Abstract
A common practice in thermal biology is to take individuals directly from the field and estimate a range of thermal traits. These estimates are then used in studies aiming to understand broad scale distributional patterns, understanding and predicting the evolution of phenotypic plasticity, and generating predictions for climate change risk. However, the use of field-caught individuals in such studies ignores the fact that many traits are phenotypically plastic and will be influenced by the thermal history of the focal individuals. The current study aims to determine the extent to which estimates of upper thermal limits (CTmax), a frequently used measure for climate change risk, are sensitive to developmental and adult acclimation temperatures and whether these two forms of plasticity are reversible. Examining a temperate and tropical population of Drosophila melanogaster we show that developmental acclimation has a larger and more lasting effect on CTmax than adult acclimation. We also find evidence for an interaction between developmental and adult acclimation, particularly when flies are acclimated for a longer period, and that these effects can be population specific. These results suggest that thermal history can have lasting effects on estimates of CTmax. In addition, we provide evidence that developmental and/or adult acclimation are unlikely to contribute to substantial shifts in CTmax and that acclimation capacity may be constrained at higher temperatures.
Keywords: heat, hardening, CTmax, developmental acclimation, adult acclimation, climate change
1. Introduction
With both mean temperature and extreme thermal events predicted to rise under climate change [1] increasing effort is being directed at better understanding and predicting species’ responses to climate change. Such efforts largely focus on characterizing ecologically meaningful measures of thermal tolerances (CTmin and CTmax) of ectothermic species [2–6]. However, estimates of upper thermal limits are often taken from individuals that are sampled directly from the field. This means that the thermal history of those individuals—that is, the physiological effects of the temperatures experienced in the field during development and as adults before collection—is not controlled or accounted for [5,7–9]. Yet, generally thermal tolerances respond plastically to changing thermal conditions [10,11]. Therefore, species collected from similar environments may be more similar in their thermal tolerances because of environmental, rather than innate, genetic effects. To account for the effects of thermal history, some researchers attempt to remove environmental effects via laboratory acclimation for varying lengths of time [8,12] or by collecting focal individuals within the same season [7] before assessing thermal limits. However, the extent to which such treatments do in fact erase the effects of thermal history remains largely unknown [13].
Prior exposure to sub-lethal temperature can result in plastic increases in thermal resistance in many ectothermic species [10,14–16]. Studies using an array of thermal treatments and exposure lengths show substantial plastic effects of temperature on thermal tolerance [10,17]. In Drosophila melanogaster the effects of adult acclimation/hardening on static heat resistance, and to a lesser degree developmental acclimation, have been well studied [10,18]; warmer developmental and adult acclimation and hardening treatments generally result in increased heat tolerance, although trade-offs between basal and heat hardening may limit the extent of the increase at warmer temperatures [18]. Similarly, seasonal shifts in temperature have also been shown to result in plastic shifts in thermal tolerance under conditions that reflect those experienced in the field [19], confirming the plastic nature of upper thermal limits. The fact that estimates of tolerance based on field-caught individuals do not always match estimates based on laboratory-reared individuals suggests complicated (plastic) effects of thermal history in flies [13] and other organisms [20].
Thermal environments that induce a plastic response can give rise to both reversible and irreversible plastic responses [21,22]. Exposure to temperature during development is predicted to produce permanent irreversible plastic responses (developmental acclimation) [23], while adult hardening (short exposures to stressful but sub-lethal temperature) and adult acclimation (longer exposures to less extreme conditions) should produce transient effects on the phenotype [14,24–26]. However, whether different forms of plasticity induce reversible or irreversible effects on upper thermal limits is not well studied in D. melanogaster or other species [27]. Further, attempts to do so have been complicated by studies using different methods that are not easily comparable. For example, acclimation treatments can encompass adult and/or developmental acclimation [10], which may produce short- and long-term effects on the phenotype [23]; yet many studies are not designed to explicitly disentangle these effects (see studies in [28]).
If developmental and adult acclimation responses to temperature result in irreversible and reversible effects on the phenotype, respectively (e.g. [21,22]) then estimates of thermal limits derived from field collected individuals will be confounded by their thermal history, thereby limiting the inferences that can be made with regards to the evolution of plasticity. While adult hardening seems to have no lasting effects on adult heat resistance or associated transcriptional responses in Drosophila [29,30], different combinations of adult acclimation treatments produced both reversible and irreversible effects on critical thermal limits in the Mediterranean fruit fly (Ceratitis capitata) [27,31]. For developmental acclimation, studies tend to suggest that its effects on thermal limits are irreversible [21,32–34]. Specifically, Chidawanyika & Terblanche [34] found developmental acclimation produced lasting effects on CTmax in the codling moth, while developmental acclimation produced irreversible effects on adult body size, morphology, and fitness traits in frogs [32] and zebrafish [33], and for lower thermal limits in adult tsetse flies [21]. However, in butterflies, the effects of developmental acclimation on egg size and recovery from cold shock were reversed by adult acclimation temperatures [35,36]. Finally, a recent study in D. melanogaster found that the effect of adult acclimation on CTmax was asymmetrical; CTmax was reversible (in the short term) after developmental acclimation at 15°C, but not developmental acclimation at 25°C [37].
In the current study we aim to investigate whether developmental and adult acclimation interact to influence estimates of CTmax and whether these forms of plasticity are reversible. We do so using a tropical and temperate population of the model species D. melanogaster, to determine whether the effect of developmental or adult acclimation, on CTmax differs across populations from different climatic zones. We address these questions by performing a partial reciprocal transplant experiment, where flies were developed at a range of temperatures and then acclimated as adults to either the same temperature at which they were developed or to 25°C and vice versa. In addition, given that some studies using field-collected [7,28] and laboratory-reared [18] individuals suggest that plasticity is reduced in warm environments possibly as a result of a trade-off between basal heat tolerance and plasticity, we also examined whether higher developmental acclimation temperatures constrain plastic responses of CTmax in adult acclimation treatments. Finally, the length of adult thermal acclimation used to remove any effects of thermal history on estimates of CTmax often varies across studies, ranging from days to weeks [28]. To determine whether the length of the adult acclimation period may influence the effects of developmental acclimation on CTmax we also examined both short and long adult acclimation periods of 5 and 23 days, respectively.
2. Material and methods
(a). Experimental populations and acclimation treatments
Tropical and temperate populations of Drosophila melanogaster were maintained as mass-bred populations at 25°C under a 12 : 12 light : dark cycle at a census population size of approximately 1 000 individuals across 3 × 250 ml bottles containing potato-dextrose-agar medium. CTmax was assessed after 11 (5-day acclimation) and 13 (23-day acclimation) generations of mass breeding. For further details of source location and initiation of experimental flies see electronic supplementary material, methods.
