Abstract
Defaunation by humans causes a loss of large animals in many ecosystems globally. Recent work has emphasized the consequences of downsizing in animal communities for ecosystem functioning. However, no study so far has integrated network theory and life-history trade-offs to mechanistically evaluate the functional consequences of defaunation in plant–animal networks. Here, we simulated an avian seed-dispersal network and its derived ecosystem function seedling recruitment to assess the relative importance of different size-related mechanisms. Specifically, we considered size matching (between bird size and seed size) and size trade-offs, which are driven by differences in plant or animal species abundance (negative size–quantity relationship) as well as in recruitment probability and disperser quality (positive size–quality relationship). Defaunation led to impoverished seedling communities in terms of diversity and seed size, but only if models accounted for size matching. In addition, size trade-off in plants, in concert with size matching, provoked rapid decays in seedling abundance in response to defaunation. These results underscore a disproportional importance of large animals for ecosystem functions. Downsizing in ecological networks will have severe consequences for ecosystem functioning, especially in interaction networks that are structured by size matching between plants and animals.
Keywords: interaction networks, biodiversity–ecosystem functioning, frugivorous animals, species traits, niche model, trait matching
1. Introduction
Biodiversity matters for ecosystem functioning, their causal relationship being a central issue of ecological research over the last decades [1,2]. This is of concern due to the ongoing and worldwide loss of biodiversity [3,4]. In particular, defaunation, defined as the human-induced decline of both abundance and species richness of animals [5], threatens ecosystem functions derived from trophic interactions [6,7]. In fact, animal loss from complex networks of ecological interactions can cause a variety of functional consequences, including diversity decays in plant communities [8,9], trophic cascades in food webs [10,11] and the collapse of entire ecological communities (i.e. ecological meltdown [12]).
During defaunation, species are usually not lost randomly [13], but depending on species traits that determine their sensitivity to disturbance (i.e. response traits [14]). For example, large species are expected to be most threatened by extinction [15] and are usually lost first [16]. Thus, the loss of animal diversity is often size-structured and leads to downsizing in impoverished communities [17]. As size also affects interspecific interactions and species' functional performance [18], accounting for size-related interaction rules in ecological networks may strongly enhance the understanding of biodiversity effects on ecosystem functions of animals [2]. First, compatibility in size (size matching) is an important determinant of the structure of interaction networks; for instance, small predators are generally unable to feed on large prey [19], so that large consumers tend to have more links than small consumers [18]. Thus, defaunation may lead to a disruption of interactions, especially for species depending on large interaction partners [17]. Second, the functional outcome of interactions will also depend on species' life-history trade-offs, in particular between size and abundance (size–quantity relationship), as large animal species usually have smaller population sizes, but stronger effects on ecosystem functions than small species (size–quality relationship) [20,21]. Defaunation could thus also lead to a reduced efficiency of interactions if large species disappear first [22]. Although some studies have related network structure and functioning to size matching [23,24] or have highlighted the functional consequences of downsizing through the alteration of interaction networks [17], no study yet has jointly analysed how size matching and size trade-offs of interacting species mediate the effects of defaunation on ecosystem functioning (but see [25,26] for conceptual frameworks).
Here, we focus on the functional relevance of size for interactions between plants and seed-dispersing animals. The final ecological outcome of their interaction, seedling recruitment, is an important component of forest regeneration [27] and represents a pivotal ecosystem function in many ecosystems [16,28]. Especially in tropical forests, most tree species depend on animals as seed dispersers [29,30]. Previous studies have shown the importance of frugivore richness for the performance of this function [31,32] and that frugivorous animals, especially those of large size, are affected by defaunation [9,33]. Size matching between plants and animals shapes the structure of seed-dispersal networks, as preferences of different-sized frugivores depend on fruit and seed size [34–36]. Moreover, size trade-offs influence seed dispersal quantity and quality [37]. On the one hand, small-seeded plants produce more seeds per unit plant biomass [38] and small-bodied frugivores are more abundant [39], resulting in high frequencies of small species in seed-dispersal networks (size–quantity relationship) [34,40]. On the other hand, size affects seed fate (i.e. seed-to-seedling transition probabilities [41]) as large seeds usually have higher survival probabilities after dispersal than small seeds (size–quality relationship), especially under adverse abiotic conditions [38,42]. Hence, plants face a trade-off between investing in many small seeds with low recruitment probability or few large seeds with high chances per seed [43,44]. Furthermore, large seed dispersers may increase the probability of seedling recruitment by moving seeds over long distances [45,46] and may be more efficient seed dispersers than small animals (size–quality relationship). Because of size matching and the size-related trade-offs of plants and animals, the local extinction of large frugivores may have a disproportionate effect on seedling recruitment and forest regeneration [47].
In this study, we combine a weighted niche model of species interactions with a simulation of animal extinction to theoretically evaluate the role of size in mediating the effects of defaunation on ecosystem functioning, here measured as seedling recruitment of animal-dispersed plants. The modelled animal and plant communities resemble tropical communities of frugivorous birds and fleshy-fruited plants that are characterized by many small and a few large plant and animal species [40,48]. We specifically focused on two mechanisms potentially important for network-mediated effects on seed dispersal in these communities: (i) size matching between plants and animals, and (ii) size trade-offs in plants and animals related to quantitative and qualitative effects on seedling recruitment. We hypothesized that both mechanisms (i.e. size matching and size trade-offs) mediate the effect of defaunation on seedling recruitment. Our model demonstrates that size matching between plants and animals in ecological networks is the crucial mechanism driving defaunation effects on ecosystem functioning, although in some cases this depends on the existence of size trade-offs in plants.
