Abstract
The performance of courtship signals provides information about the behavioural state and quality of the signaller, and females can use such information for social decision-making (e.g. mate choice). However, relatively little is known about the degree to which the perception of and preference for differences in motor performance are shaped by developmental experiences. Furthermore, the neural substrates that development could act upon to influence the processing of performance features remains largely unknown. In songbirds, females use song to identify males and select mates. Moreover, female songbirds are often sensitive to variation in male song performance. Consequently, we investigated how developmental exposure to adult male song affected behavioural and neural responses to song in a small, gregarious songbird, the zebra finch. Zebra finch males modulate their song performance when courting females, and previous work has shown that females prefer the high-performance, female-directed courtship song. However, unlike females allowed to hear and interact with an adult male during development, females reared without developmental song exposure did not demonstrate behavioural preferences for high-performance courtship songs. Additionally, auditory responses to courtship and non-courtship song were altered in adult females raised without developmental song exposure. These data highlight the critical role of developmental auditory experience in shaping the perception and processing of song performance.
Keywords: zebra finch, auditory processing, preference, EGR1, social context
1. Introduction
Across species, the performance of courtship signals provides information about the signaller that can be used by receivers for mate selection (reviewed in [1]). In songbirds, variation in the pitch, tempo and consistency of acoustic signals provides information about the species, individual identity, intention and even emotional state of the signaller [1–3]. Moreover, there is accumulating evidence that females choose mates based on variation in the performance of acoustic features that provide reliable information about the skill or vigour of the signaller and that these performance measures provide more accurate information about signaller quality and condition than ornaments alone [1,4,5].
Sexual selection theory predicts the evolution of strong discrimination abilities that allow females to readily assess male quality and distinguish between potential mates [6]. Sensory biases can shape discrimination abilities and may lead to preferences for particular signal features that arise independently of experience [7–17]. As such, the ability of females to perceive differences in motor performance might be expected to be independent of specific developmental input. Consistent with this notion, female canaries prefer songs that contain high-performance phrases, and the song preference is apparent in females raised under a variety of tutoring regimes as well as in females raised without song exposure [9,18–20]. Similarly, studies in zebra finches have found that preferences for songs with more elements or greater ‘complexity’ are not affected by developmental experiences [14]. These data suggest that female preferences for performance measures could arise independently of developmental experiences.
Developmental experiences have been shown to significantly influence female preferences. For example, female zebra finches appear to mate assortatively according to brood size, with females raised in large broods preferring songs of males raised in large broods [12,21]. However, these studies have not directly measured how developmental experiences affect the influence of specific song features, including song performance features, on song preferences. Nor have they investigated how developmental experience could affect the functional organization of neural circuits that contribute to female preferences.
In zebra finches, males sing the same vocal elements (syllables) in the same sequence during courtship and non-courtship song, but adjust their performance of those elements depending on the audience [22–24]. Females are sensitive to the changed song performance, and even females who have not had adult mating or social interactions with males prefer courtship song over non-courtship song [23]. One striking characteristic of a male zebra finch's courtship song is that syllables are produced with a high degree of consistency from rendition to rendition that is significantly greater during courtship song than during non-courtship song. Because higher vocal consistency is associated with breeding, courtship singing and greater reproductive success in other songbird species, vocal consistency has been argued to be a key performance metric that could influence female choice [25]. In line with this idea, the strength of preference for a zebra finch male's courtship song over his non-courtship song is best predicted by the difference in syllable consistency between courtship and non-courtship songs [23]. While female zebra finches are sensitive to subtle differences in song performance, it is unclear whether developmental factors shape the perception and neural processing of the performance feature of vocal consistency. Here, we investigated the degree to which preferences for high-performance courtship songs are influenced by developmental experiences.
2. Material and methods
(a). Animals
Female zebra finches were either raised in our colony (n = 58) or purchased from a breeder (n = 13). Within our colony, we raised females in one of two conditions. One set of females (normally reared) was raised with both parents and all siblings until two months of age. Thereafter, these females were removed from their nests and housed in same-sex group cages (43 × 43 × 43 cm). The second set of females (song-naive) was raised in sound-attenuating chambers (‘soundboxes’; TRA Acoustics, Cornwall, Ontario) with only their mother and siblings. Fathers were removed 5–7 days post-hatch, which is well before when females appear to memorize their father's songs [26], and male siblings were removed soon after fledging (35–40 days post-hatch). Song-naive females remained with their mothers and female siblings until two months of age, after which they were housed in all-female group cages (43 × 43 × 43 cm) inside sound-attenuating chambers. Birds were kept on a 14 L : 10 D photoperiod and provided finch seed, grit and water ad libitum, and lettuce and egg food at least once per week.
(b). Female preference testing
We assessed female preferences for songs using callback assays (e.g. [27–29]). Adult females (more than 120 days; n = 12 normally reared and 15 song-naive females from 13 different nests (electronic supplementary material, table S1)) were moved into individual cages (20 × 20 × 20 cm) in soundboxes at least 24 h prior to testing. Each soundbox was equipped with an omnidirectional microphone (Countryman Associates, California), a video camera (PalmVid, Colorado) and a speaker (Avantone, New York). Testing was performed between 09.00 and 14.00 h over the course of 7 days.
A callback test for each stimulus consisted of a 15 min silent period (pre-stimulus) followed by a ‘stimulus’ period that included a 5 min block of song playback followed by a 10 min period of silence. For each 5 min block of song playback, exemplars of an individual adult male zebra finch's courtship or non-courtship songs were played in pseudorandom order using custom-written Matlab software (Matlab, MathWorks, Natick, MA, USA). Each song stimulus was played two to three times with a 1 s interval between song exemplars. Females were tested on songs from 14 different males and received no more than four tests in a single day (e.g. courtship and non-courtship songs from two males). The orders of exposure to the songs of different males and to the songs from different social contexts were randomized both within and across days for each female. Overall levels of calling were not different between experimental groups, between first and last tests in the day and across days (see electronic supplementary material, table S2). Sound was recorded continuously throughout the entire period of testing using Sound Analysis Pro v. 2011.104 (SAP).
