Abstract
Polymeric biomaterials are of specific relevance in medical and pharmaceutical applications due to their wide range of tailorable properties and functionalities. The knowledge about interactions of biomaterials with their biological environment is of crucial importance for developing highly sophisticated medical devices. To achieve optimal in vivo performance, a description at the molecular level is required to gain better understanding about the surface of synthetic materials for tailoring their properties. This is still challenging and requires the comprehensive characterization of morphological structures, polymer chain arrangements and degradation behaviour. The review discusses selected aspects for evaluating polymeric biomaterial–environment interfaces by Langmuir monolayer methods as powerful techniques for studying interfacial properties, such as morphological and degradation processes. The combination of spectroscopic, microscopic and scattering methods with the Langmuir techniques adapted to polymers can substantially improve the understanding of their in vivo behaviour.
Keywords: Langmuir monolayer, biodegradable polymers, air–water interface, protein Langmuir layers
1. Introduction
Biomaterials are used in contact with living tissues or organisms as, for example, in medical devices [1]. Depending on their application, different demands have to be fulfilled concerning the mechanical, physical, chemical or biological functionalities. Polymeric biomaterials are of great importance for medical and pharmaceutical applications as a wide range of properties and functionalities can be achieved by specific tailoring [2–7]. They can be categorized in two groups: naturally occurring and synthetic materials. Alginates, silk and proteins, including collagens, are typical examples of biopolymers derived from natural resources [8]. Typical synthetic polymers in medical applications are aliphatic polyesters, polyanhydrides or polyamides [6,9,10]. Both groups can be further divided into two classes: non-biodegradable and biodegradable polymers. Biodegradable polymers are of special interest because of their applicability for tissue regeneration and drug delivery systems supported by their tailorable mechanical properties, morphological structures [11–13] and degradation behaviour, which are adjustable by the variation of the monomer structure, copolymerization, blending or molecular architecture. The implantation of synthetic materials is orchestrated by a sequence of the body's own (defence) mechanisms that aim to separate the implant from the rest of the body in order to prevent it from doing harm [14]. This physiological response is predominantly mediated by the immune system and should result in the encapsulation of the foreign body. Therefore, the major challenges for the application of biomaterials in medicine are to control foreign body reactions (FBRs) and simultaneously facilitate regenerative processes [15]. Interestingly, FBRs can occur immediately after implantation or be delayed, often by several years, and can have acute or chronic characteristics. The delayed reaction implies that changes occurring on the implant interface, for example, induced by degradation processes can trigger immunological reactions. However, degradation processes, especially enzymatically mediated degradation as occurring in vivo, are insufficiently understood. Furthermore, a description at the molecular level is required to gain better understanding of the interactions between biological systems and synthetic materials occurring at the material–bioenvironment interface. Additionally, the material surface topography plays an important role on the cellular response [16] and is a key factor for the differentiation of mesenchymal stem cells in vitro [17], for example. The interaction of the bioenvironment with polymer materials is commonly investigated by cell-culture techniques and afterwards characterized using, for instance, direct visualization by microscope [18], providing a wide range of information such as morphological changes, detachment forces, kinetics of cell attachment, aggregate size distribution over the time or intra- and extracellular protein expression. However, the interaction of biomolecules with a biomaterial surface at the molecular level is still challenging and requires the comprehensive characterization of surface properties. Several methods to create (ultra)-thin films are described including the self-assembled monolayer method, [19] layer-by-layer (LBL) technique [20,21], coating [22], printing [23] and Langmuir [24] techniques. The LBL technique has received much attention for the preparation of mono- and multilayers due to the mild preparation conditions from solution and the obtained layers reveal a relatively open structure allowing diffusion of components if required. An efficient and precise method to investigate interfaces is the Langmuir technique where monomolecular films are formed at the air–water interface, which are physical models of interfaces with unique properties [25]. They also enable a fast evaluation of degradation processes and a separation of chain scission kinetics from the transport processes during the degradation. Langmuir monolayers also allow the investigation of interaction between a polymer and its environment with a two-dimensional approximation (figure 1). Different types of experimental techniques to determine interfacial tension are known such as the Wilhelmy plate, the pendant drop and the capillary rise technique [26]. Langmuir layers formed through lateral compression enable the control of the film thickness, whereby their packing density, orientation and conformation can be tailored. To gain a deeper insight into interfacial behaviour of macromolecules, the description of thermodynamic and kinetic processes on the surface as well as the adsorption and ordering processes or degradation behaviour can be analysed. By spreading water-insoluble substances at the air–water interface, a Langmuir layer is formed, whereas by injecting substances able to dissolve into the subphase a Gibbs layer is formed by adsorption. The formation of a Langmuir layer from organic solvent occurs by the arrangement of amphiphilic molecules directly at the air–water interface and is studied as a function of the surface area A, whereby Gibbs layers are obtained due to the adsorption of surface-active molecules at the interface and are studied as a function of the surface concentration. The amount of molecules at the interface depends on the equilibrium between adsorption and desorption, which is adjustable, for example, by the pH value, salt concentration, ionic strength, co-components in the subphase and temperature. Both approaches allow the detailed investigation of molecules with biological relevant substances using different physical and chemical analytical techniques.
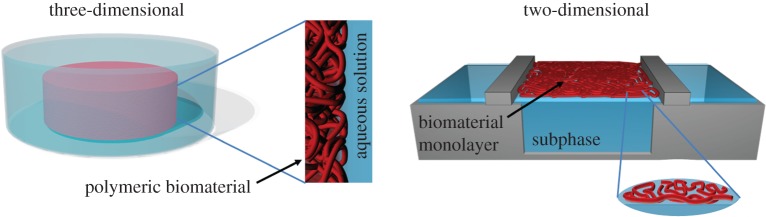
Figure 1.
Three-dimensional biomaterial–environment interface compared to the two-dimensional layer at the air–water interface.
For example, using the Langmuir method the characteristics of protein layers can be manipulated in a very controlled fashion by tuning the molecular packing and two-dimensional order. Thus, conformation transitions or morphological changes of adsorbed proteins depending on interfacial forces or subphase parameters are investigated [27,28]. A clinically relevant example is the activation of the complement cascade by synthetic surfaces in cardiovascular implants or dialysis devices [29–31]. How exactly the proteins arrange at polymeric biomaterial surfaces and what kind of features lead to conformational changes and partial or complete denaturing of proteins are open questions.
Additionally, regarding the degradation of synthetic polymers not only the degradation behaviour of the material surface has to be considered, but also the bioresponse of the released degradation fragments. Applying Langmuir techniques opens different ways to elucidate these questions. First, the formation of Langmuir or Gibbs layers based on polymers allows the investigation of their interfacial properties and the morphological re-arrangement under compression (figure 2a). Additionally, the transfer of such layers onto a solid substrate enables the characterization by diverse techniques and the implementation of cell-culture experiments. Second, the evaluation of interactions between polymer Langmuir layers and subphase components, such as enzymes or degradation fragments, simulates the biomaterial–environment interface in vivo (figure 2b). Third, the investigation of the degradation behaviour of polymers at the air–water interface avoids any diffusion processes and allows studying the degradation processes at the molecular level.
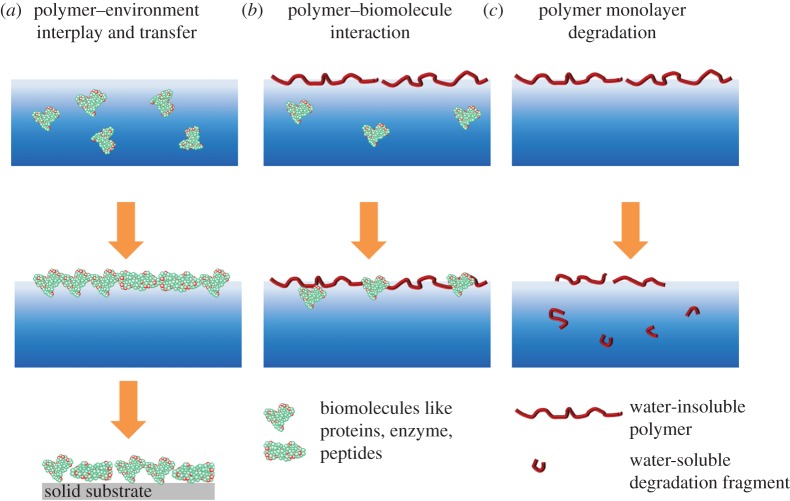
Figure 2.
(a) Well-defined Langmuir monolayers prepared from synthetic or natural (shown) polymers, which can be transferred onto a solid substrate, (b) synthetic biomaterial surface interacting with proteins and (c) polymer layer during degradation. (Online version in colour.)
This review will discuss the potentials of Langmuir monolayer methods to understand polymer monolayers, their degradation behaviour and how they can be used to investigate protein behaviour on implant interfaces. The manuscript focuses predominantly on the preparation and characterization of polymer-based Langmuir layers, while (phospho)lipid layers at the air–water interface, which are frequently discussed as a model system for cell membranes [32–36], are not considered. It is not the aim to completely cover the literature; however, previous reviews and overviews are cited to provide further information on this comprehensive topic. Representative papers have been selected revealing the special interest on protein and polymer orientations at the interface relating to their ability to interact with the environment.
2. Langmuir monolayer techniques
2.1. General principles
The Langmuir monolayer technique is based on the results of the research from Lord Rayleigh and Agnes Pockels at the end of the nineteenth century [37,38]. Basically, at constant temperature the surface pressure–area (π-A) isotherm is recorded by measuring the surface pressure π which is the difference between the surface tension of the pure subphase and the surface tension of the subphase covered by the spread monolayer during reduction or expansion of the covered surface area (A). The shape of the π-A isotherms depend, among other aspects, on the nature of amphiphiles, compression speed, spreading conditions and temperature. For classical amphiphiles, the isotherm can roughly be classified into four different regions and the corresponding transition regions: a two-dimensional gaseous (G) phase, a liquid-expanded (LE) phase, followed by liquid-condensed (LC) and a two-dimensional solid (S) phase, where the maximally reached surface pressure is called the collapse pressure (figure 3) [39,40]. Between the G and LE and the LE and LC phases, first-order transition takes place revealing a (pseudo)plateau region (coexisting region). Not all phases occur for every amphiphile and differ depending on the experimental conditions. In particular, the interpretation of polymeric π-A isotherms is usually more complex because of the macromolecular conformational flexibility and the resulting architecture such as coils, pancakes, crystals and liquid crystals [41–43].
Figure 3.
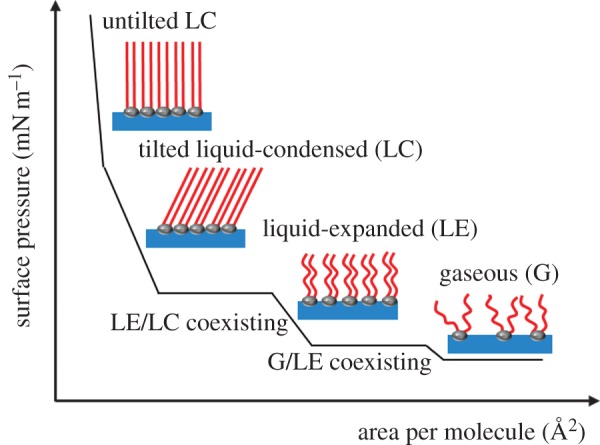
Illustration of a surface pressure–area isotherm for low molecular weight surfactants with their possible phase state. (Online version in colour.)
Low molecular weight amphiphiles, including (phospho)lipids and organic surfactants, are used initially for the formation of Langmuir layers due to their appropriate hydrophilic–hydrophobic balance, enabling the molecular orientation at the air–water interface. In the middle of the twentieth century, the idea of Langmuir monolayers was extended towards the description of the film formation of non-classical amphiphilic materials, such as (poly)peptides [44,45], proteins, [46] polysaccharides [47], nanoparticles [48], dyes [49,50], fullerenes [51–53] and (biodegradable) polymers [54,55].
A variety of sensitive methods enable the in situ characterization of the monolayers at the air–water interface, including microscopy, spectroscopy and scattering techniques. For example, Brewster angle microscopy (BAM) is used to visualize (in)homogeneities in the optical properties of the film by monitoring domain formation or variations in monolayer thickness and other morphological changes [56,57]. Dielectric properties and thicknesses of the monolayers can be obtained by (spectroscopic) ellipsometry [58,59], whereas the structural and orientational changes in the monolayer can be obtained by infrared reflection-absorption spectroscopy (IRRAS) [60–62]. Two types of spectral information are obtained from IRRAS: frequency and intensity changes. Frequency changes provide information about the molecular structure and interactions, whereas the quantitative evaluation of the measured band intensities allows the determination of the chain and group orientations. Grazing incidence X-ray diffraction provides information only about ordered domains within the monolayer [63,64], whereas specular X-ray reflectivity (XR) supplies access to the electron density distribution across the layer, allowing speculation about the monolayer profile constitution.
The transfer of monolayers from the air–water interface onto a solid substrate after the compression into a highly condensed state allows the preparation of organized mono- and multilayer structures with varying layer compositions and orientation and enables the modification of the biomaterial surface directly. A recent review summarized the LB research in surface sciences, physical chemistry, materials chemistry and nanotechnology [24]. The LB technique is also used for the elucidation of membrane surfaces, capsulation technologies, surface patterning, and for electronic and optical applications [65].
