Abstract
Opioid use disorder (OUD) is a major public health problem. High relapse rates and poor treatment retention continue to pose major challenges in OUD treatment. Of the abused opioids, oxycodone is well described to maintain self-administration and evoke the durable conditioned responses (“cue reactivity”) that result from pairing of opioid-related stimuli (e.g., paraphernalia) with repeated abuse. Serotonin (5-HT) neurotransmission, particularly through the 5-HT2C receptor (5-HT2CR), regulates psychostimulant reward and cue reactivity, and in the present experiments, we investigated the hypothesis that the selective 5-HT2CR agonist lorcaserin, which is FDA-approved for the treatment of obesity, will suppress oxycodone self-administration and oxycodone-associated cue reactivity in rats. We found that lorcaserin inhibited oxycodone intake, an effect blocked by the selective 5-HT2CR antagonist SB242084. Lorcaserin also decreased responding for the discrete cue complex (“cue reactivity”) previously associated with delivery of oxycodone (i.e., stimulus lights, infusion pump sounds) in both abstinence and extinction-reinstatement models. The selected dose range of lorcaserin (0.25–1 mg/kg) does not overtly alter spontaneous behaviors nor operant responding on inactive levers in the present study. Taken together, the ability of lorcaserin to reduce the oxycodone self-administration and decrease cue reactivity associated with relapse highlights the therapeutic potential for lorcaserin in the treatment of OUD.
INTRODUCTION
The epidemic of opioid use disorder (OUD) is one of the top public health problems in the United States.1–2 The misuse of prescription (e.g., oxycodone) and illicit opioids (e.g., heroin) can escalate into OUD with significant morbidity and mortality.3–4 Treatment goals for OUD include mitigation of opioid-induced withdrawal and attainment of long-term abstinence.5 Pharmacotherapeutics development for OUD has focused largely on μ-opioid ligands given that abused opioid analgesics share efficacy as μ-opioid receptor agonists (for review).6 Medications that act as long-acting μ-opioid agonists (e.g., methadone), partial agonists (e.g., buprenorphine) as well as antagonists (e.g., naltrexone) have been employed to promote recovery within a suitably supportive therapeutic environment.5,7 However, high relapse rates and poor treatment retention continue to pose major challenges in the treatment of OUD.
Environmental context(s) and stimuli (e.g., paraphernalia) which become associated with substance use lead to enduring conditioned responses (“cue reactivity”) that can precipitate relapse.8–9 Drug cue reactivity is measurable as conditioned physiological (e.g., elevated heart rate) and subjective responses (e.g., craving) as well as attentional orienting behaviors (e.g., drug-seeking) and limbic-corticostriatal circuit activation in humans,8,10–12 including opioid users.13–22 Furthermore, measures of cue reactivity predict relapse in abstinent heroin users following detoxification,23 while the cue-induced cortisol response correlates with subsequent opioid use in opiate-abstinent methadone and buprenorphine-treated patients.15 Thus, novel treatment strategies effective at reducing cue reactivity as well as opioid intake may be particularly effective to promote recovery from OUD.
Serotonin (5-HT) neurotransmission confers modulatory control over the limbic-corticostriatal circuitry engaged in drug reward and cue reactivity, particularly through the 5-HT2C receptor (5-HT2CR) (for reviews).24–26 Activation of the 5-HT2CR following systemic administration of the FDA-approved anti-obesity medication lorcaserin (Belviq®) or investigational, selective 5-HT2CR agonists (e.g., Ro 60–0175, WAY163909) has been shown to suppress self-administration of cocaine27–34 and nicotine in preclinical models.35–39 Similarly, selective 5-HT2CR agonists have been shown to suppress cue reactivity assessed in both cocaine28,30–32,40–41 and nicotine self-administration assays.36–37,42 However, much less is known about the involvement of the 5-HT2CR in the modulation of the reward-related behavioral effects of opioids.
One of the most commonly abused opioids in the United States is oxycodone, a semisynthetic opioid which accounts for a significant number of emergency department visits in the United States.43 Oxycodone binds to the μ-opioid receptor preferentially, but is metabolized in vivo to oxymorphone which is also a potent and highly efficacious μ-opioid agonist.44–46 Oxycodone is self-administered by rats and monkeys, and its reinforcing effects are blocked by μ-opioid antagonists (e.g., naltrexone).47–51 In the present study, we trained rats to self-administer oxycodone (0.1 mg/kg/infusion) and investigated the hypothesis that lorcaserin would suppress intake of oxycodone and cue reactivity in rats. Lorcaserin is a high-affinity, full agonist at the human 5-HT2CR with selectivity over the 5-HT2AR (18-fold) and 5-HT2BR (104-fold), sites at which lorcaserin is a partial and full agonist, respectively.52 The predicted blood concentration required to activate peripheral 5-HT2AR and 5-HT2BR would be ~250-fold and ~1,400-fold, respectively, above concentrations necessary to stimulate the full-length 5-HT2CR52 which is found only in the central nervous system.53–55
The design of most analyses of cue reactivity in rodents includes acquisition of drug self-administration followed by extinction and subsequent reinstatement sessions in the presence of the drug-associated environment.28,56–57 Cue reactivity is also assessed in the absence of extinction training.24,32,58–59 In these models, lever presses during cue reactivity test sessions are recorded and may or may not result in the delivery of drug-associated cues (for reviews).60–61 Given that the neuroadaptations determined following extinction training versus during forced abstinence are distinct,62–65 we assessed the effects of lorcaserin on cue reactivity in both the abstinence and extinction-reinstatement models. In both models, lever presses during the cue reactivity test sessions were reinforced with the discrete cue complex (i.e., stimulus lights, infusion pump sounds) which had previously been paired with oxycodone delivery. Our findings indicate that lorcaserin suppresses oxycodone intake as well as cue reactivity, which suggests that lorcaserin may be therapeutically useful for promoting abstinence and preventing relapse in OUD.
RESULTS AND DISCUSSION
Lorcaserin suppresses oxycodone self-administration
Rats acquired oxycodone self-administration (0.1 mg/kg/0.1 ml infusion) to stability, displaying <10% variation in the number of infusions received over the session (Fig. 1A). Across the last three sessions of stable oxycodone self-administration, there was no main effect of session on the number of infusions obtained [F(2,27) = 0.91; ns], active [F(2,27) = 0.84; ns] or inactive lever presses [F(2,27) = 0.76; ns]. The average daily oxycodone intake over the last three sessions of training was 2.4 ± 0.2 mg/kg (mean ± SEM).
Figure 1. Lorcaserin suppresses oxycodone self-administration.