We counted the number of calls produced by females during the pre-stimulus and stimulus periods. Calls were identified either manually on spectrograms by an observer blind to the experimental condition or using automated software custom-written in Matlab. For both approaches, we first determined syllable boundaries for all calls using an amplitude-based segmentation algorithm [22,23,30]. For automated syllable identification, we first manually labelled at least 30 call renditions for each female. We then measured seven acoustic features (mean frequency, syllable duration, frequency and amplitude slopes, spectral entropy (entropy of the spectral density, ESD), spectrotemporal entropy (STE) and amplitude entropy), which we used to create feature vectors for automated call detection. We manually verified the labelling of the first 50 calls from each day of testing for all birds. The algorithm was highly accurate and reliable, with 99.2 ± 0.3% (mean ± s.e.m.; range: 98.8–99.8%) of all calls properly labelled. All calls produced during song playback were manually labelled because calls frequently occurred during the song stimulus itself, making them difficult to identify through automated procedures.
(c). Stimuli
Stimuli were courtship and non-courtship songs from 14 males that were unfamiliar and unrelated to the females being tested. Males were either from our colony at McGill University or from a colony at the University of California, San Francisco (songs recorded between 2004 and 2010). Song recordings were performed as previously described [23,31].
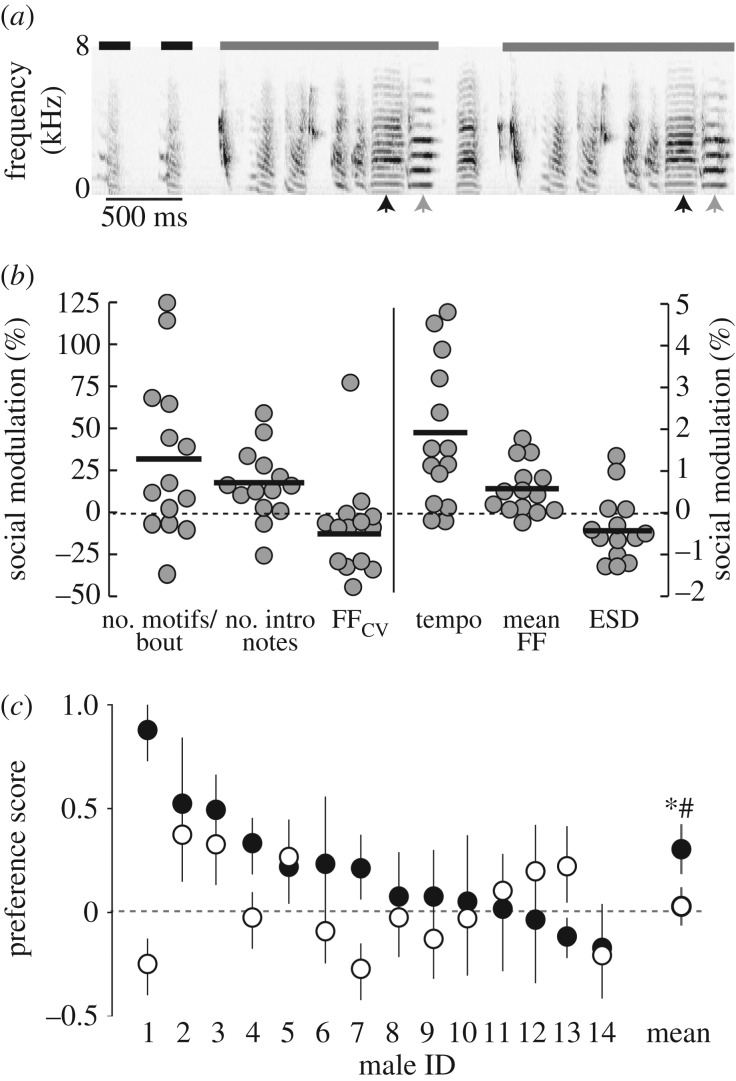
Zebra finch song consists of vocal elements (syllables) arranged in a stereotyped sequence (motif). Multiple motifs are strung together to form a ‘song bout’, and bouts are preceded by soft, simple introductory notes (figure 1a). Males sing the same syllables in the same sequence for courtship and non-courtship songs; as such, the macro-structure of courtship and non-courtship songs are highly similar. However, male zebra finches make subtle but significant adjustments to their song performance across these social contexts. We created courtship and non-courtship stimulus sets for each male by selecting four to eight renditions each of courtship and non-courtship songs, confirmed to be free of noise, as previously described [23,31]. All songs were bandpass-filtered (0.3–8.0 kHz), smoothed (Hanning window) and normalized by the maximum amplitude. We added 2 ms of silence to the beginning and end of the song bout, cosine ramped the stimuli to avoid sharp onsets and offsets in amplitude, and saved the stimulus as a wav file (44.1 kHz).
Figure 1.
Developmental song exposure shapes behavioural responses to song. (a) Spectrogram of non-courtship song from a male zebra finch. For all songs, we counted the number of introductory syllables (black bars) and number of motifs per bout (grey bars). We also measured the mean and CV of the FF across renditions of syllables with flat harmonic structure. Arrows highlight two renditions of two different syllables (black versus grey) where the FF would be the lowest frequency band. The ESD and STE were measured for all syllables. (b) Social modulation of a number of song features. Positive values indicate an increase and negative values indicate a decrease in the feature from non-courtship to courtship singing. Points are the average for each male, black lines indicate the mean across males. All features, except for the ESD (p = 0.1040), were significantly different from 0 at p < 0.05 (see Results). (c) Mean (±s.e.m.) responses of normally reared (filled circles) and song-naive females (open circles) to courtship and non-courtship songs of 14 different males (Male ID). Overall, normally reared females called more in response to courtship song than to non-courtship song, indicated by the positive preference score (difference in calling to courtship and non-courtship songs). By contrast, song-naive females did not call more in response to courtship song. Mean values across all Male IDs shown at right. *indicates significant effect of rearing condition at p < 0.05; #indicates significantly different from zero at p < 0.05.
(d). Immunocytochemistry and imaging
To investigate how developmental auditory experiences shape neural responses to courtship and non-courtship song, we analysed immediate early gene expression in response to song playback in song-naive and normally reared females. Adult song-naive and normally reared females (n = 40; more than 90 days old) that had not received any behaviour tests were placed individually in a cage inside a soundbox for at least 24 h prior to experimentation. On the morning of the experiment, lights were turned off at least 2 h prior to song playback (30 min of song at 1 s intervals) of either the courtship or non-courtship songs of one male zebra finch. After song playback, females remained in the dark, undisturbed, for 45 min to allow for protein translation [23,32]. Playback was performed in the dark to minimize female calling. Silent controls (n = 4; more than 90 days old) were females who did not receive song playback but were otherwise treated identically to birds receiving playback. Females were then deeply anaesthetized with isoflurane vapour and transcardially perfused with 25 ml heparinized saline (100 IU 100 ml−1) followed by 150 ml of 4% paraformaldehyde (pH = 7.4). Brains were collected and post-fixed for 4 h, then cryoprotected in 30% sucrose. Sagittal sections from the left and right hemispheres were cut at 40 µm (Leica Biosystems, Wetzlar, Germany) and stored in 0.025 M phosphate-buffered saline (PBS) with sodium azide at 4°C.