(i). Developmental acclimation
To distinguish between the effects of developmental acclimation and adult acclimation on CTmax we performed a partial reciprocal transplant experiment across different developmental and adult acclimation temperatures (electronic supplementary material, figure S1). To assess the effect of developmental acclimation on CTmax, eggs from both populations were placed at six constant temperatures to complete development: 16°C, 18°C, 20°C, 25°C, 28°C, and 30°C.
(ii). Adult acclimation and CTmax
Flies were placed into one of three adult acclimation treatments: (i) flies developed at 16°C, 18°C, 20°C, 25°C, 28°C, and 30°C and were acclimated as adults at the same six temperatures of development (16–30/16–30°C treatment), (ii) following development at 16°C, 18°C, 20°C, 25°C, 28°C, and 30°C flies were acclimated as adults at 25°C (16–30/25°C treatment), (iii) flies developed at 25°C and were acclimated as adults at all six temperatures 16°C, 18°C, 20°C, 25°C, 28°C, and 30°C (25/16–30°C treatment).
Following the acclimation periods, CTmax was assessed using the ramping method [38]. Thirty individual flies from each acclimation treatment were placed into a water bath pre-heated to 25°C and the temperature ramped up at a rate of 0.1°C per minute. CTmax was scored as the time/temperature at which flies had succumbed to heat stress and no movement was detected.
(b). Analyses
(i). Reaction norms
All analyses were conducted in R [39]. The effects of the different acclimation treatments were investigated in two separate analyses. The first analysis compared treatment 1 and 2 (16–30/16–30°C to 16–30/25°C) and the second analysis compared treatment 1 and 3 (16–30/16–30°C to 25/16–30°C). Thus we were effectively comparing how different developmental and adult acclimation regimes affected CTmax compared to the control treatment of developing and acclimating within the same temperature, i.e. 16–30/16–30°C. This created a balanced design that allowed us to test for an interaction between temperature and acclimation treatments. The data were analysed using a general linear model assessing the fixed effects of population, temperature (developmental or adult acclimation temperature), treatment (development or adult acclimation), and their interactions on CTmax. To account for possible technical effects of run, scorer, and day on CTmax we also included these variables as fixed factors in the model. The effects of 5- and 23-day adult acclimation on CTmax were investigated in separate analyses as these experiments were not performed at the same time. Day was only included as a factor for the 5-day model as all heat runs were performed on the same day for the 23-day experiment. Temperature was treated as a continuous variable and treatment as a factor. Model selection was performed to remove non-significant interaction terms from the full model, with model fit assessed using Akaike Information Criteria [40]. Interaction terms were removed until the model with the lowest AIC was found. Where the difference between models was less than 2.5 AIC we chose the simplest model [40]. Finally, we compared the best fit model with a model including no interaction terms (electronic supplementary material, table S1 for model AICs). Assumptions of parametric modelling were fulfilled in all models.
To determine whether developmental or adult acclimation had a larger influence on CTmax we compared the slopes of the reaction norms for each of the three treatments examining the relationship between CTmax and developmental or adult acclimation temperature (16°C, 18°C, 20°C, 25°C, 28°C, and 30°C). To compare the reaction norm slopes, within each treatment we fitted a general linear model with temperature as a continuous variable and run, scorer, and day as fixed effects (electronic supplementary material, table S2). Similar to above we performed a model selection approach, starting with the full model and removing non-significant terms comparing model fit with AIC. From these models we obtained the reaction norm slopes and their standard errors for each treatment. We then compared the reaction norm slopes between treatments with a t-test using the following formula [38].
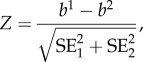 |
where b1 and b2 and 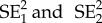 are the slope and respective standard errors for each of the regression lines.
are the slope and respective standard errors for each of the regression lines.
Similarly the t-tests from the model output were used to determine whether the reaction norm slopes were significantly different from 0. For some treatments a quadratic fit was a better fit than a linear fit (3 of 12 treatments), however because comparing the slope of a linear and quadratic regression is not possible we assumed a linear fit for all comparisons (electronic supplementary material, table S3).
A false discovery rate (FDR) was used to correct for multiple comparisons [41]. Using this method we were able to compare how the reaction norm slopes changed across the treatments to determine whether developmental or adult acclimation had lasting effects on CTmax. We compared the reaction norms for 16–30/16–30°C to 16–30/25°C and 25/16–30°C. If any period of adult acclimation reverses the effects of developmental acclimation, we predict that the reaction norm for treatment 25/16–30°C would be most similar to the 16–30/16–30°C reaction norm, and the reaction norm for 16–30/25°C should be flat and not significantly different from 0 (Prediction 1). In contrast, if developmental acclimation is not reversed by adult acclimation we expect the reaction norm for treatments 16–30/16–30°C and 16–30/25°C to be most similar, and the reaction norm for the 25/16–30°C treatment to be flat and not significantly different from 0 (Prediction 2).
(ii). Developmental and adult acclimation capacity
To examine whether the extent of plastic responses in CTmax may be constrained by innate (basal) heat tolerance we first quantified the extent of plastic response in CTmax as acclimation capacity (AC). Specifically, acclimation capacity was calculated as the difference in CTmax between the development and adult acclimation treatments (16–30/25°C – 16–30/16–30°C and 25/16–30°C – 16–30/16–30°C). Estimates of CTmax of flies developed and adult acclimated at 16–30/16–30°C represents a combination of adult and developmental effects of each temperature; CTmax of flies developed and adult acclimated at 16–30/25°C reflects developmental acclimation while controlling for adult acclimation and finally, the CTmax of flies developed and acclimated at 25/16–30°C represents adult acclimation while controlling for developmental acclimation.
To examine the extent to which adult acclimation can shift CTmax (AC1), we calculated the difference between CTmax in the 16–30/25°C treatment versus CTmax in the 16–30/16–30°C treatment, individually at each acclimation temperature (e.g. 1625–1616, 1825–1818). To examine the extent to which developmental acclimation can shift CTmax alone (AC2), we calculated the difference between CTmax at the 25/16–30°C and 16–30/16–30°C treatments, individually at each acclimation temperature (e.g. 2516–1616, 2518–1818). Calculating AC for the 25°C temperature did not make sense as this was the control temperature and therefore AC was 0.