By vertical dipping of a hydrophilic or hydrophobic solid substrate into the subphase, the film can be transferred. By repeating the transfer process, different deposition types of the multilayers are formed [66]. However, the transfer is not limited to vertical dipping method. In the Langmuir Schaefer (LS) technique, the film is transferred by horizontal dipping through stamping the solid substrate [66–68]. Moreover, the layer can be transferred from the aqueous subphase onto a solid substrate by gradually lowering the water level relative to the substrate. Compressed Langmuir monolayers are not in their thermodynamical equilibrium resulting very often in morphological changes (e.g. crystallization or aggregation) during and after the transfer process. This has to be considered for interpretation of the LB film structures and properties in comparison with the virgin Langmuir films. Almost all surface characterization methods are suitable for the description of the transferred layer, contributing to the knowledge of the monolayer assembly [24,69,70]. Several methods are used to quantify topography, thickness and morphologies, for example atomic force microscopy (AFM) [71–73], scanning electron microscopy [74,75], transmission electron microscopy (TEM) [76,77] or ellipsometry [78]. Additionally, information on interfacial properties and orientation of molecules are provided by infrared [79], UV-vis and fluorescence spectroscopy [80], X-ray techniques [81] and Fourier transform infrared spectroscopy [82].
2.2. Polymer-based Langmuir layers
A variety of synthetic and natural polymers having different chemical structures are used to form Langmuir layers at the air–water interface. To emphasize the varieties in the chain orientation of polymeric monolayers, three different polymer systems are exemplarily outlined in figure 4. For each system, small changes in the chemical structure, the composition, molecular weight distribution, hydrophilicity or the tacticity result in different chain orientation and packing motifs.
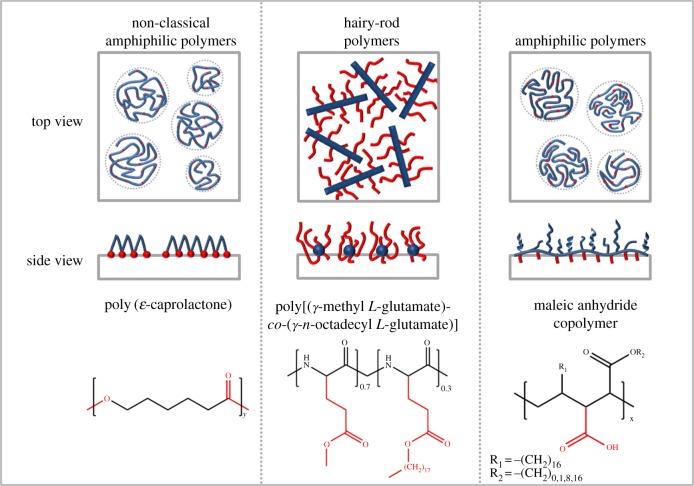
Figure 4.
The interfacial behaviour of synthetic non-classical amphiphilic polymers, hairy-rod polymers and amphiphilic polymers is shown as top and side view in a medium compressed state. One polymer is given as an example for each group by name and chemical structure [83–85]. (Online version in colour.)
In ‘amphiphilic polymers’, each repeating unit has an amphiphilic character given by the ratio of the hydrophilic to hydrophobic parts and forms high ordered structures [86,87]. Polymethacrylates [88] and poly(maleic acid) derivatives are typical polymers in this class. Polymer brushes, where the backbone and the side groups exhibit different hydrophilicity/hydrophobicity, belong to this amphiphilic polymer class. Alternatively, amphiphilic block copolymers composed of a hydrophilic segment, such as polyethylene glycol, and a hydrophobic segment, such as polystyrene, also form stable monolayers [89,90]. Under compression, such block copolymers form brushes at the interface [91]. By contrast, hairy-rod polymers are composed of rigid rod-like back bones surrounded by flexible side chains [92–94]. Cellulose derivatives [92] and glutamates [95], which form stiff α-helices by hydrogen bonding, but also aromatic polyguanamines [96] belong to hairy-rod macromolecules. Unlike hairy-rod and amphiphilic copolymers, the amphiphilic character of polymers such as aliphatic polyesters, polyanhydrides or polycarbonates, which are frequently used in clinical applications, is caused by the presence of hydrophobic parts (aliphatic chains) and hydrophilic binding groups such as ester bounds in the backbone itself. The polar groups of these macromolecules allow their nearly plane arrangement at the interface with a pancake-like structure. Varying the hydrophobic segments between hydrophilic groups in the macromolecular chain influences their spreading behaviour and packing motifs. In the following, we concentrate on those non-classical amphiphilic polymers, namely aliphatic polyesters and proteins.
3. Polymers at the air–water interface and on solid substrate
3.1. Pressure-induced morphological changes in synthetic polymer layers
Poly(ɛ-caprolactone) (PCL)-based materials reveal high degradation stability, whereby the degradation velocity can be varied by copolymerization or by the creation of advanced polymer architectures [97–99]. At the air–water interface, PCL forms closed-packed two-dimensional monolayers below the collapse point [100]. Above this point, crystallization from supersaturated solution occurs, which has been traced by in situ BAM [85]. By varying the compression speed and molecular weight of the PCL, different sized crystals are formed [85,101,102]. Expansion of the film leads to the detachment of the polymer chains from the crystalline domains, which is typically called ‘melt’ process, followed by re-formation of a monolayer. Remaining crystallites serve as nucleation centres for the next crystallization process in the second compression run. Modification of the interfacial properties as well as the film morphology of PCL-based Langmuir films has been performed by blending or copolymerization. The interfacial properties of PCL/polystyrene (PS) blends are dominated by the polyester [103], whereas the film morphology is determined by the blend composition, which is in agreement with structures observed in polymer blends of amphiphilic and non-amphiphilic polymers at the air–water interface [104]. Copolymerization prevents the phase separation. For example, the introduction of urethane junction units results in a decreased crystallization tendency and a closer film packing due to the formation of hydrogen bondings [105]. The introduction of polyethylene glycol (PEO) blocks in linear and grafted block copolymers (PCL-b-PEO) leads to the formation of a transition region before film collapse [106,107]. PEO segments dissolute in the subphase, whereby the PCL segments are still able to crystallize, which is revealed by AFM at transferred LB films. For five-arm star-shaped molecules based on PEO-b-PCL block copolymers, the PEO core units impact the PCL crystal shape in transferred LB layers, but not the crystal height (7.5 nm) [106], which is in the same range as for linear PCL crystals at the air–water interface [85]. AFM investigation of grafted copolymers of PCL and poly(glycerol adipate) (PA-g-PCL) reveals a film thickness of approximately 7.6 nm by reduced crystal size and lower crystallization rate compared to the homopolymers, which approve the high crystallization tendency of PCL chains at the air–water interface [107]. The introduction of hydrophilic poly(p-dioxanone) (PPDO) blocks giving PDC multiblock copolymer leads to an enhanced collapse tendency with increasing PPDO content, which is the result of the increased number of polar groups [108].
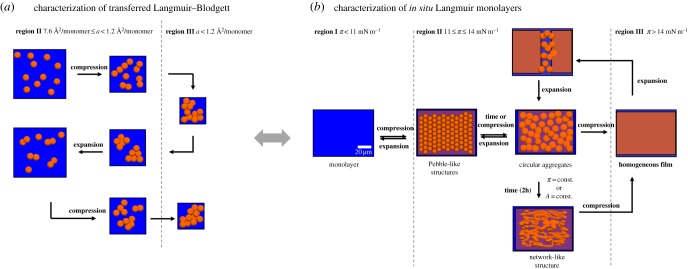
Regarding the interfacial properties of poly(lactic acid) (PLA), two different isomers have to be considered, poly(l-lactic acid) (PLLA) and poly(d-lactic acid) (PDLA) [76,109]. The π-A isotherms for PLLA and PDLA monofilms are identical; however, blends of PLLA and PDLA (rac-PLA; 50 : 50 wt%) reveal monolayers with different interfacial properties due to a different packing of the chains caused by stereo complexation [110]. This different packing of the chains is confirmed by IRRAS investigations, which show distinctions in the conformation of the helical chains in the compressed monofilms. The compression of poly[(rac-lactide)-co-glycolide] (PLGA; 50 wt% rac-PLA) monolayers reveals a more expanded behaviour with a gradual increase of the surface pressure compared with PLA monolayer [111–113]. For PLA layers, a nucleation process is observed during phase transition, while continuous wormlike structures are visualized in the condensed phase by AFM (figure 5a) for PLGAs due to the hydrophobic interactions [112]. In situ BAM investigations of PLGA monolayers confirmed circular aggregates at the air–water interface in the transition state. Moreover, these aggregates are able to form a network-like structure under constant surface pressure and surface area conditions (figure 5b). The comparison of in situ BAM measurements with ex situ AFM images evidences that transferred LB layers do not reflect quantitatively the morphology of a water swollen layer at the air–water interface. A retarded percolation of the monolayer compared to PLGA has been observed for oligo[(rac-lactide)-co-glycolide] (OLGA)-based polyesterurethanes, which are synthesized by the use of urethane junction units as chain extenders [114]. The retarded percolation is caused by a slightly increased stiffness and lower mobility caused by a higher H-bonding capacity, which has, however, no influence on the layer thickness. At the molecular level, the mechanisms responsible for the transient glass transition behaviour have been investigated by X-ray reflectivity and double-wall-ring interfacial rheometry at the air–water interface [115].
Figure 5.
Formation of a PLGA network-like structure (a) during repeated compression–expansion cycles based on Langmuir and AFM investigations and (b) at the air–water interface and its reversibility. The orange circles represent the collapsed domains; the blue and tinted orange background represent the water surface and the flat polymer monolayer area, respectively. (a) Adapted with permission from [112]. Copyright 2012 American Chemical Society. (b) Reproduced with permission from [114] © 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (Online version in colour.)
3.2. Proteins at the air–water interface
Protein-based layers are of high interest for biomedical applications as they interlink the artificial interface and the cellular compartments, including immune cells, stem cells and tissue cells. The majority of the studies investigating protein monolayers used bovine serum albumin (BSA), which is a valid model protein for fundamental studies since it is available in high amounts and well understood, whereas its clinical relevance is rather limited. Recently, in several studies monolayers of other proteins were investigated including extracellular matrix components and immunoglobulins, indicating an increasing interest and expectation to obtain new insights in protein behaviour, which might also be relevant for specific applications. For the preparation of such protein layers, several approaches have been employed including physical adsorption [116] and site-specific immobilization [117].
The interactions of biomolecules and nanomaterials with classical amphiphilic Langmuir monolayers as a model system for cell membranes were recently reviewed [32]. Therefore, the interactions of proteins with amphiphilic Langmuir layers are not considered here. We discuss a few pure protein-based Langmuir layers as representatives for biomacromolecules.
A wide range of proteins, including albumins, are water-soluble, forming Gibbs layer at the air–water interface. The flexible structures of the molecules allow conformational changes depending on the experimental conditions. Different parameters such as subphase pH value, ionic strength, temperature or spreading solvent affect the film stability at the interface [118]. Transfer of the protein layers by the LB technique requires the selection of the optimal experimental conditions in terms of temperature, surface pressure and solid substrate material. Protein solutions with high concentrations lead to unordered and loosely packed protein layers with granule structure, which could partly be denatured hindering the formation of closely packed or ordered arrays [119]. When using proteins for Langmuir monolayer studies, a few possible obstacles need to be considered. First, recombinant proteins generated by overexpression in bacteria, such as Escherichia coli, lack post-translational modifications such as glycosylation. A second aspect to be considered is the purity of proteins, since impurities caused by contaminating proteins or peptides, but also residual bacterial compounds such as endotoxins, could substantially bias the experimental readout.
3.2.1. Bovine and human serum albumin
Albumins are applied in clinical chemistry and cell-culture media [120]. Recently, the two-dimensional behaviour of serum albumins was reviewed summarizing the characterization of protein–lipid interactions, protein–ionic surfactant interactions, protein insertion into monolayers and topographical studies [46]. Owing to its stability, BSA is frequently used as an example protein for fundamental studies, but also for the development of immunosensors and for surface passivation [121,122].
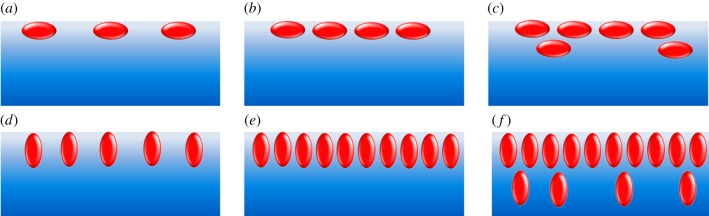
Spreading a BSA solution at the air–water interface results in an immediate jump up of the surface pressure reaching a plateau value within approximately 900 s, independent of the subphase (water, buffer, clay mineral: saponite dispersion) [123]. However, the maximum of the attained surface pressure depends on the type of subphase. Adding clay mineral or buffer, the equilibrium surface pressure increases compared to water. The adsorption process of molecules occurs in two steps. Initially, the BSA diffuses from the subphase to the interface, followed by a re-arrangement of the molecules. Afterwards, BSA molecules are able to incorporate in the existing interface layer increasing the surface pressure to positive values until an equilibrium surface pressure is reached. Figure 6 represents several possible arrangements of globular proteins such as BSA at the air–water interface [124]. Adding phosphate buffered saline (PBS) increases the surface charges and leads to a slightly higher surface tension (53 mN m−1) and increases the layer thickness, while simultaneously decreasing the refractive index and the surface density.