We analysed the expression of the immediate early gene protein EGR1 using standard immunocytochemical procedures [31,33–35]. In each immunocytochemical batch (n = 10 batches), we processed every third section from a bird from each experimental group: (i) song-naive birds that heard courtship song; (ii) song-naive birds that heard non-courtship song; (iii) normally reared birds that heard courtship song; and (iv) normally reared birds that heard non-courtship song. Females included in each batch all heard the songs of the same male. We also included a silent control bird in four immunocytochemical batches to confirm that playback increased EGR1 expression in auditory processing areas. Brain sections underwent 3×10 min rinses in 0.025 M PBS followed by a 1 h incubation in 5% donkey serum and 0.3% Triton-X. Sections were then incubated for 48 h at 4°C in primary antibody: rabbit anti-EGR1 (1 : 1000 dilution; Cat# SC-189; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and either sheep anti-tyrosine hydroxylase (TH; n = 6 batches; 1 : 1000 dilution; Cat# NB300-110, Novus Biologicals, Littleton, CO, USA) or mouse anti-NeuN (n = 3 batches; ‘Neuronal Nuclei’; 1 : 1000 dilution; Cat# MAB377; EMD Millipore, Billerica, MA, USA). Sections were then washed (3×10 min), incubated for 2 h at room temperature in donkey anti-rabbit secondary antibody conjugated to Alexa Fluor 568 (for EGR1; 5 µl ml−1; Life Sciences, Burlington, ON, Canada) and either donkey anti-mouse secondary antibody conjugated to Alexa Fluor 488 (for NeuN; 5 µl ml−1; Life Sciences) or donkey anti-sheep secondary antibody conjugated Alexa Fluor 488 (for TH; 5 µl ml−1; Life Sciences), and washed again (3×10 min). Sections were mounted and cover-slipped (ProLong Gold Antifade Reagent, Life Sciences) on chromium-aluminium subbed slides.
EGR1-labelled cells were quantified within three auditory areas, the dorsal part of the lateral mesencephalic nucleus (homologue of the inferior colliculus, IC), the caudomedial mesopallium (CMM) and the caudomedial nidopalium (NCM). Regions were identified as previously described [23,36]. Monochrome photomicrographs of EGR1 expression were taken for each region of interest in each hemisphere with a 40× objective using a Zeiss Axio Imager upright microscope and an AxioCam MRm Zeiss camera (Carl Zeiss, Jena, Germany). Using Fiji imaging software (NIH), EGR1-immunoreactive (EGR1-ir) neurons were manually counted in a 225 × 170 µm window on multiple sections (6.0 ± 1.0 sections region−1), and counts were averaged across sections within each hemisphere.
(e). Analysis
(i). Callback tests
We characterized a female's response to song as the per cent change in calling behaviour in response to stimulus playback:
If a female failed to call at least five times during both the pre-stimulus and stimulus periods for a callback test, her responses for that male's song were dropped from the analysis. All females heard courtship and non-courtship songs from multiple males. We calculated the difference in call responses between courtship and non-courtship songs for each male stimulus set (e.g. response to courtship song minus response to non-courtship song for male A), and used this difference as our ‘preference score’. In other words,
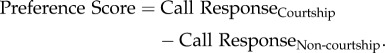 |
Positive preference scores indicate more calling for courtship songs and suggest courtship song preferences, whereas negative preference scores indicate more calling for non-courtship songs and suggest preferences for non-courtship songs.
We investigated the effects of developmental song exposure and male song stimuli (n = 14 male stimuli) on preference scores using a mixed-effects model. In this model, rearing condition, male ID and the interaction were independent variables, while preference score was the dependent variable. We included female ID as a random variable as females were tested on multiple stimuli. Nest of origin did not influence preference scores so was not included in the model (electronic supplementary material, tables S1 and S2). In a separate analysis to investigate agreement in preference between rearing conditions, we averaged the preference scores for a male's song across females within each rearing condition and assessed the Pearson's product-moment correlations between the average preference scores of song-naive females and of normally reared females. To analyse agreement in preference within each rearing condition, we compared the coefficient of variation (CV; standard deviation/mean) of preference scores for each male stimulus of normally reared females and song-naive females using a paired t-test.
(ii). Song analysis
Studies of motor performance have focused on measures of the quantity and quality of song performance [5,37]. Zebra finches alter both types of measures across courtship and non-courtship song. In particular, they produce longer, faster songs with more consistent and less noisy syllable structure during courtship singing (reviewed in [38]). To investigate the measures that explain variation in preference scores, we analysed the degree to which changes in song features from non-courtship to courtship song for each male covaried with mean preference scores.
We measured seven features of our stimuli to summarize context-dependent differences in the quantity and quality of performance. Specifically, we used two song features as measures of context-dependent changes in song quantity: we counted the number of introductory notes and the number of motifs per bout (figure 1a). To summarize context-dependent changes in song quality, we analysed four spectral features and one temporal feature. The four spectral features of syllables included the mean and variability of the fundamental frequency (FF), the mean entropy of the spectral density (ESD) and the mean STE. We measured the FF in syllables with flat, harmonic structure and calculated the mean (FFmean) and coefficient of variation (standard deviation/mean) of the FF across renditions (FFcv; figure 1a; [22,23,39]). We measured the ESD and STE for all syllables [40,41]. While such measures of entropy have not been historically discussed as performance measures (e.g. [4]), they exhibit many of the hallmarks of features of high-performance signals. For example, ESD and STE of individual syllables decrease over the course of learning and are precisely controlled by neural inputs to the syrinx from song control circuitry [41–46]. Moreover, the ESD is modulated by social context in adults and regulated by activity in brain areas that control other song performance features [41]. ESD is the entropy of the Fourier transform of the entire syllable collapsed across time, and normalized ESD values range between 0 (pure tones) and 1 (white noise). STE is the entropy of the distribution of power in the time-frequency representation, calculated using non-overlapping 8 ms Hanning windows. Finally, in addition to these four spectral features, we also measured context-dependent changes in motif duration (i.e. duration from the onset of the first syllable of the motif to the onset of the last syllable of the motif: [22,23]) as a temporal measure of song quality.
