Abstract
Mimicry is one of the best-studied examples of adaptation, and recent studies have provided new insights into the role of mimicry in speciation and diversification. Classical Müllerian mimicry theory predicts convergence in warning signal among protected species, yet tropical butterflies are exuberantly diverse in warning colour patterns, even within communities. We tested the hypothesis that microhabitat partitioning in aposematic butterflies and insectivorous birds can lead to selection for different colour patterns in different microhabitats and thus help maintain mimicry diversity. We measured distribution across flight height and topography for 64 species of clearwing butterflies (Ithomiini) and their co-mimics, and 127 species of insectivorous birds, in an Amazon rainforest community. For the majority of bird species, estimated encounter rates were non-random for the two most abundant mimicry rings. Furthermore, most butterfly species in these two mimicry rings displayed the warning colour pattern predicted to be optimal for anti-predator defence in their preferred microhabitats. These conclusions were supported by a field trial using butterfly specimens, which showed significantly different predation rates on colour patterns in two microhabitats. We therefore provide the first direct evidence to support the hypothesis that different mimicry patterns can represent stable, community-level adaptations to differing biotic environments.
Keywords: adaptation, anti-predator defence, Müllerian mimicry, niche
1. Introduction
One of the most intensively studied examples of adaptation is Müllerian mimicry, where groups of unpalatable species display a common warning colour pattern and thereby share the cost incurred through predator learning [1]. Butterflies provide many examples of mimicry ‘rings’ comprising multiple species with extremely similar patterns (e.g. [2]) that have evolved through convergence [3]. Furthermore, numerous field experiments have demonstrated very strong stabilizing selection, which explains this convergence as predicted by classical Müllerian mimicry theory [1,4]. However, surprisingly, mimicry patterns are also highly diverse [5], both across space and within communities, with more than 10 butterfly mimicry rings occurring at a single Amazonian locality [2]. Shifts between mimicry patterns have long been considered a likely cause of ecological speciation [6], as sexual selection on colour pattern and natural selection against hybrid individuals can rapidly result in reproductive isolation, even in the presence of gene flow [7–15].
Spatial variation in predator communities over distances of a few kilometres to hundreds of kilometres is a likely factor in maintaining intraspecific variation in warning colour patterns [16–21], with strong natural selection driving narrow geographical colour pattern clines [17,22,23]. Seasonal variation in predators also facilitates the maintenance of alternative defensive strategies [24]. However, the processes responsible for maintaining mimicry diversity within communities are less well understood. Two classes of hypotheses have been proposed. In the first, colour pattern diversity is viewed as an unstable phenomenon, resulting either from geographical overlap between largely allopatric mimicry patterns or from rapid evolution of new patterns that, once abundant, experience only weak selection for convergence [5]. Such communities represent a dynamic equilibrium, either because mimicry rings offering less protection are continuously ‘rescued’ by immigration [25,26] or because selection constantly drives convergence but is counter-balanced by rapid diversification.
By contrast, the second class of hypotheses views distinct mimicry rings as adaptations to varying abiotic or biotic environments, and thus as representing stable niches related to predator defence. Several studies have confirmed height stratification of mimicry rings in ithomiine [3,27,28] and nocturnally roosting Heliconius butterflies [29]. Mimicry rings may also be segregated with respect to forest disturbance [3,29–32] and topography [3]. Species that share host plants often mimic each other, probably because adults are constrained to fly in similar microhabitats by the distribution of their host plants [27,33].
There are several possible explanations for such microhabitat segregation in mimicry rings. Papageorgis [34] proposed that diverse warning colour patterns in Amazonian butterflies might be maintained by different patterns having a ‘dual-signal’ function of both camouflage and warning against different vegetation backgrounds related to flight height and ambient light. An alternative hypothesis is that different patterns represent adaptations not to physical variation among microhabitats, but to ecological variation in the predator community. If predators show microhabitat segregation similar to mimicry rings, then different predator species may be most familiar with different mimicry patterns, and selection for convergence across microhabitats will be low [5,27,29,30].
Gompert et al. [35] provided theoretical support for the hypothesis that microhabitat preferences in predators and prey can promote mimicry diversity. In that study, strong microhabitat segregation in predators drove microhabitat segregation among mimicry rings, and thereby fostered coexistence of several mimicry rings [35]. Furthermore, on a broader scale, it has been shown that habitat patches several kilometres apart can represent alternative mimicry optima, supporting polymorphisms within species [16,26,36]. However, the optimality of mimicry patterns in finer-scale microhabitats has never been tested. Here, we test this hypothesis for the first time by studying ithomiine butterflies (Nymphalidae: Ithomiini) and their avian predators in a diverse Amazonian rainforest community in eastern Ecuador. Ithomiines, known as ‘clearwing butterflies’ after the transparent wings of many species, inhabit Neotropical forests below 3000 m, with some 60 species in the most diverse communities of the western Amazon. All species are believed to be unpalatable [37] and participate in mimicry ‘rings’ with other ithomiines or putatively unpalatable butterflies, especially the Heliconiinae [38], in addition to presumed palatable Batesian mimics. Ithomiine butterflies dominate these mimicry rings in both species diversity and abundance [2]. Although there are almost no published observations of predation on ithomiines, the primary predators driving the evolution of mimicry are believed to be insectivorous birds. These are the only abundant predators with sufficiently developed colour vision to explain precise mimicry [13,39–42].
We adopted two approaches to test our hypothesis. First, in our study community, we measured the distribution of butterflies and birds with respect to two principal microhabitat axes, flight height and topography, which are known to influence ithomiine mimicry pattern abundance at the study site [3]. We then used these data to estimate the relative encounter rates between mimicry patterns and individual bird species, and therefore test whether butterfly species were, on average, most likely to encounter birds that were most familiar with their colour pattern. Second, we conducted an experimental field trial with dead butterfly specimens to directly measure predation rates on colour patterns in different microhabitats. We use the resulting data to address the question of whether microhabitat segregation in birds and butterflies can lead to the stable coexistence of multiple mimicry patterns.
2. Material and methods
(a). Study groups
Our study group included all ithomiines and co-mimetic butterflies (co-mimics). In the absence of data on butterfly palatability to a range of insectivorous birds, we assumed that all non-ithomiine co-mimics might potentially be Müllerian mimics. Eight mimicry rings involving ithomiines were recognized based on similarity in wing pattern characters and parallel geographical variation in wing pattern [2,3,33,43]. Although human and bird vision differ, we assume that shared wing pattern characters visible to us and used to classify mimicry must also be important cues for predators because these characters show convergent evolution. Moreover, experiments show that birds learn, after attacking unpalatable butterflies, to avoid palatable butterflies that humans classify as co-mimics [44,45], and models of animal vision suggest that birds are unlikely to be able to discriminate between butterflies that are regarded as mimetic by humans [46].
Several bird species present at the study site are known to be predators of butterflies, such as jacamars (Galbulidae) [47,48] (J.C.R.W. 1997–2016, personal observation) and some flycatchers [42,49,50], but these are species characteristic of forest edges, large light gaps or forest canopy. We know of no published evidence of insectivorous birds that are regular predators of butterflies in the understorey, yet some must be to drive the evolution of understorey mimicry rings. We therefore assumed that all potentially insectivorous birds could be important selective agents, and used Ridgely & Greenfield's guide [51] to determine such insectivorous birds (see electronic supplementary material, S3). Manakins (Pipridae) are predominantly frugivorous but also eat insects and were thus included in our analyses.