2. Material and methods
(a). Overall approach
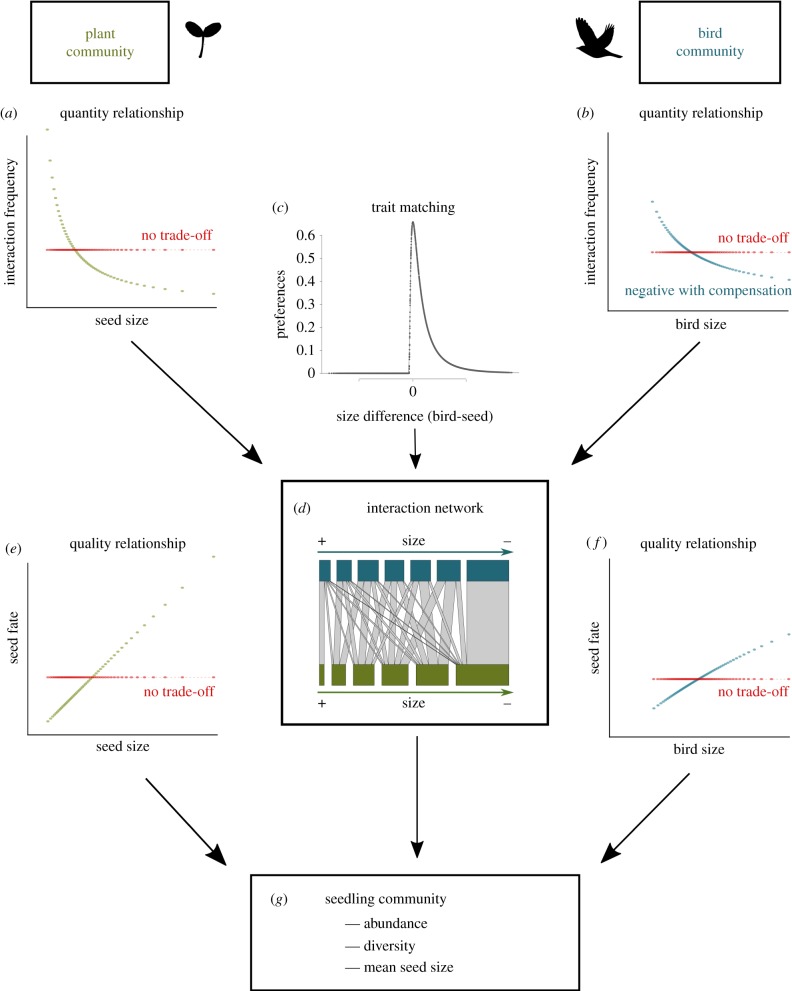
In order to predict the size-mediated effect of defaunation on ecosystem functioning, we followed a general framework (figure 1; see also [25]), considering species size matching and size trade-offs of a community of fleshy-fruited plants and avian seed dispersers. Zoochory by frugivorous birds is important to maintain the structure and diversity of plant communities, especially in tropical forests [29,49], where birds disperse up to 75% of plant species [33]. For each of six scenarios (see below), we simulated a generic seed-dispersal network emerging from a mechanistic model, which avoids possible sampling effects, and informed this model with the trait distributions from empirical tropical bird and plant communities that are dominated by many species of small and moderate size [40,48]. Size effects entered the model in several steps: first, species interacted according to size-related or neutral interaction rules (i.e. size matching versus no size matching), leading to different structure of the network (figure 1c,d; electronic supplementary material, appendix S1). With neutral interactions, only species abundances structured the network. Second, we assumed two sources by which species life-history trade-offs of plants and frugivores could affect the functional outcome of plant–frugivore interactions. Plant and bird size determined species' abundance, and thus their total interaction frequencies (size–quantity relationship, figure 1a,b). Moreover, we assumed a relationship between size and seed fate imposed by plants (i.e. large seeds survive better than small seeds [38,50]) and/or by birds (i.e. large birds disperse seeds over longer distances [51] and promote higher seed survival than small birds [52]) (figure 1e,f). Size–quantity and size–quality relationships together constituted potential size trade-offs for both plants and birds.
Figure 1.
Flow diagram representing the different analytical steps to estimate seedling recruitment from seed-dispersal networks. Size–quantity relationships (i.e. a higher interaction frequency of small compared to large species) were considered for both (a) plant and (b) bird communities. (c) Size matching was derived from a right-skewed niche shape of trait matching as a function of trait distances between species (bird–seed size). (d) An example of a seed-dispersal network (subset of six plant (green) and seven bird (blue) species) structured by size trade-offs and size matching; species are ordered by decreasing size and increasing interaction frequencies. To quantify seedling recruitment from seed-dispersal networks, size–quality relationships were considered for both (e) plant and (f) bird species. (g) By multiplying realized interaction frequencies with the product of bird and seed quality values, we computed the abundance, diversity and mean seed size of the resulting seedling communities. We quantified the effect of size matching and the respective size trade-offs by disabling the respective mechanism in the model.
We considered eight different scenarios: size matching versus no size matching, crossed with size trade-off in plants only, birds only, both or none. For simplicity, we do not present the trade-off scenarios ‘plants only’ and ‘birds only’ for scenarios without size matching as these did not differ from the other neutral scenarios; this reduces the potential eight scenarios to six. For each scenario, we compared the decay of ecosystem functioning between a random and a deterministic (size-dependent) sequence of bird species extinction from the ecological network. With this model, we demonstrate how the effect of defaunation on seedling recruitment is influenced by the different size-related mechanisms. All analyses and simulations were performed using R v. 3.1.2 [53] and the analysis code can be found in electronic supplementary material, appendix S3 (and is accessible online at https://github.com/JochenFruend/Defaunation_SeedDispersalWebs).
(b). Plant and animal communities
We considered starting communities composed of 50 species of plants (i) and 60 species of birds (j), respectively. This is similar to the ratio and magnitude found for empirical seed-dispersal networks from the tropics (e.g. [40,54]). For the sake of simplicity, our model only focused on size, represented by a single variable for plants and birds, respectively. Hence, to create species trait values (vectors x for plants, y for birds), we constructed an idealized lognormal distribution from equidistant quantiles [55], resulting in communities with many small and a few large species. Trait distributions match the empirical distributions of fruit volume and bird body mass presented in [40], with μ = 1.86 and σ = 0.63 (mean and s.d. on log-scale) for the plant community and μ = 1.18 and σ = 0.36 for the bird community. Trait variation among species was large (e.g. ranging from 0.2 to 29 cm in fruit length for plants and from 7 to 1400 g for birds), so that minor variation of traits within species was not relevant [56]. Note that trait matching in this kind of interaction is usually based on fruit size (diameter) and bird gape width (e.g. [35]), whereas recruitment success and bird movement are related to seed size and avian body mass, respectively [57,58]. Since fruit size and seed size [59], as well as avian gape width and body mass [60], are positively correlated, using a single size variable for each group (seed size, bird size) is reasonable for our model.
(c). Size–quantity relationships
Once trait values were defined for plant and bird species, we calculated overall expected interaction frequencies for each species. For this purpose, we considered a negative relationship between a species's size and its expected interaction frequency [34,38,39]. For plants, we assumed the relationship
| 2.1 |
where xi represents the seed size value for plant i and fi represents the expected plant interaction frequency (figure 1a).
In the case of birds, we assumed undercompensation (i.e. interaction frequency decreases less rapidly than bird size increases) as larger species tend to consume more fruits per individual [61]. Thus, expected bird species interaction frequencies followed the relationship
| 2.2 |
with yj representing the bird size value for bird j, gj representing the expected bird interaction frequency and β being an undercompensation parameter, set to 10% of the maximum value of 1/y (figure 1b). Results were essentially the same for other values of β (see electronic supplementary material, appendix S2).