For all song features, we calculated, for each male, the mean value within each singing context (courtship versus non-courtship). We then calculated the degree of ‘social modulation’ for each song feature as the per cent change between courtship and non-courtship song for each male:
We defined the social modulation of song tempo as the negative of the social modulation of motif duration (i.e. a decrease in the motif duration = a faster song tempo). For the FFmean, FFcv, ESD and STE, we calculated the social modulation for multiple syllables for each male (two to three syllables per male for FF measures, 5–12 syllables per male for the ESD and STE) then took the average social modulation across syllables for each male. For each song feature, we used Wilcoxon's signed-rank tests to identify whether the social modulation was significantly different from 0.
We investigated whether preference scores for an individual male's courtship song were correlated with the degree of social modulation of each song feature using full-factorial ANCOVAs. For this model, rearing condition (normal versus song-naive), the social modulation of that feature for each male (n = 14 males) and the interaction were independent variables. Mean preference score associated with each male's song was the dependent variable. When the interaction between rearing and the degree of social modulation of a song feature was significant, we performed linear regressions of social modulation in the song feature and preference scores independently for song-naive and normally reared females.
(iii). EGR1 expression
We quantified EGR1 expression in left and right hemispheres and then analysed whether rearing condition or stimulus context (courtship versus non-courtship) influenced EGR1 expression. We used a full-factorial design with stimulus context, rearing condition, hemisphere and all possible interactions as the independent variables. We included batch and bird ID nested within batch as random variables and analysed the number of EGR1-ir cells µm−2 as the dependent variable. We found no significant effects of hemisphere for any of the brain regions and, thus, report only the results for stimulus context, rearing condition and their interaction. We did not include silent controls in the statistical analyses but present them for visual comparison in the figures. However, we confirmed that song playback increased EGR1 expression in auditory processing areas (IC: F1,28 = 520.8 p < 0.0001; CMM: F1,22 = 33.0, p < 0.0001; NCM: F1,22 = 5.5, p = 0.0287).
For all analyses, statistics were done using JMP 11.2.0 (Cary, NC, USA). For all mixed-effects models, we used unbounded variance components and the restricted maximum-likelihood method to fit the data. We used least-squared Tukey's HSD, within the mixed model, for all post hoc tests. We used t-tests (normally distributed data) or Wilcoxon's signed-rank tests (non-normally distributed data) to test whether distribution means were significantly different from zero and set α < 0.05 for all tests unless otherwise noted.
3. Results
(a). Normally reared, but not song-naive, females consistently prefer courtship song
We analysed the responses of normally reared and song-naive females to playback of the courtship and non-courtship songs of 14 males. On average, the courtship songs of these males were longer (number of motifs per bout: W1,13 = 28.0, p = 0.0269) and preceded by more introductory notes (W1,13 = 27.5, p = 0.0574) than non-courtship songs. In addition, their courtship songs contained syllables with higher FFmean (W1,13 = 36.5, p = 0.0081), lower FFcv (W1,13 = −32.5, p = 0.0215) and lower ESD (W1,13 = −26.5, p = 0.1040), and were produced at a faster tempo (W1,13 = −37.5, p = 0.0061) than non-courtship songs (figure 1b).
We then investigated whether rearing condition affected behavioural responses to courtship and non-courtship songs. Consistent with an effect of developmental auditory experience on song preferences, we found that preference scores (difference in calling response between courtship and non-courtship song stimuli; see Material and methods) were significantly higher for normally reared than for song-naive females (electronic supplementary material, table S3; figure 1c; F1,25 = 6.4; p = 0.0181). Moreover, preference scores of normally reared females were significantly greater than zero (t1,13 = 2.5; p = 0.0257), suggesting an overall preference for courtship song. By contrast, preference scores of song-naive females were both positive and negative and not different from zero (t1,13 = −0.2; p = 0.8475), indicating no overall direction of the preferences. Thus, normally reared but not song-naive females consistently prefer the high-performance courtship song.
Previous work has found decreased response magnitudes by song-naive birds [47]. One possibility is that the difference in preference between normally reared and song-naive birds could arise if song-naive females show similar response patterns to individual stimuli as normally reared birds, but with diminished response magnitudes. To investigate this possibility, we correlated the preference scores of normally reared and song-naive females for each of the 14 male songs but found no significant correlations between females from the different experimental groups (R2 = −0.0009, p = 0.9185). Together with the analysis of mean preference scores, this analysis indicates that the response profiles of song-naive females were distinct from those of normally reared females.
While there were differences between the rearing conditions in the preference for courtship song, we found that within each rearing condition females expressed similar preferences. In particular, the variance (CV) of preference scores within each rearing condition was generally low and did not differ between normally reared and song-naive females (electronic supplementary material, figure S1; W1,13 = 8.5; p = 0.6257).
(b). Developmental song exposure affects the relationship between song preferences and the social modulation of syllable structure
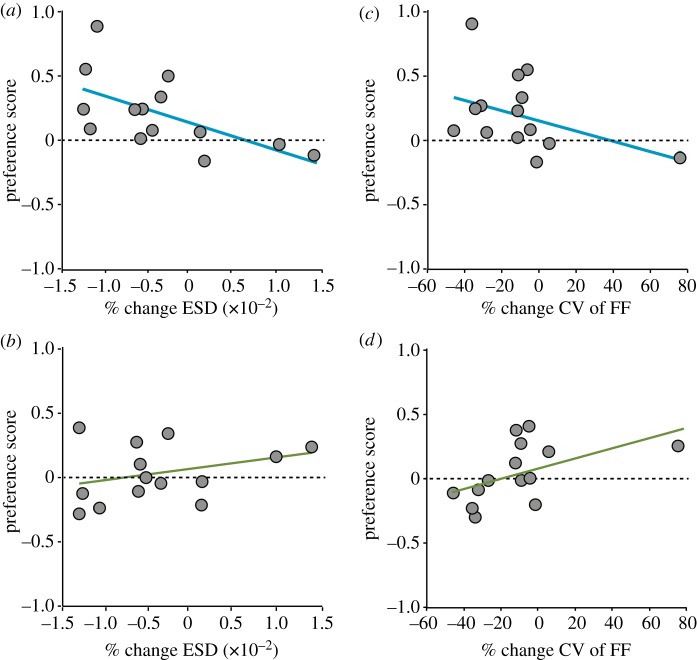
Male zebra finches differ in the degree to which they alter distinct song features across social contexts (figure 1a; [23]), and developmental auditory experience could shape how variation in the degree of social modulation of song features relates to variation in female responses. We found that the relationship between the degree of social modulation in ESD and preference score varied between normally reared and song-naive females (interaction: F1,12 = 9.4; p = 0.0098; electronic supplementary material, table S3). In particular, preferences for courtship song were stronger when the courtship song syllables were less noisy (had lower ESD) than non-courtship renditions for normally reared (figure 2a; t1,13 = −2.5; p = 0.0276; R2 = 0.34) but not for song-naive females (figure 2b; t1,13 = 1.1; p = 0.2825; R2 = 0.10).