(b). Study location
The study was conducted at the Napo Wildlife Center, Orellana, Ecuador, a topographically variable area with relatively undisturbed forest (see electronic supplementary material, S1). Data on the topographic distribution of butterflies were obtained by K.R.W., M.E. and C.D.J. sampling eight 30 m-diameter plots, located in pairs with one on a ridge and one in the adjacent valley, along the ‘Parrot Trail’ (240–300 m, 0°31′ S, 76°23′ W). Birds were sampled by J.C.R.W. in the same eight plots as butterflies and during additional timed transect walks along ridge and valley trails between and near the plots.
(c). Species abundance and distribution
Fieldwork was conducted from 16 October to 15 December 2005. We recorded butterfly distribution and abundance during 30 min sampling and observation periods in each plot, from 08.00 to 17.00. Ridge plots were surveyed for a total of 23 h among all plots, and the same for valley plots. Plots were patrolled continuously during the 30 min sampling period and attempts made to capture all mimetic butterflies using hand-nets, up to 9 m above the ground. Specimens were either killed and retained or marked and released, and we recorded the time of day, species or mimicry pattern (if unidentified), sex and initial flight height. Observers carried entomological nets with metal handles composed of up to 12 sections each 0.6 m in length, with these gradated handles facilitating flight height estimation.
Birds were recorded in the same plots from 04.20 to 18.00 h, concentrating in particular between 06.30–11.00 and 15.00–17.00 h when bird activity was greatest. A total of 44 h observation time was spent in ridge plots and the same time in valley plots. Birds were also recorded during timed walks along ridge and stream trails between Parrot Trail plots (15 h spent in ridge walks and the same in valley walks). Species were identified by sight and call using prior experience and Ridgely & Greenfield's guide [51]. For all individuals, we recorded time of day, species, and initial flight or perch height.
(d). Mimicry pattern encounter rates for bird species
To characterize butterfly and bird distribution, we assigned each butterfly and bird individual to one of eight microhabitats, representing combinations of two topography categories (ridge and valley) with four flight height categories (0–1 m, 1–2 m, 2–3 m, above 3 m). Flight height intervals were based on our observations of significant differences in mimicry rings between the ground and 3 m, and a sharp decline in observed numbers of butterfly individuals above 3 m. These data were used to estimate the relative frequency of encounters between each bird species and each of the eight mimicry patterns. For a given bird species (k) and mimicry pattern (i), we summed the product of bird abundance (Ikj) and butterfly abundance (Bij) in each of the eight microhabitats (j) and divided by the sum of these products across all mimicry patterns, as an estimate of the relative encounter rate (Mik) of that mimicry pattern in comparison with others.
Therefore, the relative mimicry pattern encounter rate for bird species k and mimicry pattern i is
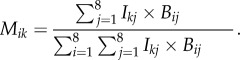 |
We then used a permutation approach to test for non-random encounter rates between different mimicry patterns and individual bird species, which might occur due to microhabitat segregation. We permuted mimicry pattern among butterfly individuals to generate 500 ‘null’ communities, maintaining the same numbers of individuals in each mimicry ring and the same butterfly abundance distribution among microhabitats. For each null community, we calculated relative mimicry encounter rates (Mik) as above, for the 25 most abundant bird species (greater than 10 individuals recorded) in the community. We then compared our empirical values of Mik with those in the 500 null communities to address two questions:
H1. Within individual bird species, do the encounter rates of different mimicry patterns differ significantly from those expected if there were no microhabitat segregation of mimicry patterns? We focused on the mimicry pattern encountered most frequently by each bird species since that is the pattern that species is most likely to avoid, and therefore the pattern that should be optimal for co-existing butterflies to display. For each bird species k and mimicry pattern i, the frequency of null communities with the highest Mik equal or greater than the highest empirical Mik represents the probability of such a high encounter rate being the result of chance (a one-tailed test).
H2. Within the entire bird community, do a significant number of bird species most frequently encounter a mimicry pattern other than the most abundant pattern? The two most abundant mimicry patterns recorded were ‘eurimedia’ (37%) and ‘hermias’ (31%) (see Results; electronic supplementary material, S4), so to simplify analyses, we focused on encounter rates of these two patterns. Empirically, 19 out of 25 bird species had the highest empirical encounter rate for the less abundant pattern ‘hermias’ (see Results; electronic supplementary material, S5). The frequency of null communities with 19 or more bird species having Mhermias k as the highest pattern encounter rate represents the probability of such a biased community being the result of chance (a one-tailed test).
(e). Birds encountered by butterfly species
We then examined whether butterfly species tend to most often encounter birds that are most familiar with their colour pattern, and hence that are most likely to avoid them. First, we calculated the weighted average rate of encounters (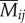 ) of each of the eight mimicry rings (i) within each of the eight microhabitats (j) across all bird individuals (all bird species, k = 1–129) observed in a given microhabitat (Ij).
) of each of the eight mimicry rings (i) within each of the eight microhabitats (j) across all bird individuals (all bird species, k = 1–129) observed in a given microhabitat (Ij).
The average relative encounter rate for mimicry pattern i of birds occurring in microhabitat j is
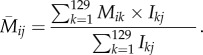 |
Finally, for each butterfly species (h), we calculated weighted average mimicry encounter rates (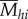 ) of birds occurring in the microhabitats where that butterfly species was recorded, by weighting bird-mimicry encounter frequencies (
) of birds occurring in the microhabitats where that butterfly species was recorded, by weighting bird-mimicry encounter frequencies (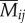 ) with observed butterfly abundance (Bhj) in each microhabitat and summing across microhabitat.
) with observed butterfly abundance (Bhj) in each microhabitat and summing across microhabitat.
The average relative encounter rate for mimicry pattern i of birds encountering butterfly species h is
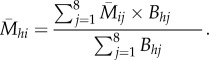 |
These final data indicate the mimicry pattern that will be most familiar to birds encountering a given butterfly species, and the pattern with the highest (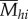 ) should be the optimal pattern for that butterfly species. As we found that the second most abundant pattern ‘hermias’ was predicted to be optimal for 15 of the 21 species with that colour pattern (see Results; electronic supplementary material, S6), we tested whether the association between predicted and actual optimal patterns was greater than expected by chance, by permuting predicted optimal patterns among all butterfly species 500 times.
) should be the optimal pattern for that butterfly species. As we found that the second most abundant pattern ‘hermias’ was predicted to be optimal for 15 of the 21 species with that colour pattern (see Results; electronic supplementary material, S6), we tested whether the association between predicted and actual optimal patterns was greater than expected by chance, by permuting predicted optimal patterns among all butterfly species 500 times.