To examine how AC shifted as temperatures increased, we regressed AC onto acclimation temperatures using a linear model in R [39]. We did not regress AC back onto CTmax, as this would violate one of the major assumptions of linear models, that is, of independence. This is because CTmax is used to estimate AC, and regressing AC against CTmax will thus result in significant but spurious relationships between AC and CTmax [42]. Finally, as the 5- and 23-day acclimation treatments produced comparable results, we provide the analysis of acclimation capacity using the 5-day data only.
3. Results
(a). 5-Day adult acclimation
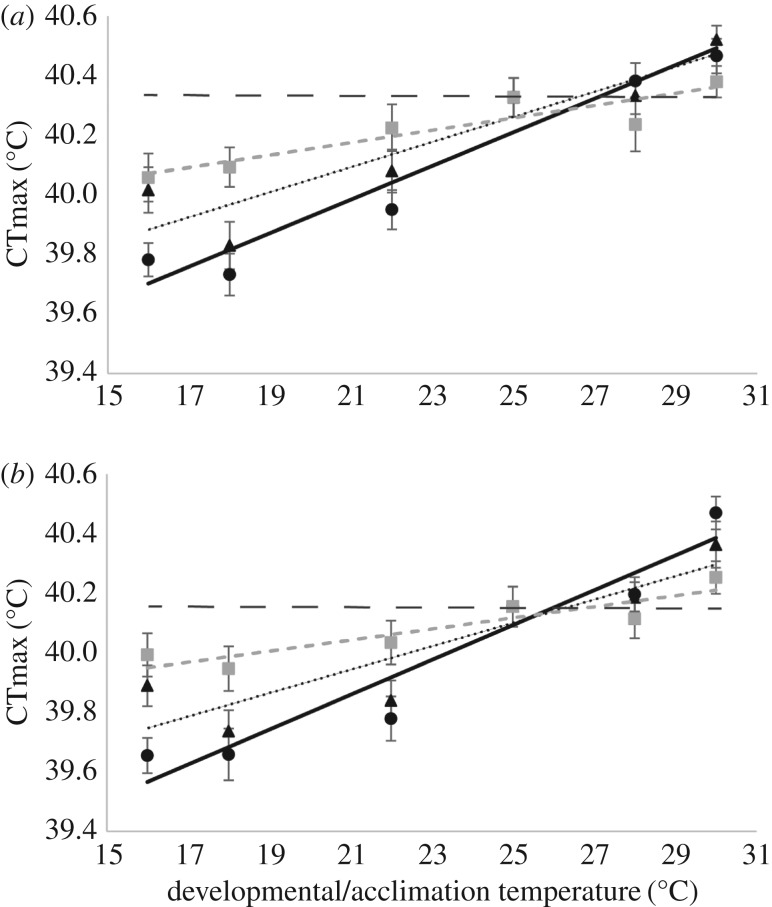
Regardless of which comparisons were performed (16–30/16–30°C versus 16–30/25°C or 16–30/16–30°C versus 25/16–30°C) CTmax was significantly higher in the tropical population (table 1a; figure 1a), although the difference in mean CTmax between the populations was small (across all treatments 0.14°C). Scorer did not always have a significant effect on CTmax, while day and run were both significant (table 1). Irrespective of whether we compared the 16–30/16–30°C treatment to the 16–30/25°C or 25/16–30°C treatments, we found that acclimation temperature, be that developmental or adult had a significant effect on the mean of CTmax—CTmax increased as acclimation temperature increased (table 1; figure 1). The increase in CTmax across acclimation temperatures was small and typically less than 1°C (figure 1). Treatment (development versus adult acclimation) also had a significant effect on CTmax regardless of the comparison being made (table 1). The significant temperature×treatment interaction results from the fact that developmental acclimation resulted in larger shifts in the mean of CTmax than adult acclimation when treatments 2 and 3 were compared (0.62–0.69°C, treatment 1: 16–30/25°C c.f. 0.31–0.32°C, treatment 2: 25/16–30°C; figure 1), however the largest increase in mean CTmax was observed for treatment 3 (16–30/16–30°C treatment, 0.73–0.84°C). We found significant two-way interactions between temperature and the technical effects (run, day, and scorer). However, we found no significant higher order interactions indicating that the temperature × treatment interaction was robust to these technical effects.
Table 1.
Analysis of variance examining the effects of temperature (development and adult acclimation) on flies developed and adult acclimated under three different treatments 16–30/16–30°C, 16–30/25°C, and 25/16–30°C on CTmax for northern and southern populations of D. melanogaster and adult acclimated for 5 days.
| treatment | fixed effects | d.f. | MS | F |
|---|---|---|---|---|
| 16–30/16–30 versus 16–30/25 | population | 1 | 3.483 | 37.301*** |
| temperature (acclimation) | 1 | 43.119 | 461.827*** | |
| treatment | 1 | 0.655 | 7.020** | |
| run | 2 | 10.594 | 113.466*** | |
| scorer | 2 | 0.446 | 4.779** | |
| day | 2 | 3.064 | 32.817*** | |
| temperature × treatment | 1 | 1.417 | 15.181*** | |
| temperature × scorer | 2 | 0.343 | 3.673* | |
| temperature × day | 2 | 0.906 | 9.702*** | |
| error | 693 | 0.093 | ||
| 16–30/16–30 versus 25/16–30 | population | 1 | 3.115 | 33.075*** |
| temperature (acclimation) | 1 | 26.542 | 281.804*** | |
| treatment | 1 | 1.991 | 21.135*** | |
| run | 2 | 11.574 | 122.879*** | |
| day | 2 | 3.698 | 39.260*** | |
| temperature × treatment | 1 | 6.158 | 65.379*** | |
| temperature × day | 2 | 0.725 | 7.693*** | |
| error | 698 | 0.094 |
***p < 0.001, **p < 0.001, *p < 0.01.
Figure 1.
5-Day adult acclimation for (a) tropical and (b) temperate populations of D. melanogaster. Solid black line (circles) flies developed 16–30°C and acclimated as adults at 16–30°C (16–30/16–30°C), dotted black line (triangle) flies developed at 16–30°C and acclimated as adults at 25°C (16–30/25°C) and dashed grey line (square) flies developed at 25°C and acclimated as adults at 16–30°C (25/16–30°C). Dashed black line represents 25°C/25°C.