(d). Size matching in seed-dispersal networks
The occurrence of species interactions will depend on species preferences (based on the matching between sizes of plant and bird species, i.e. xi and yj from the previous section, figure 1c). Therefore, to generate seed-dispersal networks (figure 1d) based on a size matching rule, we used a quantitative niche model modified from Fründ et al. [55] to first calculate species' preference matrices. Thus, the preference of bird species j for a certain plant species i (Pi,j) was determined as a function of the pairwise difference in trait values between bird and plant species as follows:
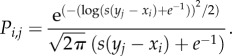 |
2.3 |
This represents a right-skewed niche shape, where yj and xi are bird size and seed size, respectively, and parameter s controls the degree of specialization (figure 1c). The standard lognormal distribution (with μ = 0 and σ = 1) has its mode at e−1, which we shifted to a mode at yj − xi = 0 to achieve a skewed trait matching function that is maximized for perfect matching. We calculated Pi,j using the R function dlnorm() as follows:
| 2.4 |
which gives the density and returns zero for all cases with s(yj − xi) + e−1 ≤ 0. All Pi,j were set to 1 for the scenarios without size matching. Parameter s was set to 10 for the size-matching scenarios, generating size-structured networks. We selected a right-skewed shape to account for the fact that negative size matching (bird < seed) renders interactions impossible (‘forbidden links' sensu [62]), whereas positive size matching (bird > seed) only makes interactions less likely. This function phenomenologically describes the asymmetry in size matching between plants and birds in real-world seed-dispersal networks [50]. Results were qualitatively identical for varying degrees of specialization (parameter s). Using a symmetric (Gaussian) niche shape instead of the skewed trait matching function also produced similar results, except for a lower sensitivity of seedling diversity to the loss of large frugivores (see electronic supplementary material, appendix S2).
In our model, birds choose among plants depending on plant frequency and size-dependent preferences. For each bird j, its per-visit interaction probability 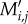 with plant i was calculated as
with plant i was calculated as
| 2.5 |
where (fi) represents interaction frequency of plant i and Pi,j the size-dependent preference of bird j. As we modelled interactions as a bird's choice among plants, realized bird frequencies were fixed to their expected interaction frequencies (gj), whereas realized plant frequencies could vary depending on bird preferences (i.e. how ‘popular’ a plant species was with the given set of birds). The output Mi,j = gj · 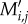 represents a weighted network matrix (with continuous values that sum to gj for each bird species j). For each matrix, we computed complementary specialization (H2′), a metric ranging from 0 (no specialization) to 1 (complete specialization), and weighted connectance (i.e. linkage density divided by the number of species in the network), using the R package bipartite v. 2.05 [63]. As expected, H2′ values of our modelled networks were equal to 0 for scenarios without size matching and ranged between 0.16 and 0.24 for the scenarios with size matching. Likewise, values of weighted connectance were greater than 0.45 for scenarios without size matching, and less than 0.3 for those considering size matching. This illustrates that networks accounting for size matching were less connected and exhibited a higher degree of niche partitioning than networks in the neutral scenarios, where abundance was the only driver of structure.
represents a weighted network matrix (with continuous values that sum to gj for each bird species j). For each matrix, we computed complementary specialization (H2′), a metric ranging from 0 (no specialization) to 1 (complete specialization), and weighted connectance (i.e. linkage density divided by the number of species in the network), using the R package bipartite v. 2.05 [63]. As expected, H2′ values of our modelled networks were equal to 0 for scenarios without size matching and ranged between 0.16 and 0.24 for the scenarios with size matching. Likewise, values of weighted connectance were greater than 0.45 for scenarios without size matching, and less than 0.3 for those considering size matching. This illustrates that networks accounting for size matching were less connected and exhibited a higher degree of niche partitioning than networks in the neutral scenarios, where abundance was the only driver of structure.
(e). Size–quality relationships
We assumed a positive relationship between seed and bird size and seed fates (i.e. seed-to-seedling transition probabilities) [38,45,50], and constructed mathematical functions that caused balanced trade-offs. That is, effects of size on quantity were expected to cancel out effects on quality (i.e. the net effect of size on seedling recruitment would be neutral without accounting for network complexity). The relationship between size and seed fate reflects abiotic and biotic processes that affect seedling recruitment, and relates to environmental filtering of large seeds under certain conditions [42,50], as well as to biotic processes, such as predation or intra-specific competition, that vary in response to dispersal distance [29,52,64]. Seed fate depending on plant traits was calculated as
| 2.6 |
where xi represents the seed size value for plant i, qi corresponds to the seed fate component imposed by the plant and |x| is the mean of all xi (figure 1e).
For birds, the balancing effect was achieved with a Michaelis–Menten function:
| 2.7 |
and
| 2.8 |
where yj represents the bird size value for bird j, β is the undercompensation parameter in equation (2.2), rj corresponds to the seed fate imposed by the bird, which was derived from raw values r′j scaled by their mean |r′| (figure 1f).
For the scenarios without trade-offs, fi and qi (for plants) or gj and rj (for birds) were set to a value of 1.
(f). Seedling recruitment
For the six scenarios, we transformed seed dispersal matrices into seedling recruitment by accounting for species-specific seed fates depending on size. Specifically, we multiplied realized interaction frequencies with the product of bird and plant quality values (seed fate components) to obtain the abundance of seedlings of each plant attributed to each seed dispersal agent, from which we calculated the total seedling abundance per plant species:
| 2.9 |
From vector Ri across plants, we finally calculated three different dimensions of seedling recruitment across the entire plant community: (i) abundance or total number of seedlings, estimated as the sum of Ri across plant species; (ii) diversity of seedlings, defined as the Shannon index (H) of seedling community Ri; and (iii) mean seed size of the seeds that were finally recruited, calculated as the community weighted mean (xi weighted by Ri). These three variables represent complementary aspects of seedling recruitment, in terms of the number of individuals, the distribution of individuals among species (reflecting their degree of dominance or evenness) and a characterization of the recruited community based on a species trait. Thus, we could explore if different dimensions of seedling recruitment vary in their response to defaunation.
(g). Defaunation scenarios
We evaluated changes in functional decay of seedling recruitment, comparing between a scenario representing a random sequence of seed disperser extinction (mean of 10 000 random sequences) and another scenario representing a deterministic extinction sequence based on bird species' size (i.e. removing bird species from the largest to the smallest one; size-structured defaunation). For all scenarios, we calculated the three dimensions of seedling recruitment corresponding to each value of bird richness. We assumed there was no density compensation (i.e. the abundance of remaining seed dispersers did not change after extinctions), as it remains questionable for real-world communities [65,66]. We scaled each extinction sequence relative to the starting value, which allowed us to analyse relative changes and to easily compare the shapes of the functional decay between scenarios as well as between dimensions of seedling recruitment.