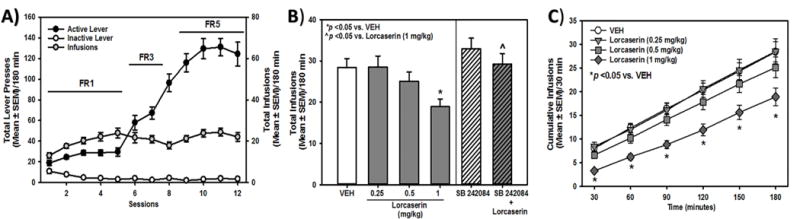
[A] Total presses (mean ± SEM) on the active (black circles) or inactive lever (white circles; left X-axis), and total number of oxycodone infusions obtained (gray circles; right X-axis) are presented for the acquisition of oxycodone self-administration. [B] The effects and specificity of lorcaserin (0.25, 0.5, 1 mg/kg) to alter total oxycodone infusions (mean ± SEM) are presented. Lorcaserin (1.0 mg/kg) suppressed oxycodone intake relative to vehicle (*p < 0.05 vs. VEH; left panel) while pretreatment with SB 242084 (0.5 mg/kg; right panel) reversed the effects of lorcaserin (1.0 mg/kg) (ˆ p < 0.05 vs. 1 mg/kg of lorcaserin). [C] The effects of lorcaserin (0.25, 0.5, 1 mg/kg) or vehicle (VEH) on cumulative oxycodone infusions (mean ± SEM) are presented. Lorcaserin (1.0 mg/kg) suppressed oxycodone intake across all time bins (* p < 0.05 vs. VEH).
Lorcaserin (0.25, 0.5 or 1 mg/kg; s.c.) or vehicle was administered 15 min prior to self-administration sessions while SB 242084 (0.5 mg/kg; i.p., 30 min) pretreatment was administered prior to vehicle or lorcaserin (1 mg/kg, i.p.; 15 min). A one-way ANOVA revealed a main effect of pretreatment on oxycodone infusions obtained [F(5,54) = 4.10; p < 0.05] and active [F(5,54) = 5.64; p < 0.05], but not inactive lever, presses [F(5,54) = 1.28; ns]. A priori comparisons revealed that lorcaserin (1 mg/kg) significantly reduced oxycodone infusions obtained (p < 0.05; Fig. 1B, left panel) and active lever presses (data not shown) relative to vehicle; inactive lever presses were unaffected (data not shown). The selective 5-HT2CR antagonist SB 242084 (0.5 mg/kg; i.p.) blocked the effects of lorcaserin (1 mg/kg) on oxycodone infusions obtained (p < 0.05, Fig. 1B, right panel) and active lever presses (p < 0.05; data not shown); inactive lever presses were not altered (ns; data not shown).
Figure 1C illustrates the effects of the three test doses of lorcaserin (0.25–1 mg/kg) on the cumulative infusions across session time. A mixed model ANOVA revealed a main effect of lorcaserin dose [F(3,36) = 6.77; p < 0.05] and time [F(5,36 = 418.91; p < 0.05], but no lorcaserin dose X time interaction [F(15,180) = 1.58; ns], on oxycodone infusions obtained. A priori comparisons revealed that lorcaserin (1 mg/kg) significantly reduced oxycodone infusions obtained relative to vehicle at all time points (Fig. 1C). This time course for the efficacy of lorcaserin to suppress oxycodone intake over the 180-min session is consistent with the reported pharmacokinetic parameters for lorcaserin (1–2 mg/kg) in male Sprague-Dawley rats [half-life (t1/2) ~2–3.5 h].35,68
Lorcaserin suppresses oxycodone cue reactivity in an abstinence model
We tested the hypothesis that lorcaserin will suppress cue reactivity 24 hours after the last oxycodone self-administration session. Rats readily acquired oxycodone self-administration to stability (Fig. 2A). Across the last three sessions of stable oxycodone self-administration, there was no main effect of session on infusions [F(2,87) = 0.64, ns], active [F(2,87) = 0.59, ns] or inactive lever presses [F(2,87) = 0.87, ns]. The average daily oxycodone intake over the last three sessions of training was 1.9 ± 0.1 mg/kg (mean ± SEM).
Figure 2. Lorcaserin suppresses oxycodone cue reactivity in the abstinence model.
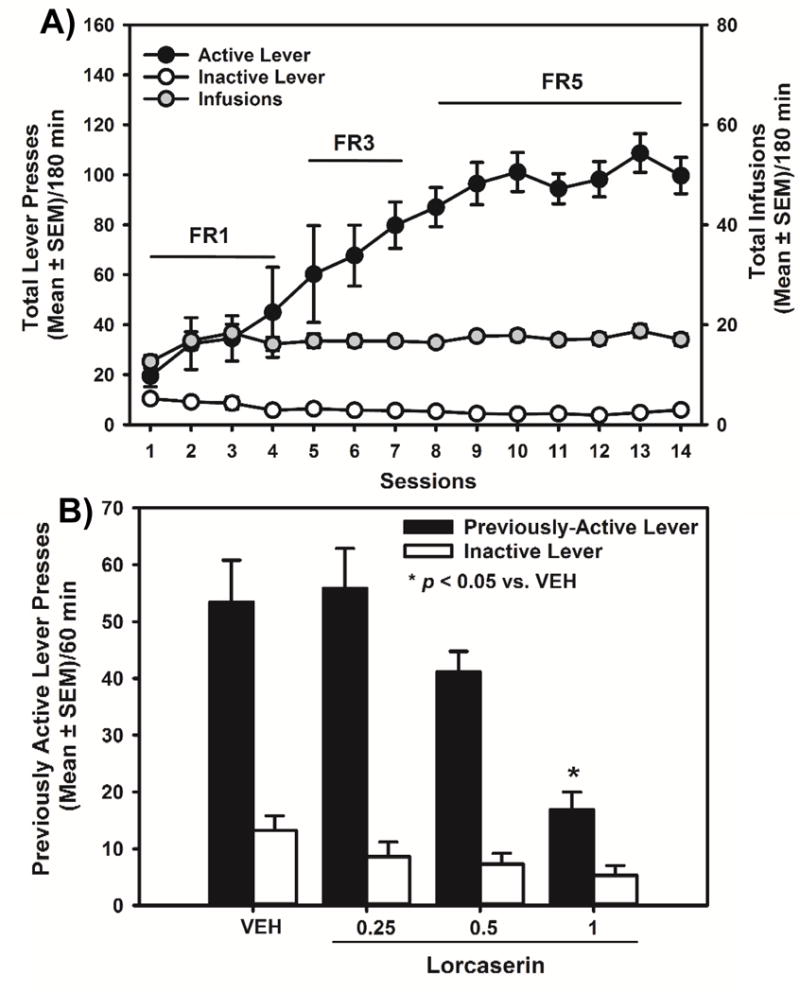
[A] Total presses (mean ± SEM) on the active (black circles) or inactive lever (white circles; left X-axis), and total number of oxycodone infusions obtained (gray circles; right X-axis) are presented for the acquisition of oxycodone self-administration. [B] The effects of lorcaserin (0.25, 0.5, 1 mg/kg) on previously active and inactive lever presses (mean ± SEM) at 24 hrs of abstinence from the last self-administration session are presented. Lorcaserin (1.0 mg/kg) suppressed previously active, but not inactive, lever presses, relative to vehicle (*p < 0.05 vs. VEH).