Figure 2.
Developmental song exposure alters responses to song performance measures. The average social modulation of all syllables measured for the ESD (a,b) or FFcv (c,d) for an individual male versus the average preference scores of females tested on songs from that male for normally reared (a,c) and song-naive (b,d) females. Positive values for social modulation indicate that syllables were more ‘noisy’ (a,b) or more variable across renditions (c,d) during courtship than non-courtship singing. Positive values for the preference score indicate greater increases in calling to courtship versus non-courtship song. (Online version in colour.)
Similarly, there was an influence of rearing condition on the relationship between the social modulation of pitch variability (i.e. per cent difference in FFcv between courtship and non-courtship song) and preference scores (electronic supplementary material, table S3; interaction: F1,12 = 8.6; p = 0.0127). In normally reared females, there was no significant relationship between song preference and the social modulation of FFcv (figure 2c; t1,13 = −1.6; p = 0.1391; R2 = 0.17). By contrast, there was a trend for song-naive females to show stronger preferences for a male's courtship song when it was more variable across renditions than his non-courtship song (figure 2d; t1,13 = 2.0; p = 0.0671; R2 = 0.25). Rearing condition, however, did not significantly affect the relationship between female preference scores and the social modulation of other song features (electronic supplementary material, table S3).
(c). Developmental song exposure modulates EGR1 responses to courtship and non-courtship songs
The behavioural data indicate that developmental song exposure dramatically shapes the preference for courtship and non-courtship songs. In order to gain insight into the neural locus underlying this perceptual difference, we examined how developmental auditory experience affected auditory responses to courtship and non-courtship songs (i.e. stimulus context).
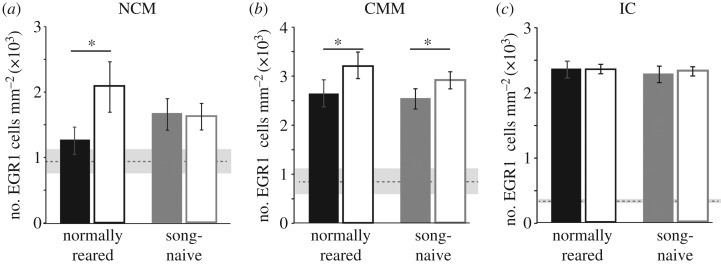
Developmental song exposure and stimulus context significantly interacted to affect EGR1 responses in the caudomedial nidopallium (NCM; F1,27.3 = 9.90; p = 0.0040). Specifically, EGR1 expression was modulated by the stimulus context in normally reared but not in song-naive birds (figure 3a). In normally reared birds, EGR1 expression was significantly higher following playback of courtship song than following playback of non-courtship song (p = 0.0006). However, in song-naive birds EGR1 expression was not significantly different in response to courtship or non-courtship song (p = 0.9998).
Figure 3.
EGR1 expression is differentially modulated by social context and developmental song exposure across brain areas. (a) In the NCM, EGR1 expression was modulated by the social context of the stimulus in normally reared females but not in song-naive females. *indicates a significant difference at p < 0.05. (b) In the CMM, EGR1 expression was significantly higher in response to courtship song than non-courtship song in both normally reared and song-naive females. *indicates significant effects of stimulus context but not rearing condition at p < 0.05. (c) In the IC, there were no effects of the social context of the stimulus on EGR1 expression in either normally reared or song-naive birds. Plotted are mean ± s.e.m. For all plots, open bars are responses to courtship song, filled bars are for non-courtship songs. Horizontal dashed lines with grey shading indicate the mean ± s.d. for EGR1 expression in silent controls.
In the CMM, stimulus context but not developmental song exposure affected EGR1 expression (figure 3b). Overall, EGR1 expression was significantly higher when normally reared and song-naive birds heard courtship song than when they heard non-courtship song (F1,26.8 = 11.5; p = 0.0021).
Finally, neither developmental song exposure nor stimulus context affected EGR1 expression in the IC (all p > 0.20; figure 3c).
4. Discussion
Vocal performance is hypothesized to represent a reliable measure of male quality, and females have been found to preferentially respond to features indicating higher-quality vocal performance [1,4,5,25,48,49]. However, little is known about the degree to which developmental auditory experiences shape a receiver's ability to recognize and discriminate such performance variation. Here, we demonstrated that developmental exposure to song significantly shapes behavioural and neural responses to socially modulated variation in vocal performance in zebra finches. A number of performance measures are significantly different between courtship and non-courtship songs (figure 1b; [22–24,41,50]). We found that normally reared females but not song-naive females systematically preferred courtship songs over non-courtship songs (figure 1c). Furthermore, we found that the social modulation of distinct performance measures differentially explained variation in song preference for normally reared and song-naive females (figure 2). These behavioural differences mapped onto differences in song-induced expression of EGR1 in a region of the secondary auditory cortex (figure 3). Specifically, in normally reared females but not in song-naive females, hearing courtship song led to greater EGR1 expression in the NCM than hearing non-courtship song. These data indicate that behavioural and neural responses to context-dependent song modulation are critically shaped by developmental auditory experiences.
Given that performance metrics can provide important insight into male fitness, sexual selection theory predicts the evolution of strong discrimination abilities in females that allow them to readily assess male performance and prefer signals that reflect heightened performance [6]. As such, the ability of females to perceive differences in motor performance might be expected to be independent of specific developmental input. Vocal consistency has been argued to reflect motor performance across a range of songbird species, including zebra finches [37]. As in a previous study [23], we observed that normally reared females preferred songs with greater spectral consistency. In particular, normally reared females showed stronger preferences for courtship songs when courtship song syllables were performed with greater spectral consistency (and lower spectral entropy) than non-courtship songs. However, in contrast with the view that preferences for high-performance songs are independent of development, song-naive females did not show preferences for more consistent songs. If anything, our data suggest that females raised without developmental exposure to song prefer noisier, more variable songs. As such, our data underscore the importance of developmental auditory experiences to the perception of and preference for performance measures of courtship signals.
