( 10.1098/rspb.2005.3056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narita S, Nomura M, Kato Y, Fukatsu T. 2006. Genetic structure of sibling butterfly species affected by Wolbachia infection sweep: evolutionary and biogeographical implications. Mol. Ecol. 15, 1095–1108. ( 10.1111/j.1365-294X.2006.02857.x) [DOI] [PubMed] [Google Scholar]
- 6.Hiroki M, Tagami Y, Miura K, Kato Y. 2004. Multiple infection with Wolbachia inducing different reproductive manipulations in the butterfly Eurema hecabe. Proc. R. Soc. Lond. B 271, 1751–1755. ( 10.1098/rspb.2004.2769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narita S, Kageyama D, Hiroki M, Sanpei T, Hashimoto S, Kamitoh T, Kato Y. 2011. Wolbachia-induced feminisation newly found in Eurema hecabe, a sibling species of Eurema mandarina (Lepidoptera: Pieridae). Ecol. Entomol. 36, 309–317. ( 10.1111/j.1365-2311.2011.01274.x) [DOI] [Google Scholar]
- 8.Hiroki M, Kato Y, Kamito T, Miura K. 2002. Feminization of genetic males by a symbiotic bacterium in a butterfly, Eurema hecabe (Lepidoptera: Pieridae). Naturwissenschaften 89, 167–170. ( 10.1007/s00114-002-0303-5) [DOI] [PubMed] [Google Scholar]
- 9.Narita S, Kageyama D, Nomura M, Fukatsu T. 2007. Unexpected mechanism of symbiont-induced reversal of insect sex: feminizing Wolbachia continuously acts on the butterfly Eurema hecabe during larval development. Appl. Environ. Microbiol. 73, 4332–4341. ( 10.1128/AEM.00145-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narita S, Nomura M, Kageyama D. 2007. Naturally occurring single and double infection with Wolbachia strains in the butterfly Eurema hecabe: transmission efficiencies and population density dynamics of each Wolbachia strain. FEMS Microbiol. Ecol. 61, 235–245. ( 10.1111/j.1574-6941.2007.00333.x) [DOI] [PubMed] [Google Scholar]
- 11.Kern P, Cook JM, Kageyama D, Riegler M.. 2015. Double trouble: combined action of meiotic drive and Wolbachia feminization in Eurema butterflies. Biol. Lett. 11, 20150095 ( 10.1098/rsbl.2015.0095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narita S, Nomura M, Kato Y, Yata O, Kageyama D. 2007. Molecular phylogeography of two sibling species of Eurema butterflies. Genetica 131, 241–253. ( 10.1007/s10709-006-9134-1) [DOI] [PubMed] [Google Scholar]
- 13.Narita S, Nomura M, Kageyama D. 2007. A natural population of the butterfly Eurema hecabe with Wolbachia-induced female-biased sex ratio not by feminization. Genome 50, 365–372. ( 10.1139/g07-020) [DOI] [PubMed] [Google Scholar]
- 14.Kageyama D, Traut W. 2004. Opposite sex-specific effects of Wolbachia and interference with the sex determination of its host Ostrinia scapulalis. Proc. R. Soc. Lond. B 271, 251–258. ( 10.1098/rspb.2003.2604) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugimoto TN, Ishikawa Y. 2012. A male-killing Wolbachia carries a feminizing factor and is associated with degradation of the sex-determining system of its host. Biol. Lett. 8, 412–415. ( 10.1098/rsbl.2011.1114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuler H, et al. 2016. The hitchhiker's guide to Europe: the infection dynamics of an ongoing Wolbachia invasion and mitochondrial selective sweep in Rhagoletis cerasi. Mol. Ecol. 25, 1595–1609. ( 10.1111/mec.13571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyata M, Konagaya T, Yukuhiro K, Nomura M, Kageyama D. 2017. Data from: Wolbachia-induced meiotic drive and feminization is associated with an independent occurrence of selective mitochondrial sweep in a butterfly. Dryad Digital Repository. 10.5061/dryad.7qf5f [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Miyata M, Konagaya T, Yukuhiro K, Nomura M, Kageyama D. 2017. Data from: Wolbachia-induced meiotic drive and feminization is associated with an independent occurrence of selective mitochondrial sweep in a butterfly. Dryad Digital Repository. 10.5061/dryad.7qf5f [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Sequences of Tpi, COI and COIII were submitted to DDBJ/EMBL/GenBank (electronic supplementary material, S2). Information for butterfly samples was deposited in Dryad (http://dx.doi.org/10.5061/dryad.7qf5f) [17].