Lorcaserin (0.25–1 mg/kg) or vehicle was administered 15 min prior to the test session. A one-way ANOVA revealed a main effect of lorcaserin pretreatment on previously active [F(3,26) = 8.65; p < 0.05], but not inactive lever presses [F(3,26) = 2.29; ns]. A priori comparisons revealed that pretreatment with lorcaserin (1 mg/kg) significantly decreased previously active lever presses relative to vehicle (p < 0.05; Fig 2B). The latency to the first lever press following pretreatment with lorcaserin was unaltered [F(3,26) = 0.93; ns; data not shown]. These data suggest that lorcaserin suppresses oxycodone cue reactivity in a model that does not include extinction training.
Lorcaserin suppresses cue reactivity in the extinction-reinstatement model
We tested the hypothesis that lorcaserin will suppress cue reactivity after extinction from oxycodone self-administration. Rats were trained to self-administer oxycodone to stability (Fig. 3A). Across the last three sessions of stable oxycodone self-administration, there was no main effect of session on infusions [F(2,27) = 0.13, ns], active [F(2,27) = 0.18, ns] or inactive lever presses [F(2,27) = 0.64, ns]. The average daily oxycodone intake over the last three sessions of training was 1.9 ± 0.2 mg/kg (mean ± SEM).
Figure 3. Lorcaserin suppresses cue reactivity in the extinction-reinstatement model.
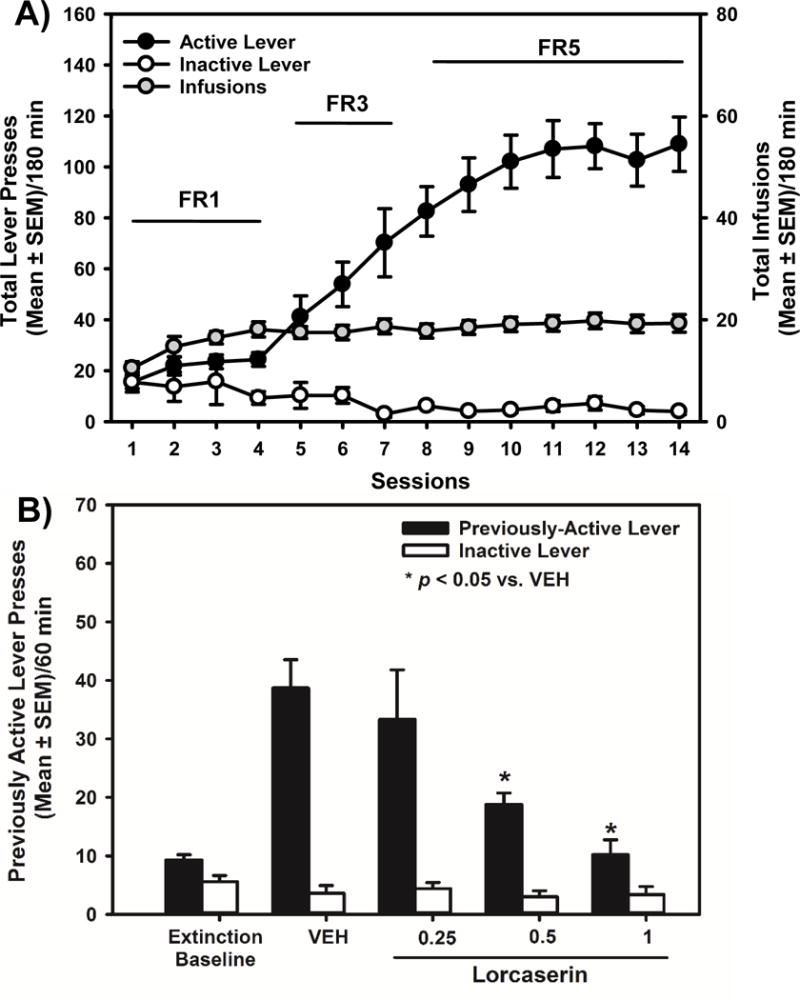
[A] Total presses (mean ± SEM) on the active (black circles) or inactive lever (white circles; left X-axis), and total number of oxycodone infusions obtained (gray circles; right X-axis) are presented for the acquisition of oxycodone self-administration. [B] The effects of lorcaserin (0.25, 0.5, 1 mg/kg) on previously active and inactive lever presses (mean ± SEM) following extinction training are presented. The “extinction baseline” was calculated as the mean total lever presses of all rats on the active (9.2 ± 2.9) or inactive lever (5.8 ± 1.1) during the last 60-min extinction session. Lorcaserin (0.5 and 1 mg/kg) significantly reduced previously active lever presses relative to vehicle (*p < 0.05 vs. VEH).
Stable acquisition of oxycodone self-administration in this cohort was followed by daily, 60-min extinction sessions; during these sessions, active and inactive lever presses were recorded but had no scheduled consequences. Presses on the previously active lever decreased across extinction sessions with all rats achieving extinction criteria (<15 total responses/hour for three consecutive sessions) (data not shown). A main effect of extinction session on previously active [F(11,84) = 6.88; p < 0.05], but not inactive lever, presses [F(11,84) = 1.07; ns] was observed. The “extinction baseline” was calculated as the mean total lever presses of all rats on the active (9.2 ± 2.9) or inactive lever (5.8 ± 1.1) during the last 60-min extinction session (Fig. 3B).
Lorcaserin (0.25–1 mg/kg) or vehicle was administered 15 min prior to the test session. A one-way ANOVA revealed a main effect of lorcaserin pretreatment on previously active [F(3,36) = 8.39; p < 0.05], but not inactive, lever presses [F(3,36) = 0.75; ns]. A priori comparisons revealed that pretreatment with lorcaserin (0.5 and 1 mg/kg) significantly reduced previously active lever presses relative to vehicle (p < 0.05; Fig. 3B). The latency to the first lever press following pretreatment with lorcaserin was unaltered [F(3,36) = 2.44; ns] (data not shown). In contrast to the abstinence model, greater sensitivity of cue reactivity to lorcaserin-evoked suppression is evident following extinction training.