(h). Sensitivity to choice of parameter values
We explored how variability in the parameters selected for the trait-matching function and the undercompensation function (in the case of bird species) impacted our results. We thus repeated the same simulations with different values for the specialization parameter (s = 2 and s = 50, representing low and extremely high specialization, respectively) and the undercompensation parameter β (β = 0 and β = 50% of the maximum value of 1/yj, representing no and high degrees of undercompensation, respectively). Results were robust to the choice of parameters s and β (see electronic supplementary material, appendix S2).
3. Results
(a). Size-matching effect on seedling recruitment
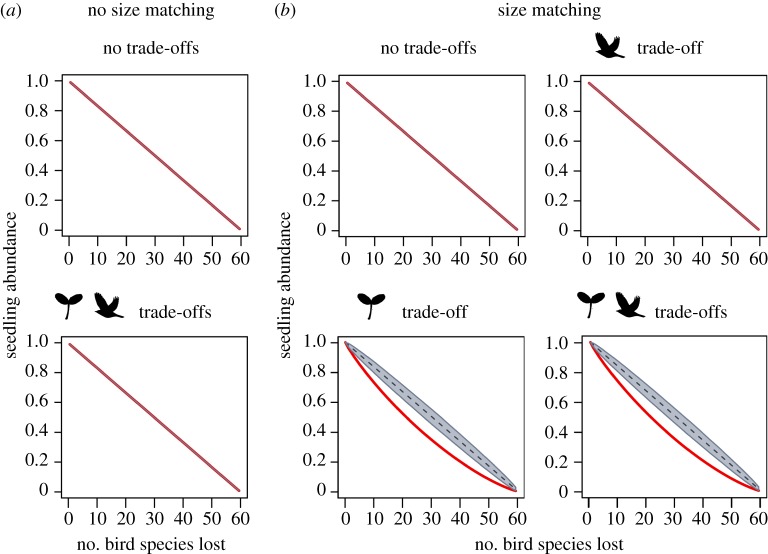
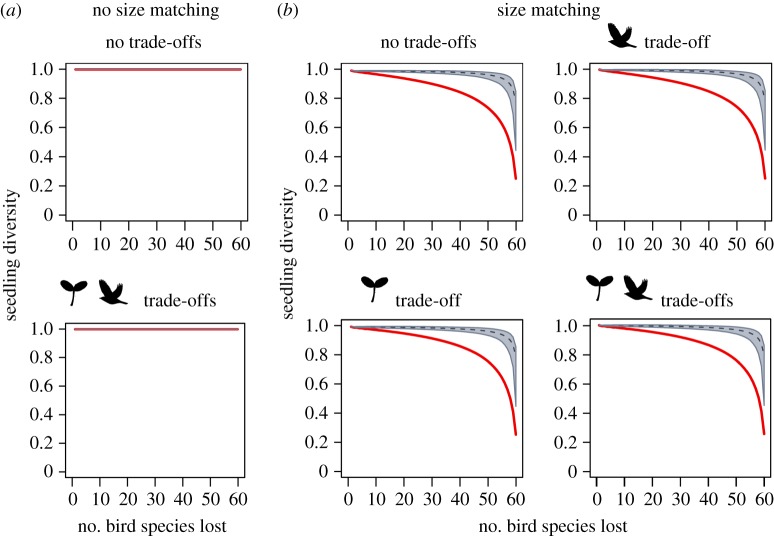
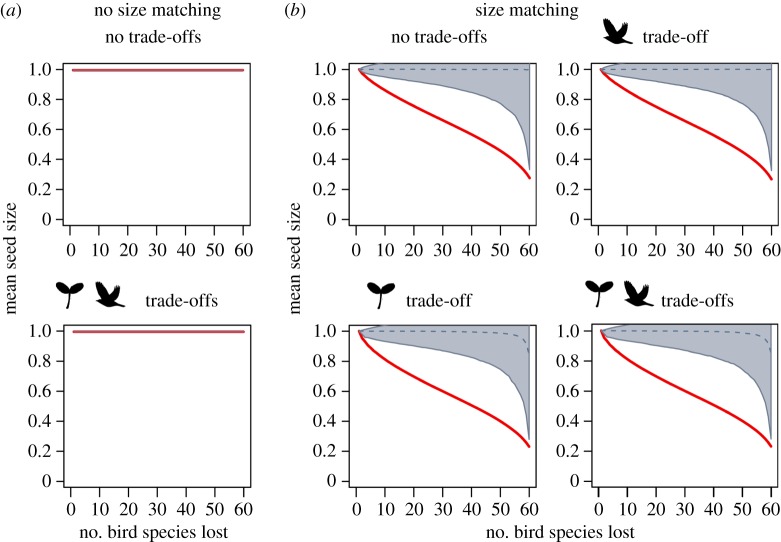
Size-structured defaunation of avian frugivores led to decays in seedling recruitment, but these defaunation effects differed among scenarios and different dimensions of seedling recruitment (figures 2–4). Defaunation effects on seedling abundance were independent of size matching between plants and birds, particularly in scenarios that did not account for size trade-offs in plants (figure 2). By contrast, defaunation effects on seedling diversity (figure 3) and mean seed size (figure 4) consistently depended on size matching. In scenarios with size matching, seedling diversity declined weakly when bird species were lost, especially under random extinction, but declined sharply after about 80% of bird species were removed (figure 3). The mean seed size rapidly decayed in response to size-structured defaunation and followed a sigmoidal functional decay curve. By contrast, the mean seed size hardly changed under random extinction. Hence, the reduction in seed size along the extinction sequence was driven by the interactive effects between size-structured defaunation and size matching between plants and birds (figure 4).
Figure 2.
Defaunation effects on seedling abundance under different scenarios of size matching and size trade-offs. We compared consequences of size-structured bird extinction (red line; defaunation) and random extinction (black dashed line, with grey areas representing the 95% confidence intervals across 10 000 iterations). Shown are model scenarios for (a) absence and (b) presence of size matching and different combinations of plant and bird size trade-offs (negative size–quantity and positive size–quality relationships). In the case of no size matching (a), scenarios with trade-offs only for plants or only for birds showed identical results and are not shown. In all cases, values on the y-axis were scaled to a starting value of 1 to facilitate comparisons among scenarios.
Figure 3.
Defaunation effects on seedling diversity under different scenarios of size matching and size trade-offs. We compared consequences of size-structured bird extinction (red line; defaunation) and random extinction (black dashed line, with grey areas representing the confidence intervals). Model scenarios were defined as explained in figure 2.
Figure 4.