Figure 6.
Scheme of conceivable BSA arrangements at the air–water interface: (a) loosely packed monolayer in a side-on orientation, (b) closed packed monolayer in a side-on orientation, (c) the same as (b) having an additional loosely packed second layer, (d), (e) and (f) represent the orientations of (a), (b) and (c) in edge-on orientation, respectively. Reprinted from [124], Copyright 2003, with permission from Elsevier. (Online version in colour.)
Variation of the subphase pH leads to a modified time to reach the surface pressure equilibrium [125]. Below the isoelectric point (pH 3.8) BSA is positively charged and in its F form, where most hydrophobic residues are located at the outer shell, leading to a high driving force directed to the interface. Close to the isoelectric point (pH 5.1) BSA is in a compactly packed N form and at pH 8.2 (above the isoelectric point) the BSA molecules are negatively charged and in the B form. The B form is more expanded compared with the N form, exposing the less hydrophobic segments to the subphase. The negative surface pressure values after spreading indicate directly that the rearrangement of BSA leads to a reduction of projected surface area per molecule. Surface dilational elasticity measurements do not indicate significant conformational differences of BSA over a broad pH range around the equilibrium surface pressures [126]. At different pH values and temperatures, BSA and β-lactoglobulin mixed monolayers have been evaluated using compression experiments revealing an electrostatic character of the interaction between the proteins [127]. Desorption phenomena during compression have to be considered evaluating the intermolecular forces.
The understanding of the adsorption behaviour of proteins at the interface has been investigated using BSA with sum-frequency generation (SFG) and ellipsometry [128]. The layer thickness of adsorbed BSA reaches its maximum value close to the isoelectric point, revealing the formation of an amorphous multilayer. At the same pH value, SFG amplitudes show a minimum in the BSA and H2O-related bands, which are attributed to the absence of the electric field at the interface at this pH value. Recent developments using SFG for studying different protein layers (BSA, lysozyme and β-lactoglobulin) at air–water interfaces have been summarized under consideration of the interpretation of SFG spectra [129].
The effect arising from different BSA concentrations or denaturation on the adsorption behaviour, the surface tension is investigated by tensiometry, ellipsometry and IRRAS [124]. The steady-state surface tension of 50 mN m−1 (water) is basically independent of concentration but decreases dramatically after thermal denaturation (40 mN m−1) caused by different hydrophobicities. Ellipsometry and IRRAS revealed a higher surface density and layer thickness (up to 16 nm) with increasing concentration. Ellipsometry (change in the thickness 9–16 nm and refractive indices 1.42–1.39) and IRRAS support the assumption that at higher concentrations a second BSA layer is formed.
The amino acid sequence of BSA and HSA, which share 76% sequence homology, has no major impact on the overall range of surface excess (Ã). This is related to the surface area, the Avogadro's constant (Ã = 1/A Na) and the thickness of the layers [130]. For HSA, the extrapolated surface area at the zero surface pressure is in the range of 1 m2 mg−1 [131]. The π-A isotherm exhibits a pseudo-plateau between 19 and 24 mN m−1, which is attributed to a change from an unfolded to a coiled conformation. BAM investigations reveal very small bright circular domains at low surface pressures (π = 2.5 mN m−1), which grow by compression and form grouping rows (π > 19.2 mN m−1) in the pseudo-plateau region caused by the packing of ‘loop’ structures. The relative film thickness d increases slowly under compression until π = 15 mN m−1. At higher surface pressures, the fast increasing of d corresponds to packing of the ‘loops’ achieving a maximum value of 4 nm.
The structure of BSA and HSA monolayers adsorbed at the air–water interface has been studied by neutron specular reflection [130,132]. The surface excess Γ increases sharply with increasing concentration where 5 × 10−2 g dm−3 tends to be the respective saturation limit at pH values close to the isoelectric point. The determined protein layer thicknesses are in the range of the short axial length of the globular protein solution structure (40 Å) over almost all investigated conditions, suggesting an adsorption of the molecules with their long axes parallel to the water surface. The macroscopic film morphology and the spreading dynamics of the defatted form of HSA have been investigated by ellipsometry, neutron reflectometry, X-ray reflectometry and BAM, revealing an alteration from a loose network to a more homogeneous film with increasing concentration [133].
The in situ conformational changes in protein layers (β-casein, BSA, lysozyme and fibrinogen) at the air–water interface have been investigated by circular dichroism (CD) spectroscopy technique. The existing α-helices of BSA in solution vanished at the air–water interface and a disordered protein layer is formed. Lysozyme and fibrinogen, being α + β-type proteins in solution, exist in β-sheet conformation at the air–water interface [134].
The fibril formation in BSA and human insulin (HI) monolayers has been evaluated by epifluorescence microscopy at the air–water interface as a faster, cheaper and easier to use alternative to CD spectroscopy and AFM [135]. Time-dependent π-A isotherm characterization reveals a shift of the lift-off point to smaller surface areas (from 3000 to 2500 Å2) within 48 h, indicating a slightly decreased fibrillization.
The BSA unfolding is studied by dilational surface rheology at the interface using guanidine hydochloride (G.HCl) to determine the critical denaturant concentration [126]. Low concentrations of G.HCl (less than or equal to 0.2 M) lead to a decrease in the electrostatic adsorption barrier. Increasing G.HCl concentrations result in changes in the shape of the dynamic surface elasticity curves due to the formation of loops and tails in the surface layer. The maximum of the dynamic surface elasticity indicates the destruction of the tertiary and secondary structures of BSA at the interface, which takes place at lower concentrations compared to the bulk phase. The unfolding process leads to a re-spreading of the macromolecules requiring a larger surface area. BSA transferred at 10 mN m−1 yields a homogeneous layer of protein particles with a diameter of 25 nm and a height of below 5 nm [119] representing the individual molecules (4 × 4 × 14 nm3) [123]. BSA and glutaraldehyde (GA)-treated BSA layers at the air–water interface have been transferred to a solid substrate regarding the ability to react with A-β-galactosidase [136]. The change in the absorbance for GA-treated BSA layer reveals the formation of an antibody film at the surface.
3.2.2. Other proteins
The addition of ionic surfactants has a strong impact on the kinetic dependence of the dynamic surface properties, as shown for β-casein [137]. The hydrophilic and hydrophobic parts of β-casein form loops and tails at the interface, while for β-casein/ionic surfactant mixtures the β-casein is displaced from the adsorption layer. Fibronectin (FN) is a glycoprotein of the ECM, which facilitates the binding of cells. The impact of Ca2+ and Na+ ions and the pH value on a fibronectin monolayer is compared to the monolayer behaviour of bovine submaxillary mucin (BMS) [138]. The specific molecular area is unchanged for BMS by alteration of the ionic strength, but, for fibronectin, the specific molecular area increases linearly with the square root of the ionic strength. The behaviour of FN is attributed to an unfolding structure due to the diminished electrostatic intramolecular interaction. Both proteins exhibit a change in the specific area depending on the pH value.
FN monolayers of high molecular cohesion with defined surface density have been transferred onto polydimethylsiloxane (PDMS) substrates by the LS-method to improve stem cell adhesion behaviour [139]. LS layers are shown to be uniform and homogeneous as indicated by AFM and immunofluorescence images, whereas layers prepared by solution deposition method are rather heterogeneous with the appearance of resembling protein aggregates. Owing to the well-defined organization of the FN LS layers, human mesenchymal stem cells seeded on PDMS showed a reduced absolute number of adherent cells, but a higher and more homogenously vinculin expression in comparison with a PDMS surface equipped with a solution deposition layer, indicating a more in vivo like behaviour.
Lysozymes form stable and homogeneous layers at the air–water interface, which can be adjusted by the pH value and the salt concentration [140]. The protein adsorption rate decreases with increasing the positive charge of lysozyme [141]. The equilibrium time period depends on the subphase ion in the following order: buffer < water < saponite dispersion. The homogeneity of the layer is proved by π-A isotherms, UV-vis and fluorescence spectroscopy [123]. In time-dependent experiments, a decrease in the surface pressure from 0 to −1 mN m−1 during the first hour is attributed to the solubility of the lysozyme molecules or exchanges with subphase components. Afterwards, π increases within 3–10 h reflecting the formation of a monolayer.
The effect of Hofmeister anion (NaX, X = I, Br, Cl, F) on the adsorption kinetics of the positively charged lysozyme (below the isoelectric point of 11.35) is evaluated by surface tension measurements and time-resolved X-ray reflectometry [141]. The presence of salt increases the protein adsorption rate following an inverse Hofmeister series caused by the interaction of strongly polarized halide anion Br- with the local electric field of the layer which is not the case for F−. The initially adsorbed lysozyme molecules have a flat unfolded structure on a pure water subphase. Adding salt leads to the unfolding of the lysozyme molecules during adsorption as a result of protein–protein rearrangements in the following order F− < Br− ≈ I− < Cl−.
External reflection FTIR spectroscopy has been used to investigate and to compare the conformational changes of three globular proteins, lysozyme, BSA and β-lactoglobulin, adsorbed at the air–water interface [142]. The surface pressure kinetics of the lysozyme adsorption is relatively slow, equilibrium surface pressure is reached after about 2.5 h, while the secondary structure alters within 10 min. Lysozymes form network-like adsorbed structures of unfolded protein layers composed of high content of antiparallel β-sheets. The structural change and the slow gradual adsorption rate are caused by hydrophobic interactions and are associated with a preferred adsorbed orientation or changes in the protein structure.
By contrast, the native structure of BSA and β-lactoglobulin remains stable after the fast adsorption (equilibrium time approx. 10 min) to the interface, which is probably the reason for their higher emulsion efficiency.
The interfacial rheological properties, the protein conformation, and the interaction between adsorbed proteins are reviewed for a variety of proteins such as casein, ovalbumin and β-lactoglobulin [143,144]. For several positively and negatively charged proteins, it has been shown that the adsorption kinetics are strongly influenced by the energetics of interaction of proteins with the interface and not only diffusion-controlled [145]. In general, positively charged proteins exhibit adsorption rates, which are an order of magnitude slower than their respective bulk diffusivities due to the energy barrier for adsorption on the surface. In contrast, for negatively charged proteins the adsorption rates are in the same range than their bulk diffusivities due to their attraction towards the air–water interface. X-ray and neutron reflectometry measurements revealed that adsorbed β-lactoglobulin keeps its globular structure and forms monolayers at the interface (layer thickness 36 Å) [146].
AFM investigations of LB films reveal that the monolayers of lysozyme are homogeneously composed of small, soft round aggregates with a diameter of 25 nm and a height of below 3 nm when transferred at 5 mN m−1 [123].
3.2.3. Peptides
Besides proteins or enzymes, a variety of oligopeptides and polypeptides have been investigated at the air–water interface [44,147,148]. Because of the complexity, only a few examples related to oligopeptides and polypeptides are mentioned here. A more detailed summary of the interactions of β-amyloid-, antimicrobial-, fusion peptides and peptides responsible for protein interaction with membranes has recently been published [32]. Rod-like α-helical polypeptides based on poly-γ-alkyl-l-glutamate form aggregates at the air–water interface during spreading [149,150]. With increasing surface density, poly-γ-benzyl-l-glutamate (PBLG) forms solid-like films with increasing fibre diameters [150]. Moreover, the morphological variation of a stimuli-responsive elastin-like polypeptide (ELP) is investigated as a function of temperature and compression [151]. Stable ELP layers are formed at the air–water interface, whereby an increasing temperature induces a shift of the isotherm to larger surface areas indicating a phase transition followed by the formation of aggregates. The temperature changes of the secondary structure are investigated by surface-enhanced Raman (SERS) scattering and with atomic force microscopy for LB layers. By circular dichroism (CD) spectroscopy, it is shown that a temperature increase from 21.5°C to 41.5°C leads to the formation of a plateau region in the π-A isotherm caused by a change in the helix orientation [152]. Based on the results obtained from π-A isotherm, CD spectroscopy and AFM, a structural model is derived where the horizontally oriented helices partially alter their orientation to vertical upon compression in the plateau region.
The most important pharmaceutical peptide for diabetes treatment is insulin. To understand the physical/chemical properties of insulin at interfaces, the investigation of its aggregation behaviour is important. The recent progress in this field is summarized for different interfaces focusing the Langmuir technique [153]. Shortly, insulin forms homogenous layers at the air–water interface after spreading on a pure subphase, which is loosely packed before compression. No aggregates are observed by BAM and fluorescein isothiocyanate-labelled insulin epifluorescence [154]. Moreover, zinc concentration, pH, ionic strength, and proteins in the subphase lead to aggregation of insulin at the air–water interface. Spread from acid solution insulin forms homogeneous layers at 20°C [154]. Acidic pH values (pH = 1) result in a more expanded conformation compared to pH 5.7, indicating the presence of an increased amount of monomeric insulin. By contrast, association of insulin molecules has been observed at pH 10 forming a more compact and rigid monolayer with smaller mean molecular surface area.