We found that the selective 5-HT2CR agonist lorcaserin inhibited oxycodone intake, an effect blocked by the selective 5-HT2CR antagonist SB242084. Lorcaserin also decreased responding for the discrete cue complex previously associated with delivery of oxycodone in both the abstinence and extinction-reinstatement models in rodents. The selected dose range of lorcaserin (0.25–1 mg/kg) employed here did not overtly alter spontaneous behaviors37–38 nor operant responding on inactive levers or the latency to the first press in the present study. Taken together, these data suggest that lorcaserin reduced reward-related effects of oxycodone which cannot be explained by non-specific, rate-decreasing effects of lorcaserin.
Serotonin-enhancing drugs have been shown to modulate opioid-induced behavioral effects in animals. Administration of the selective serotonin reuptake inhibitor fluoxetine during withdrawal from chronic opioid exposure, a period during which brain 5-HT levels are diminished,69 ameliorated physical signs of naloxone-precipitated withdrawal70 and subjective measures (i.e., preference for an opioid-associated environment and anxiety-like measures) in opioid-withdrawn rodents.71 Systemic administration of 5-HT releasers (i.e., dexfenfluramine, fenfluramine) suppressed heroin self-administration in rats72–73 and attenuated the discriminative stimulus effects of morphine in monkeys,74 behavioral effects that might involve the 5-HT1R and 5-HT2R subtypes.72–73 Interestingly, recent studies found that lorcaserin reduced opioid-induced behavioral sensitization and ameliorated physical signs of naloxone-precipitated withdrawal following chronic opioid exposure in mice.75–76 These studies suggest that 5-HT neurotransmission can critically influence aspects of opioid-evoked behaviors potentially via engagement of the brain 5-HT2CR system in keeping with the present study. Future experiments are required to extend these observations to a broader dose range for oxycodone self-administration and lorcaserin pretreatment as well as analysis of the effects of lorcaserin upon chronic administration.
Opioids bind with high affinity to μ-opioid receptors localized to ventral tegmental area (VTA) dopamine neurons which innervate the nucleus accumbens (NAc) to comprise a key component of the neural circuitry underlying both drug and natural reward-motivated behaviors (for reviews).77–80 A μ-opioid receptor agonist (e.g., morphine) microinjected into the VTA supports self-administration,81–83 while intra-VTA infusion of a μ-opioid receptor antagonist resulted in a compensatory enhancement of heroin self-administration.84 Opioid agonists selective for the μ-opioid receptor hyperpolarize VTA γ-aminobutyric acid (GABA) interneurons in the VTA resulting in a loss of tonic GABA inhibition over VTA neurons,85 increased firing rates of VTA dopamine neurons and enhanced dopamine release in the NAc.85–86 This indirect mechanism of opioid receptor regulation of VTA output is widely considered to mediate the reward-related behavioral effects of opioid analgesics77–80 (but see).87
The μ-opioid receptor and the 5-HT2CR could functionally interface at the level of the VTA to control oxycodone self-administration. The 5-HT2CR is expressed on VTA GABA interneurons and projection neurons.88–91 Stimulation of the 5-HT2CR triggers a signaling cascade that results in membrane depolarization and neuronal firing,92–94 and has been shown to increase the firing rate of GABA interneurons,88, 95 enhance basal GABA release in the VTA,96 and decrease firing rates of VTA dopamine neurons.88, 95 The 5-HT2CR is also expressed on a subset of dopamine VTA neurons89–90 and functions as a key regulator of their physiology in mice.97 These findings suggest the possibility that lorcaserin may directly regulate oxycodone self-administration via actions at the 5-HT2CR localized to the VTA,98 although further experimentation is required to explore the functional mechanisms through which the VTA μ-opioid receptor and the 5-HT2CR interact. This site of action for the 5-HT2CR may also control oxycodone cue reactivity as suggested for cocaine,99 although corticostriatal involvement should be considered given that the functional status of the 5-HT2CR system in the medial prefrontal cortex is a key contributor to cocaine cue reactivity.24,32,41
The efficacy of lorcaserin to reduce oxycodone self-administration and decrease cue reactivity associated with relapse highlights the therapeutic potential for lorcaserin in the treatment of OUD. Furthermore, the observation that selective 5-HT2CR agonists suppress self-administration and cue reactivity associated with abused drugs across psychostimulant and opioid classes is intriguing, and future studies are required to appreciate the overlapping mechanisms and sites of action for the 5-HT2CR to control reward-related behaviors.
METHODS
Animals
Male Sprague-Dawley rats (n=72; Harlan, Inc., Houston, TX) weighing 250–325 g at the start of experiments were used. Rats were acclimated for seven days in a colony room maintained at a constant temperature (21–23°C) and humidity (45–50%) on a 12-hour light-dark cycle (lights on 0600–1800 h). Rats were housed two/cage and handled daily throughout the study. Food and water were available ad libitum throughout all phases of the studies. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011) and with approval from the University of Texas Medical Branch Institutional Animal Care and Use Committee.
Drugs
Oxycodone hydrochloride [Sigma, Research Triangle Park, NC] and lorcaserin hydrochloride (Hangzhou Trylead Chemical Technology Co., Ltd., Hangzhou, China) were dissolved in 0.9% NaCl. SB 242084 (6-chloro-5-methyl-1-[[2-(2-methylpyrid-3-yloxy) pyrid-5-yl]carbamoyl]indolinedihydrochloride; Sigma Chemical Co., St. Louis, MO, USA) was dissolved in 0.9% NaCl containing 10 mM citric acid (Sigma Chemical Co.) and 8% 2-hydroxypropyl-β-cyclodextrin (Trappsol®, Cyclodextrin Technologies Development Inc., High Springs, FL, USA) with the final pH of the solution adjusted to 5.6.
Surgical implantation of intravenous catheters
Implantations of intravenous catheters with back mounts were performed under anesthesia with a cocktail containing 8.6 mg/kg of xylazine, 1.5 mg/kg of acepromazine, and 43 mg/kg of ketamine in bacteriostatic saline and allowed to recover for 5–7 days.24,28,41,66 Catheter patency was maintained by daily flushes with a solution of 0.1 mL bacteriostatic saline containing heparin sodium (10 U/mL; American Pharmaceutical Partners, East Schaumburg, IL), streptokinase (0.67 mg/mL; Sigma Chemical), and ticarcillin disodium (66.67 mg/mL; Research Products International, Mt. Prospect, IL) immediately following daily oxycodone self-administration sessions. Proper catheter function was verified periodically throughout experiments by intravenous administration of 10 mg/kg of methohexital sodium (Monarch Pharmaceuticals Inc., Bristol, TN), a dose sufficient to briefly anesthetize the animal only when administered intravenously. All rats were allowed 5–7 days of recovery after surgery before initiation of self-administration training.