Defaunation effects on mean seed size of recruited seedlings under different scenarios of size matching and size trade-offs. We compared consequences of size-structured bird extinction (red line; defaunation) and random extinction (black dashed line; grey areas representing the confidence intervals, which extend to outside the plotting area). Model scenarios were defined as explained in figure 2.
(b). Size trade-offs effect on seedling recruitment
The effect of size-structured defaunation on seedling diversity was hardly influenced by size trade-offs in plants and birds that account for the notion that large size corresponds to low frequency, but high recruitment probability in seed-dispersal systems (figure 3). However, the size trade-off in plants slightly amplified the functional decay of mean seed size in response to bird extinction (figure 4). By contrast, the functional decay of seedling abundance was notably changed by the size trade-off in plants (figure 2). Seedling abundance declined linearly along extinction sequences in most scenarios, both with and without size matching and for both random and size-structured defaunation. However, when size matching acted together with size trade-offs in plants, seedling abundance declined faster than linear, following a negative exponential curve in response to size-structured defaunation (figure 2).
4. Discussion
Here, we have illustrated how size-related mechanisms condition the effect of defaunation on ecosystem functioning. We simulated how size matching and size trade-offs in plants and animals mediate the consequences of animal loss from seed-dispersal networks on seedling recruitment. Overall, we found that effects of defaunation on seedling recruitment occurred only if size matching structured the interaction networks. Size trade-off in plants exacerbated these effects in the case of seedling abundance. Our results demonstrate a disproportionate importance of large frugivores. Thus, size matching and, to a lesser extent, size trade-off in plants mediated defaunation effects on ecosystem functioning.
(a). Size matching is crucial for defaunation effects on seedling recruitment
Consistent with our hypothesis, we found that the functional decay in response to size-structured defaunation depends on size matching between species. Hence, defaunation effects on seedling recruitment depend on the interacting effects between size as response and matching trait in seed-dispersal networks [50,54]. Our results imply that in communities where all frugivores may consume all plant species, no effect of frugivore loss on seedling diversity and mean seed size would be expected until complete defaunation. However, this is an unlikely scenario, as trait matching is a general phenomenon in ecological networks [67,68]. Although matching traits could also relate to other types of traits, such as the spatial or phenological matching between plant and animal species [69], size-related traits are generally most important for the matching between plants and animals in these types of interactions [34,35].
Seedling diversity gradually declined along both random extinction and defaunation sequences. Functional redundancy among frugivore species was especially pronounced under a random extinction sequence. Functional redundancy could thus buffer seedling diversity against frugivore loss, leading to a saturating biodiversity–ecosystem functioning relationship [25]. Redundancy can be explained by the relatively low values of complementary specialization (H2′) in the modelled networks that account for the size matching between plants and birds. Since most empirical seed-dispersal networks are indeed rather generalized [25,70], seedling diversity may often be relatively unaffected by moderate levels of defaunation. The dominance of small-seeded plant species in the modelled and in empirical plant communities [35] further contributes to this effect because small-seeded plants can be dispersed by both big and small frugivores [36].
Different from seedling diversity, the decay of mean seed size was sigmoidal (i.e. seed size declined rapidly already at the beginning of the size-structured defaunation sequence). Hence, the extinction of large bird species had a disproportionate impact on this dimension of seedling recruitment, as large-seeded plant species remained undispersed after the loss of large frugivores. The disproportionate functional role of large frugivores has been demonstrated in empirical studies of large-seeded plants [35]. The loss of large frugivores can result in functional collapse [71] and rapid evolutionary change [27] in such communities. In our simulations, downsizing of animal communities imposed a quick downsizing in the recruiting plant community. Such linked responses between plant and animal communities may also amplify effects of downsizing in other types of ecological systems [72].
(b). Size trade-offs can modify defaunation effects on seedling recruitment
Defaunation effects on seedling abundance were strongest if a size trade-off in plants was combined with size matching between plants and animals. Under random extinction, seedling abundance declined proportionally to the number of lost frugivore species. This is expected for biodiversity–ecosystem functioning relationships if species loss is not followed by density compensation [25]. A linear decay of function was also found for most scenarios with size-structured defaunation because balanced trade-offs led to the same expected number of recruited seedlings per dispersing animal species. Similarly, size matching between plants and birds had no influence on seedling abundance in scenarios without trade-off in plants because the identity of dispersed plant species did not matter for seedling abundance. However, if a trade-off in plants coincided with size matching, seedling abundance decayed more rapidly in response to size-structured defaunation. Similar nonlinear, accelerating decays have been predicted for biodiversity–ecosystem functioning relationships when there is a positive correlation between response and effect traits in the species providing the function [21,25]. Here, a related effect occurred because of a novel mechanism that increased the contribution of large frugivores to seedling abundance.
Given both size trade-off in plants and size matching between plants and animals, large frugivores contributed disproportionately to the recruiting seedling community. Although the number of dispersed seeds per bird species was fixed in the scenario without size trade-off in birds, large frugivores contributed more to seedling recruitment than small frugivores, if (i) large seeds had a higher recruitment probability than small seeds and if (ii) large seeds could only be dispersed by large frugivores. Therefore, in this scenario, a higher number of recruited seedlings traces back to large than to small frugivores. Consequently, although the size of the seed pool declines linearly with defaunation, the loss of large frugivores leads to a disproportionate loss of large seeds with a high recruitment probability. With increasing dominance of small frugivores, small seeds dominate the seed pool and their low recruitment probability leads to a disproportionate decline of seedling abundance. The effect of size trade-offs in plants should thus be considered for estimating the consequences of the extirpation of large seed dispersers. If size matching operates together with size trade-off in plants, vegetation dynamics might be more sensitive to the loss of a few large frugivores than if mechanisms structuring the network are independent of seed size. In particular, applications primarily interested in the number of recruits (e.g. reforestation) should pay attention to interactive effects of different size-related mechanisms. Moreover, the mechanism that large frugivores have a higher dispersal efficiency than small frugivores not due to their own effect traits (e.g. because they disperse seeds over longer distances [45]) but because they disperse seeds that are more valuable for the aggregate function can be used to formulate novel hypotheses also for other ecosystem functions. For example, it may apply to other functions derived from trophic relationships, such as pollination [68] or detritus cycling [73], where both size matching and size-related trade-offs of the interacting partners have been found to operate.