The human insulin (HI) Langmuir monolayers are investigated in the presence and absence of Zn(II) ions depending on the subphase pH value, revealing different conformation by spectroscopic data [153,155]. The addition of zinc ions influences the lifting up of the molecular surface area and the transition state [156]. Surface pressure–area and surface potential-area isotherms suggest aggregation of the HI molecules at the air–water interface in the presence of Zn(II) ions [155]. Infrared absorption and CD spectroscopy reveal a higher content of β-sheet and β-strands, as aggregation occurred under basic conditions. Compression–decompression cycles show that the HI aggregates are not stable and tend to spread to a monomolecular monolayer, which is in accordance with UV-vis and fluorescence spectroscopy analysis. IRRAS obtained mainly α-helix and less β-sheet structures at different surface pressures; however, the spectral bands differ from those obtained in solution. Reasons for the conformational change of the insulin probably are a lower degree of freedom of the molecules and misfolding of the hydrophobic residues and their exposing to the air, while the hydrophilic segments submerge into the subphase.
4. Polymer–protein interactions at the air–water interface
The Langmuir technique proved to be a powerful tool for the investigation of in situ molecular interaction between the surface layer and subphase compounds. The interaction between polymer and biomolecules leads to functional changes of the material–environment interface. Such modification can be investigated by applying the Langmuir monolayer approach. For example, the surfaces of a spin-casted PLA film, functionalized by LB-layers of amphiphilic AB or ABA block copolymers of PLA as A block and either PEO, α-methoxy-ω-hydroxy PEO, α-carboxy-ω-hydroxy PEO or poly(l-aspartic acid) as B block, improve the protein and cell adhesion [157]. The simulation of the biomolecule impact, such as proteins or enzymes, on a pre-existing synthetic polymer layer is discussed in this section on the microscopic and macroscopic level.
PLA is often used in medical applications and for tissue regeneration [158,159]. Its hydrophobic character is the reason for non-specific protein adsorption. The modification of the interfacial properties of PLA has been shown using Langmuir monolayer technique by applying mixed monolayers with hydrophilic PEO-polypropylene oxide triblock copolymers (PEO-b-PPO-b-PEO) [160]. Thus, the interaction with BSA molecules (adsorbed/penetrated) at the air–water interface of the PEO-b-PPO-b-PEO-modified-PLA monolayer is tuned by changing the surface density and chain length of PEO. The hydrophilization with long PEO segments reduces the protein adsorption compared to pure PLA monolayers due to the formation of a thick (twice as thick as a radius of gyration RG) PEO layer immersed into the subphase.
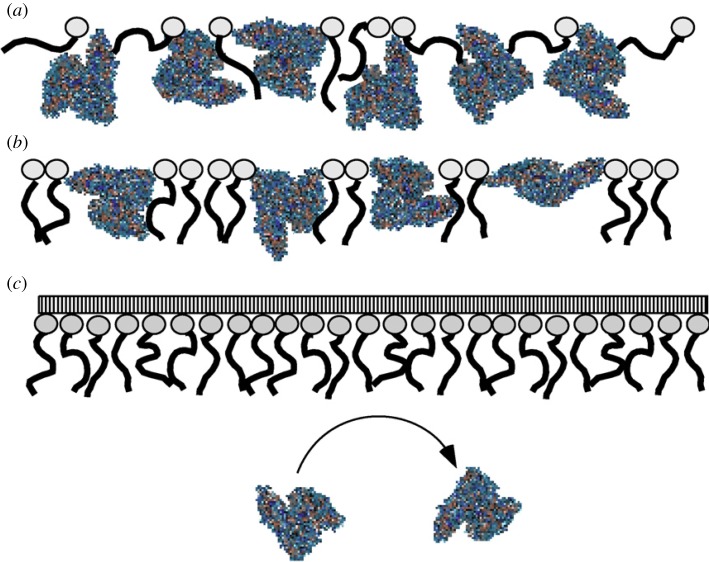
The formation of poly(styrene)-b-poly(ethylene oxide) (PS-b-PEO) monolayers inhibits the HSA adsorption to the air–water interface [161]. The layer thickness of PS-b-PEO monolayer in the presence and absence of albumin is determined by in situ surface plasmon resonance (SPR) measurements. The closed packed PEO ‘brushes’ operate as a shield and prevent albumin adsorption. An effective inhibition is obtained only after the formation of brush-like structures, as illustrated in figure 7. At lower PEO surface density, the surface pressure kinetics of albumin insertion reveals two different situations: albumin binds to the air–water interface very rapidly when PEO is below the ‘pancake-brush’ transition and is able to induce this transition. Above the transition, albumin penetrates in between free spaces of the PS-b-PEO monolayer.
Figure 7.
Possible description of the HSA penetration into a PS-b-PEO monolayer at different surface densities (a) at low packing density of PS-b-PEO: HSA easily penetrates into the monolayer, (b) medium packing density of PS-b-PEO: the HSA adsorption leads to a pancake–brush-transition and (c) high packing density of PS-b-PEO: PEO brushes inhibit HSA penetration. Reprinted from [161], Copyright 2007, with permission from Elsevier. (Online version in colour.)
Mixed monolayers based on PLA50 and BSA are studied to evaluate the film organization considering composition and compression state, whereas the influence of each component is successfully described by the Maxwell's model [162]. Up to approximately 15 mN m−1 (phase transition), the shape of the isotherm is governed by PLA50. During the phase transition of PLA50, a continuous BSA phase is formed from then on defining the rheological properties of the monolayer. AFM of corresponding LB films transferred at 16 mN m−1 shows separate aggregates on a homogeneous layer, suggesting a condensed PLA50 phase. Langmuir and LB experiments suggest that BSA molecules are present between PLA50 aggregates, preventing the interaction of the polyester molecules [76].
Concerning the degradation of polymer layers at the air–water interface, the interaction of polymer layers with enzymes and degradation fragments is a major topic. It is assumed that degradation fragments immediately dissolve into the subphase and have not been considered in the degradation process anymore. Recently, the impacts of the intermediate hydrolysis products of PLGA consisting of both water-soluble and water-insoluble components have been investigated [163]. Monolayers based on water-insoluble degradation OLGA fragments reveal highly compressible film behaviour, which significantly differs from the complex behaviour observed for pristine PLGA. An increased elasticity modulus of the Langmuir layer is obtained as a consequence of improved packing abilities and increased water-solubility. Water-soluble degradation fragments are surface-active. On the contrary, lactic acid and glycolic acid do not influence the surface tension of water, but are able to form weakly interacting by H-bonds reducing the required monolayer surface area by looping effect in combination with a PLGA layer.
5. Langmuir monolayer degradation of polymer layers
5.1. General principles
Biodegradation of polymers is usually observed by time-consuming in vitro or in vivo methods using three-dimensional samples considering diffusion processes for the evaluation of the degradation kinetics. Using Langmuir monolayer methods, all polymer chains are in direct contact with the air–water interface. Therefore, diffusion processes of the degrading components (like water) in the surrounding fluid and the degradation fragments out of the sample are excluded as kinetically relevant. By applying the Langmuir monolayer degradation (LMD) technique, the hydrolytic as well as enzymatic degradation of polymers can be investigated. Degradation conditions can easily be varied (temperature, pH value, enzyme addition, salt type and concentration) allowing a versatile access to the degradation properties of plenty of different materials. In this section, the degradation of polymer layers at the air–water interface is considered.
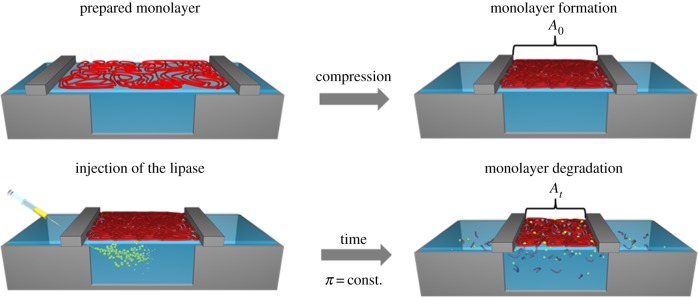
During LMD experiments, the reduction of the covered surface area with time is recorded under constant surface pressure (barostatic) conditions. The experimental setup for an enzymatic degradation experiment is shown in figure 8. In the first step, a closed packed monolayer is formed by compression of the polymer film, which is typically the case at the inflection point of the π-A isotherm. The hydrolysis of esters, amides or other bonds in the polymer main chain results in the formation of water-soluble low molecular weight fragments (oligomers and monomers), which are able to leak out of the polymer monolayer into the subphase. To keep the surface pressure constant, the surface area has to be decreased as a result of the reduced amount of molecules at the interface. This time-dependent surface area reduction indicates the degradation process of the polymer. The depiction of the area reduction versus the time is called degradation curve, allowing the correlation between the reduction of the covered surface area and the formation of water-soluble molecules. Different descriptions are used for the surface area reduction data. In a simplified way, the surface area reduction is represented only by dividing the covered surface area (At) after the degradation time interval t by the initially required surface area of the compressed monolayer (A0) [164]. Alternatively, the surface area reduction is described by the total area loss (ΔA(t)) at a certain time point where ΔA(t) is the difference between A0 and At [165]. Based on this, the relative area change ΔArel(t) is given as follows:
| 5.1 |
Figure 8.
Schematic of the implementation of an LMD experiment at the air–water interface: monolayer formation by compression, injection of the enzyme into the subphase beneath the monolayer and monolayer degradation by fragmentation under constant surface pressure conditions. A0 and At correspond to equation (5.2).
Applying the dynamic fragmentation model, the corrected surface area reduction (ΔAcorr(t)), calculated according to equation (5.2), ΔArel(t) is often used for the description of the degradation kinetics. This model considers the correction of small surface areas value [166].
| 5.2 |
By applying this modelling approach, two degradation mechanisms can be distinguished based on the characteristic release kinetics of water-soluble degradation fragments leaving the air–water interface into the subphase: random chain scission and chain-end cut mechanism [166].
In analogy to the bulk degradation, random chain scission is characterized by an exponential slope, and chain-end scission mechanism by linear slope (figure 9) [167,168]. Considering the bond cleavage mechanisms of macromolecules at the air–water interface, the hydrolytic degradation and enzymatic degradation play the most prominent role. Therefore, the kind of polymers, which are applicable for the monolayer degradation approach is limited, and most established examples belong to the family of polyesters, like PLA- and PCL-based (co)polymers.
Figure 9.
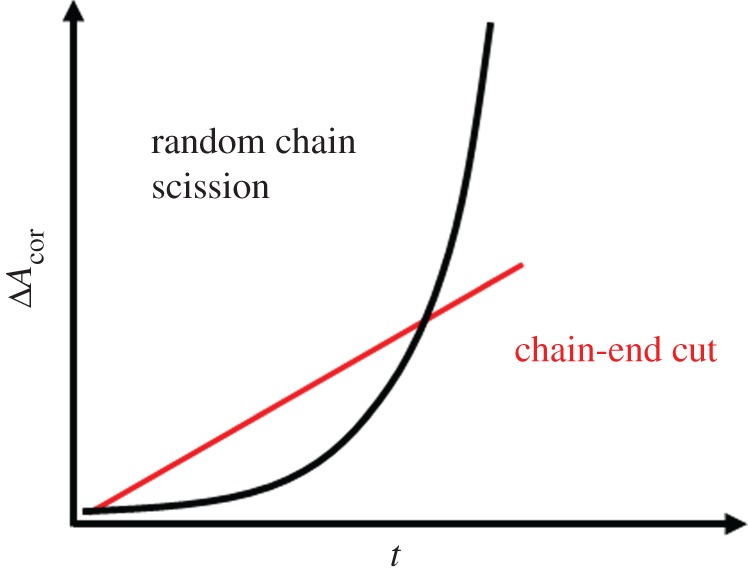
Schematic of two-dimensional degradation curve by random chain scission and chain-end cut mechanism. (Online version in colour.)
5.2. Langmuir monolayer degradation investigations of polyester-based monolayers
The degradation of polyesters and blended polyesters monolayers has been analysed with an emphasis on the composition, the degrading medium and its pH value. A sigmoid-shaped degradation curve is obtained for the hydrolytic degradation of rac-PLA monolayers using the change of surface area ratio (A/A0) on an alkaline subphase (pH = 10.5) [169]. Moreover, the extent of surface area ratio reduction increases with increasing surface pressure (π = 4, 7, 10 mN m−1). Under alkaline conditions, an instantaneous linear surface area reduction is obtained in LMD experiments (ΔAcorr(t)) revealing a chain-end scission mechanism for rac-PLA and PLGA [165]. These results are supported by three-dimensional degradation investigations of oligolactides terminated with hydroxyl functions, which are preferably hydrolytic degraded by a backbiting mechanism [170].