Self-administration training
Standard operant conditioning chambers (Med-Associates, Inc., St. Albans, VT, USA) housed in ventilated, sound-attenuating cubicles with fans (Med-Associates, Inc.) were utilized for oxycodone self-administration studies. Each chamber was equipped with a pellet receptacle flanked by two retractable response levers, a stimulus light above each response lever, and a house light opposite the levers. Oxycodone infusions were delivered via syringes attached to infusion pumps (Med-Associates, Inc.) located outside the cubicle. The infusion pumps were connected to liquid swivels (Instech, Plymouth Meeting, PA) that were fastened to the catheters via polyethylene 20 tubing encased inside a metal spring leash (Plastics One, Roanoke, VA).
Freely fed rats were trained to lever press for oxycodone infusions (0.1 mg/kg/0.1 mL infusion) using established methods.24,28,41,66 The training dose for oxycodone (0.1 mg/kg/0.1 mL infusion) was chosen according to previous studies;49–50 preliminary data demonstrated this dose maintained stable self-administration in rats. Oxycodone self-administration training consisted of daily 180-min sessions during which rats were trained to press the active lever to obtain an infusion on a fixed ratio (FR) 1–3 schedule of reinforcement before progressing to an FR5 schedule. Schedule completions on the active lever resulted in delivery of an oxycodone infusion over a 6-sec period paired simultaneously with illumination of the house and stimulus lights and activation of the infusion pump (discrete cue complex); inactive lever presses produced no scheduled consequences. Following reinforcer delivery, the stimulus light and infusion pump were inactivated; the house light remained on for an additional 20 sec to indicate a timeout period during which lever presses had no scheduled consequences. The criterion for stable self-administration (<10% variation in the number of infusions obtained for three consecutive sessions) was achieved prior to initiation of test sessions. Cue reactivity test sessions proceeded with the rats tethered to catheters and placed in chambers as in self-administration sessions; previously active lever presses resulted in delivery of cues without fluid delivery. Due to diminished catheter patency in some rats (n=22), n=50 rats were included in the analyses.
RESEARCH DESIGN
Lorcaserin effects on oxycodone self-administration
In rats (n=10) trained to self-administer oxycodone (0.1 mg/kg/inf) to stability, the efficacy and specificity of lorcaserin to alter oxycodone intake were assessed. Lorcaserin (0.25, 0.5 or 1 mg/kg) or vehicle was injected subcutaneously (s.c.) 15 min prior to the oxycodone self-administration session in a within subjects design. Doses and pretreatment times for lorcaserin were chosen based on previous studies,27,35–38 and tests were administered in a pseudorandomized order with a minimum of two intervening sessions of oxycodone self-administration to assure stability of baseline responding. To investigate the dependency of effects on the 5-HT2CR, the selective 5-HT2CR antagonist SB 242084 (0.5 mg/kg; s.c.)28 was administered at 30 min prior, followed by lorcaserin (1 mg/kg, i.p.) or vehicle intraperitoneally (i.p.) 15 min prior to the self-administration test session.
Lorcaserin effects on oxycodone cue reactivity
In rats trained to self-administer oxycodone to stability, we assessed the effects of lorcaserin on cue reactivity in both the abstinence and extinction-reinstatement models. In the abstinence cohort (n=30), lorcaserin (0.25, 0.5, or 1 mg/kg) or vehicle was administered s.c. 15 min prior to the 60-min test session in a between subjects design. Cue reactivity tests were conducted at 24 hours of abstinence from oxycodone self-administration. Rats were placed in operant chambers and lever presses on the previously active lever were reinforced by the discrete cue complex (house and stimulus light illuminated, infusion pump activated) on an FR1 schedule; presses on the inactive lever were recorded, but produced no scheduled consequences.
Rats in the second cohort (n=10) were subjected to daily 60-min extinction sessions after achieving stable self-administration. Extinction sessions were conducted during which active and inactive lever presses were recorded, but had no scheduled consequences. Once rats achieved the extinction criterion of response rates <15 total responses/hour for three consecutive sessions, lorcaserin (0.25, 0.5, or 1 mg/kg) or vehicle was injected s.c. 15 min prior to the 60-min session in a within subjects design. A single, non-response contingent presentation of the discrete cue complex initiated the cue reactivity test. During the session, responses on the previously active lever were reinforced by the discrete cue complex on an FR1 schedule; presses on the inactive lever were recorded but produced no scheduled consequences. Rats in this cohort completed all test sessions; the order of injections was pseudorandomized with a minimum of three intervening extinction sessions occurring between each drug challenge to assure stability of extinction criterion (<15 total responses/hr).
Statistical analyses
All pharmacological manipulations were conducted by an experimenter blinded to the test conditions. A one-way analysis of variance (ANOVA) for a within subjects design was employed to assess (a) oxycodone infusions, (b) active or previously active, and (c) inactive lever responses during self-administration for all cohorts. A mixed model ANOVA for the factors of lorcaserin dose and session time bin (30–180 mins) across the oxycodone self-administration test session was conducted. In the abstinence cohort, a one-way ANOVA for a between subjects design was employed to assess (a) previously active, (b) inactive lever presses, and (c) latency to respond on the previously active lever during cue reactivity test sessions. A one-way ANOVA for a within subjects design was used to assess (a) previously active, and (b) inactive lever presses during extinction sessions and cue reactivity tests after extinction. In the abstinence cohort, the between subjects design indicated a lorcaserin-induced suppression of oxycodone cue reactivity; hence, the within subjects design was employed in the extinction-reinstatement cohort to increase power and reduce the number of rats required. A priori comparisons were defined prior to the start of experimentation and conducted by Dunnett’s procedure or Student–Newman–Keuls test.67 Analyses were conducted with SAS for Windows (Version 9.4, SAS Institute, Inc., Cary, NC) with an experiment-wise error rate set at α=0.05.
Acknowledgments
We thank Drs. Jane Acri, Aidan Hampson, and Tatiana Ramey of the Division of Therapeutics and Medical Consequences of the National Institute of Drug Abuse for their thoughtful and constructive feedback throughout the project. We also thank Dr. Marcy B. Jordan for her helpful discussions and editorial assistance.
Funding
This work was supported by NIDA grants U54 DA037632 (F.G.M.), K05 DA020087 (K.A.C.), T32 DA07287 (E.D.H.), K99/R00 DA033374 (N.C.A.) and by the Center for Addiction Research at the University of Texas Medical Branch.