(f). Differential predation
We tested whether predation rates differed between mimicry patterns and microhabitats using dead butterfly specimens. Because we found differences in the two predominant mimicry patterns (‘eurimedia’ and ‘hermias’) across topography (between ridges and valleys), we designed the experiment to test whether topography had an effect on predation rate of these two patterns. Ithomiines belonging to ‘eurimedia’ and ‘hermias’ mimicry rings were collected outside of predation study plots and at random with respect to species, killed and attached with cyanoacrylate glue to the tips of sticks that were driven into the ground (electronic supplementary material, S7A,B). The bottom of each stick was sprayed with locally obtained insect repellent up to 10 cm above the ground to deter terrestrial scavengers. Butterflies were placed alternately with wings open or closed (both natural postures for resting ithomiines), from 1 to 1.5 m above the ground. At each of 10 sites (five on a ridge and five in the adjacent valley, each separated by 100–200 m), we placed two pairs of ‘eurimedia’ and ‘hermias’ butterflies approximately 5 m apart, with each pair containing one ‘eurimedia’ and one ‘hermias’ individual approximately 1 m apart (electronic supplementary material, S7D), with individuals randomized with respect to species. Predation study sites were located outside of study plots but in similar microhabitats, where ithomiines were observed flying. A total of 10 butterfly individuals of each mimicry pattern were thus distributed across five ridge sites, and a further 10 individuals of each mimicry pattern were distributed across five adjacent stream sites. The study was conducted during the latter part of the same fieldwork period in which butterfly and bird surveys were conducted, and sites were checked twice daily, once at dawn and once at dusk. At each check, the number and pattern of predated individuals were recorded. Bird predation was inferred where wings were observed to be torn (e.g. electronic supplementary material, S7C) or entirely missing, with the body intact, or when the body was observed to be torn consistent with a bird (rather than arthropod) attack. No other scavengers were observed attacking the specimens. Damaged or missing specimens were replaced, and sites were moved approximately 20 m each day to reduce predator habituation. Specimens were checked 34 times in total.
We used a maximum-likelihood approach to test for differences in predation with respect to the two mimicry patterns and the two microhabitats, pooling data for all specimens within these four categories. Given the low numbers of predation events, we felt that it would be unreasonable to attempt to include additional parameters (such as study site and wing position) into modelling variation in predation rate. We calculated the values of the predation probabilities of the two patterns within one microhabitat, or of one pattern across two microhabitats, that maximized the log-likelihood functions for the observed predation results, and we computed the corresponding maximum log-likelihood value. Next, we calculated the maximum log-likelihood under the assumption that these predation probabilities were the same (the null hypothesis) and compared that with the maximum log-likelihood where predation probabilities were allowed to differ. A likelihood ratio test was used to test for the significance of the difference between the two log-likelihood scores, with d.f. = 1. See electronic supplementary material, S8 for further details.
3. Results
In our eight study plots (electronic supplementary material, S1), we recorded 656 individuals of 64 species of butterflies, distributed across eight mimicry rings (figure 1; electronic supplementary material, S2). Very similar relative abundances were recorded for the most common mimicry rings by different observers. Dominant groups were Ithomiini (49 species) and Heliconiinae (five species). A total of 127 species and 893 individuals of birds were recorded and identified as potential predators of Lepidoptera (see electronic supplementary material, S3). Dominant families included Tyrannidae, Thamnophilidae, Furnariidae, Thraupidae, Bucconidae and Picidae, representing 63% of all species.
Figure 1.
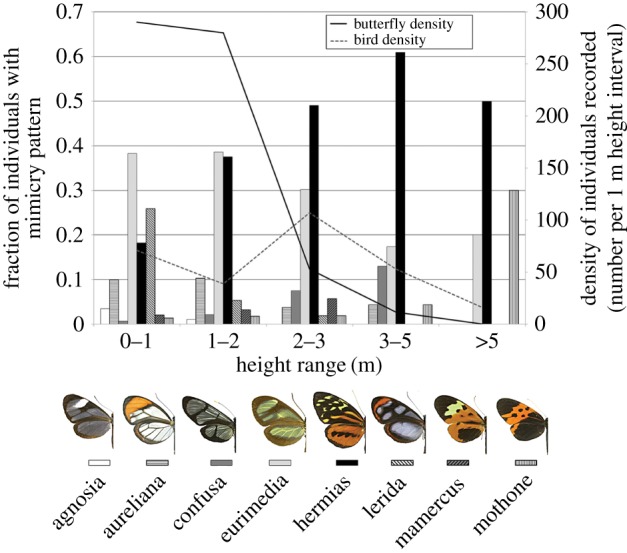
Relative butterfly mimicry pattern abundance (bars) and butterfly and bird density (numbers of individuals per 1 m height band) (lines) recorded at different heights above the ground.
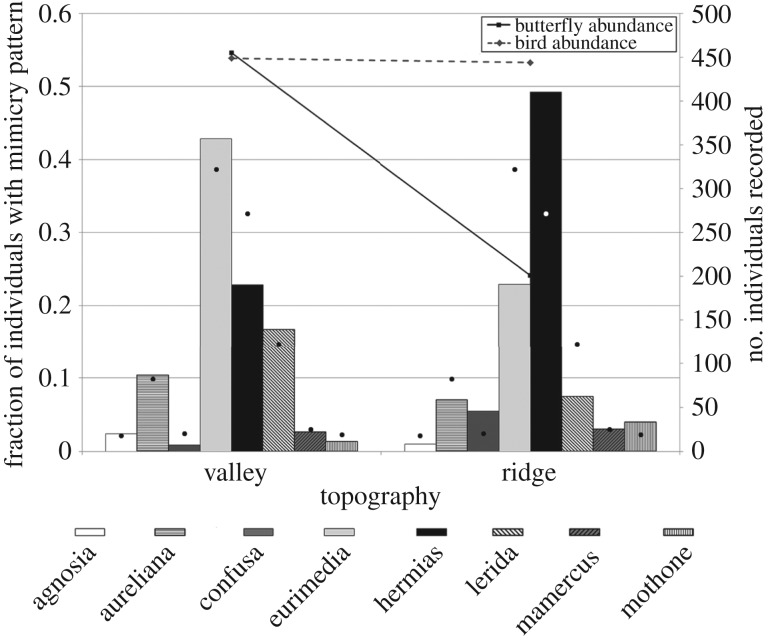
Aposematic butterfly density declined sharply with height above 3 m, whereas bird density showed a peak at 2–3 m and a more gradual decline with height (figure 1). Overall, ‘eurimedia’ was the dominant mimicry pattern, comprising 241 (37%) of all individuals, followed by ‘hermias’ (203, 31%) and ‘lerida’ (91, 14%), but the fraction of the community occupied by these patterns varied across height. ‘Lerida’ and ‘eurimedia’ patterns were dominant from 0 to 1 m, ‘eurimedia’ and ‘hermias’ equally dominant from 1 to 2 m, and ‘hermias’ dominant in height categories above 2 m, reaching more than 60% of the community from 3 to 5 m (figure 1). With respect to topography, more than twice as many butterflies were recorded in valley sites compared with ridge sites, whereas bird abundance was similar across these two categories (figure 2). ‘Eurimedia’ was the dominant pattern (43% of individuals) in valley sites, followed by ‘hermias’ (23%), whereas ‘hermias’ was dominant (49%) in ridge sites, followed by ‘eurimedia’ (23%) (figure 2).
Figure 2.
Relative butterfly mimicry pattern abundance (bars) and numbers of butterfly and bird individuals recorded (lines) in valley and ridge sites. Black and white dots represent relative abundances of mimicry patterns that would be expected if butterflies were distributed randomly with respect to topography.