Alkaline pH values of the subphase are necessary to increase the degradation velocity in a time period suitable for the Langmuir technique in the case of PLA. At acidic pH values (pH 1.9 and 3.5), no degradation is observed within a time range of 100 min [169,171]. Particularly, for PLA-based materials, the specification of the stereochemistry has a comprehensive effect on the biological and physical properties. Three- and two-dimensional degradation reveals a significant faster degradation of amorphous rac-PLA compared to the semi-crystalline PLLA and PDLA [172,173]. Moreover, reduced hydrolysis rates of PLLA/PDLA stereocomplexes arise from a higher degree of crystallinity as well as a stronger interaction in the formed stereocomplex compared to PLLA homopolymer [174]. Stereocomplex formation in enantiomeric PLA Langmuir monolayers also causes stereo-selective enzymatic degradation by proteinase K [173], where equimolar mixed monolayers of PLLA and PDLA blends degrade substantially slower compared to its homopolymers. The blending of crystallizable polymers like PLLA and PCL results in a faster degradation of the layer revealing the importance of the orientation and crystallization behaviour [164,175]. In blends, the hydrolysis is enhanced by the decreased order of the chains in the monolayer. The two-dimensional enzymatic hydrolysis of PLA-co-PEO occurs with constant hydrolysis rates in accordance with the random fragmentation mechanism predicted for PLA. Accumulation of charged, water-insoluble PLA fragments is detected by surface potential measurements [176]. Considering the uniqueness of polymers, it is obvious that the degradation velocity depends on their molecular weight. For low molecular weight polymers, the surface area reduction occurs faster due to the faster formation of water-soluble degradation fragments, which has been confirmed for PLA- and PCL-based Langmuir monolayer in accordance with three-dimensional degradation studies [165,177]. For PCL-based monolayers, the largest water-soluble degradation fragments are tetramers [178].
The hydrolytic chain scission rate of random PLGA copolymers increases compared to rac-PLA at the air–water interface due to the introduction of more hydrophilic PGA units as it is known from bulk investigations [171,179]. In contrast, the slightly more hydrophobic poly-(R)-3-hydroxybutyrate (P3HB, composed of an additional CH2-group per monomer unit) forms more hydrolytically stable monolayers [180,181], which is attributed to a reduced submerging of the polymer chains into the subphase. The access of the functional units defines the ability of interaction between polymer backbone and a biomolecule regarding the degradation behaviour as well as for adsorption studies. In two-dimensional enzymatic degradation studies of P3HB and PLA, a reduced enzymatic degradation velocity compared to alkaline degradation is the result of a lower accessibility of the functional units caused by the large size of the enzyme compared to the alkaline ions [180]. The PCL backbone is more hydrophobic compared to PLA and P3HB, which results in significant longer hydrolytic degradation times [164,175,182], but the enzymatic degradation is accelerated by a variety of enzymes [178,183,184]. A random chain scission of the PCL chains has been obtained at the air–water interface using the lipase from Humicola lanuginosa and the lipase from Pseudomonas cepacia [166,178,185]. Surface potential measurements reveal charged degradation fragments at the interface [178].
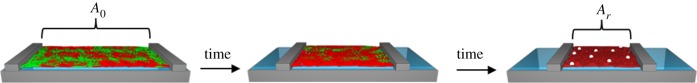
The degradation behaviour of PCL is modified by the introduction of enzymatic non-degradable (related to the lipase from Pseudomonas cepacia) segments. The multiblock copolymers (PDC) composed of PCL and PPDO segments linked by TMDI reveal an almost linear weight loss, which is described by a random chain scission process using a dangling chain model [108]. The enzymatic degradation of the PCL segments proceeds faster compared to the hydrolytic bond cleavage in the PPDO blocks. By changing the chemical composition (the ratio of PPDO to PCL segments), the degradation rates are adjusted in two-dimensional as well as in three-dimensional degradation experiments [186]. Regarding the enzymatic degradation of copolymer-based monolayers composed of degradable and of hydrophobic, non-degradable segments, the polymer–enzyme interaction behaviour has to be considered. Using the example of multiblock copolymers, PDLCL constituted of oligo(ɛ-caprolactone) (OCL) segments and the hydrophobic oligo(ω-pentadecalactone) (OPDL) segments, which are non-degradable by this specific lipase from P. cepacia (figure 10). The introduction of a hydrophobic segment (OPDL) reduces the degradation rates of PCL-based multiblock copolymers (PDLCL) as a result of polymer–enzyme interactions [187]. At the beginning, the copolymer forms a homogeneous layer consisting in equal shares of OCL and OPDL segments requiring the surface area A0. With ongoing time, the amount of OCL fragments at the interface decreases due to the formation of water-soluble OCL degradation fragments by enzymatic bond cleavage. As OPDL cannot be degraded by the lipase from P. cepacia, a defined surface area has to remain (Ar) after the entire degradation of the OCL segments. Moreover, the surface-active lipase is able to interact with the non-degradable OPDL segments. Therefore, Ar is the sum of the required surface area of OPDL segments and the surface-active lipase. The enrichment of lipase at the polymer–water interface induces morphological changes expanding the required surface area of the polymer layer [187]. Consequently, the surface area versus time correlation of the PDLCL monolayer consists of the induced change of the required surface area due to the polymer–enzyme interactions (OPDL segments) and the formation of water-soluble degradable fragments (OCL segments).
Figure 10.
Depiction of the PDLCL degradation at the air–water interface as a result of two parallel processes: film degradation (green OCL segments) and polymer–enzymes interaction (red OPDL segments). The enzyme is represented by the white bullets.
In contrast to PPDO segments, the OPDL segments stay at the air–water interface independent of the degree of degradation of the OCL segments enabling unrestricted interaction with the surface-active lipase decreasing the apparent surface area reduction. While the PPDO segments promote the surface area reduction in PDC, for PDLCL the formation of water-soluble fragments is reduced, since small OCL degradation fragments, not connected to OPDL segments, can be solved in the subphase only.
For the degradation mechanism as well as for the interaction with other molecules in the subphase, the influence of the end groups is an important topic in three- as well as in two-dimensional systems. Functional end groups, for example, can act as linker units and therefore support/prevent adsorption of molecules to the interface due to sterically hindrance or hydrophilic/hydrophobic interaction/repulsion, respectively. For PLA monolayers based on end-capped and non-end-capped polymers, the same degradation rates are obtained revealing the influence of transport process in bulk degradation investigations, which can be neglected in two-dimensional systems [165]. End-capped OCL with phenylboronic acid pinacol ester or phenylboronic acid are investigated using the LMD technique regarding the enzymatic degradation behaviour [188]. While the layer compression behaviour of end-capped OCL layers is less affected, the injection of the lipase from P. cepacia leads to a retarded film degradation compared to pure OCL layers by incorporation of the enzyme molecules into the layer due to end group effects.
Besides hydrophilicity and end groups, the polymer architecture affects the degradation behaviour. Using star-shaped PCLs, the overall hydrophilicity is increased, while the molecular weight is constant [189]. The increase of the hydrophilicity of star-shaped polymers has an impact on the chain packing behaviour and therefore on the degradation behaviour of the monolayer. Moreover, the chain scission by the lipase from P. cepacia is prevented by the introduction of randomly distributed urethane linkers in the PCL chain.
6. Summary and outlook
This article provides an overview of the capabilities of Langmuir techniques for elucidating the polymeric biomaterial–environment interface. It is focused on polymer films at the air–water interface to study the interaction with proteins as well as the ability of proteins to form Langmuir or Gibbs layers interacting with other substances in the subphase. The Langmuir monolayer technique reveals a high potential for investigating both morphological and degradation behaviour of natural and synthetic polymers, evolving several of new attractive topics. The interfacial properties of polymers are significantly influenced by their composition and architecture, which determine crystallization and aggregation processes. The Langmuir monolayer degradation technique provides monomolecular layers at the interface, which allows their investigation excluding diffusion processes. The LMD approach refers to the formation of water-soluble degradation fragments, based on bond cleavages in the polymer backbone and their submerging of low molecular weight fragments into the subphase. The impact of hydrophilic water-insoluble segments on the surface area reduction is also accessible. Thereby, the degradation conditions can be varied allowing a versatile access to the hydrolytic and enzymatic degradation properties of plenty of different polymers. Moreover, the Langmuir approach enables the parallel analyses of both the polymer–biomolecule interactions and the degradation process at the molecular level. The interactions between the polymer material and its degradation products influencing its degradation behaviour are exhibited by the Langmuir technique.
For future investigations, a variety of surface-specific spectroscopic and microscopic techniques are available to gain new information on orientation, packing motifs and intermolecular interactions. For example, IRRAS, which has so far only been performed for a limited number of proteins and synthetic polymers, could provide access to structural and orientational changes. Moreover, degradation processes and hydrolysis kinetics of monolayers are accessible and can occur without the formation of water-soluble degradation fragments. The enrichment of surface-active degradation products and water-soluble proteins/enzymes at the pure or covered surface can be monitored providing information about real-time conformational changes related to interactions with surface-active enzymes and absorption kinetics.
A key challenge is the identification of water-soluble degradation fragments and the determination of the concentration in the subphase due to their low concentration. Electrospray ionization mass spectrometry (ESI-MS) may allow the determination of fragment traces giving new insights into the interactions of water-soluble molecules and polymer layers degradation processes. Chemical changes in the monolayer during degradation without the formation of water-soluble fragments can be identified by the shape and position of the IR bands corresponding to the stretching and deformation vibrations. Besides IRRAS, surface potential measurements provide access to the enrichment of water-insoluble and surface-active subphase components.
Rheological properties of the Langmuir layer got recently into special interest. The investigation of interfacial rheology is applied to understand the viscoelastic properties, which provide the modulus and relaxation behaviour [190] and to gain information about the structure–rheology interplay. The two-dimensional properties for a variety of systems like synthetic (co)polymers [115,191,192], mixed protein layers [193], biomolecules [45,194,195] and low molecular weight molecules [196] have been performed. However, investigations on pure protein layers and their interaction with water-soluble molecules are still challenging. The degradation kinetics as well as the adsorption kinetics of degradation fragments has to be understood in more detail for future applications of polymer-based biomaterials. More detailed information about the molecular arrangement is provided by spectroscopic ellipsometry (layer thickness) and in situ synchrotron X-ray scattering methods, which enable the evaluation of crystalline regions.
To judge experimental data and to gain a complete understanding at the monomolecular level, computer simulation studies are required including molecular dynamic and quantum chemical analyses applied in combination with the results obtained in Langmuir investigations, as it has been done for the hydrolytic degradation of polymer-based biomaterials [197]. This knowledge-based approach is required to enable the tailored design of biodegradable polymeric materials.
The combination of a variety of analytical methods, including microscopy, spectroscopy and scattering techniques, with the Langmuir technique applied on polymers and biomolecules is challenging, but opens a tool box, which will provide new insights for the evaluation of biomaterial interface science. Conclusively, the described Langmuir techniques together with improved analytical methods cannot only be used for fundamental investigation of the polymer behaviour on the air–water interface, but may also be applicable for investigation that aim to understand the in vivo behaviour of polymers and their interaction with proteins.
Authors' contributions
A.-C.S., T.R., B.S. and A.L. wrote the review article.
Competing interests
We declare we have no competing interests.
Funding
The authors thank the Helmholtz Association for partially funding through programme-oriented funding and grant no. VH-VI-423 (Helmholtz Virtual Institute, Multifunctional Biomaterials for Medicine).