ABBREVIATIONS
- 5-HT
serotonin (5-hydroxytryptamine)
- 5-HT1R
5-HT1 receptor
- 5-HT2CR
5-HT2C receptor
- OUD
Opioid use disorder
- NAc
nucleus accumbens
- VTA
ventral tegmental area
Footnotes
Dr. Cunningham is an uncompensated consultant for Arena Pharmaceuticals. Dr. Haney has received research support from Insys Therapeutics for investigator-initiated studies. Dr. Comer has consulted, received research funding and/or medication from Alkermes, AstraZeneca, Braeburn, Camurus Cerecor, Clinilabs, Daiichi Sankyo, Egalet, Endo, Heptares, Indivior/Reckitt, Janssen, KemPharm, Medicinova, NeRRe, Omeros, Opiant/Lightlakeo, Pfizer, and Shire over the last three years.
The remaining authors declare no competing financial interests.
Author Contributions
H.N. carried out behavioral pharmacological evaluations and drafted the manuscript. E.D.H. conducted statistical analyses and edited the manuscript. R.G.F. and S.J.S. carried out behavioral pharmacological evaluations. N.C.A. and K.A.C. supervised the experimental design, conduct and interpretation of studies. S.D.C., M.H., F.G.M., N.C.A. and K.A.C. conceptualized the project, designed, analyzed and interpreted experiments, and wrote/edited the manuscript.
References
- 1.Substance Abuse Mental Health Services Administration, Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: 2014. [Google Scholar]
- 2.Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med. 2016;374(2):154–63. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies–tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–6. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- 4.Jamison RN, Mao J. Opioid Analgesics. Mayo Clin Proc. 2015;90(7):957–68. doi: 10.1016/j.mayocp.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. Factors influencing the selection of hydrocodone and oxycodone as primary opioids in substance abusers seeking treatment in the United States. Pain. 2013;154(12):2639–48. doi: 10.1016/j.pain.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Pierce RC, O’Brien CP, Kenny PJ, Vanderschuren LJ. Rational development of addiction pharmacotherapies: successes, failures, and prospects. Cold Spring Harb Perspect Med. 2012;2(6):a012880. doi: 10.1101/cshperspect.a012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL, Hasson AL, Huang Z, Jacobs P, Kosinski AS, Lindblad R, McCance-Katz EF, Provost SE, Selzer J, Somoza EC, Sonne SC, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–46. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12(1):15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 9.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- 10.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 11.Carter BL, Tiffany ST. Cue-reactivity and the future of addiction research. Addiction. 1999;94(3):349–351. [PubMed] [Google Scholar]
- 12.Field M, Marhe R, Franken IH. The clinical relevance of attentional bias in substance use disorders. CNS Spectr. 2014;19(3):225–30. doi: 10.1017/S1092852913000321. [DOI] [PubMed] [Google Scholar]
- 13.Jones JD, Vadhan NP, Luba RR, Comer SD. The effects of heroin administration and drug cues on impulsivity. J Clin Exp Neuropsychol. 2016;38(6):709–20. doi: 10.1080/13803395.2016.1156652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Back SE, Gros DF, McCauley JL, Flanagan JC, Cox E, Barth KS, Brady KT. Laboratory-induced cue reactivity among individuals with prescription opioid dependence. Addict Behav. 2014;39(8):1217–23. doi: 10.1016/j.addbeh.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fatseas M, Denis C, Massida Z, Verger M, Franques-Reneric P, Auriacombe M. Cue-induced reactivity, cortisol response and substance use outcome in treated heroin dependent individuals. Biol Psychiatry. 2011;70(8):720–727. doi: 10.1016/j.biopsych.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Franken IH, de Haan HA, van der Meer CW, Haffmans PM, Hendriks VM. Cue reactivity and effects of cue exposure in abstinent posttreatment drug users. J Subst Abuse Treat. 1999;16(1):81–85. doi: 10.1016/s0740-5472(98)00004-x. [DOI] [PubMed] [Google Scholar]
- 17.Garland EL, Froeliger BE, Passik SD, Howard MO. Attentional bias for prescription opioid cues among opioid dependent chronic pain patients. J Behav Med. 2013;36(6):611–20. doi: 10.1007/s10865-012-9455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McHugh RK, Park S, Weiss RD. Cue-induced craving in dependence upon prescription opioids and heroin. Am J Addict. 2014;23(5):453–8. doi: 10.1111/j.1521-0391.2014.12129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell J, Gray JA, Bradley BP, Kasvikis Y, Strang J, Barratt L, Marks I. The effects of exposure to drug-related cues in detoxified opiate addicts: a theoretical review and some new data. Addict Behav. 1990;15(4):339–54. doi: 10.1016/0306-4603(90)90044-x. [DOI] [PubMed] [Google Scholar]
- 20.Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, Brewer C, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry. 2001;158(10):1680–6. doi: 10.1176/appi.ajp.158.10.1680. [DOI] [PubMed] [Google Scholar]
- 21.Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O’Brien CP, Childress AR. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165(3):390–4. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- 22.Zijlstra F, Veltman DJ, Booij J, van den Brink W, Franken IH. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug Alcohol Depend. 2009;99(1–3):183–92. doi: 10.1016/j.drugalcdep.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Marissen MA, Franken IH, Waters AJ, Blanken P, van den Brink W, Hendriks VM. Attentional bias predicts heroin relapse following treatment. Addiction. 2006;101(9):1306–12. doi: 10.1111/j.1360-0443.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 24.Anastasio NC, Stutz SJ, Fox RG, Sears RM, Emeson RB, DiLeone RJ, O’Neil RT, Fink LH, Li D, Green TA, Moeller FG, Cunningham KA. Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology. 2014;39(2):370–82. doi: 10.1038/npp.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67(1):176–97. doi: 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins GA, Fletcher PJ. Therapeutic Potential of 5-HT2C Receptor Agonists for Addictive Disorders. ACS Chem Neurosci. 2015;6(7):1071–88. doi: 10.1021/acschemneuro.5b00025. [DOI] [PubMed] [Google Scholar]
- 27.Collins GT, Gerak LR, Javors MA, France CP. Lorcaserin reduces the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2016;356(1):85–95. doi: 10.1124/jpet.115.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG, Gilbertson SR, Rosenzweig-Lipson S. Selective serotonin 5-HT2C receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology. 2011;61(3):513–523. doi: 10.1016/j.neuropharm.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT(2C) receptor agonist RO 60–0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine and contextual cues. Neuropsychopharmacology. 2008;33(6):1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- 30.Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther. 2000;295(3):1183–1191. [PubMed] [Google Scholar]
- 31.Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol. 2007;18(8):791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]