Based on the distribution of birds and butterflies (electronic supplementary material, S4) among the eight topography-flight height microhabitats, 19 of the 25 most abundant bird species were estimated to encounter the second most abundant mimicry pattern (‘hermias’) most frequently, and 16 of these species encountered ‘hermias’ significantly more often than expected by chance (H1, p < 0.05) (electronic supplementary material, S5; e.g. figure 3). Out of the six bird species encountering ‘eurimedia’ most frequently, two encountered ‘eurimedia’ significantly more often than expected by chance (H1, p < 0.05) (electronic supplementary material, S5; e.g. figure 3). As a community, the number of bird species encountering ‘hermias’ most frequently was significantly higher than in null communities without microhabitat segregation of mimicry patterns (H2, p = 0.04 of finding this number, i.e. 19, or more in 500 permuted communities).
Figure 3.
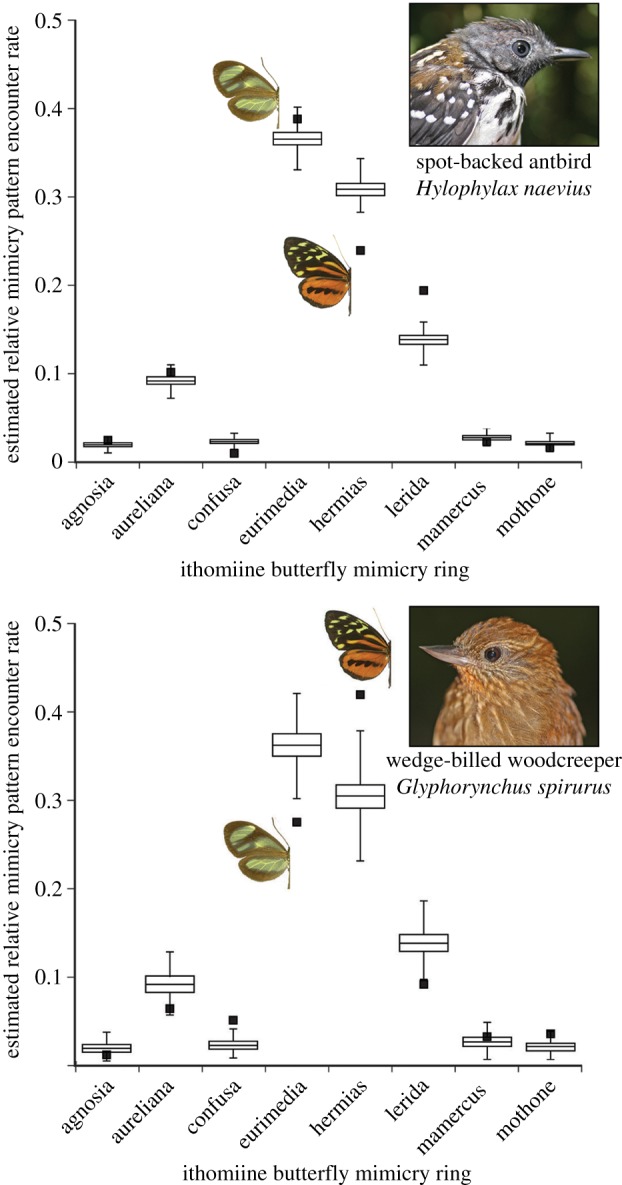
Estimated relative encounter rates of eight ithomiine butterfly mimicry rings by two potential bird predators during the study. Black squares show observed values and standard box-plots represent a distribution of values generated under a null model of no microhabitat segregation of mimicry rings (500 simulations). The low-flying spot-backed antbird was estimated to encounter more ‘eurimedia’ and fewer ‘hermias’ in the field than expected under the null model, while the opposite was true for wedge-billed woodcreeper, which showed a preference for midstorey and ridge-tops where the ‘hermias’ mimicry ring tended to fly. (Online version in colour.)
Estimates of the average mimicry encounter rates of predators co-occurring with each butterfly species resulted in 10 of 12 species in the most abundant mimicry ring (‘eurimedia’) being predicted to have the optimal colour pattern for predator avoidance (electronic supplementary material, S6). In other words, the average bird predator encountering these ‘eurimedia’ species is more likely to have previously encountered that colour pattern than any other. Of the 21 species within the second most abundant mimicry ring (‘hermias’), 15 were predicted to have the optimal colour pattern for predator defence (p < 0.01 of finding 15 or more ‘hermias’ with an optimal pattern when expected optimal patterns were permuted among all butterfly species 500 times). Within ‘hermias’, there was no correspondence between rarity and species identified as having suboptimal patterns; most notably, the optimal pattern for the most abundant ‘hermias’ species, Hypothyris semifulva, was predicted to be ‘eurimedia’, as this was a relatively low-flying valley species that overlapped most with other non-mimic ‘eurimedia’ species. Species in all other mimicry rings were predicted to have suboptimal patterns, as their optimal patterns were predicted to be either ‘eurimedia’ or ‘hermias’ (electronic supplementary material, S6).
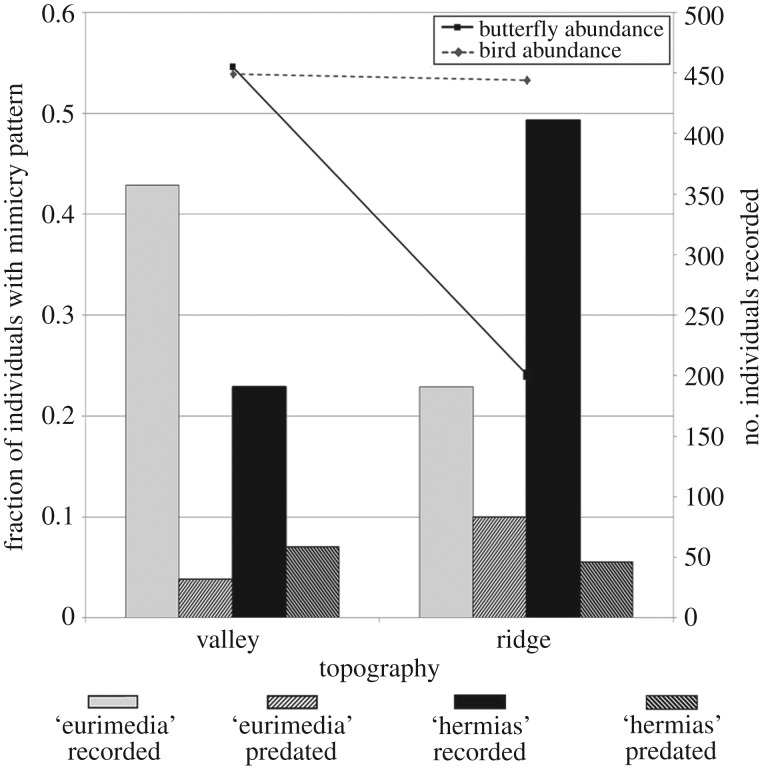
In the predation study, a total of 340 trials (checks for predation of specimens) were conducted for each combination of mimicry pattern and microhabitat. At ridge sites, 34 ‘eurimedia’ and 19 ‘hermias’ were predated, while at valley sites, 24 ‘hermias’ and 13 ‘eurimedia’ were predated (figure 4). In terms of predation on different mimicry patterns, ‘eurimedia’ was significantly more predated on ridges than ‘hermias’ (PH = 0.056 and PE = 0.100; p = 0.03), while ‘hermias’ was more predated at streams than ‘eurimedia’ (PH = 0.071 and PE = 0.038; p = 0.06). Across microhabitats, ‘eurimedia’ was significantly more predated on ridges than in valleys (p = 0.001), while predation rates on ‘hermias’, although higher at streams, did not differ significantly across microhabitats (p = 0.4). Because the morning and evening checks of specimens were conducted in the same site, prior to moving specimens to another site, some independence of data is potentially lost because a single bird is more likely to be responsible for predation events recorded at those two times. We therefore analysed the morning and evening data independently, as above. Most predation events were recorded at the morning check of sites (61 out of 90 predation events); ‘eurimedia’ was significantly more predated on ridges than ‘hermias’ (PH = 0.029 and PE = 0.067; p = 0.020), while ‘hermias’ was significantly more predated at streams than ‘eurimedia’ (PH = 0.058 and PE = 0.023; p = 0.020). Across microhabitats, ‘eurimedia’ was significantly more predated on ridges than in valleys (p = 0.004), while predation rates on ‘hermias’, although higher at streams, did not differ significantly across microhabitats (p = 0.06). All comparisons were non-significant for the smaller evening dataset.