Our results indicate that developmental acclimation had lasting effects on CTmax regardless of adult acclimation (figure 1). The slope of the reaction norm for flies developed at 16–30°C and adult acclimated at 25°C (16–30/25°C) was not significantly different from the reaction norm for flies that developed and adult acclimated at 16–30°C (16–30/16–30°C) for the tropical population (table 2; figure 1a), suggesting that adult acclimation at 25°C did not have a large effect on CTmax. Interestingly, this was not the case for the temperate population where the reaction norm slopes for the 16–30/25°C and 16–30/16–30°C treatments were significantly different from each other (table 2; figure 1b). However, the CTmax of flies in the 16–30/25°C treatment were not more similar to the CTmax of the 25/25°C treatment, which we would expect if adult acclimation had a larger effect on CTmax than developmental acclimation. Instead the reaction norm slope fell between treatments 16–30/16–30°C and 25/25°C suggesting both developmental and adult acclimation contributed to CTmax (figure 1b). Finally, if adult acclimation erased the effects of developmental acclimation on CTmax we would expect the CTmax of flies in treatment 25/16–30°C to resemble the CTmax of flies in the 16–30/16–30°C treatment, and the reaction norm slopes of these two treatments to be the same. However, the reaction norm slopes for these two treatments for both populations were significantly different from each other (table 2) suggesting that developmental acclimation has lasting effects on CTmax.
Table 2.
t-tests comparing whether the reaction norm slope for each of the three treatments differs significantly from 0 and from each other for the tropical and temperate populations of D. melanogaster and whether the results fit with Prediction 1 that adult acclimation has lasting effects on CTmax (P.1), or Prediction 2 that developmental acclimation has lasting effects on CTmax (P.2).
| comparison to 0 |
comparison to each other |
|||||||
|---|---|---|---|---|---|---|---|---|
| treatment | slope | t | P.1 | P.2 | treatment | t | P.1 | P.2 |
| 5 days—tropical | ||||||||
| 16–30/25 | 0.042 ± 0.006*** | 7.848 | 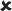 |
✓ | 16–30/16–30 versus 16–30/25 | 1.921 | 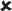 |
✓ |
| 25/16–30 | 0.021 ± 0.006*** | 3.747 | ✓ | 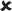 |
16–30/16–30 versus 25/16–30 | 4.481** | 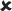 |
✓ |
| 5 days—temperate | ||||||||
| 16–30/25 | 0.041 ± 0.006*** | 7.496 | 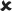 |
✓ | 16–30/16–30 versus 16–30/25 | 2.121* | ✓ | 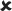 |
| 25/16–30 | 0.020 ± 0.005*** | 3.844 | ✓ | 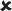 |
16–30/16–30 versus 25/16–30 | 4.993** | 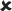 |
✓ |
| 23 days—tropical | ||||||||
| 16–30/25 | 0.006 ± 0.006 | 1.046 | ✓ |  |
16–30/16–30 versus 16–30/25 | 2.60* | ✓ | 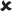 |
| 25/16–30 | 0.005 ± 0.007 | 0.683 | 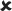 |
✓ | 16–30/16–30 versus 25/16–30 | 2.539* | 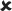 |
✓ |
| 23 days—temperate | ||||||||
| 16–30/25 | 0.021 ± 0.005*** | 4.251 | 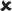 |
✓ | 16–30/16–30 versus 16–30/25 | 2.234* | ✓ |  |
| 25/16–30 | −0.005 ± 0.006 | 0.819 | 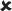 |
✓ | 16–30/16–30 versus 25/16–30 | 3.606** | 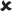 |
✓ |
***p < 0.001, **p < 0.001, *p < 0.01. p-values corrected for multiple comparison using a false discovery rate (FDR).
Adult acclimation did not erase previous thermal history; rather it contributed to an increase in CTmax with increasing temperature for both populations (figure 1). The reaction norms for the 16–30/25°C and 25/16–30°C treatments were significantly different from zero in both populations (table 2), meaning that both developmental and adult acclimation played a role in shaping CTmax across treatments.
(b). 23-Day adult acclimation
Overall, the results from the 23-day adult acclimation experiment support the general conclusion that developmental acclimation plays a larger role in shaping CTmax than adult acclimation, although the results did not parallel those seen for the 5-day acclimation experiment. Unlike the 5-day treatment, populations did not differ in their CTmax (table 3; figure 2). Run was significant in both models while scorer was significant for only the 16–30/16–30 versus 16–30/25 (table 3) (same scorers were used for both experiments). Similar to the 5-day experiment, increasing developmental and adult acclimation temperatures significantly increased CTmax for both populations (figure 2; table 3). Finally, a significant interaction between acclimation temperature and treatment was also found, driven by the fact that the 16–30/16–30 treatment resulted in the largest shift in CTmax across both populations (figure 2a,b). Once again, we found significant two-way interactions between the main effects of temperature and population and the technical effects (run, day, and scorer). However, no higher order interactions were significant suggesting that the temperature x treatment interaction was robust to these technical effects.
Table 3.
Analysis of variance examining the effects of temperature (development and adult acclimation) on flies developed and adult acclimated under three different treatments 16–30/16–30°C, 16–30/25°C, and 25/16–30°C on CTmax for northern and southern populations of D. melanogaster and adult acclimated for 23 days.
| treatment | fixed effects | d.f | MS | F |
|---|---|---|---|---|
| 16–30/16–30 versus 16–30/25 | population | 1 | 0.229 | 1.069 |
| temperature (acclimation) | 1 | 12.482 | 58.210*** | |
| treatment | 1 | 5.943 | 27.715*** | |
| run | 4 | 2.106 | 9.823*** | |
| scorer | 3 | 0.580 | 2.704* | |
| temperature × treatment | 1 | 2.548 | 11.883*** | |
| temperature × run | 4 | 1.444 | 6.735*** | |
| population × scorer | 3 | 0.728 | 3.395* | |
| error | 685 | 0.214 | ||
| 16–30/16–30 versus 25/16–30 | population | 1 | 0.299 | 1.186 |
| temperature | 1 | 6.555 | 26.019*** | |
| treatment | 1 | 8.327 | 33.055*** | |
| run | 4 | 2.303 | 9.142*** | |
| scorer | 3 | 0.472 | 1.874 | |
| temperature × treatment | 1 | 6.378 | 25.315*** | |
| temperature × run | 4 | 1.648 | 6.541*** | |
| temperature × scorer | 3 | 1.030 | 4.087** | |
| error | 682 | 0.252 |
Figure 2.
23-Day adult acclimation for (a) tropical and (b) temperate populations of D. melanogaster. Solid black line (circle) flies developed at 16–30°C and acclimated at 16–30°C (16–30/16–30°C), dotted black line (triangle) flies developed at 16–30°C and acclimated at 25°C (16–30/25°C), and dashed grey line (square) flies developed at 25°C and acclimated at 16–30°C (25/16–30°C). Dashed black line represents 25°C/25°C.