Overall, size trade-offs in plants and birds were less important than size matching for conditioning defaunation effects on seedling recruitment. Here, we simulated scenarios where size trade-offs in birds and plants were fully balanced (i.e. different life-history strategies were equivalent since quantitative and qualitative effects of size on seedling recruitment cancelled each other out). Although this is a reasonable and conservative approach [74], real-world communities may deviate from balanced trade-offs [37,41]. For instance, seed size may increase in importance in environments where large-seeded plant species have a higher recruitment probability than small-seeded plant species, such as in dense forests [42,58]. Similarly, large size of seed dispersers and long-distance seed dispersal may be especially advantageous if biotic processes related to density- and distance-dependent mortality of offspring prevail [52,64]. Under such conditions, size trade-offs are expected to influence defaunation effects on seedling recruitment and forest regeneration most strongly. If the increase in functional quality with size is stronger than the decrease in abundance (e.g. [61]), we would expect that large animals have even stronger disproportionate effects on ecosystem functions than small animals.
5. Conclusion
Here, we shed light on the mechanisms underpinning the effects of animal downsizing on ecosystem functioning. Namely, we identify size matching in ecological networks as the crucial mechanism mediating defaunation effects on ecosystem functions by animals, such as seed dispersal and subsequent seedling recruitment. By combining a niche model of species interactions with simulations of species' extinction, we demonstrate how a disproportionate importance of large animals for seedling recruitment follows from different size-related mechanisms. In particular, the subtle interplay between size matching and size trade-offs in plants highlights that defaunation will have severe and unforeseen consequences for coupled plant and animal communities, and their pivotal ecosystem functions.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Matthias Dehling and Irene M. A. Bender for providing empirical trait data collected in a Peruvian tropical forest, and Katrin Böhning-Gaese and the research groups at the Senckenberg Biodiversity and Climate Research Center (BiK-F). We are thankful to J. Rodríguez, J.P. González-Varó, K. Cazelles, M. McGeoch and three anonymous referees for their valuable comments on previous manuscript versions.
Data accessibility
The underlying R code has been uploaded as part of the electronic supplementary material, appendix S3, and is accessible online at https://github.com/JochenFruend/Defaunation_SeedDispersalWebs.
Authors' contributions
All the authors designed the study. J.F. provided the quantitative niche model used as a baseline for modelling work. I.D. performed the analysis with inputs from J.F. and M.S. I.D. drafted the manuscript and all authors contributed equally to interpretation and writing.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the Spanish National Program of Research and Development (MICINN CGL2011-28430 and MinECo CGL2015-68963-C2-2-R grants to D.G.) and the FPI Program—European Social Fund (BES2012-052863 and EEBB-I-15-0973 mobility grant to I.D.). J.F. was supported by a DFG research fellowship (FR3364/2-1).
References
- 1.Loreau M, et al. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808. ( 10.1126/science.1064088) [DOI] [PubMed] [Google Scholar]
- 2.Brose U, Hillebrand H. 2016. Biodiversity and ecosystem functioning in dynamic landscapes. Phil. Trans. R. Soc. B 371, 20150267 ( 10.1098/rstb.2015.0267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galetti M, Dirzo R. 2013. Ecological and evolutionary consequences of living in a defaunated world. Biol. Conserv. 163, 1–6. ( 10.1016/j.biocon.2013.04.020) [DOI] [Google Scholar]
- 4.Kurten E, Wright S, Carson W. 2015. Hunting alters seedling functional trait composition in a Neotropical forest. Ecology 96, 1923–1932. ( 10.1890/14-1735.1) [DOI] [PubMed] [Google Scholar]
- 5.Dirzo R, Miranda Á. 1990. Contemporary Neotropical defaunation and forest structure, function, and diversity: a sequel to John Terborgh. Conserv. Biol. 4, 444–447. ( 10.1111/j.1523-1739.1990.tb00320.x) [DOI] [Google Scholar]
- 6.Allhoff KT, Drossel B. 2016. Biodiversity and ecosystem functioning in evolving food webs. Phil. Trans. R. Soc. B 371, 20150281 ( 10.1098/rstb.2015.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes A, Weigelt P, Jochum M, Ott D, Hodapp D, Haneda N, Brose U. 2016. Species richness and biomass explain spatial turnover in ecosystem functioning across tropical and temperate ecosystems. Phil. Trans. R. Soc. B 371, 20150279 ( 10.1098/rstb.2015.0279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran C, Catterall C, Kanowski J. 2009. Reduced dispersal of native plant species as a consequence of the reduced abundance of frugivore species in fragmented rainforest. Biol. Conserv. 142, 541–552. ( 10.1016/j.biocon.2008.11.006) [DOI] [Google Scholar]
- 9.Harrison R, Tan S, Plotkin J, Slik F, Detto M, Brenes T, Itoh A, Davies S. 2013. Consequences of defaunation for a tropical tree community. Ecol. Lett. 16, 687–694. ( 10.1111/ele.12102) [DOI] [PubMed] [Google Scholar]
- 10.Srivastava D, Bell T. 2009. Reducing horizontal and vertical diversity in a foodweb triggers extinctions and impacts functions. Ecol. Lett. 12, 1016–1028. ( 10.1111/j.1461-0248.2009.01357.x) [DOI] [PubMed] [Google Scholar]
- 11.Kurten E. 2013. Cascading effects of contemporaneous defaunation on tropical forest communities. Biol. Conserv. 163, 22–32. ( 10.1016/j.biocon.2013.04.025) [DOI] [Google Scholar]
- 12.Estes JA, Palmisano JF. 1974. Sea otters: their role in structuring nearshore communities. Science 185, 1058–1060. ( 10.1126/science.185.4156.1058) [DOI] [PubMed] [Google Scholar]
- 13.Hooper DU, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 14.Lavorel S, Garnier E. 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol. 16, 545–556. ( 10.1046/j.1365-2435.2002.00664.x) [DOI] [Google Scholar]