Figure 4.
Results of experimental field predation study. Relative butterfly mimicry pattern abundance recorded in surveys (solid bars), fraction of butterflies attacked in field trials (hatched bars), and numbers of butterfly and bird individuals recorded in surveys (lines) in valley and ridge sites.
4. Discussion
Our study provides the first empirical support for the hypothesis that microhabitat segregation in warningly coloured butterflies and avian insectivores can maintain a diversity of Müllerian mimetic warning colour patterns within communities [27,29,32,35]. This is the first time that both mimetic butterflies and their predators have been studied together at the microhabitat scale, and both our analytical approach and experimental results support the idea that different warning colour patterns can be optimal for anti-predator defence in different microhabitats. Our study thus extends research on how variation in predator communities helps maintain warning colour pattern polymorphisms within prey species at larger spatial scales [16–22,26], to show that predator community structure can also promote warning colour pattern diversity across species within a single prey community.
It is likely that height and topography, through their effects on microclimate variables such as temperature and humidity [52], affect two important aspects of ithomiine ecology: choice of host plant and male mate-locating sites. Ithomiine caterpillars feed almost exclusively on Solanaceae plants, and different clades of butterflies have specialized on particular plant clades [53]. Host plants are regarded as significant in determining ithomiine flight height [27] and spatial distribution [33], and we also documented marked preferences for ridge or valley sites among ithomiine host plants (K.R.W. & M.E. 2005, unpublished data) that may help explain specific preferences for topographic microhabitats. Furthermore, we also noted that males tended to maintain territories where they awaited females (termed ‘perching’ by Scott [54]) at similar heights and in similar topographic microhabitats to those where their mimicry rings typically fly.
Birds also showed distinct preferences for vertical foraging stratum and topography, consistent with previous studies (e.g. [55]). The similar height and topographic distributions of birds and mimicry patterns resulted in the encounter rates of different mimicry patterns being significantly different for individual bird species. The great majority of the most abundant insectivorous birds occurred in the midstorey and canopy, and thus were estimated to preferentially encounter the second most abundant but highest flying mimicry pattern (‘hermias’). A smaller number of understorey birds were estimated to most frequently encounter the most common understorey mimicry ring (‘eurimedia’). Topographic preferences among predators and prey strengthened these patterns, since high-flying species of both birds and butterflies also tended to occur more commonly on ridge-tops. As a consequence, both the most abundant (‘eurimedia’) and the second most abundant pattern (‘hermias’) were predicted to be optimal in different microhabitats for the majority of species in each mimicry ring.
If colour patterns serve as microhabitat-specific anti-predator defences, then the differing abundance in ridge and valley sites of the two most common patterns, ‘hermias’ and ‘eurimedia’, leads to clear predictions of relative predation rates in the field trials. In all comparisons (between mimicry patterns within a single microhabitat and between microhabitats within a single mimicry pattern), empirical predation rates were, as expected, inversely related to observed abundances of mimicry patterns. Most notably, at ridge sites, ‘hermias’ was approximately twice as abundant as ‘eurimedia’, and predation rates on ‘eurimedia’ were overall 1.8 times as high as for ‘hermias’. The opposite was observed in valleys, where ‘eurimedia’ was approximately twice as abundant as ‘hermias’, and predation rates on ‘hermias’ were 1.9 times as high as for ‘eurimedia’. These data thus support the conclusion that the ‘eurimedia’ pattern was optimal in valley sites and the ‘hermias’ pattern optimal on ridges, despite these microhabitats being only 100–300 m apart (see electronic supplementary material, S1).
The overall dominance of the two most abundant mimicry patterns and segregation by flight height that we observed were consistent with three other studies at Ecuadorian Amazonian sites spaced across a three-decade period [27,32,56]. These broad patterns of abundance and flight height partitioning thus seem to be a general feature of ithomiine communities in this region. However, even though microhabitat segregation can help maintain the coexistence of these two most common mimicry patterns, the remaining six patterns were never predicted to be optimal in our analysis of butterfly and bird microhabitat distributions. It is likely that at least some patterns, such as ‘agnosia’, might prove to be optimal in marginal microhabitats, such as forest edges and secondary growth, that were not well represented in our study. In addition, we did not consider temporal changes in bird and butterfly distribution. For example, the lowest-flying mimicry ring, ‘lerida’, was most active early in the morning, perhaps exposing it to a distinct suite of predators in comparison with later-flying mimicry rings. Furthermore, temporal partitioning of mimicry rings also occurs throughout the year [30], which may also be coincident with seasonal changes in the predatory bird fauna, perhaps selecting for different optimal defences at different times of year (e.g. [24]). Our first analysis also assumes equivalence among insectivorous birds and among mimicry patterns. Neither of these are likely to be true, and some bird species are likely to account for a disproportionate number of attacks. Nevertheless, our data suggest that the majority of bird species, regardless of abundance, are sufficiently restricted in microhabitat as to encounter particular mimicry patterns at non-random rates. Furthermore, we made the simplifying assumption that the most abundant patterns are the best protected, but differences in unpalatability, detectability and escaping ability are also likely to be significant [57]. Finally, because of predator generalization, some rare patterns (e.g. ‘mamercus’) may be avoided by predators that have been educated by encounters with similar but more abundant (e.g. ‘hermias’) mimicry rings.
Alternatively, suboptimal patterns may be maintained because there is little selection for a mimicry switch [5,32]. If community composition shifts over time, weak selective pressures for convergence may not persist long enough to effect change and similarly protected patterns may coexist indefinitely [58]. Similar shifts in community composition may occur over space; some of the rarer mimicry rings in our study community may be maintained by continuous migration from source regions where they are more abundant, such that patterns that are apparently suboptimal in one area are optimal in another [16,17,19,20,26,36,59].