In contrast to the 5-day adult acclimation treatment, the CTmax of tropical flies developed at 16–30°C and adult acclimated at 25°C (16–30/25°C) resembled the CTmax of flies developed and adult acclimated at 25°C (25/25°C); that is, the reaction norm slope for 16–30/16/30°C did not differ significantly from 0 (table 2; figure 2a). This suggests that adult acclimation at 25°C for 23 days negated the effects of developmental acclimation on CTmax in tropical flies. However, the reaction norm slope for tropical flies developed at 25°C and adult acclimated at 16–30°C (25/16–30°C) was also not significantly different from 0 (table 2; figure 2a)—if adult acclimation had a larger effect on CTmax than developmental acclimation we would expect CTmax to increase with increasing adult acclimation temperatures regardless of developmental temperature and would thus observe a significant reaction norm slope for this treatment (i.e. the reaction norm slope should be more similar to the 16–30/16–30°C treatment). Instead there was little evidence for adult acclimation effects on CTmax (table 2; figure 2a). Rather, the results for the tropical population suggest that longer term adult acclimation and developmental acclimation interact in complex ways to affect CTmax.
In contrast, there was stronger support for developmental acclimation having a large influence on CTmax in the temperate population (table 2). Specifically, the reaction norm slope of the 16–30°C/25°C treatment was significantly different from 0 (figure 2b; table 2), which is not consistent with our expected response if adult acclimation predominately drove CTmax. In addition, the CTmax of flies in the 25°C/16–30°C treatment were more similar to the CTmax of flies in the 25°C/25°C treatment (25°C/16–30°C reaction norm slope not significantly different from zero) consistent with developmental acclimation having a larger effect on CTmax than adult acclimation (table 2; figure 2b). These results suggest population-specific effects of longer term adult acclimation on estimates of CTmax.
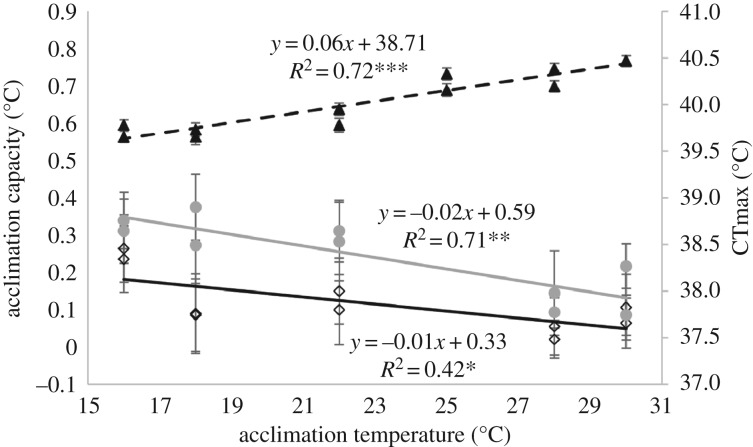
Finally, we found that acclimation capacity for developmental and/or adult acclimation decreased with increasing temperature (figure 3). This suggests that the capacity to increase CTmax via plasticity decreases with increasing temperature. In contrast, CTmax increased with developmental temperature, suggesting that increases in plastic responses to adult and developmental thermal acclimation may be constrained by higher basal CTmax at warmer temperatures, although the current experimental design does not allow us to statistically test this relationship.
Figure 3.
Relationship between acclimation capacity, CTmax, and acclimation temperature for AC1: adult acclimation (diamonds), AC2: developmental acclimation (circles) and CTmax (triangles). Error bars represent standard errors. *p < 0.05, **p < 0.01, ***p < 0.001.
4. Discussion
Studies that seek to characterize thermal limits and thermal plasticity on non-model organisms predominately use estimates of thermal limits (generally measured as CTmax) from field individuals [2,5,7,28,43,44], even though thermal tolerances are highly plastic [11]. Such studies assume that short acclimation periods in the laboratory will remove any effects of thermal history (developmental and/or acclimation) on CTmax, or that thermal history has no effect on estimates of CTmax. While thermal tolerances can shift when field collected individuals experience different adult acclimation conditions in the laboratory [28], the extent to which thermal history influences acclimation responses is poorly understood. The current study investigates the relative contributions of developmental and adult acclimation to upper thermal limits in D. melanogaster to determine the degree to which the effects of developmental and adult acclimation on estimates of upper thermal limits are reversible.
Irrespective of the length of adult acclimation (5 or 23 days), developmental acclimation had lasting effects on upper thermal limits for both populations. Altering the length of adult acclimation from 5 to 23 days changed the patterns in CTmax between the treatments and populations but did not change the overall conclusion that developmental acclimation tended to produce irreversible effects on CTmax in D. melanogaster, in line with current theoretical predictions [23]. This result was consistent across populations, with the tropical and temperate populations showing similar responses to developmental and adult acclimation. Although a lack of divergence in CTmax between temperate and tropical populations is not consistent with clinal studies that have shown clinal patterns in heat [45,46], it may simply reflect the limited power of detecting divergence in traits with only two populations. A lack of divergence in CTmax and plasticity in these populations may also reflect convergent evolution driven by laboratory rearing. However, it is unclear how laboratory adaptation will affect CTmax and its plasticity, and there is no evidence of laboratory adaptation influencing estimates of heat resistance in Drosophila species [47].
Our knowledge of the mechanisms underlying plasticity in heat resistance and the timescales at which they work is mostly restricted to heat shock proteins (hsps) and short-term plastic responses (hardening). In D. melanogaster, the adult hardening response is reversible at both the molecular and phenotypic level [10,48]. However acclimation responses, be that developmental or adult, are less well studied at the mechanistic level. Once again hsps have been implicated in adult acclimation responses but little is known about the timing and reversibility of these responses [49], although cellular membrane phospholipids have been associated with thermal acclimation and cold resistance [50–52]. Developmental acclimation can also induce widespread transcriptome changes, with 80% of genes showing an effect of acclimation in D. melanogaster [53], but these changes were not linked to heat resistance phenotypes or studied through time. Widespread transcriptome changes [53] may be consistent with our results that show increasing developmental temperatures increased CTmax more so than increasing adult acclimation temperatures, but further work is needed to understand the timing and reversibility of acclimation responses (but see, [27,31,37]).