- 15.Newbold T, Scharlemann J, Butchart S, Şekercioğlu Ç, Alkemade R, Booth H, Purves D. 2013. Ecological traits affect the response of tropical forest bird species to land-use intensity. Proc. R. Soc. B 280, 20122131 ( 10.1098/rspb.2012.2131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401–406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 17.Brose U, et al. 2016. Predicting the consequences of species loss using size-structured biodiversity approaches. Biol. Rev. 92, 684–697. ( 10.1111/brv.12250) [DOI] [PubMed] [Google Scholar]
- 18.Woodward G, et al. 2005. Body size in ecological networks. Trends Ecol. Evol. 20, 402–409. ( 10.1016/j.tree.2005.04.005) [DOI] [PubMed] [Google Scholar]
- 19.Gravel D, Albouy C, Thuiller W. 2016. The meaning of functional trait composition of food webs for ecosystem functioning. Phil. Trans. R. Soc. B 371, 20 150 268 ( 10.1098/rstb.2015.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White E, Ernest SK, Kerkhoff A, Enquist B. 2007. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 22, 323–330. ( 10.1016/j.tree.2007.03.007) [DOI] [PubMed] [Google Scholar]
- 21.Larsen T, Williams N, Kremen C. 2005. Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecol. Lett. 8, 538–547. ( 10.1111/j.1461-0248.2005.00749.x) [DOI] [PubMed] [Google Scholar]
- 22.Hagen M, et al. 2012. Biodiversity, species interactions and ecological networks in a fragmented world. Adv. Ecol. Res. 46, 89–120. ( 10.1016/B978-0-12-396992-7.00002-2) [DOI] [Google Scholar]
- 23.Allesina S, Alonso D, Pascual M. 2008. A general model for food web structure. Science 320, 658–661. ( 10.1126/science.1156269) [DOI] [PubMed] [Google Scholar]
- 24.Gravel D, Poisot T, Albouy C, Velez L, Mouillot D. 2013. Inferring food web structure from predator–prey body size relationships. Methods Ecol. Evol. 4, 1083–1090. ( 10.1111/2041-210X.12103) [DOI] [Google Scholar]
- 25.Schleuning M, Fründ J, García D. 2015. Predicting ecosystem functions from biodiversity and mutualistic networks: an extension of trait-based concepts to plant–animal interactions. Ecography 38, 1–13. ( 10.1111/ecog.00983) [DOI] [Google Scholar]
- 26.Bartomeus I, Gravel D, Tylianakis JM, Aizen M, Dickie I, Bernard-Verdier M. 2016. A common framework for identifying linkage rules across different types of interactions. Funct. Ecol. 30, 1894–1903. ( 10.1111/1365-2435.12666) [DOI] [Google Scholar]
- 27.Galetti M, et al. 2013. Functional extinction of birds drives rapid evolutionary changes in seed size. Science 340, 1086–1090. ( 10.1126/science.1233774) [DOI] [PubMed] [Google Scholar]
- 28.Farwig N, Berens D. 2012. Imagine a world without seed dispersers: a review of threats, consequences and future directions. Basic Appl. Ecol. 13, 109–115. ( 10.1016/j.baae.2012.02.006) [DOI] [Google Scholar]
- 29.Howe HF, Smallwood J. 1982. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 13, 201–228. ( 10.1146/annurev.es.13.110182.001221) [DOI] [Google Scholar]
- 30.Stoner KE, Henry M. 2009. Seed dispersal and frugivory in tropical ecosystems. In Tropical biology and conservation management, volume V: ecology (eds Del Claro K, Oliveira PS, Rico-Gray V), pp. 176–193. Oxford, UK: Eolss Publisher/UNESCO. [Google Scholar]
- 31.García D, Martínez D. 2012. Species richness matters for the quality of ecosystem services: a test using seed dispersal by frugivorous birds. Proc. R. Soc. B 279, 3106–3113. ( 10.1098/rspb.2012.0175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferger S, Dulle H, Schleuning M, Böhning-Gaese K. 2016. Frugivore diversity increases frugivory rates along a large elevational gradient. Oikos 125, 245–253. ( 10.1111/oik.02296) [DOI] [Google Scholar]
- 33.Wenny DG, Sekercioglu C, Cordeiro NJ, Rogers HS, Kelly D. 2016. Seed dispersal by fruit-eating birds. In Why birds matter: avian ecological function and ecosystem services Sekercioglu (C¸H, Wenny DG, Whelan CJ), pp. 107–145. Chicago, IL: University of Chicago Press. [Google Scholar]
- 34.González-Castro A, Yang S, Nogales M, Carlo TA. 2015. Relative importance of phenotypic trait matching and species' abundances in determining plant–avian seed dispersal interactions in a small insular community. AoB Plants 7, plv017 ( 10.1093/aobpla/plv017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dehling D, Jordano P, Schaefer H, Böhning-Gaese K, Schleuning M. 2016. Morphology predicts species' functional roles and their degree of specialization in plant–frugivore interactions. Proc. R. Soc. B 283, 20152444 ( 10.1098/rspb.2015.2444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wheelwright NT. 1985. Fruit size, gape width, and the diets of fruit-eating birds. Ecology 66, 808–818. ( 10.2307/1940542) [DOI] [Google Scholar]
- 37.Schupp E, Jordano P, Gómez J. 2010. Seed dispersal effectiveness revisited: a conceptual review. New Phytol. 188, 333–353. ( 10.1111/j.1469-8137.2010.03402.x) [DOI] [PubMed] [Google Scholar]
- 38.Moles A, Ackerly D, Webb C, Tweddle J, Dickie J, Westoby M. 2005. A brief history of seed size. Science 307, 576–580. ( 10.1126/science.1104863) [DOI] [PubMed] [Google Scholar]
- 39.Cotgreave P. 1993. The relationship between body size and population abundance in animals. Trends Ecol. Evol. 8, 244–248. ( 10.1016/0169-5347(93)90199-Y) [DOI] [PubMed] [Google Scholar]
- 40.Dehling M, Töpfer T, Schaefer M, Jordano P, Böhning-Gaese K, Schleuning M. 2014. Functional relationships beyond species richness patterns: trait matching in plant–bird mutualisms across scales. Global Ecol. Biogeogr. 23, 1085–1093. ( 10.1111/geb.12193) [DOI] [Google Scholar]
- 41.Donoso I, García D, Rodríguez-Pérez J, Martínez D. 2016. Incorporating seed fate into plant–frugivore networks increases interaction diversity across plant regeneration stages. Oikos 125, 1762–1771. ( 10.1111/oik.02509) [DOI] [Google Scholar]
- 42.Leishman MR, Wright IJ, Moles AT, Westoby M. 2000. The evolutionary ecology of seed size. In Seeds: the ecology of regeneration in plant communities (ed. Fenner M.), pp. 31–57. Wallingford, UK: CAB International. [Google Scholar]
- 43.Turnbull LA, Rees M, Crawley MJ. 1999. Seed mass and the competition/colonization trade-off: a sowing experiment. J. Ecol. 87, 899–912. ( 10.1046/j.1365-2745.1999.00405.x) [DOI] [Google Scholar]
- 44.Lebrija-Trejos E, Reich P, Hernández A, Wright S. 2016. Species with greater seed mass are more tolerant of conspecific neighbours: a key driver of early survival and future abundances in a tropical forest. Ecol. Lett. 19, 1071–1080. ( 10.1111/ele.12643) [DOI] [PubMed] [Google Scholar]