Our study supports the idea that any ecological shift that results in a mimetic butterfly species being exposed to new suites of predators, such as a change in host plant or mate-locating strategy linked to different microhabitats, probably initiates selection for phenotypic change that could ultimately lead to speciation. Previous studies of Neotropical butterflies have shown that predation has morphological, physiological and behavioural consequences [38,60]. Here, we confirm that shifts in microhabitat are likely to also result in strong selection on warning colour patterns. Concerted changes in wing pattern and microhabitat have indeed apparently occurred multiple times in the ithomiine community that we studied [3], driving ecological convergence. Furthermore, shifts in mimetic wing pattern are associated with both pre-zygotic and post-zygotic reproductive isolation in mimetic butterflies [10,12,13,15]. Our results suggest that at least some coexisting mimetic patterns can also be considered ecological niches in their own right [61], rather than by-products of processes operating at larger spatial and temporal scales. Partitioning of species among these niches should help to maintain community species richness in some of the most biologically diverse ecosystems in the world.
Finally, our research contributes to knowledge of the complexity of ecological interactions linking plants, herbivores and predators, and adds to a growing body of literature showing that diversity in anti-predator defence can be maintained by differing abiotic and biotic microenvironments [62–69]. Although competition has usually been seen as the principal driving force for ecological divergence and adaptive radiation [70], predation is likely to be just as important, if not more so, in communities where competition is minimal [71–75].
Supplementary Material
Acknowledgements
We thank R. Aldaz, A. Toporov, A. Zölei, G. Papp and C. Giraldo Sánchez for help in the field, the Ecuadorian Ministerio del Ambiente and Museo Ecuatoriano de Ciencias Naturales for providing research permits, and the Napo Wildlife Center for logistic support in the field. We also thank W. Haber, G. Beccaloni and J. Mallet for stimulating discussions and inspiration from their publications.
Ethics
Research and collection permits were provided by the Ministerio del Ambiente, Ecuador, with the support and collaboration of the Museo Ecuatoriano de Ciencias Naturales, Quito, Ecuador.
Data accessibility
Distribution databases for butterflies and birds recorded in this study are included as electronic supplementary material.
Authors' contributions
C.D.J., K.R.W. and J.C.R.W. conceived the study; all authors contributed to the design of the field experiments; K.R.W., M.E. and C.D.J. collected field data on butterflies and microhabitats; J.C.R.W. collected field data on birds; K.R.W. and M.E. carried out statistical analyses; K.R.W. wrote the first draft of the manuscript; and all authors contributed to significantly revising and improving the draft. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the Leverhulme Trust F/00158/AK, F/09 364F (UK) and additionally supported by the Centre National de la Recherche Scientifique (ATIP grant to M.E.), the Muséum National d'Histoire Naturelle and the University of Florida.
References
- 1.Müller F. 1879. Ituna and Thyridia; a remarkable case of mimicry in butterflies. Trans. Entomol. Soc. Lond. 1879, xx–xxix. [Google Scholar]
- 2.Beccaloni GW. 1997. Ecology, natural history and behavior of ithomiine butterflies and their mimics in Ecuador (Lepidoptera: Nymphalidae: Ithomiinae). Trop. Lepid. 8, 103–124. [Google Scholar]
- 3.Elias M, Gompert Z, Jiggins C, Willmott KR. 2008. Mutualistic interactions drive ecological niche convergence in a diverse butterfly community. PLoS Biol. 6, 2642–2649. ( 10.1371/journal.pbio.0060300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherratt TN. 2008. The evolution of Mullerian mimicry. Naturwissenschaften 95, 681–695. ( 10.1007/s00114-008-0403-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joron M, Mallet J. 1998. Diversity in mimicry: paradox or paradigm? Trends Ecol. Evol. 13, 461–466. ( 10.1016/S0169-5347(98)01483-9) [DOI] [PubMed] [Google Scholar]
- 6.Bates HW. 1862. Contributions to an insect fauna of the Amazon Valley: Lepidoptera: Heliconidae. Trans. Linn. Soc. Lond. 23, 495–566. ( 10.1111/j.1096-3642.1860.tb00146.x) [DOI] [Google Scholar]
- 7.Jiggins CD, Estrada C, Rodrigues A. 2004. Mimicry and the evolution of premating isolation in Heliconius melpomene Linnaeus. J. Evol. Biol. 17, 680–691. ( 10.1111/j.1420-9101.2004.00675.x). [DOI] [PubMed] [Google Scholar]
- 8.Kronforst MR, Young LG, Kapan DD, McNeely C, O'Neill RJ, Gilbert LE. 2006. Linkage of butterfly mate preference and wing color preference cue at the genomic location of wingless. Proc. Natl Acad. Sci. USA 103, 6575–6580. ( 10.1073/pnas.0509685103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavarez J, Salazar CA, Bermingham E, Salcedo C, Jiggins CD, Linares M. 2006. Speciation by hybridization in Heliconius butterflies. Nature 441, 868–871. ( 10.1038/nature04738) [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain NL, Hill RI, Kapan DD, Gilbert LE, Kronforst MR. 2009. Polymorphic butterfly reveals the missing link in ecological speciation. Science 326, 847–850. ( 10.1126/science.1179141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muñoz AG, Salazar C, Castaño J, Jiggins CD, Linares M. 2010. Multiple sources of reproductive isolation in a bimodal butterfly hybrid zone. J. Evol. Biol. 23, 1312–1320. ( 10.1111/j.1420-9101.2010.02001.x) [DOI] [PubMed] [Google Scholar]
- 12.Merrill RM, Gompert Z, Dembeck LM, Kronforst MR. 2011. Mate preference across the speciation continuum in a clade of mimetic butterflies. Evolution 65, 1489–1500. ( 10.1111/j.1558-5646.2010.01216.x) [DOI] [PubMed] [Google Scholar]
- 13.Merrill RM, Wallbank RW, Bull V, Salazar PC, Mallet J, Stevens M, Jiggins CD. 2012. Disruptive ecological selection on a mating cue. Proc. R. Soc. B 279, 4907–4913. ( 10.1098/rspb.2012.1968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkbeiner SD, Briscoe AD, Reed RD. 2014. Warning signals are seductive: relative contributions of color and pattern to predator avoidance and mate attraction in Heliconius butterflies. Evolution 68, 3410–3420. ( 10.1111/evo.12524) [DOI] [PubMed] [Google Scholar]