We found that any improvement to CTmax via adult acclimation occurred within the first 5 days and that longer adult acclimation periods did not further increase CTmax, consistent with recent research [27,37]. Longer acclimation periods are therefore unlikely to erase the effects of thermal history on CTmax in short-lived species like D. melanogaster. We did however find that the patterns of acclimation responses between the 5- and 23-day treatments did differ. Adult acclimation may account for some of this change, but CTmax did not always shift in the direction that would be expected if just adult acclimation could account for these differences. Age effects are known to contribute to declining stress resistance in Drosophila [54]. Unfortunately, we could not explicitly test for age effects as the experiments were run in separate generations, making the experiments not directly comparable. However, we do not believe age effects contributed largely to the observed patterns in the 23-day treatment as the CTmax of flies reared and developed at 16–30°C did not differ between the 5- and 23-day experiments. This result is in stark contrast to a recent study [55] where age effects were implicated in declines of upwards of 4°C in CTmax when flies were developed and acclimated at 25°C for 24 days. Nevertheless, an interaction between developmental acclimation, adult acclimation, and age cannot be ruled out with the current design and it is possible age effects contribute to the complex relationship between developmental and adult acclimation reported here. Further work investigating the effects of age on plasticity is warranted, particularly as age effects will be inherent in any study on field individuals and may further obscure the effects of acclimation [56].
The significant interaction between adult and developmental acclimation in the current study suggests that developmental temperature, and thus thermal history can influence the capacity for adult acclimation. Moreover, estimates of acclimation capacity/plasticity from field individuals [7,28] likely underestimate the capacity for plastic responses in species because developmental acclimation is largely ignored. The effects of thermal history, may also be compounded if thermal plastic responses trade-off with basal thermal tolerances [7,18]. In line with van Heewaarden et al. [18] we found that acclimation capacity for developmental and/or adult acclimation decreased with increasing temperature (figure 3) suggesting that the capacity to increase CTmax via plasticity decreases with increasing temperature. In contrast, CTmax increased with developmental temperature, suggesting that increases in adult and developmental acclimation capacity may come at a cost of high basal CTmax at warmer temperatures (figure 3). Moreover, the relationship between acclimation capacity and temperature means that estimates of plasticity/acclimation responses taken from field individuals will be inherently biased by temperatures at the source location; that is, individuals collected from warm environments will have a higher basal CTmax and a lower acclimation capacity than individuals collected from cool environments.
Developmental and adult acclimation effects on CTmax were small (less than 1°C), consistent with previous results using Drosophila [18,57,58]. Although acclimation effects of less than 1°C may not dramatically alter estimates of climate change risk under current models, whether a 0.5°C change in heat tolerance may be important in the short term is less clear. Current projections of climate change responses tend to focus on long-term effects; however, increases in temperature will be incremental [59]. As such small acclimation effects may be enough to buffer species in the short term and provide opportunity for selection to shift trait means [60]. Moreover, Bush et al. [61] showed an evolved shift of only 0.5°C has the potential to reduce projected range losses in Drosophila by up to 33% by 2105, which suggests that small plastic responses may contribute significantly to reducing the impact of climate change risk.
In the current study we demonstrate that the effects of developmental temperatures on upper thermal limits are larger, and more lasting, than adult acclimation effects in the model species D. melanogaster. Given that the heat shock response is quite conserved across ectothermic species [62], these results suggest that CTmax measured on field-caught individuals may be confounded by thermal history. An interaction between developmental and adult acclimation temperatures presented here and in previous studies [32,33] could further complicate the interpretation of plasticity estimates based on field collected individuals, particularly as developmental acclimation had a larger effect on CTmax than adult acclimation. In addition, we show that acclimation capacity (developmental/adult) decreases with increasing temperature suggesting a trade-off between plasticity in CTmax and basal CTmax. Having said that, it is important to acknowledge that conducting controlled common garden experiments on laboratory-reared individuals to explicitly eliminate/untangle genetic and plastic effects is simply not possible for many species. Nevertheless, we need to be aware of the potential error thermal history may introduce into estimates of critical thermal limits and be careful how we interpret estimates of plasticity from field-caught individuals.
Supplementary Material
Acknowledgements
We like to thank Fiona Cockerell, Katherine Sutton, Lindsey Heffernan, Florencia Camus, Clementine Lasne, Allannah Clemson, Marina Telonis-Scott, and Tarmo Ketola for technical support.
Authors' contributions
V.K., B.v.H., and C.M.S. designed the experiment; V.K. and B.v.H. performed the experiments; C.M.S. provided equipment for the experiments; V.K., B.v.H., and C.M.S. wrote the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
We thank the Australian Research Council for funding through their discovery schemes to V.K., B.v.H., and C.M.S., the Science and Industry Endowment Fund for funding to C.M.S. and Monash University for financial support to V.K., B.v.H., and C.M.S.
References
- 1.IPCC. 2014. Climate change 2014: mitigation of climate change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addo-Bediako A, Chown SL, Gaston KJ. 2000. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B 267, 739–745. ( 10.1098/rspb.2000.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kellermann V, Overgaard J, Hoffmann AA, Flojgaard C, Svenning JC, Loeschcke V. 2012. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc. Natl Acad. Sci. USA 109, 16 228–16 233. ( 10.1073/pnas.1207553109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond SE, Sorger DM, Hulcr J, Pelini SL, Del Toro I, Hirsch C, Oberg E, Dunn RR. 2012. Who likes it hot? A global analysis of the climatic, ecological, and evolutionary determinants of warming tolerance in ants. Glob. Change Biol. 18, 448–456. ( 10.1111/j.1365-2486.2011.02542.x) [DOI] [Google Scholar]
- 6.Stevenson RD. 1985. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am. Nat. 126, 362–386. ( 10.1086/284423) [DOI] [Google Scholar]
- 7.Stillman JH. 2003. Acclimation capacity underlies susceptibility to climate change. Science 301, 65 ( 10.1126/science.1083073) [DOI] [PubMed] [Google Scholar]