References
- 1.Williams D. 2014. Essential biomaterials science. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Rydz J, Sikorska W, Kyulavska M, Christova D. 2014. Polyester-based (bio)degradable polymers as environmentally friendly materials for sustainable development. Int. J. Mol. Sci. 16, 564–596. ( 10.3390/ijms16010564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohane DS, Langer R. 2008. Polymeric biomaterials in tissue engineering. Pediatr. Res. 63, 487–491. ( 10.1203/01.pdr.0000305937.26105.e7) [DOI] [PubMed] [Google Scholar]
- 4.Campoccia D, Montanaro L, Arciola CR. 2013. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 34, 8533–8554. ( 10.1016/j.biomaterials.2013.07.089) [DOI] [PubMed] [Google Scholar]
- 5.Gupta B, Revagade N, Hilborn J. 2007. Poly(lactic acid) fiber: an overview. Prog. Polym. Sci. 32, 455–482. ( 10.1016/j.progpolymsci.2007.01.005) [DOI] [Google Scholar]
- 6.Ulery BD, Nair LS, Laurencin CT. 2011. Biomedical applications of biodegradable polymers. J. Polym. Sci. B Polym. Phys. 49, 832–864. ( 10.1002/polb.22259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang Y, Li L, Scott RA, Kiick KL. 2017. 50th anniversary perspective: polymeric biomaterials: diverse functions enabled by advances in macromolecular chemistry. Macromolecules 50, 483–502. ( 10.1021/acs.macromol.6b02389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ha TLB, Quan TM, Vu DN, Si DM. 2013. Naturally derived biomaterials: preparation and application. In Regenerative medicine and tissue engineering (ed. JA Andrades), pp. 247–274. Rijeka, Croatia: InTech. [Google Scholar]
- 9.Teo AJT, Mishra A, Park I, Kim Y-J, Park W-T, Yoon Y-J. 2016. Polymeric biomaterials for medical implants and devices. ACS Biomater. Sci. Eng. 2, 454–472. ( 10.1021/acsbiomaterials.5b00429) [DOI] [PubMed] [Google Scholar]
- 10.Chew SA, Danti S. 2017. Biomaterial-based implantable devices for cancer therapy. Adv. Healthcare Mater. 6, 1600766 ( 10.1002/adhm.201600766) [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Cheng J, Zhuo R. 2016. Amphiphilic hyperbranched polymers with a biodegradable hyperbranched poly(ɛ-caprolactone) core prepared from homologous AB2 macromonomer. RSC Adv. 6, 52 334–52 338. ( 10.1039/C6RA08531H) [DOI] [Google Scholar]
- 12.Dash TK, Konkimalla VB. 2012. Polymeric modification and its implication in drug delivery: poly-ɛ-caprolactone (PCL) as a model polymer. Mol. Pharmaceut. 9, 2365–2379. ( 10.1021/mp3001952) [DOI] [PubMed] [Google Scholar]
- 13.Mondal D, Griffith M, Venkatraman SS. 2016. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: current scenario and challenges. Int. J. Polym. Mater. 65, 255–265. ( 10.1080/00914037.2015.1103241) [DOI] [Google Scholar]
- 14.Anderson JM. 2001. Biological responses to materials. Annu. Rev. Mater. Res. 31, 81–110. ( 10.1146/annurev.matsci.31.1.81) [DOI] [Google Scholar]
- 15.Prasad Shastri V, Lendlein A. 2010. Engineering materials for regenerative medicine. MRS Bull. 35, 571–577. ( 10.1557/mrs2010.524) [DOI] [Google Scholar]
- 16.Unadkat HV, et al. 2011. An algorithm-based topographical biomaterials library to instruct cell fate. Proc. Natl Acad. Sci. USA 108, 16 565–16 570. ( 10.1073/pnas.1109861108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metavarayuth K, Sitasuwan P, Zhao X, Lin Y, Wang Q. 2016. Influence of surface topographical cues on the differentiation of mesenchymal stem cells in vitro. ACS Biomater. Sci. Eng. 2, 142–151. ( 10.1021/acsbiomaterials.5b00377) [DOI] [PubMed] [Google Scholar]
- 18.Saltzman WM. 2000. Cell interactions with polymer. In Principles of tissue engineering (eds Lanza RP, Langer R, Vacanti J), 2nd edn New York, NY: Academic Press. [Google Scholar]
- 19.Watson S, Nie M, Wang L, Stokes K. 2015. Challenges and developments of self-assembled monolayers and polymer brushes as a green lubrication solution for tribological applications. RSC Adv. 5, 89 698–89 730. ( 10.1039/C5RA17468F) [DOI] [Google Scholar]
- 20.Decher G. 2012. Layer-by-layer assembly (putting molecules to work). In Multilayer thin films pp. 1–21. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA. [Google Scholar]
- 21.Saums MK, Wang WF, Han B, Madhavan L, Han L, Lee D, Wells RG. 2014. Mechanically and chemically tunable cell culture system for studying the myofibroblast phenotype. Langmuir 30, 5481–5487. ( 10.1021/la4047758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bose S, Keller SS, Alstrøm TS, Boisen A, Almdal K. 2013. Process optimization of ultrasonic spray coating of polymer films. Langmuir 29, 6911–6919. ( 10.1021/la4010246) [DOI] [PubMed] [Google Scholar]
- 23.Krebs FC. 2009. Fabrication and processing of polymer solar cells: a review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 93, 394–412. ( 10.1016/j.solmat.2008.10.004) [DOI] [Google Scholar]
- 24.Ariga K, Yamauchi Y, Mori T, Hill JP. 2013. 25th anniversary article: What can be done with the Langmuir-Blodgett method? Recent developments and its critical role in materials science. Adv. Mater. 25, 6477–6512. ( 10.1002/adma.201302283) [DOI] [PubMed] [Google Scholar]
- 25.Giner-Casares JJ, Brezesinski G, Mohwald H. 2014. Langmuir monolayers as unique physical models. Curr. Opin. Colloid Interface Sci. 19, 176–182. ( 10.1016/j.cocis.2013.07.006) [DOI] [Google Scholar]
- 26.Berry JD, Neeson MJ, Dagastine RR, Chan DYC, Tabor RF. 2015. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interface Sci. 454, 226–237. ( 10.1016/j.jcis.2015.05.012) [DOI] [PubMed] [Google Scholar]
- 27.Wang K-H, Lin W-D, Wu J-Y, Lee Y-L. 2013. Conformation transitions of adsorbed proteins by interfacial forces at an air-liquid interface and their effect on the catalytic activity of proteins. Soft Matter 9, 2717–2722. ( 10.1039/C2SM27371C) [DOI] [Google Scholar]
- 28.Xu R, Dickinson E, Murray BS. 2007. Morphological changes in adsorbed protein films at the air-water interface subjected to large area variations, as observed by Brewster angle microscopy. Langmuir 23, 5005–5013. ( 10.1021/la063280q) [DOI] [PubMed] [Google Scholar]
- 29.Chenoweth DE. 1984. Complement activation during hemodialysis: clinical observations, proposed mechanisms, and theoretical implications. Artif. Organs 8, 281–287. ( 10.1111/j.1525-1594.1984.tb04291.x) [DOI] [PubMed] [Google Scholar]
- 30.Johnson RJ. 1994. Complement activation during extracorporeal therapy: biochemistry, cell biology and clinical relevance. Nephrol. Dial. Transplant. 9(Suppl 2), 36–45. [PubMed] [Google Scholar]
- 31.Shepard AD, Gelfand JA, Callow AD, O'Donnell TF Jr. 1984. Complement activation by synthetic vascular prostheses. J. Vasc. Surg. 1, 829–838. ( 10.1016/0741-5214(84)90015-6) [DOI] [PubMed] [Google Scholar]
- 32.Nobre TM, Pavinatto FJ, Caseli L, Barros-Timmons A, Dynarowicz-Łątka P, Oliveira ON Jr. 2015. Interactions of bioactive molecules & nanomaterials with Langmuir monolayers as cell membrane models. Thin Solid Films 593, 158–188. ( 10.1016/j.tsf.2015.09.047) [DOI] [Google Scholar]
- 33.Stefaniu C, Brezesinski G, Möhwald H. 2014. Langmuir monolayers as models to study processes at membrane surfaces. Adv. Colloid Interface Sci. 208, 197–213. ( 10.1016/j.cis.2014.02.013) [DOI] [PubMed] [Google Scholar]
- 34.Travkova OG, Andra J, Mohwald H, Brezesinski G. 2010. Conformational properties of arenicins: from the bulk to the air-water interface. Chemphyschem 11, 3262–3268. ( 10.1002/cphc.201000472) [DOI] [PubMed] [Google Scholar]
- 35.Travkova OG, Andra J, Mohwald H, Brezesinski G. 2013. Influence of Arenicin on phase transitions and ordering of lipids in 2D model membranes. Langmuir 29, 12 203–12 211. ( 10.1021/la402340d) [DOI] [PubMed] [Google Scholar]
- 36.Travkova OG, Brezesinski G. 2013. Adsorption of the antimicrobial peptide arenicin and its linear derivative to model membranes - a maximum insertion pressure study. Chem. Phys. Lipids 167, 43–50. ( 10.1016/j.chemphyslip.2013.01.010) [DOI] [PubMed] [Google Scholar]
- 37.Rayleigh L. 1889. Measurements of the amount of oil necessary in order to check the motions of camphor upon water. Proc. R. Soc. Lond. 47, 364–367. ( 10.1098/rspl.1889.0099) [DOI] [Google Scholar]
- 38.Pockels A. 1891. Surface tension. Nature 43, 437–439. ( 10.1038/043437b0) [DOI] [Google Scholar]
- 39.Kaganer VM, Mohwald H, Dutta P. 1999. Structure and phase transitions in Langmuir monolayers. Rev. Mod. Phys. 71, 779–819. ( 10.1103/RevModPhys.71.779) [DOI] [Google Scholar]
- 40.Phan MD, Lee J, Shin K. 2016. Collapsed STATES of Langmuir monolayers. J. Oleo Sci. 65, 385–397. ( 10.5650/jos.ess15261) [DOI] [PubMed] [Google Scholar]
- 41.Kumaki J, Kajitani T, Nagai K, Okoshi K, Yashima E. 2010. Visualization of polymer chain conformations in amorphous polyisocyanide Langmuir-Blodgett films by atomic force microscopy. J. Am. Chem. Soc. 132, 5604–5606. ( 10.1021/ja908426u) [DOI] [PubMed] [Google Scholar]
- 42.Maestro A, Hilles HM, Ortega F, Rubio RG, Langevin D, Monroy F. 2010. Reptation in langmuir polymer monolayers. Soft Matter 6, 4407–4412. ( 10.1039/c0sm00250j) [DOI] [Google Scholar]
- 43.Gavranovic GT, Deutsch JM, Fuller GG. 2005. Two-dimensional melts: polymer chains at the air-water interface. Macromolecules 38, 6672–6679. ( 10.1021/ma050061n) [DOI] [Google Scholar]
- 44.Lhor M, Bernier SC, Horchani H, Bussieres S, Cantin L, Desbat B, Salesse C. 2014. Comparison between the behavior of different hydrophobic peptides allowing membrane anchoring of proteins. Adv. Colloid Interface Sci. 207, 223–239. ( 10.1016/j.cis.2014.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caruso B, Ambroggio EE, Wilke N, Fidelio GD. 2016. The rheological properties of beta amyloid Langmuir monolayers: comparative studies with melittin peptide. Colloids Surf. B 146, 180–187. ( 10.1016/j.colsurfb.2016.06.003) [DOI] [PubMed] [Google Scholar]
- 46.Crawford NF, Leblanc RM. 2014. Serum albumin in 2D: a Langmuir monolayer approach. Adv. Colloid Interface Sci. 207, 131–138. ( 10.1016/j.cis.2013.10.021) [DOI] [PubMed] [Google Scholar]
- 47.Lopez RF, Nobre TM, Accardo CD, Pernambuco PC, Nader HB, Lopes CC, Caseli L. 2013. Effect of carrageenans of different chemical structures in biointerfaces: a Langmuir film study. Colloids Surf. B 111, 530–535. ( 10.1016/j.colsurfb.2013.06.039) [DOI] [PubMed] [Google Scholar]
- 48.Toulemon D, Liu Y, Cattoën X, Leuvrey C, Bégin-Colin S, Pichon BP. 2016. Enhanced collective magnetic properties in 2D monolayers of iron oxide nanoparticles favored by local order and local 1D shape anisotropy. Langmuir 32, 1621–1628. ( 10.1021/acs.langmuir.5b04145) [DOI] [PubMed] [Google Scholar]
- 49.Tsukanova V, Slyadneva O, Inoue T, Harata A, Ogawa T. 1999. Compression of the dye monolayer at the air/water interface studied by second harmonic generation. Chem. Phys. 250, 207–215. ( 10.1016/S0301-0104(99)00257-8) [DOI] [Google Scholar]
- 50.Sergeeva TI, Gromov SP, Vedernikov AI, Kapichnikova MS, Alfimov MV, Möbius D, Zaitsev SY. 2005. Organisation in monolayers at the air–water interface of butadienyl dyes containing benzodithiacrown-ether or dimethoxybenzene. Colloids Surf. A 264, 207–214. ( 10.1016/j.colsurfa.2005.05.024) [DOI] [Google Scholar]
- 51.Bulhoes LOS, Obeng YS, Bard AJ. 1993. Langmuir-Blodgett and electrochemical studies of fullerene films. Chem. Mater. 5, 110–114. ( 10.1021/cm00025a021) [DOI] [Google Scholar]
- 52.Liu WJ, Jeng U, Lin TL, Lai SH, Shih MC, Tsao CS, Wang LY, Chiang LY, Sung LP. 2000. Adsorption of dodecahydroxylated-fullerene monolayers at the air–water interface. Phys. B (Amsterdam, Neth.) 283, 49–52. ( 10.1016/S0921-4526(99)01890-6) [DOI] [Google Scholar]
- 53.Jin J, et al. 1999. Structural characterizations of C60-derivative Langmuir−Blodgett films and their photovoltaic behaviors. Langmuir 15, 4565–4569. ( 10.1021/la981459y) [DOI] [Google Scholar]