- 15.Jiggins CD, Naisbit RE L. CR, Mallet J. 2001. Reproductive isolation caused by color pattern mimicry. Nature 411, 302–305. ( 10.1038/35077075) [DOI] [PubMed] [Google Scholar]
- 16.Kapan DD. 2001. Three-butterfly mimicry system provides a field test of Müllerian mimicry. Nature 409, 338–340. ( 10.1038/35053066) [DOI] [PubMed] [Google Scholar]
- 17.Chouteau M, Angers B. 2011. The role of predators in maintaining the geographic organization of aposematic signals. Am. Nat. 178, 810–817. ( 10.1086/662667) [DOI] [PubMed] [Google Scholar]
- 18.Valkonen JK, Nokelainen O, Niskanen M, Kilpimaa J, Björklund M, Mappes J. 2012. Variation in predator species abundance can cause variable selection pressure on warning signaling prey. Ecol. Evol. 2, 1971–1976. ( 10.1002/ece3.315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nokelainen O, Valkonen J, Lindsted C, Mappes J. 2014. Changes in predator community structure shifts the efficacy of two warning signals in Arctiid moths. J. Anim. Ecol. 83, 598–605. ( 10.1111/1365-2656.12169) [DOI] [PubMed] [Google Scholar]
- 20.Willink B, García-Rodríguez A, Bolaños F, Pröhl H. 2014. The interplay between multiple predators and prey color divergence. Biol. J. Linn. Soc. 113, 580–589. ( 10.1111/bij.12355) [DOI] [Google Scholar]
- 21.Hegna RH, Mappes J. 2014. Influences of geographic differentiation in the forewing warning signal of the wood tiger moth in Alaska. Evol. Ecol. 28, 1003–1017. ( 10.1007/s10682-014-9734-7) [DOI] [Google Scholar]
- 22.Mallet J, Barton NH. 1989. Strong natural selection in a warning-color hybrid zone. Evolution 43, 421–431. ( 10.2307/2409217) [DOI] [PubMed] [Google Scholar]
- 23.Sherratt TN. 2006. Spatial mosaic formation through frequency-dependent selection in Müllerian mimicry complexes. J. Theor. Biol. 240, 165–174. ( 10.1016/j.jtbi.2005.09.017) [DOI] [PubMed] [Google Scholar]
- 24.Mappes J, Kokko H, Ojala K, Lindström L. 2014. Seasonal changes in predator community switch the direction of selection for prey defences. Nat. Commun. 5, 1–7. ( 10.1038/ncomms6016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joron M, Iwasa Y. 2005. The evolution of a Mullerian mimic in a spatially distributed community. J. Theor. Biol. 237, 87–103. ( 10.1016/j.jtbi.2005.04.005) [DOI] [PubMed] [Google Scholar]
- 26.Joron M, Wynne IR, Lamas G, Mallet J. 1999. Variable selection and the coexistence of multiple mimetic forms of the butterfly Heliconius numata. Evol. Ecol. 13, 721–754. ( 10.1023/A:1010875213123) [DOI] [Google Scholar]
- 27.Beccaloni GW. 1997. Vertical stratification of ithomiine butterfly (Nymphalidae: Ithomiinae) mimicry complexes: the relationship between adult flight height and larval host-plant height. Biol. J. Linn. Soc. 62, 313–341. ( 10.1006/bijl.1997.0165) [DOI] [Google Scholar]
- 28.Medina MC, Robbins RK, Lamas G. 1996. Vertical stratification of flight by ithomiine butterflies (Lepidoptera: Nymphalidae) at Pakitza, Manu: national Park, Perú. In Manu: the biodiversity of southeastern Peru (eds Wilson DE, Sandoval A), pp. 211–216. Washington, DC: Smithsonian Institution. [Google Scholar]
- 29.Mallet J, Gilbert L. 1995. Why are there so many mimicry rings? Correlations between habitat, behavior and mimicry in Heliconius butterflies. Biol. J. Linn. Soc. 55, 159–180. ( 10.1111/j.1095-8312.1995.tb01057.x) [DOI] [Google Scholar]
- 30.DeVries PJ, Lande R, Murray D. 1999. Associations of co-mimetic ithomiine butterflies on small spatial and temporal scales in a neotropical rainforest. Biol. J. Linn. Soc. 67, 73–85. ( 10.1006/bijl.1998.0288) [DOI] [Google Scholar]
- 31.Estrada C, Jiggins CD. 2002. Patterns of pollen feeding and habitat preference among Heliconius species. Ecol. Entomol. 27, 448–456. ( 10.1046/j.1365-2311.2002.00434.x) [DOI] [Google Scholar]
- 32.Hill RI. 2009. Habitat segregation among mimetic ithomiine butterflies (Nymphalidae). Evol. Ecol. 24, 273–285. ( 10.1007/s10682-009-9305-5) [DOI] [Google Scholar]
- 33.Willmott KR, Mallet J. 2004. Correlations between adult mimicry and larval host plants in ithomiine butterflies. Proc. R. Soc. Lond. B 271, S266–S269. ( 10.1098/rsbl.2004.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papageorgis C. 1975. Mimicry in neotropical butterflies. Am. Sci. 63, 522–532. [Google Scholar]
- 35.Gompert Z, Willmott KR, Elias M. 2011. Heterogeneity in predator micro-habitat use and the maintenance of Müllerian mimicry diversity. J. Theor. Biol. 281, 39–46. ( 10.1016/j.jtbi.2011.04.024) [DOI] [PubMed] [Google Scholar]
- 36.Chouteau M, Arias M, Joron M. 2016. Warning signals are under positive frequency-dependent selection in nature. Proc. Natl Acad. Sci. USA 113, 2164–2169. ( 10.1073/pnas.1519216113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown KS. 1984. Adult obtained pyrrolizidine alkaloids defend ithomiine butterflies against a spider predator. Nature 309, 707–709. ( 10.1038/309707a0) [DOI] [Google Scholar]
- 38.Chai P, Syrgley RB. 1990. Predation and the flight, morphology, and temperature of Neotropical rain-forest butterflies. Am. Nat. 135, 748–765. ( 10.1086/285072) [DOI] [Google Scholar]
- 39.Chai P. 1986. Field observations and feeding experiments on the responses of rufous-tailed jacamars (Galbula ruficauda) to free-flying butterflies in a tropical rain forest. Biol. J. Linn. Soc. 29, 161–189. ( 10.1111/j.1095-8312.1986.tb01772.x) [DOI] [Google Scholar]
- 40.Finkbeiner SD, Briscoe AD, Reed R. 2012. The benefit of being a social butterfly: communal roosting deters predation. Proc. R. Soc. B 279, 2769–2776. ( 10.1098/rspb.2012.0203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langham GM. 2005. Rufous-tailed jacamars and aposematic butterflies: do older birds attack novel prey? Behav. Ecol. 17, 285–290. ( 10.1093/beheco/arj027) [DOI] [Google Scholar]
- 42.Pinheiro CEG. 2011. On the evolution of warning coloration, Batesian and Müllerian mimicry in Neotropical butterflies: the role of jacamars (Galbulidae) and tyrant-flycatchers (Tyrannidae). J. Avian Biol. 42, 277–281. ( 10.1111/j.1600-048X.2011.05435.x) [DOI] [Google Scholar]
- 43.Jiggins CD, Mallarino R, Willmott KR, Bermingham E. 2006. The phylogenetic pattern of speciation and wing pattern change in Neotropical Ithomia butterflies (Lepidoptera: Nymphalidae). Evolution 60, 1454–1466. ( 10.1111/j.0014-3820.2006.tb01224.x) [DOI] [PubMed] [Google Scholar]
- 44.Brower JVZ. 1958. Experimental studies of mimicry in some North American butterflies. Part I. The Monarch, Danaus plexippus, and Viceroy, Limenitis archippus archippus. Evolution 12, 32–47. ( 10.2307/2405902) [DOI] [Google Scholar]