- 8.Magozzi S, Calosi P. 2015. Integrating metabolic performance, thermal tolerance, and plasticity enables for more accurate predictions on species vulnerability to acute and chronic effects of global warming. Glob. Change Biol. 21, 181–194. ( 10.1111/gcb.12695) [DOI] [PubMed] [Google Scholar]
- 9.Artacho P, Saravia J, Perret S, Bartheld JL, Le Galliard JF. 2017. Geographic variation and acclimation effects on thermoregulation behavior in the widespread lizard Liolaemus pictus. J. Therm. Biol. 63, 78–87. ( 10.1016/j.jtherbio.2016.11.001) [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann AA, Sorensen JG, Loeschcke V. 2003. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J. Therm. Biol. 28, 175–216. ( 10.1016/S0306-4565(02)00057-8) [DOI] [Google Scholar]
- 11.Angilletta MJ. 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Slabber S, Worland MR, Leinaas HP, Chown SL. 2007. Acclimation effects on thermal tolerances of springtails from sub-Antarctic Marion Island: indigenous and invasive species. J. Insect Physiol. 53, 113–125. ( 10.1016/j.jinsphys.2006.10.010) [DOI] [PubMed] [Google Scholar]
- 13.Schiffer M, Hangartner S, Hoffmann AA. 2013. Assessing the relative importance of environmental effects, carry-over effects and species differences in thermal stress resistance: a comparison of Drosophilids across field and laboratory generations. J. Exp. Biol. 216, 3790–3798. ( 10.1242/jeb.085126) [DOI] [PubMed] [Google Scholar]
- 14.Cossins AR, Bowler K. 1987. Temperature biology of animals. New York, NY: Chapman and Hall. [Google Scholar]
- 15.Leroi AM, Bennett AF, Lenski RE. 1994. Temperature acclimation and competitive fitness—an experimental test of the beneficial acclimation assumption. Proc. Natl Acad. Sci. USA 91, 1917–1921. ( 10.1073/pnas.91.5.1917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huey RB, Berrigan D, Gilchrist GW, Herron JC. 1999. Testing the adaptive significance of acclimation: a strong inference approach. Am. Zool. 39, 323–336. ( 10.1093/icb/39.2.323) [DOI] [Google Scholar]
- 17.Chown SL, Slabber S, McGeoch MA, Janion C, Leinaas HP. 2007. Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc. R. Soc. B 274, 2531–2537. ( 10.1098/rspb.2007.0772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Heerwaarden B, Kellermann V, Sgro CM. 2016. Limited scope for plasticity to increase upper thermal limits. Funct. Ecol. 30, 1947–1956. ( 10.1111/1365-2435.12687) [DOI] [Google Scholar]
- 19.Hoffmann AA, Shirriffs J, Scott M. 2005. Relative importance of plastic vs genetic factors in adaptive differentiation: geographical variation for stress resistance in Drosophila melanogaster from eastern Australia. Funct. Ecol. 19, 222–227. ( 10.1111/j.1365-2435.2005.00959.x) [DOI] [Google Scholar]
- 20.Terblanche J, Klok CJ, Krafsur ES, Chown SL. 2006. Phenotypic plasticity and geographic variation in thermal tolerance and water loss of the tsetse Glossina pallidipes (Diptera: Glossinidae): implications for distribution modelling (vol. 74, pg 786, 2006). Am. J. Trop. Med. Hyg. 75, 186–186. [PMC free article] [PubMed] [Google Scholar]
- 21.Terblanche JS, Chown SL. 2006. The relative contributions of developmental plasticity and adult acclimation to physiological variation in the tsetse fly, Glossina pallidipes (Diptera, Glossinidae). J. Exp. Biol. 209, 1064–1073. ( 10.1242/jeb.02129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khazaeli AA, Tatar M, Pletcher SD, Curtsinger JW. 1997. Heat-induced longevity extension in Drosophila. 1. Heat treatment, mortality, and thermotolerance. J. Gerontol. A Biol. Sci. Med. Sci. 52, B48–B52. ( 10.1093/gerona/52A.1.B48) [DOI] [PubMed] [Google Scholar]
- 23.Piersma T, Drent J. 2003. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 18, 228–233. ( 10.1016/s0169-5347(03)00036-3) [DOI] [Google Scholar]
- 24.Lindquist S. 1986. The heat-shock response. Annu. Rev. Biochem. 55, 1151–1191. ( 10.1146/annurev.bi.55.070186.005443) [DOI] [PubMed] [Google Scholar]
- 25.Gabriel W. 2006. Selective advantage of irreversible and reversible phenotypic plasticity. Arch. Hydrobiol. 167, 1–20. ( 10.1127/0003-9136/2006/0167-0001) [DOI] [Google Scholar]
- 26.Sgro CM, Terblanche JS, Hoffmann AA. 2016. What can plasticity contribute to insect responses to climate change? Annu. Rev. Entomol. 61, 433–451. ( 10.1146/annurev-ento-010715-023859) [DOI] [PubMed] [Google Scholar]
- 27.Weldon CW, Terblanche JS, Chown SL. 2011. Time-course for attainment and reversal of acclimation to constant temperature in two Ceratitis species. J. Therm. Biol. 36, 479–485. ( 10.1016/j.jtherbio.2011.08.005) [DOI] [Google Scholar]
- 28.Gunderson AR, Stillman JH. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401 ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krebs RA, Loeschcke V. 1995. Resistance to thermal stress in adult Drosophila buzzatii: acclimation and variation among populations. Biol. J. Linn. Soc. 56, 505–515. ( 10.1111/j.1095-8312.1995.tb01107.x) [DOI] [Google Scholar]
- 30.Telonis-Scott M, Clemson AS, Johnson TK, Sgro CM. 2014. Spatial analysis of gene regulation reveals new insights into the molecular basis of upper thermal limits. Mol. Ecol. 23, 6135–6151. ( 10.1111/mec.13000) [DOI] [PubMed] [Google Scholar]
- 31.Nyamukondiwa C, Terblanche JS. 2010. Within-generation variation of critical thermal limits in adult Mediterranean and Natal fruit flies Ceratitis capitata and Ceratitis rosa: thermal history affects short-term responses to temperature. Physiol. Entomol. 35, 255–264. ( 10.1111/j.1365-3032.2010.00736.x) [DOI] [Google Scholar]
- 32.Watkins TB, Vraspir J. 2006. Both incubation temperature and posthatching temperature affect swimming performance and morphology of wood frog tadpoles (Rana sylvatica). Physiol. Biochem. Zool. 79, 140–149. ( 10.1086/498182) [DOI] [PubMed] [Google Scholar]
- 33.Schaefer J, Ryan A. 2006. Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. J. Fish Biol. 69, 722–734. ( 10.1111/j.1095-8649.2006.01145.x) [DOI] [Google Scholar]
- 34.Chidawanyika F, Terblanche JS. 2011. Costs and benefits of thermal acclimation for codling moth, Cydia pomonella (Lepidoptera: Tortricidae): implications for pest control and the sterile insect release programme. Evol. Appl. 4, 534–544. ( 10.1111/j.1752-4571.2010.00168.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeilstra I, Fischer K. 2005. Cold tolerance in relation to developmental and adult temperature in a butterfly. Physiol. Entomol. 30, 92–95. ( 10.1111/j.0307-6962.2005.00430.x) [DOI] [Google Scholar]
- 36.Fischer K, Eenhoorn E, Bot ANM, Brakefield PM, Zwaan BJ. 2003. Cooler butterflies lay larger eggs: developmental plasticity versus acclimation. Proc. R. Soc. Lond. B 270, 2051–2056. ( 10.1098/rspb.2003.2470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slotsbo S, Schou MF, Kristensen TN, Loeschcke V. 2016. Reversibility of developmental heat and cold plasticity is asymmetric and has long lasting consequences for adult thermal tolerance. J. Exp. Biol. 219, 2726–2732. ( 10.1242/jeb.143750) [DOI] [PubMed] [Google Scholar]
- 38.Cohen P, West SG, Aiken LS. 2003. Applied multiple regression/correlation analysis for behavioural sciences. NJ: Lawrence Erlbaum Associates. [Google Scholar]
- 39.R Core Team. 2014. R: a language and environment for statistical computing.