- 54.Moon HK, Choi YS, Lee JK, Ha CS, Lee WK, Gardella JA. 2009. Miscibility and hydrolytic behavior of poly(trimethylene carbonate) and poly(L-lactide) and their blends in monolayers at the air/water interface. Langmuir 25, 4478–4483. ( 10.1021/la8032435) [DOI] [PubMed] [Google Scholar]
- 55.Deschênes L, Lyklema J, Danis C, Saint-Germain F. 2015. Phase transitions in polymer monolayers: application of the Clapeyron equation to PEO in PPO–PEO Langmuir films. Adv. Colloid Interface Sci. 222, 199–214. ( 10.1016/j.cis.2014.11.002) [DOI] [PubMed] [Google Scholar]
- 56.Vollhardt D. 2014. Brewster angle microscopy: a preferential method for mesoscopic characterization of monolayers at the air/water interface. Curr. Opin. Colloid Interface Sci. 19, 183–197. ( 10.1016/j.cocis.2014.02.001) [DOI] [Google Scholar]
- 57.Hoenig D, Moebius D. 1991. Direct visualization of monolayers at the air-water interface by Brewster angle microscopy. J. Phys. Chem. 95, 4590–4592. ( 10.1021/j100165a003) [DOI] [Google Scholar]
- 58.Kawaguchi M, Tohyama M, Mutoh Y, Takahashi A. 1988. Ellipsometric study of polymer monolayers spread at the air water interface.1. Thickness of monolayers. Langmuir 4, 407–410. ( 10.1021/la00080a025) [DOI] [Google Scholar]
- 59.Reiter R, Motschmann H, Orendi H, Nemetz A, Knoll W. 1992. Ellipsometric microscopy - imaging monomolecular surfactant layers at the air-water-interface. Langmuir 8, 1784–1788. ( 10.1021/la00043a017) [DOI] [Google Scholar]
- 60.Kim YS, Snively CM, Liu YJ, Rabolt JF, Chase DB. 2008. Real-time imaging of crystallization in polylactide enantiomeric monolayers at the air-water interface. Langmuir 24, 10 791–10 796. ( 10.1021/la801747k) [DOI] [PubMed] [Google Scholar]
- 61.Mendelsohn R, Mao G, Flach CR. 2010. Infrared reflection–absorption spectroscopy: principles and applications to lipid–protein interaction in Langmuir films. Biochim. Biophys. Acta, Biomembr. 1798, 788–800. ( 10.1016/j.bbamem.2009.11.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bourque H, Laurin I, Pézolet M, Klass JM, Lennox RB, Brown GR. 2001. Investigation of the poly(l-lactide)/poly(d-lactide) stereocomplex at the air−water interface by polarization modulation infrared reflection absorption spectroscopy. Langmuir 17, 5842–5849. ( 10.1021/la0009792) [DOI] [Google Scholar]
- 63.Weidemann G, Brezesinski G, Vollhardt D, Mohwald H. 1998. Texture change separate from the transition between two tilted phases in Langmuir monolayers. J. Phys. Chem. B 102, 1224–1228. ( 10.1021/jp972777t) [DOI] [Google Scholar]
- 64.Broniatowski M, Flasiński M, Dynarowicz-Ła¸tka P, Majewski J. 2010. Grazing incidence diffraction and X-ray reflectivity studies of the interactions of inorganic mercury salts with membrane lipids in Langmuir monolayers at the air/water interface. J. Phys. Chem. B 114, 9474–9484. ( 10.1021/jp101668n) [DOI] [PubMed] [Google Scholar]
- 65.Zhavnerko G, Marletta G. 2010. Developing Langmuir-Blodgett strategies towards practical devices. Mater. Sci. Eng. B 169, 43–48. ( 10.1016/j.mseb.2009.12.005) [DOI] [Google Scholar]
- 66.Ulman A. 1991. Part Two - Langmuir–Blodgett films. In An introduction to ultrathin organic films (ed. Ulman A.), pp. 101–236. San Diego, CA: Academic Press. [Google Scholar]
- 67.Langmuir I, Schaefer VJ. 1938. Activities of urease and pepsin monolayers. J. Am. Chem. Soc. 60, 1351–1360. ( 10.1021/ja01273a023) [DOI] [Google Scholar]
- 68.Pohjakallio M, Aho T, Kontturi K, Kontturi E. 2011. Morphology of poly(methyl methacrylate) and polystyrene blends upon Langmuir-Schaefer deposition. Soft Matter 7, 743–748. ( 10.1039/C0SM00360C) [DOI] [Google Scholar]
- 69.Petty MC. 1996. Langmuir-Blodgett films: an introduction. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 70.Roberts GG. 1990. Langmuir-Blodgett films. New York, NY: Springer US. [Google Scholar]
- 71.Kumaki J. 2016. Observation of polymer chain structures in two-dimensional films by atomic force microscopy. Polym. J. 48, 3–14. ( 10.1038/pj.2015.67) [DOI] [Google Scholar]
- 72.Wang X, Wen G, Huang C, Wang Z, Shi Y. 2014. Aggregation behavior of the blends of PS-b-PEO-b-PS and PS-b-PMMA at the air/water interface. RSC Adv. 4, 49 219–49 227. ( 10.1039/C4RA08579E) [DOI] [Google Scholar]
- 73.Claro PCD, Coustet ME, Diaz C, Maza E, Cortizo MS, Requejo FG, Pietrasanta LI, Ceolin M, Azzaroni O. 2013. Self-assembly of PBzMA-b-PDMAEMA diblock copolymer films at the air-water interface and deposition on solid substrates via Langmuir-Blodgett transfer. Soft Matter 9, 10 899–10 912. ( 10.1039/c3sm52336e) [DOI] [Google Scholar]
- 74.Pandey RK, Upadhyay C, Prakash R. 2013. Pressure dependent surface morphology and Raman studies of semicrystalline poly(indole-5-carboxylic acid) by the Langmuir-Blodgett technique. RSC Adv. 3, 15 712–15 718. ( 10.1039/c3ra41895b) [DOI] [Google Scholar]
- 75.Matharu Z, Sumana G, Gupta V, Malhotra BD. 2010. Langmuir–Blodgett films of polyaniline for low density lipoprotein detection. Thin Solid Films 519, 1110–1114. ( 10.1016/j.tsf.2010.08.053) [DOI] [Google Scholar]
- 76.Boury F, Gulik A, Dedieu JC, Proust JE. 1994. First-order transition in a polymer monolayer: structural analysis by transmission electronic microscopy and atomic force microscopy. Langmuir 10, 1654–1656. ( 10.1021/la00018a006) [DOI] [Google Scholar]
- 77.Price EW, Guo Y, Wang CW, Moffitt MG. 2009. Block copolymer strands with internal microphase separation structure via self-assembly at the air−water interface. Langmuir 25, 6398–6406. ( 10.1021/la804317s) [DOI] [PubMed] [Google Scholar]
- 78.Geiß G, Hickel W, Lupo D, Praß W, Scheunemann U. 1991. Ellipsometry on anisotropic Langmuir-Blodgett films. Ber. Bunsen-Ges. 95, 1345–1349. ( 10.1002/bbpc.19910951106) [DOI] [Google Scholar]
- 79.Haro M, Gascón I, Aroca R, López MC, Royo FM. 2008. Structural characterization and properties of an azopolymer arranged in Langmuir and Langmuir–Blodgett films. J. Colloid Interface Sci. 319, 277–286. ( 10.1016/j.jcis.2007.10.009) [DOI] [PubMed] [Google Scholar]
- 80.Debnath P, Chakraborty S, Deb S, Nath J, Bhattacharjee D, Hussain SA. 2015. Reversible transition between excimer and J-aggregate of indocarbocyanine dye in Langmuir–Blodgett (LB) films. J. Phys. Chem. C 119, 9429–9441. ( 10.1021/acs.jpcc.5b02111) [DOI] [Google Scholar]
- 81.Huie Z, Yu G, Shunsuke Y, Tokuji M, Masaya M. 2016. Angle-resolved X-ray photoelectron spectroscopy study of poly(vinylidene fluoride)/poly(N -dodecylacrylamide) Langmuir–Blodgett nanofilms. Jpn. J. Appl. Phys. 55, 03DD11. ( 10.7567/JJAP.55.03DD11) [DOI] [Google Scholar]
- 82.Ulman A. 1990. Fourier Transform infrared spectroscopy of Langmuir—Blodgett and self-assembled films. In ACS Symposium Series on Fourier transform infrared spectroscopy in colloid and interface science, pp. 144–159. Washington, DC: American Chemical Society. [Google Scholar]
- 83.Tredgold RH. 1987. Langmuir-Blodgett films made from preformed polymers. Thin Solid Films 152, 223–230. ( 10.1016/0040-6090(87)90418-4) [DOI] [Google Scholar]
- 84.Schmidt A, Mathauer K, Reiter G, Foster MD, Stamm M, Wegner G, Knoll W. 1994. Self-diffusion of hairy rod molecules in Langmuir-Blodgett-Kuhn multilayers probed with neutron and X-Ray reflectometry. Langmuir 10, 3820–3826. ( 10.1021/la00022a070) [DOI] [Google Scholar]
- 85.Li B, Wu Y, Liu M, Esker AR. 2006. Brewster angle microscopy study of poly(ɛ-caprolactone) crystal growth in Langmuir films at the air/water interface. Langmuir 22, 4902–4905. ( 10.1021/la060048b) [DOI] [PubMed] [Google Scholar]
- 86.Tredgold RH, Vickers AJ, Hoorfar A, Hodge P, Khoshdel E. 1985. X-ray analysis of some porphyrin and polymer Langmuir-Blodgett films. J. Phys. D: Appl. Phys. 18, 1139–1145. ( 10.1088/0022-3727/18/6/017) [DOI] [Google Scholar]
- 87.Schulz B, Dietzel B, Kaminorz Y. 2001. Supramolecular structure and electro-optical properties of functionalized maleic anhydride copolymers. Macromol. Symp. 164, 457–464. ( 10.1002/1521-3900(200102)164:1%3C457::AID-MASY457%3E3.0.CO;2-E) [DOI] [Google Scholar]
- 88.Bernardini C, Stuart MAC, Stoyanov SD, Arnaudov LN, Leermakers FAM. 2012. Polymer compatibility in two dimensions. Modeling of phase behavior of mixed polymethacrylate Langmuir films. Langmuir 28, 5614–5621. ( 10.1021/la2040642) [DOI] [PubMed] [Google Scholar]
- 89.Glagola CP, Miceli LM, Milchak MA, Halle EH, Logan JL. 2012. Polystyrene–poly(ethylene oxide) diblock copolymer: the effect of polystyrene and spreading concentration at the air/water interface. Langmuir 28, 5048–5058. ( 10.1021/la204100d) [DOI] [PubMed] [Google Scholar]
- 90.Deschênes L, Bousmina M, Ritcey AM. 2008. Micellization of PEO/PS block copolymers at the air/water interface: a simple model for predicting the size and aggregation number of circular surface micelles. Langmuir 24, 3699–3708. ( 10.1021/la702141h) [DOI] [PubMed] [Google Scholar]
- 91.Kim HC, Lee H, Khetan J, Won Y-Y. 2015. Surface mechanical and rheological behaviors of biocompatible poly((d,l-lactic acid-ran-glycolic acid)-block-ethylene glycol) (PLGA–PEG) and poly((d,l-lactic acid-ran-glycolic acid-ran-ɛ-caprolactone)-block-ethylene glycol) (PLGACL–PEG) block copolymers at the air–water interface. Langmuir 31, 13 821–13 833. ( 10.1021/acs.langmuir.5b03622) [DOI] [PubMed] [Google Scholar]
- 92.Schulze M, Seufert M, Fakirov C, Tebbe H, Buchholz V, Wegner G. 1997. Supramolecular architectures of cellulose derivatives. Macromol. Symp. 120, 237–245. ( 10.1002/masy.19971200124) [DOI] [Google Scholar]
- 93.Tredgold RH. 1994. More complex structures formed by the Langmuir–Blodgett technique. In Order in thin organic films (ed. Tredgold RH.). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 94.Mabuchi M, Kobata S, Ito S, Yamamoto M, Schmidt A, Knoll W. 1998. Preparation and characterization of the Langmuir−Blodgett films made of hairy-rod polyglutamates bearing various chromophores in the side chain. Langmuir 14, 7260–7266. ( 10.1021/la980555w) [DOI] [Google Scholar]
- 95.Schwiegk S, Vahlenkamp T, Xu Y, Wegner G. 1992. Origin of orientation phenomena observed in layered Langmuir-Blodgett structures of hairy-rod polymers. Macromolecules 25, 2513–2525. ( 10.1021/ma00035a034) [DOI] [Google Scholar]
- 96.Fujimori A, Miura S, Kikkawa T, Shibasaki Y. 2015. Fine structural analysis, formation of interfacial particle films, and accurate estimation of orientation in polyguanamine derivatives with a high refractive index. J. Polym. Sci. B Polym. Phys. 53, 999–1009. ( 10.1002/polb.23728) [DOI] [Google Scholar]
- 97.Woodruff MA, Hutmacher DW. 2010. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 35, 1217 ( 10.1016/j.progpolymsci.2010.04.002) [DOI] [Google Scholar]
- 98.Sisson AL, Ekinci D, Lendlein A. 2013. The contemporary role of ɛ-caprolactone chemistry to create advanced polymer architectures. Polymer 54, 4333–4350. ( 10.1016/j.polymer.2013.04.045) [DOI] [Google Scholar]
- 99.Huang MH, Li SM, Hutmacher DW, Schantz JT, Vacanti CA, Braud C, Vert M. 2004. Degradation and cell culture studies on block copolymers prepared by ring opening polymerization of epsilon-caprolactone in the presence of poly(ethylene glycol). J. Biomed. Mater. Res. A 69a, 417–427. ( 10.1002/jbm.a.30008) [DOI] [PubMed] [Google Scholar]
- 100.Leiva A, Gargallo L, Radić D. 2004. Interfacial properties of poly(caprolactone) and derivatives. J. Macromol. Sci. A Pure Appl. Chem. 41, 577–583. ( 10.1081/MA-120030926) [DOI] [Google Scholar]
- 101.Li B. 2004. Surface Characterization Of Poly(ɛ-caprolactone) at the air/water interface. Master thesis, Virginia, Virginia Polytechnic Institute and State University, Blacksburg.