Female preferences for courtship signals have been proposed to depend on a combination of innate predispositions and ‘reference templates’ [51,52]. For example, a number of studies have found that female zebra finches raised without exposure to conspecific song continue to show preferences for zebra finch song over canary song [12]. However, song-naive female zebra finches failed to demonstrate preferences for conspecific song when tested against heterospecific songs that were closer in acoustic structure to zebra finch song [53]. These studies suggest that whereas the auditory system of song-naive females may have relatively crude innate filters for song discrimination, developmental exposure to song is important for more fine-tuned song responses. Our data support this contention. We find that normally reared but not song-naive females behaviourally discriminate between courtship and non-courtship songs, which consist of the same syllables produced in the same sequences but differ subtly in consistency, spectral entropy and tempo. While our data underscore that the temporal or spectral statistics of male song shape auditory processing circuits in female zebra finches, they leave open questions of when and what kind of song exposure is necessary for the development of preferences for high-performance courtship songs. The lack of preferential responses to courtship song could arise because of the lack of developmental song exposure or because of enhanced tuning to the other sounds available in the auditory scene (e.g. mother's calls, siblings untutored plastic song). Furthermore, it remains unclear whether there is a definitive ‘critical’ period for auditory preferences in female birds, and it will be interesting to determine if adult auditory exposure can ameliorate perceptual deficits in song-naive females. Social and reproductive experiences with males in adulthood have been found to affect the magnitude of preferences for courtship song [23,31], suggesting the possibility that adult social experiences could also alter the behavioural responses of song-naive females.
In addition to suggesting that developmental song exposure is important for fine-tuning behavioural responses, our data also indicate that different parts of the auditory system demonstrate different degrees of developmental plasticity. We found that EGR1 responses in the IC and CMM were not affected by developmental song exposure. By contrast, EGR1 responses in the NCM were affected by developmental song exposure. Consequently, our data support the contention that ascending auditory regions may be less sensitive to developmental auditory experiences (e.g. [53–55]) and that developmental influences on fine-tuned auditory responses are likely to be mediated by developmental influences on forebrain circuitry. However, it remains unclear how the dynamic responses in NCM influence activity throughout the neural circuits that underlie behavioural responses to song. We propose that activity in NCM could be more influential in such social decisions because NCM neurons, but not CMM neurons, project to more lateral parts of the caudal nidopallium that have been implicated in decision-making and that connect to arcopallial motor output regions [56–59].
The NCM has been found to be critical for auditory learning and memory [60–62]. Both neurophysiological activity and EGR1 expression in the NCM show rapid and lasting habituation to repeated playback of songs from the same male, and this habituation is thought to reflect a memory trace of the stimulus [62]. Our finding that EGR1 expression in the NCM is differentially affected by courtship and non-courtship song in normally reared females but not in song-naive females raises the intriguing possibility that song-naive birds may also exhibit deficits in auditory learning and memory. Revealing the degree to which normally reared and song-naive females differ in sensory learning could lend further insight into developmental contributions to brain organization, sensory processing and cognition.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgement
We thank Jon Sakata for helpful suggestions on the manuscript, and Vivian Ng, Michele Kim and Lily Su for help collecting behavioural data.
Ethics
All procedures adhered to Canadian Council on Animal Care guidelines for the care and use of animals, and were approved by the McGill University Animal Care Committee.
Data accessibility
Datasets supporting this article can be found in Dryad repository: http://dx.doi.org/10.5061/dryad.22v00 [63].
Authors' contributions
S.C.W. conceived the study, performed the statistical analyses, wrote the draft and created the figures. O.C. helped design the study, collected and analysed the behavioural data. Y.C. helped design the study and performed the immunocytochemistry, imaging and analysis. All authors edited and commented on the manuscript.
Competing interests
We have no competing interests.
Funding
This work was supported by RGPIN402186 (S.C.W.) and RGPIN402417 (Jon Sakata) from the National Sciences and Engineering Research Council.
References
- 1.Bradbury J, Vehrencamp S. 2011. Principles of animal communication. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 2.Morton ES. 1977. On the occurrence and significance of motivation-structural rules in some bird and mammal sounds. Am. Nat. 111, 855–869. ( 10.1086/283219) [DOI] [Google Scholar]
- 3.Zann RA. 1996. The zebra finch: a synthesis of field and laboratory studies. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Byers J, Hebets E, Podos J. 2010. Female mate choice based upon male motor performance. Anim. Behav. 79, 771–778. ( 10.1016/j.anbehav.2010.01.009) [DOI] [Google Scholar]
- 5.Podos J, Lahti DC, Moseley DL. 2009. Vocal performance and sensorimotor learning in songbirds. Adv. Stud. Behav. 40, 159–195. ( 10.1016/S0065-3454(09)40005-6) [DOI] [Google Scholar]
- 6.Trivers R. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man, pp. 136–179. New York, NY: Aldine de Gruyter. [Google Scholar]
- 7.Ryan MJ, Cummings ME. 2013. Perceptual biases and mate choice. Annu. Rev. Ecol. Evol. Syst. 44, 437–459. ( 10.1146/annurev-ecolsys-110512-135901) [DOI] [Google Scholar]
- 8.Collins SA. 1999. Is female preference for male repertoires due to sensory bias? Proc. R. Soc. Lond. B 266, 2309–2314. ( 10.1098/rspb.1999.0924) [DOI] [Google Scholar]