- 32.Swinford-Jackson SE, Anastasio NC, Fox RG, Stutz SJ, Cunningham KA. Incubation of cocaine cue reactivity associates with neuroadaptations in the cortical serotonin (5-HT) 5-HT2C receptor (5-HT2CR) system. Neuroscience. 2016;324:50–61. doi: 10.1016/j.neuroscience.2016.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manvich DF, Kimmel HL, Cooper DA, Howell LL. The serotonin 2C receptor antagonist SB 242084 exhibits abuse-related effects typical of stimulants in squirrel monkeys. J Pharmacol Exp Ther. 2012;342(3):761–769. doi: 10.1124/jpet.112.195156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manvich DF, Kimmel HL, Howell LL. Effects of serotonin 2C receptor agonists on the behavioral and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2012;341(2):424–434. doi: 10.1124/jpet.111.186981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins GA, Silenieks LB, Lau W, de LI, Lee DK, Izhakova J, Coen K, Le AD, Fletcher PJ. Evaluation of chemically diverse 5-HT2C receptor agonists on behaviours motivated by food and nicotine and on side effect profiles. Psychopharmacology (Berl) 2013;226(3):475–490. doi: 10.1007/s00213-012-2919-2. [DOI] [PubMed] [Google Scholar]
- 36.Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ. The 5-HT2C receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology. 2016;101:237–45. doi: 10.1016/j.neuropharm.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 37.Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology. 2012;37(5):1177–1191. doi: 10.1038/npp.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin ED, Johnson JE, Slade S, Wells C, Cauley M, Petro A, Rose JE. Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. J Pharmacol Exp Ther. 2011;338(3):890–896. doi: 10.1124/jpet.111.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grottick AJ, Corrigall WA, Higgins GA. Activation of 5-HT2C receptors reduces the locomotor and rewarding effects of nicotine. Psychopharmacology (Berl) 2001;157(3):292–298. doi: 10.1007/s002130100801. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT2A receptor antagonist M100,907 and the 5-HT2C receptor antagonist SB242,084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27(4):576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 41.Anastasio NC, Liu S, Maili L, Swinford SE, Lane SD, Fox RG, Hamon SC, Nielsen DA, Cunningham KA, Moeller FG. Variation within the serotonin (5-HT) 5-HT2C receptor system aligns with vulnerability to cocaine cue reactivity. Transl Psychiatry. 2014;4:e369. doi: 10.1038/tp.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fletcher PJ, Rizos Z, Noble K, Soko AD, Silenieks LB, Le AD, Higgins GA. Effects of the 5-HT2C receptor agonist Ro60-0175 and the 5-HT2A receptor antagonist M100907 on nicotine self-administration and reinstatement. Neuropharmacology. 2012;62(7):2288–2298. doi: 10.1016/j.neuropharm.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 43.Crane EH. The CBHSQ Report. Rockville (MD): 2013. Emergency Department Visits Involving Narcotic Pain Relievers. [PubMed] [Google Scholar]
- 44.Yoburn BC, Shah S, Chan K, Duttaroy A, Davis T. Supersensitivity to opioid analgesics following chronic opioid antagonist treatment: relationship to receptor selectivity. Pharmacol Biochem Behav. 1995;51(2–3):535–9. doi: 10.1016/0091-3057(94)00375-s. [DOI] [PubMed] [Google Scholar]
- 45.Lester PA, Traynor JR. Comparison of the in vitro efficacy of mu, delta, kappa and ORL1 receptor agonists and non-selective opioid agonists in dog brain membranes. Brain Res. 2006;1073–1074:290–6. doi: 10.1016/j.brainres.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 46.Thompson CM, Wojno H, Greiner E, May EL, Rice KC, Selley DE. Activation of G-proteins by morphine and codeine congeners: insights to the relevance of O- and N-demethylated metabolites at mu- and delta-opioid receptors. J Pharmacol Exp Ther. 2004;308(2):547–54. doi: 10.1124/jpet.103.058602. [DOI] [PubMed] [Google Scholar]
- 47.Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology. 2015;40(2):421–8. doi: 10.1038/npp.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS. Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol. 2004;12(3):163–72. doi: 10.1037/1064-1297.12.3.163. [DOI] [PubMed] [Google Scholar]
- 49.Pravetoni M, Pentel PR, Potter DN, Chartoff EH, Tally L, LeSage MG. Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS One. 2014;9(7):e101807. doi: 10.1371/journal.pone.0101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leri F, Burns LH. Ultra-low-dose naltrexone reduces the rewarding potency of oxycodone and relapse vulnerability in rats. Pharmacol Biochem Behav. 2005;82(2):252–62. doi: 10.1016/j.pbb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Olmstead MC, Burns LH. Ultra-low-dose naltrexone suppresses rewarding effects of opiates and aversive effects of opiate withdrawal in rats. Psychopharmacology (Berl) 2005;181(3):576–81. doi: 10.1007/s00213-005-0022-7. [DOI] [PubMed] [Google Scholar]
- 52.Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325(2):577–587. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- 53.Anastasio NC, Lanfranco MF, Bubar MJ, Seitz PK, Stutz SJ, McGinnis AG, Watson CS, Cunningham KA. Serotonin 5-HT2C receptor protein expression is enriched in synaptosomal and post-synaptic compartments of rat cortex. J Neurochem. 2010;113(6):1504–1515. doi: 10.1111/j.1471-4159.2010.06694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Julius D, MacDermott AB, Axel R, Jessell JM. Molecular characterization of a functional cDNA encoding the serotonin 1C receptor. Science. 1988;241:558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- 55.Julius D, MacDermott AB, Jessel TM, Huang K, Molineaux S, Schieren I, Axel R. Functional expression of the 5-HT1c receptor in neuronal and nonneuronal cells. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):385–393. doi: 10.1101/sqb.1988.053.01.046. [DOI] [PubMed] [Google Scholar]
- 56.Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195(2):223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- 57.Nic Dhonnchadha BA, Fox RG, Stutz SJ, Rice KC, Cunningham KA. Blockade of the serotonin 5-HT2A receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci. 2009;123(2):382–396. doi: 10.1037/a0014592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology (Berl) 1998;135(2):151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- 59.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412(6843):141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 61.Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2014;76(Pt B):460–78. doi: 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Ciano P, Everitt BJ. Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol. 2002;13(5–6):397–405. doi: 10.1097/00008877-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt EF, Sutton MA, Schad CA, Karanian DA, Brodkin ES, Self DW. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self-administration. J Neurosci. 2001;21(7):RC137. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11(5):648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421(6918):70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 66.Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, Watson CS, Gilbertson SR, Rice KC, Rosenzweig-Lipson S, Moeller FG. Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci. 2013;4(1):110–121. doi: 10.1021/cn300072u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keppel G. Design and Analysis: A Researcher’s Handbook. Prentice-Hall, Inc; Englewood Cliffs, NJ: 1973. [Google Scholar]