- 45.Brower JVZ. 1958. Experimental studies of mimicry in some North American butterflies. Part II. Battus philenor and Papilio troilus, P. polyxenes and P. glaucus. Evolution 12, 123–136. ( 10.2307/2406023) [DOI] [Google Scholar]
- 46.Llaurens V, Joron M, Thery M. 2014. Cryptic differences in colour among Müllerian mimics: how can the visual capacities of predators and prey shape the evolution of wing colours? J. Evol. Biol. 27, 531–540. ( 10.1111/jeb.12317) [DOI] [PubMed] [Google Scholar]
- 47.Burton PJK. 1976. Feeding behavior in the paradise jacamar and the swallow-wing. Living Bird 15, 223–238. [Google Scholar]
- 48.Chai P. 1996. Butterfly visual characteristics and ontogeny of responses to butterflies by a specialized tropical bird. Biol. J. Linn. Soc. 59, 37–67. ( 10.1006/bijl.1996.0053) [DOI] [Google Scholar]
- 49.Pinheiro CEG. 1996. Palatability and escaping ability in neotropical butterflies: tests with wild kingbirds (Tyrannus melancholicus, Tyrannidae). Biol. J. Linn. Soc. 59, 351–365. ( 10.1006/bijl.1996.0069) [DOI] [Google Scholar]
- 50.Pinheiro CEG. 2003. Does Müllerian mimicry work in nature? Experiments with butterflies and birds (Tyrannidae). Biotropica 35, 356 ( 10.1646/02128) [DOI] [Google Scholar]
- 51.Ridgely RS, Greenfield PJ. 2001. The birds of Ecuador, volume II: field guide. London, UK: Christopher Helm. [Google Scholar]
- 52.Checa MF, Rodriguez J, Willmott KR, Liger B. 2014. Microclimate variability significantly affects the composition, abundance and phenology of butterfly communities in a highly threatened Neotropical dry forest. Fla. Entomol. 97, 1–13. ( 10.1653/024.097.0101) [DOI] [Google Scholar]
- 53.Willmott KR, Freitas AVL. 2006. Higher-level phylogeny of the Ithomiinae (Lepidoptera: Nymphalidae): classification, patterns of larval hostplant colonisation and diversification. Cladistics 22, 297–368. ( 10.1111/j.1096-0031.2006.00108.x) [DOI] [PubMed] [Google Scholar]
- 54.Scott JA. 1976. Mate locating behavior of the western North American butterflies. J. Res. Lepid. 14, 1–40. ( 10.1016/B978-0-12-024914-5.50008-5) [DOI] [Google Scholar]
- 55.Stotz DF, Fitzpatrick JW, Parker TA, Moskovits DK. 1996. Neotropical birds: ecology and conservation. Chicago, IL: Chicago University Press. [Google Scholar]
- 56.Drummond BA. 1976. Comparative ecology and mimetic relationships of ithomiine butterflies in Eastern Ecuador. PhD thesis, University of Florida, Gainesville, FL. [Google Scholar]
- 57.Mallet J. 2001. Causes and consequences of a lack of coevolution in Müllerian mimicry. Evol. Ecol. 13, 777–806. ( 10.1023/A:1011060330515) [DOI] [Google Scholar]
- 58.Chouteau M, Angers B. 2012. Wright's shifting balance theory and the diversification of aposematic signals. PLoS ONE 7, e34028 ( 10.1371/journal.pone.0034028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown KS, Benson WW. 1974. Adaptive polymorphism associated with multiple Müllerian mimicry in Heliconius numata (Lepid. Nymph.). Biotropica 6, 205–228. ( 10.2307/2989666) [DOI] [Google Scholar]
- 60.Srygley RB, Chai P. 1990. Predation and the elevation of thoracic temperature in brightly-colored, neotropical butterflies. Am. Nat. 135, 766–787. ( 10.1086/285073) [DOI] [Google Scholar]
- 61.Chazot N, Willmott KR, Paola SE, Toporov A, Hill R, Jiggins C, Elias M. 2014. Mutualistic mimicry and filtering by altitude shape the structure of Andean butterfly communities. Am. Nat. 183, 26–39. ( 10.1086/674100) [DOI] [PubMed] [Google Scholar]
- 62.Agrawal AA, Fishbein M. 2006. Plant defence syndromes. Ecology 87(Suppl.), S132–S149. ( 10.1890/0012-9658%282006%2987%5B132%3APDS%5D2.0.CO%3B2) [DOI] [PubMed] [Google Scholar]
- 63.Bursell J, Dyck J. 2003. Background matching and evolution of cryptic colors of selected passerines in deciduous woodlands. Lundiana 4, 511–559. [Google Scholar]
- 64.Caro TM, Graham CM, Stoner CJ, Vargas JK. 2006. Adaptive significance of antipredator behavior in artiodactyls. Anim. Behav. 67, 205–228. ( 10.1016/j.anbehav.2002.12.007) [DOI] [Google Scholar]
- 65.Cooper WE, Whiting MJ. 2007. Universal optimization of flight initiation distance and habitat-driven variation in escape tactics in a Namibian lizard assemblage. Ethology 113, 661–672. ( 10.1111/j.1439-0310.2007.01363.x) [DOI] [Google Scholar]
- 66.Fine PVA, Miller ZJ, Mesones I, Irazuzta S, Appel HM, Stevens MHH, Sääksjärvi I, Schultz JC, Coley PC. 2006. The growth-defence trade-off and habitat specialization by plants in Amazonian forests. Ecology 87(Suppl), S150–S162 ( 10.1890/0012-9658%282006%2987%5B150%3ATGTAHS%5D2.0.CO%3B2) [DOI] [PubMed] [Google Scholar]
- 67.Mikolajewski DJ, De Block M, Rolff J, Johansson F, Beckerman AP, Stoks R. 2010. Predator-driven trait diversification in a dragonfly genus: covariation in behavioral and morphological antipredator defence. Evolution 64, 3327–3335. ( 10.1111/j.1558-5646.2010.01078.x) [DOI] [PubMed] [Google Scholar]
- 68.Petrin Z, Schilling EG, Loftin CS, Johansson F. 2010. Predators shape distribution and promote diversification of morphological defences in Leucorrhinia, Odonata. Evol. Ecol. 24, 1003–1016. ( 10.1007/s10682-010-9361-x) [DOI] [Google Scholar]
- 69.Walsh MR, Post DM. 2011. Interpopulation variation in a fish predator drives evolutionary divergence in prey in lakes. Proc. R. Soc. B 278, 2628–2637. ( 10.1098/rspb.2010.2634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 71.Abrams PA. 2000. Character shifts of prey species that share predators. Am. Nat. 156, S45–S61. ( 10.1086/303415) [DOI] [PubMed] [Google Scholar]
- 72.Meyer JR, Kassen R. 2007. The effects of competition and predation on diversification in a model adaptive radiation. Nature 446, 432–435. ( 10.1038/nature05599) [DOI] [PubMed] [Google Scholar]
- 73.Nosil P, Crespi BJ. 2006. Experimental evidence that predation promotes divergence in adaptive radiation. Proc. Natl Acad. Sci. USA 103, 9090–9095. ( 10.1073/pnas.0601575103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vamosi SM. 2005. On the role of enemies in divergence and diversification of prey: a review and synthesis. Can. J. Zool. 83, 894–910. ( 10.1139/z05-063) [DOI] [Google Scholar]
- 75.Arbuckle K, Speed MP. 2015. Antipredator defenses predict diversification rates. Proc. Natl Acad. Sci. USA 112, 13 598–13 602. ( 10.1073/pnas.1509811112) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Distribution databases for butterflies and birds recorded in this study are included as electronic supplementary material.