- 9.Nagle L, Kreutzer M. 1997. Adult female domesticated canaries can modify their song preferences. Can. J. Zool. 75, 1346–1350. ( 10.1139/z97-759) [DOI] [Google Scholar]
- 10.Drăgănoiu TI, Nagle L, Kreutzer M. 2002. Directional female preference for an exaggerated male trait in canary (Serinusanaria) song. Proc. R. Soc. Lond. B 269, 2525–2531. ( 10.1098/rspb.2002.2192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vyas A, Harding C, Borg L, Bogdan D. 2009. Acoustic characteristics, early experience, and endocrine status interact to modulate female zebra finches’ behavioural responses to songs. Horm. Behav. 55, 50–59. ( 10.1016/j.yhbeh.2008.08.005) [DOI] [PubMed] [Google Scholar]
- 12.Lauay C, Gerlach NM, Adkins-Regan E, DeVoogd TJ. 2004. Female zebra finches require early song exposure to prefer high-quality song as adults. Anim. Behav. 68, 1249–1255. ( 10.1016/j.anbehav.2003.12.025) [DOI] [Google Scholar]
- 13.Hernandez AM, MacDougall-Shackleton SA. 2004. Effects of early song experience on song preferences and song control and auditory brain regions in female house finches (Carpodacus mexicanus). J. Neurobiol. 59, 247–258. ( 10.1002/neu.10312) [DOI] [PubMed] [Google Scholar]
- 14.Woodgate JL, Leitner S, Catchpole CK, Berg ML, Bennett AT, Buchanan KL. 2011. Developmental stressors that impair song learning in males do not appear to affect female preferences for song complexity in the zebra finch. Behav. Ecol. 22, 566–573. ( 10.1093/beheco/arr006) [DOI] [Google Scholar]
- 15.Braaten RF, Reynolds K. 1999. Auditory preference for conspecific song in isolation-reared zebra finches. Anim. Behav. 58, 105–111. ( 10.1006/anbe.1999.1134) [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal GG. 2007. Spatiotemporal dimensions of visual signals in animal communication. Annu. Rev. Ecol. Evol. Syst. 38, 155–178. ( 10.1146/annurev.ecolsys.38.091206.095745) [DOI] [Google Scholar]
- 17.Schielzeth H, Bolund E, Forstmeier W. 2010. Heritability of and early environment effects on variation in mating preferences. Evolution 64, 998–1006. ( 10.1111/j.1558-5646.2009.00890.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasteau M, Nagle L, Kreutzer M. 2004. Preferences and predispositions for intra-syllabic diversity in female canaries (Serinus canaria). Behaviour 141, 571–583. ( 10.1163/1568539041166735) [DOI] [Google Scholar]
- 19.Pasteau M, Nagle L, Kreutzer M. 2007. Influences of learning and predispositions on frequency level preferences on female canaries (Serinus canaria). Behaviour 144, 1103–1118. ( 10.1163/156853907781871798) [DOI] [Google Scholar]
- 20.Pasteau M, Nagle L, Monbureau M, Kreutzer M. 2009. Aviary experience has no effect on predisposition of female common canaries (Serinus canaria) for longer sexy phrases. Auk 126, 383–388. ( 10.1525/auk.2009.08101) [DOI] [Google Scholar]
- 21.Holveck M-J, Riebel K. 2009. Low-quality females prefer low-quality males when choosing a mate. Proc. R. Soc. B 277, 153–160. ( 10.1098/rspb.2009.1222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao MH, Brainard MS. 2006. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J. Neurophysiol. 96, 1441–1455. ( 10.1152/jn.01138.2005) [DOI] [PubMed] [Google Scholar]
- 23.Woolley SC, Doupe AJ. 2008. Social context-induced song variation affects female behaviour and gene expression. PLoS Biol. 6, e62 ( 10.1371/journal.pbio.0060062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sossinka R, Böhner J. 1980. Song types in the zebra finch Poephila guttata castanotis. Z. Tierpsychol. 53, 123–132. [Google Scholar]
- 25.Byers BE. 2007. Extrapair paternity in chestnut-sided warblers is correlated with consistent vocal performance. Behav. Ecol. 18, 130–136. ( 10.1093/beheco/arl058) [DOI] [Google Scholar]
- 26.Riebel K. 2003. Developmental influences on auditory perception in female zebra finches---is there a sensitive phase for song preference learning? Anim. Biol. 53, 73–87. ( 10.1163/157075603769700304) [DOI] [Google Scholar]
- 27.Clayton N. 1988. Song discrimination learning in zebra finches. Anim. Behav. 36, 1016–1024. ( 10.1016/S0003-3472(88)80061-7) [DOI] [Google Scholar]
- 28.Nagle L, Kreutzer M, Vallet E. 2002. Adult female canaries respond to male song by calling. Ethology 108, 463–472. ( 10.1046/j.1439-0310.2002.00790.x) [DOI] [Google Scholar]
- 29.Dunning JL, Pant S, Bass A, Coburn Z, Prather JF. 2014. Mate choice in adult female Bengalese finches: females express consistent preferences for individual males and prefer female-directed song performances. PLoS ONE 9, e89438 ( 10.1371/journal.pone.0089438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woolley SC, Rajan R, Joshua M, Doupe AJ. 2014. Emergence of context-dependent variability across a basal ganglia network. Neuron 82, 208–223. ( 10.1016/j.neuron.2014.01.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schubloom HE, Woolley SC. 2016. Variation in social relationships relates to song preferences and EGR1 expression in a female songbird. Dev. Neurobiol. 76, 1029–1040. ( 10.1002/dneu.22373) [DOI] [PubMed] [Google Scholar]
- 32.Mello CV, Ribeiro S. 1998. Z ENK protein regulation by song in the brain of songbirds. J. Comp. Neurol. 393, 426–438. ( 10.1002/(SICI)1096-9861(19980420)393:4%3C426::AID-CNE3%3E3.0.CO;2-2) [DOI] [PubMed] [Google Scholar]
- 33.Bharati IS, Goodson JL. 2006. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience 143, 661–670. ( 10.1016/j.neuroscience.2006.08.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matheson LE, Sakata JT. 2015. Catecholaminergic contributions to vocal communication signals. Eur. J. Neurosci. 41, 1180–1194. ( 10.1111/ejn.12885) [DOI] [PubMed] [Google Scholar]
- 35.Matheson LE, Sun H, Sakata JT. 2015. Forebrain circuits underlying the social modulation of vocal communication signals. Dev. Neurobiol. 76, 47–63. ( 10.1002/dneu.22298) [DOI] [PubMed] [Google Scholar]
- 36.Sockman KW, Gentner TQ, Ball GF. 2005. Complementary neural systems for the experience-dependent integration of mate-choice cues in European starlings. J. Neurobiol. 62, 72–81. ( 10.1002/neu.20068) [DOI] [PubMed] [Google Scholar]
- 37.Sakata JT, Vehrencamp SL. 2012. Integrating perspectives on vocal performance and consistency. J. Exp. Biol. 215, 201–209. ( 10.1242/jeb.056911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woolley S, Kao M. 2015. Variability in action: contributions of a songbird cortical-basal ganglia circuit to vocal motor learning and control. Neuroscience 296, 39–47. ( 10.1016/j.neuroscience.2014.10.010) [DOI] [PubMed] [Google Scholar]