- 40.Burnham KP, Anderson DR. 2004. Multimodel inference—understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304. ( 10.1177/0049124104268644) [DOI] [Google Scholar]
- 41.Benjamini Y, Yekutieli D. 2001. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 29, 1165–1188. ( 10.1214/aos/1013699998) [DOI] [Google Scholar]
- 42.Kelly C, Price TD. 2005. Correcting for regression to the mean in behavior and ecology. Am. Nat. 166, 700–707. ( 10.1086/497402) [DOI] [PubMed] [Google Scholar]
- 43.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calosi P, Bilton DT, Spicer JI, Votier SC, Atfield A. 2010. What determines a species' geographical range? Thermal biology and latitudinal range size relationships in European diving beetles (Coleoptera: Dytiscidae). J. Anim. Ecol. 79, 194–204. ( 10.1111/j.1365-2656.2009.01611.x) [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann AA, Anderson A, Hallas R. 2002. Opposing clines for high and low temperature resistance in Drosophila melanogaster. Ecol. Lett. 5, 614–618. ( 10.1046/j.1461-0248.2002.00367.x) [DOI] [Google Scholar]
- 46.Sgro CM, Overgaard J, Kristensen TN, Mitchell KA, Cockerell FE, Hoffmann AA. 2010. A comprehensive assessment of geographic variation in heat tolerance and hardening capacity in populations of Drosophila melanogaster from eastern Australia. J. Evol. Biol. 23, 2484–2493. ( 10.1111/j.1420-9101.2010.02110.x) [DOI] [PubMed] [Google Scholar]
- 47.Griffiths JA, Schiffer M, Hoffmann AA. 2005. Clinal variation and laboratory adaptation in the rainforest species Drosophila birchii for stress resistance, wing size, wing shape and development time. J. Evol. Biol. 18, 213–222. ( 10.1111/j.1420-9101.2004.00782.x) [DOI] [PubMed] [Google Scholar]
- 48.Clemson AS, Sgro CM, Telonis-Scott M. 2016. Thermal plasticity in D. melanogaster from eastern Australia: quantitative traits to transcripts. J. Evol. Biol. 29, 2447–2463. ( 10.1111/jeb.12969) [DOI] [PubMed] [Google Scholar]
- 49.Colinet H, Overgaard J, Com E, Sorensen JG. 2013. Proteomic profiling of thermal acclimation in Drosophila melanogaster. Insect Biochem. Mol. Biol. 43, 352–365. ( 10.1016/j.ibmb.2013.01.006) [DOI] [PubMed] [Google Scholar]
- 50.Hazel JR. 1995. Thermal adaptation in biological membranes—is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 57, 19–42. ( 10.1146/annurev.ph.57.030195.000315) [DOI] [PubMed] [Google Scholar]
- 51.Overgaard J, Tomcala A, Sorensen JG, Holmstrup M, Krogh PH, Simek P, Kostal V. 2008. Effects of acclimation temperature on thermal tolerance and membrane phospholipid composition in the fruit fly Drosophila melanogaster. J. Insect Physiol. 54, 619–629. ( 10.1016/j.jinsphys.2007.12.011) [DOI] [PubMed] [Google Scholar]
- 52.Cooper BS, Hammad LA, Fisher NP, Karty JA, Montooth KL. 2012. In a variable thermal environment selection favors greater plasticity of cell membranes in Drosophila melanogaster. Evolution 66, 1976–1984. ( 10.1111/j.1558-5646.2011.01566.x) [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Nolte V, Schlotterer C. 2015. Temperature related reaction norms of gene expression: regulatory architecture and functional implications. Mol. Biol. Evol. 32, 2393–2402. ( 10.1093/molbev/msv120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colinet H, Siaussat D, Bozzolan F, Bowler K. 2013. Rapid decline of cold tolerance at young age is associated with expression of stress genes in Drosophila melanogaster. J. Exp. Biol. 216, 253–259. ( 10.1242/jeb.076216) [DOI] [PubMed] [Google Scholar]
- 55.Schou MF, Kristensen TN, Kellermann V, Schloetterer C, Loeschcke V. 2014. A Drosophila laboratory evolution experiment points to low evolutionary potential under increased temperatures likely to be experienced in the future. J. Evol. Biol. 27, 1859–1868. ( 10.1111/jeb.12436) [DOI] [PubMed] [Google Scholar]
- 56.Davison TF. 1969. Changes in temperature tolerance during the life cycle of Calliphora erythrocephala. J. Insect Physiol. 15, 977–988. ( 10.1016/0022-1910(69)90138-3) [DOI] [Google Scholar]
- 57.Overgaard J, Kristensen TN, Mitchell KA, Hoffmann AA. 2011. Thermal tolerance in widespread and tropical Drosophila species: does phenotypic plasticity increase with latitude? Am. Nat. 178, S80–S96. ( 10.1086/661780) [DOI] [PubMed] [Google Scholar]
- 58.Mitchell KA, Sgro CM, Hoffmann AA. 2011. Phenotypic plasticity in upper thermal limits is weakly related to Drosophila species distributions. Funct. Ecol. 25, 661–670. ( 10.1111/j.1365-2435.2010.01821.x) [DOI] [Google Scholar]
- 59.Maclean IMD, Suggitt AJ, Wilson RJ, Duffy JP, Bennie JJ. 2017. Fine-scale climate change: modelling spatial variation in biologically meaningful rates of warming. Glob. Change Biol. 23, 256–268. ( 10.1111/gcb.13343) [DOI] [PubMed] [Google Scholar]
- 60.Hoffmann AA, Sgro CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 61.Bush A, Mokany K, Catullo R, Hoffmann A, Kellermann V, Sgro C, McEvey S, Ferrier S. 2016. Incorporating evolutionary adaptation in species distribution modelling reduces projected vulnerability to climate change. Ecol. Lett. 19, 1468–1478. ( 10.1111/ele.12696) [DOI] [PubMed] [Google Scholar]
- 62.Lindquist S, Craig EA. 1988. The heat-shock proteins. Annu. Rev. Genet. 22, 631–677. ( 10.1146/annurev.ge.22.120188.003215) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.