- 45.Jordano P, García C, Godoy JA, García-Castaño JL. 2007. Differential contribution of frugivores to complex seed dispersal patterns. Proc. Natl Acad. Sci. USA 104, 3278–3282. ( 10.1073/pnas.0606793104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wotton DM, Kelly D. 2011. Frugivore loss limits recruitment of large-seeded trees. Proc. R. Soc. B 278, 3345–3354. ( 10.1098/rspb.2011.0185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vidal M, Hasui E, Pizo M, Tamashiro J, Silva W, Guimarães P. 2014. Frugivores at higher risk of extinction are the key elements of a mutualistic network. Ecology 95, 3440–3447. ( 10.1890/13-1584.1) [DOI] [Google Scholar]
- 48.Wheelwright NT. 1988. Fruit-eating birds and bird-dispersed plants in the tropics and temperate zone. Trends Ecol. Evol. 3, 270–274. ( 10.1016/0169-5347(88)90061-4) [DOI] [PubMed] [Google Scholar]
- 49.Herrera CM, Pyllmyr O. 2009. Plant animal interactions: an evolutionary approach. Oxford, UK: Blackwell. [Google Scholar]
- 50.Muñoz M, Schaefer H, Böhning-Gaese K, Schleuning M. In press. Importance of animal and plant traits for fruit removal and seedling recruitment in a tropical forest. Oikos 126, 823–832. ( 10.1111/oik.03547) [DOI] [Google Scholar]
- 51.Schurr FM., Spiegel O, Steinitz O, Trakhtenbrot A, Tsoar A, Nathan R. 2009. Long-distance seed dispersal. Annu. Plants Rev. 38, 204–237. [DOI] [PubMed] [Google Scholar]
- 52.McConkey KR, Brockelman WY. 2011. Nonredundancy in the dispersal network of a generalist tropical forest tree. Ecology 92, 1492–1502. ( 10.1890/10-1255.1) [DOI] [PubMed] [Google Scholar]
- 53.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 54.Schleuning M, et al. 2014. Ecological, historical and evolutionary determinants of modularity in weighted seed-dispersal networks. Ecol. Lett. 17, 454–463. ( 10.1111/ele.12245) [DOI] [PubMed] [Google Scholar]
- 55.Fründ J, McCann K, Williams N. 2016. Sampling bias is a challenge for quantifying specialization and network structure: lessons from a quantitative niche model. Oikos 125, 502–513. ( 10.1111/oik.02256) [DOI] [Google Scholar]
- 56.González-Varo J, Traveset A. 2016. The labile limits of forbidden interactions. Trends Ecol. Evol. 31, 700–710. ( 10.1016/j.tree.2016.06.009) [DOI] [PubMed] [Google Scholar]
- 57.Sutherland GD, Harestad AS, Price K, Lertzman KP. 2000. Scaling of natal dispersal distances in terrestial birds and mammals. Conserv. Ecol. 4, 16 ( 10.5751/ES-00184-040116) [DOI] [Google Scholar]
- 58.Moles AT, Westoby M. 2004. Seedling survival and seed size: a synthesis of the literature. J. Ecol. 92, 372–383. ( 10.1111/j.0022-0477.2004.00884.x) [DOI] [Google Scholar]
- 59.Wright I, et al. 2007. Relationships among ecologically important dimensions of plant trait variation in seven neotropical forests. Ann. Bot. 99, 1003–1015. ( 10.1093/aob/mcl066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moran C, Catterall C. 2010. Can functional traits predict ecological interactions? A case study using rain forest frugivores and plants in Australia. Biotropica 42, 318–326. ( 10.1111/j.1744-7429.2009.00594.x) [DOI] [Google Scholar]
- 61.García D, Martínez D, Stouffer D, Tylianakis J. 2014. Exotic birds increase generalization and compensate for native bird decline in plant–frugivore assemblages. J. Anim. Ecol. 83, 1441–1450. ( 10.1111/1365-2656.12237) [DOI] [PubMed] [Google Scholar]
- 62.Burns KC. 2013. What causes size coupling in fruit–frugivore interaction webs? Ecology 94, 295–300. ( 10.1890/12-1161.1) [DOI] [PubMed] [Google Scholar]
- 63.Dormann C, Frund J, Bluthgen N, Gruber B. 2009. Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol. J. 2, 7–24. ( 10.2174/1874213000902010007) [DOI] [Google Scholar]
- 64.Fricke EC, Tewksbury JJ, Rogers HS. 2014. Multiple natural enemies cause distance-dependent mortality at the seed-to-seedling transition. Ecol. Lett. 17, 593–598. ( 10.1111/ele.12261) [DOI] [PubMed] [Google Scholar]
- 65.Winfree R. 2013. Global change, biodiversity, and ecosystem services: what can we learn from studies of pollination? Basic Appl. Ecol. 14, 453–460. ( 10.1016/j.baae.2013.07.004) [DOI] [Google Scholar]
- 66.Bello C, Galetti M, Pizo M, Magnago L, Rocha M, Lima R, Peres C, Ovaskainen O, Jordano P. 2015. Defaunation affects carbon storage in tropical forests. Sci. Adv. 1, e1501105 ( 10.1126/sciadv.1501105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eklöf A, et al. 2013. The dimensionality of ecological networks. Ecol. Lett. 16, 577–583. ( 10.1111/ele.12081) [DOI] [PubMed] [Google Scholar]
- 68.Garibaldi L, et al. 2015. Trait matching of flower visitors and crops predicts fruit set better than trait diversity. J. Appl. Ecol. 52, 1436–1444. ( 10.1111/1365-2664.12530) [DOI] [Google Scholar]
- 69.Ramos-Robles M, Andresen E, Díaz-Castelazo C. 2016. Temporal changes in the structure of a plant–frugivore network are influenced by bird migration and fruit availability. PeerJ 4, e2048 ( 10.7717/peerj.2048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Correa S, Arujo J, Penha J, Cunha C, Bobier K, Anderson J. 2016. Stability and generalization in seed dispersal networks: a case study of frugivorous fish in Neotropical wetlands. Proc. R. Soc. B 283, 20161267 ( 10.1098/rspb.2016.1267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pérez-Méndez N, Jordano P, Valido A. 2015. Downsized mutualisms: consequences of seed dispersers' body-size reduction for early plant recruitment. Perspect. Plant Ecol. Evol. Syst. 17, 151–159. ( 10.1016/j.ppees.2014.12.001) [DOI] [Google Scholar]
- 72.Sheridan J, Bickford D. 2011. Shrinking body size as an ecological response to climate change. Nat. Clim. Change 1, 401–406. ( 10.1038/nclimate1259) [DOI] [Google Scholar]
- 73.Nichols E, Peres C, Hawes J, Naeem S. 2016. Multitrophic diversity effects of network degradation. Ecol. Evol. 6, 4936–4946. ( 10.1002/ece3.2253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chave J. 2004. Neutral theory and community ecology. Ecol. Lett. 7, 241–253. ( 10.1111/j.1461-0248.2003.00566.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The underlying R code has been uploaded as part of the electronic supplementary material, appendix S3, and is accessible online at https://github.com/JochenFruend/Defaunation_SeedDispersalWebs.