- 68.Subramanian M, Kurawattimath V, Pocha K, Freeden C, Rao I, Mariappan TT, Marathe PH, Vikramadithyan RK, Abraham P, Kulkarni CP, Katnapally P, Nutakki R, Paruchury S, Bhutani P, Mandlekar S. Role of hepatic blood flow and metabolism in the pharmacokinetics of ten drugs in lean, aged and obese rats. Xenobiotica. 2014;44(12):1108–16. doi: 10.3109/00498254.2014.932470. [DOI] [PubMed] [Google Scholar]
- 69.Tao R, Ma Z, Auerbach SB. Alteration in regulation of serotonin release in rat dorsal raphe nucleus after prolonged exposure to morphine. J Pharmacol Exp Ther. 1998;286(1):481–8. [PubMed] [Google Scholar]
- 70.Singh VP, Jain NK, Kulkarni SK. Fluoxetine suppresses morphine tolerance and dependence: modulation of NO-cGMP/DA/serotoninergic pathways. Methods Find Exp Clin Pharmacol. 2003;25(4):273–80. doi: 10.1358/mf.2003.25.4.769675. [DOI] [PubMed] [Google Scholar]
- 71.Harris GC, Aston-Jones G. Augmented accumbal serotonin levels decrease the preference for a morphine associated environment during withdrawal. Neuropsychopharmacology. 2001;24(1):75–85. doi: 10.1016/S0893-133X(00)00184-6. [DOI] [PubMed] [Google Scholar]
- 72.Higgins GA, Wang Y, Corrigall WA, Sellers EM. Influence of 5-HT3 receptor antagonists and the indirect 5-HT agonist, dexfenfluramine, on heroin self-administration in rats. Psychopharmacology (Berl) 1994;114(4):611–9. doi: 10.1007/BF02244992. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Joharchi N, Fletcher PJ, Sellers EM, Higgins GA. Further-Studies to Examine the Nature of Dexfenfluramine-Induced Suppression of Heroin Self-Administration. Psychopharmacology (Berl) 1995;120(2):134–141. doi: 10.1007/BF02246185. [DOI] [PubMed] [Google Scholar]
- 74.Li JX, Koek W, Rice KC, France CP. Effects of direct- and indirect-acting serotonin receptor agonists on the antinociceptive and discriminative stimulus effects of morphine in rhesus monkeys. Neuropsychopharmacology. 2011;36(5):940–9. doi: 10.1038/npp.2010.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu X, Pang G, Zhang YM, Li G, Xu S, Dong L, Stackman RW, Jr, Zhang G. Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in heroin-treated mice. Neurosci Lett. 2015;607:23–8. doi: 10.1016/j.neulet.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang G, Wu X, Zhang YM, Liu H, Jiang Q, Pang G, Tao X, Dong L, Stackman RW., Jr Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-dependent mice. Neuropharmacology. 2016;101:246–54. doi: 10.1016/j.neuropharm.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fields HL, Margolis EB. Understanding opioid reward. Trends Neurosci. 2015;38(4):217–25. doi: 10.1016/j.tins.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6(2):243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 79.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays. 2010;32(9):748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Welzl H, Kuhn G, Huston JP. Self-administration of small amounts of morphine through glass micropipettes into the ventral tegmental area of the rat. Neuropharmacology. 1989;28(10):1017–23. doi: 10.1016/0028-3908(89)90112-3. [DOI] [PubMed] [Google Scholar]
- 82.Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28(5):551–5. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- 83.Zangen A, Ikemoto S, Zadina JE, Wise RA. Rewarding and psychomotor stimulant effects of endomorphin-1: anteroposterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. J Neurosci. 2002;22(16):7225–33. doi: 10.1523/JNEUROSCI.22-16-07225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Britt MD, Wise RA. Ventral tegmental site of opiate reward: antagonism by a hydrophilic opiate receptor blocker. Brain Res. 1983;258(1):105–8. doi: 10.1016/0006-8993(83)91232-5. [DOI] [PubMed] [Google Scholar]
- 85.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12(2):483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jalabert M, Bourdy R, Courtin J, Veinante P, Manzoni OJ, Barrot M, Georges F. Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci U S A. 2011;108(39):16446–50. doi: 10.1073/pnas.1105418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Margolis EB, Hjelmstad GO, Fujita W, Fields HL. Direct bidirectional mu-opioid control of midbrain dopamine neurons. J Neurosci. 2014;34(44):14707–16. doi: 10.1523/JNEUROSCI.2144-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Giovanni G, Di MV, La GV, Esposito E. m-Chlorophenylpiperazine excites non-dopaminergic neurons in the rat substantia nigra and ventral tegmental area by activating serotonin-2C receptors. Neuroscience. 2001;103(1):111–116. doi: 10.1016/s0306-4522(00)00561-3. [DOI] [PubMed] [Google Scholar]
- 89.Bubar MJ, Cunningham KA. Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience. 2007;146(1):286–277. doi: 10.1016/j.neuroscience.2006.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bubar MJ, Stutz SJ, Cunningham KA. 5-HT(2C) receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PLoS One. 2011;6(6):e20508. doi: 10.1371/journal.pone.0020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113(2):296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stanford IM, Kantaria MA, Chahal HS, Loucif KC, Wilson CL. 5-Hydroxytryptamine induced excitation and inhibition in the subthalamic nucleus: action at 5-HT(2C), 5-HT(4) and 5-HT(1A) receptors. Neuropharmacology. 2005;49(8):1228–1234. doi: 10.1016/j.neuropharm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 93.Yamamoto R, Hatano N, Sugai T, Kato N. Serotonin induces depolarization in lateral amygdala neurons by activation of TRPC-like current and inhibition of GIRK current depending on 5-HT(2C) receptor. Neuropharmacology. 2014;82:49–58. doi: 10.1016/j.neuropharm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 94.Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71(3):488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prisco S, Pagannone S, Esposito E. Serotonin-dopamine interaction in the rat ventral tegmental area: An electrophysiological study in vivo. J Pharmacol Exp Ther. 1994;271:83–90. [PubMed] [Google Scholar]
- 96.Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Role of 5-hydroxytryptamine2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J Pharmacol Exp Ther. 2009;329(2):625–633. doi: 10.1124/jpet.108.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu P, He Y, Cao X, Valencia-Torres L, Yan X, Saito K, Wang C, Yang Y, Hinton A, Jr, Zhu L, Shu G, Myers MG, Jr, Wu Q, Tong Q, Heisler LK, Xu Y. Activation of serotonin 2C receptors in dopamine neurons inhibits binge-like eating in mice. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.06.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. Injection of the 5-HT2C receptor agonist Ro60-0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology. 2004;29(2):308–318. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- 99.Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, Thiel KJ, Neisewander JL. Stimulation of medial prefrontal cortex serotonin 5-HT2C receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology. 2010;35(10):2037–2048. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]