- 39.James LS, Sakata JT. 2015. Predicting plasticity: acute context-dependent changes to vocal performance predict long-term age-dependent changes. J. Neurophysiol. 114, 2328–2339. ( 10.1152/jn.00688.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakata JT, Brainard MS. 2006. Real-time contributions of auditory feedback to avian vocal motor control. J. Neurosci. 26, 9619–9628. ( 10.1523/JNEUROSCI.2027-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stepanek L, Doupe AJ. 2010. Activity in a cortical-basal ganglia circuit for song is required for social context-dependent vocal variability. J. Neurophysiol. 104, 2474–2486. ( 10.1152/jn.00977.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamaguchi K, Mooney R. 2012. Recurrent interactions between the input and output of a songbird cortico-basal ganglia pathway are implicated in vocal sequence variability. J. Neurosci. 32, 11 671–11 687. ( 10.1523/JNEUROSCI.1666-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Secora KR, Peterson JR, Urbano CM, Chung B, Okanoya K, Cooper BG. 2012. Syringeal specialization of frequency control during song production in the Bengalese Finch (Lonchura striata domestica). PLoS ONE 7, e34135 ( 10.1371/journal.pone.0034135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tchernichovski O, Mitra PP, Lints T, Nottebohm F. 2001. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science 291, 2564–2569. ( 10.1126/science.1058522) [DOI] [PubMed] [Google Scholar]
- 45.Kojima S, Doupe AJ. 2007. Song selectivity in the pallial-basal ganglia song circuit of zebra finches raised without tutor song exposure. J. Neurophysiol. 98, 2099–2109. ( 10.1152/jn.00916.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams H, Crane LA, Hale TK, Esposito MA, Nottebohm F. 1992. Right-side dominance for song control in the zebra finch. J. Neurobiol. 23, 1006–1020. ( 10.1002/neu.480230807) [DOI] [PubMed] [Google Scholar]
- 47.Woodgate JL, Bennett AT, Leitner S, Catchpole CK, Buchanan KL. 2010. Developmental stress and female mate choice behaviour in the zebra finch. Acad. Emerg. Med. 79, 1381–1390. ( 10.1016/j.anbehav.2010.03.018) [DOI] [Google Scholar]
- 48.Vallet E, Kreutzer M. 1995. Female canaries are sexually responsive to special song phrases. Acad. Emerg. Med. 49, 1603–1610. ( 10.1016/0003-3472(95)90082-9) [DOI] [Google Scholar]
- 49.Ballentine B, Hyman J, Nowicki S. 2004. Vocal performance influences female response to male bird song: an experimental test. Behav. Ecol. 15, 163–168. ( 10.1093/beheco/arg090) [DOI] [Google Scholar]
- 50.Cooper BG, Goller F. 2006. Physiological insights into the social-context-dependent changes in the rhythm of the song motor program. J. Neurophysiol. 95, 3798–3809. ( 10.1152/jn.01123.2005) [DOI] [PubMed] [Google Scholar]
- 51.Widemo F, Sæther SA. 1999. Beauty is in the eye of the beholder: causes and consequences of variation in mating preferences. Trends Ecol. Evolut. 14, 26–31. ( 10.1016/S0169-5347(98)01531-6) [DOI] [PubMed] [Google Scholar]
- 52.Jennions MD, Petrie M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. 72, 283–327. ( 10.1017/S0006323196005014) [DOI] [PubMed] [Google Scholar]
- 53.Hauber ME, Woolley SM, Cassey P, Theunissen FE. 2013. Experience dependence of neural responses to different classes of male songs in the primary auditory forebrain of female songbirds. Behav. Brain Res. 243, 184–190. ( 10.1016/j.bbr.2013.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amin N, Gastpar M, Theunissen FE. 2013. Selective and efficient neural coding of communication signals depends on early acoustic and social environment. PLoS ONE 8, e61417 ( 10.1371/journal.pone.0061417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maul KK, Voss HU, Parra LC, Salgado-Commissariat D, Ballon D, Tchernichovski O, Helekar SA. 2010. The development of stimulus-specific auditory responses requires song exposure in male but not female zebra finches. Dev. Neurobiol. 70, 28–40. ( 10.1002/dneu.20751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vates GE, Broome BM, Mello CV, Nottebohm F. 1996. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taenopygia guttata). J. Comp. Neurol. 366, 613–642. ( 10.1002/(SICI)1096-9861(19960318)366:4%3C613::AID-CNE5%3E3.0.CO;2-7) [DOI] [PubMed] [Google Scholar]
- 57.Bottjer SW, Brady JD, Cribbs B. 2000. Connections of a motor cortical region in zebra finches: relation to pathways for vocal learning. J. Comp. Neurol. 420, 244–260. ( 10.1002/(SICI)1096-9861(20000501)420:2%3C244::AID-CNE7%3E3.0.CO;2-M) [DOI] [PubMed] [Google Scholar]
- 58.De Groof G, George I, Touj S, Stacho M, Jonckers E, Cousillas H, Hausberger M, Güntürkün O, Van der Linden A. 2016. A three-dimensional digital atlas of the starling brain. Brain Struct. Funct. 221, 1899–1909. ( 10.1007/s00429-015-1011-1) [DOI] [PubMed] [Google Scholar]
- 59.Güntürkün O. 2005. The avian ‘prefrontal cortex'and cognition. Curr. Opin Neurobiol. 15, 686–693. ( 10.1016/j.conb.2005.10.003) [DOI] [PubMed] [Google Scholar]
- 60.Mello CV, Velho TA, Pinaud R. 2004. Song-induced gene expression: a window on song auditory processing and perception. Ann. NY Acad. Sci. 1016, 263–281. ( 10.1196/annals.1298.021) [DOI] [PubMed] [Google Scholar]
- 61.Yanagihara S, Yazaki-Sugiyama Y. 2016. Auditory experience-dependent cortical circuit shaping for memory formation in bird song learning. Nat. Commun. 7, 11946 ( 10.1038/ncomms11946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dong S, Clayton DF. 2009. Habituation in songbirds. Neurobiol. Learn. Mem. 92, 183–188. ( 10.1016/j.nlm.2008.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y, Clark O, Woolley SC. 2017. Data from: Courtship song preferences in female zebra finches are shaped by developmental auditory experience. Dryad Digital Repository. ( 10.5061/dryad.22v00) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Chen Y, Clark O, Woolley SC. 2017. Data from: Courtship song preferences in female zebra finches are shaped by developmental auditory experience. Dryad Digital Repository. ( 10.5061/dryad.22v00) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Datasets supporting this article can be found in Dryad repository: http://dx.doi.org/10.5061/dryad.22v00 [63].