- 102.Li BB, Esker AR. 2007. Molar mass dependent growth of poly(epsilon-caprolactone) crystals in Langmuir films. Langmuir 23, 2546–2554. ( 10.1021/la062563f) [DOI] [PubMed] [Google Scholar]
- 103.Li B, Esker AR. 2006. Blends of poly(ɛ-caprolactone) and intermediate molar mass polystyrene as Langmuir films at the air/water interface. Langmuir 23, 574–581. ( 10.1021/la0625291) [DOI] [PubMed] [Google Scholar]
- 104.Hottle JR, Deng J, Kim H-J, Farmer-Creely CE, Viers BD, Esker AR. 2005. Blends of amphiphilic poly(dimethylsiloxane) and nonamphiphilic octaisobutyl-POSS at the air/water interface. Langmuir 21, 2250–2259. ( 10.1021/la047565j) [DOI] [PubMed] [Google Scholar]
- 105.Schöne A-C, Kratz K, Schulz B, Reiche J, Santer S, Lendlein A. 2015. Surface pressure-induced isothermal 2D- to 3D-transitions in Langmuir films of poly(ɛ-caprolactone)s and oligo(ɛ-caprolactone) based polyesterurethanes. Polym. Adv. Technol. 26, 1411–1420. ( 10.1002/pat.3638) [DOI] [Google Scholar]
- 106.Joncheray TJ, Denoncourt KM, Mathieu C, Meier MAR, Schubert US, Duran RS. 2006. Langmuir and Langmuir−Blodgett Films of Poly(ethylene oxide)-b-Poly(ɛ-caprolactone) star-shaped block copolymers. Langmuir 22, 9264–9271. ( 10.1021/la061290l) [DOI] [PubMed] [Google Scholar]
- 107.Naolou T, Busse K, Lechner B-D, Kressler J. 2014. The behavior of poly(ɛ-caprolactone) and poly(ethylene oxide)-b-poly(ɛ-caprolactone) grafted to a poly(glycerol adipate) backbone at the air/water interface. Colloid Polym. Sci. 292, 1199–1208. ( 10.1007/s00396-014-3168-1) [DOI] [Google Scholar]
- 108.Reiche J, Kulkarni A, Kratz K, Lendlein A. 2008. Enzymatic monolayer degradation study of multiblock copolymers consisting of poly(ɛ-caprolactone) and poly(p-dioxanone) blocks. Thin Solid Films 516, 8821–8828. ( 10.1016/j.tsf.2007.11.053) [DOI] [Google Scholar]
- 109.Ringard-Lefebvre C, Baszkin A. 1994. Main transition-like feature in polylactic acid Langmuir films. Langmuir 10, 2376–2381. ( 10.1021/la00019a057) [DOI] [Google Scholar]
- 110.Klass JM, Lennox RB, Brown GR, Bourque H, Pézolet M. 2003. Enantiomeric polylactides at the air−water interface: π−A isotherms and PM-IRRAS studies of enantiomers and their blend. Langmuir 19, 333–340. ( 10.1021/la020606w) [DOI] [Google Scholar]
- 111.Boury F, Olivier E, Proust JE, Benoit JP. 1993. A study of poly(α-hydroxy acid)s monolayers spread at the air/water interface: influence of the d,l-lactic acid/glycolic acid ratio. J. Colloid Interface Sci. 160, 1–9. ( 10.1006/jcis.1993.1361) [DOI] [Google Scholar]
- 112.Park HW, Choi J, Ohn K, Lee H, Kim JW, Won YY. 2012. Study of the air-water interfacial properties of biodegradable polyesters and their block copolymers with poly(ethylene glycol). Langmuir 28, 11 555–11 566. ( 10.1021/La300810q) [DOI] [PubMed] [Google Scholar]
- 113.Boury F, Saulnier P, Proust JE, Panaı¨otov I, Ivanova T, Postel C, Abillon O. 1999. Characterization of the morphology of poly(α-hydroxy acid)s Langmuir–Blodgett films by atomic force microscopy measurements. Colloids Surf. A 155, 117–129. ( 10.1016/S0927-7757(98)00705-5) [DOI] [Google Scholar]
- 114.Schöne AC, Richau K, Kratz K, Schulz B, Lendlein A. 2015. Influence of diurethane linkers on the Langmuir layer behavior of oligo[(rac-lactide)-co-glycolide]-based polyesterurethanes. Macromol. Rapid Commun. 36, 1910–1915. ( 10.1002/marc.201500316) [DOI] [PubMed] [Google Scholar]
- 115.Kim HC, Lee H, Jung H, Choi YH, Meron M, Lin B, Bang J, Won Y-Y. 2015. Humidity-dependent compression-induced glass transition of the air-water interfacial Langmuir films of poly(d,l-lactic acid-ran-glycolic acid) (PLGA). Soft Matter 11, 5666–5677. ( 10.1039/C4SM02535K) [DOI] [PubMed] [Google Scholar]
- 116.Wang K, Zhou C, Hong Y, Zhang X. 2012. A review of protein adsorption on bioceramics. Interface Focus 2, 259–277. ( 10.1098/rsfs.2012.0012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Camarero JA. 2008. Recent developments in the site-specific immobilization of proteins onto solid supports. Pept. Sci. 90, 450–458. ( 10.1002/bip.20803) [DOI] [PubMed] [Google Scholar]
- 118.Cumper CWN, Alexander AE. 1950. The surface chemistry of proteins. Trans. Faraday Soc. 46, 235–253. ( 10.1039/tf9504600235) [DOI] [Google Scholar]
- 119.Furuno T. 2014. Atomic force microscopy study on the unfolding of globular proteins in the Langmuir films. Thin Solid Films 552, 170–179. ( 10.1016/j.tsf.2013.12.052) [DOI] [Google Scholar]
- 120.Gani SA, Mukherjee DC, Chattoraj DK. 1999. Adsorption of biopolymer at solid-liquid interfaces. 2. Interaction of BSA and DNA with casein. Langmuir 15, 7139–7144. ( 10.1021/la970687%2B) [DOI] [Google Scholar]
- 121.Sanchez-Gonzalez J, Ruiz-Garcia J, Galvez-Ruiz MJ. 2003. Langmuir-Blodgett films of biopolymers: a method to obtain protein multilayers. J. Colloid Interface Sci. 267, 286–293. ( 10.1016/S0021-9797(03)00754-9) [DOI] [PubMed] [Google Scholar]
- 122.Reviakine I, Jung F, Braune S, Brash JL, Latour R, Gorbet M, van Oeveren W. 2017. Stirred, shaken, or stagnant: What goes on at the blood–biomaterial interface. Blood Rev. 31, 11–21. ( 10.1016/j.blre.2016.07.003) [DOI] [PubMed] [Google Scholar]
- 123.Miao S, Leeman H, De Feyter S, Schoonheydt RA. 2010. Facile preparation of Langmuir-Blodgett films of water-soluble proteins and hybrid protein-clay films. J. Mater. Chem. 20, 698–705. ( 10.1039/b913659b) [DOI] [Google Scholar]
- 124.McClellan SJ, Franses EI. 2003. Effect of concentration and denaturation on adsorption and surface tension of bovine serum albumin. Colloids Surf. B 28, 63–75. ( 10.1016/S0927-7765(02)00131-5) [DOI] [Google Scholar]
- 125.Pedraz P, Montes FJ, Cerro RL, Diaz ME. 2012. Characterization of Langmuir biofilms built by the biospecific interaction of arachidic acid with bovine serum albumin. Thin Solid Films 525, 121–131. ( 10.1016/j.tsf.2012.10.055) [DOI] [Google Scholar]
- 126.Noskov BA, Mikhailovskaya AA, Lin SY, Loglio G, Miller R. 2010. Bovine serum albumin unfolding at the air/water interface as studied by dilational surface rheology. Langmuir 26, 17 225–17 231. ( 10.1021/la103360h) [DOI] [PubMed] [Google Scholar]
- 127.Sanchez-Gonzalez J, Cabrerizo-Vilchez MA, Galvez-Ruiz MJ. 2001. Interactions, desorption and mixing thermodynamics in mixed monolayers of beta-lactoglobulin and bovine serum albumin. Colloids Surf. B 21, 19–27. ( 10.1016/S0927-7765(01)00180-1) [DOI] [PubMed] [Google Scholar]
- 128.Engelhardt K, Rumpel A, Walter J, Dombrowski J, Kulozik U, Braunschweig B, Peukert W. 2012. Protein adsorption at the electrified air-water interface: implications on foam stability. Langmuir 28, 7780–7787. ( 10.1021/la301368v) [DOI] [PubMed] [Google Scholar]
- 129.Engelhardt K, Peukert W, Braunschweig B. 2014. Vibrational sum-frequency generation at protein modified air-water interfaces: effects of molecular structure and surface charging. Curr. Opin. Colloid Interface Sci. 19, 207–215. ( 10.1016/j.cocis.2014.03.008) [DOI] [Google Scholar]
- 130.Lu JR, Su TJ, Penfold J. 1999. Adsorption of serum albumins at the air/water interface. Langmuir 15, 6975–6983. ( 10.1021/la990131h) [DOI] [Google Scholar]
- 131.Toimil P, Prieto G, Miñones J Jr, Trillo JM, Sarmiento F. 2012. Monolayer and Brewster angle microscopy study of human serum albumin—Dipalmitoyl phosphatidyl choline mixtures at the air–water interface. Colloids Surf. B 92, 64–73. ( 10.1016/j.colsurfb.2011.11.022) [DOI] [PubMed] [Google Scholar]
- 132.Lu JR, Su TJ, Thomas RK. 1999. Structural conformation of bovine serum albumin layers at the air-water interface studied by neutron reflection. J. Colloid Interface Sci. 213, 426–437. ( 10.1006/jcis.1999.6157) [DOI] [PubMed] [Google Scholar]
- 133.Campbell RA, Ang JC, Sebastiani F, Tummino A, White JW. 2015. Spread films of human serum albumin at the air-water interface: optimization, morphology, and durability. Langmuir 31, 13 535–13 542. ( 10.1021/acs.langmuir.5b03349) [DOI] [PubMed] [Google Scholar]
- 134.Damodaran S. 2003. In situ measurement of conformational changes in proteins at liquid interfaces by circular dichroism spectroscopy. Anal. Bioanal. Chem. 376, 182–188. ( 10.1007/s00216-003-1873-6) [DOI] [PubMed] [Google Scholar]
- 135.Sessions K, Sacks S, Li SH, Leblanc RM. 2014. epi-Fluorescence imaging at the air-water interface of fibrillization of bovine serum albumin and human insulin. Chem. Commun. 50, 8955–8957. ( 10.1039/c4cc04030a) [DOI] [PubMed] [Google Scholar]
- 136.Owaku K, Shinohara H, Ikariyama Y, Aizawa M. 1989. Preparation and characterization of protein Langmuir-Blodgett films. Thin Solid Films 180, 61–64. ( 10.1016/0040-6090(89)90054-0) [DOI] [Google Scholar]
- 137.Latnikova AV, Lin SY, Loglio G, Miller R, Noskov BA. 2008. Impact of surfactant additions on dynamic properties of beta-casein adsorption layers. J. Phys. Chem. C 112, 6126–6131. ( 10.1021/jp712107n) [DOI] [Google Scholar]
- 138.Holly FJ, Dolowy K, Yamada KM. 1984. comparative surface chemical studies of cellular fibronectin and submaxillary mucin monolayers - effects of ph, ionic-strength, and presence of calcium-ions. J. Colloid Interface Sci. 100, 210–215. ( 10.1016/0021-9797(84)90427-2) [DOI] [Google Scholar]
- 139.Bhuvanesh T, et al. In press. Langmuir–Schaefer films of fibronectin as designed biointerfaces for culturing stem cells. Polym. Adv. Technol. ( 10.1002/pat.3910) [DOI] [Google Scholar]
- 140.Thakur G, Wang CS, Leblanc RA. 2008. Surface chemistry and in situ spectroscopy of a lysozyme Langmuir monolayer. Langmuir 24, 4888–4893. ( 10.1021/la703893m) [DOI] [PubMed] [Google Scholar]
- 141.Yano YF, Kobayashi Y, Ina T, Nitta K, Uruga T. 2016. Hofmeister anion effects on protein adsorption at an air-water interface. Langmuir 32, 9892–9898. ( 10.1021/acs.langmuir.6b02352) [DOI] [PubMed] [Google Scholar]
- 142.Lad MD, Birembaut F, Matthew JM, Frazier RA, Green RJ. 2006. The adsorbed conformation of globular proteins at the air/water interface. Phys. Chem. Chem. Phys. 8, 2179–2186. ( 10.1039/b515934b) [DOI] [PubMed] [Google Scholar]
- 143.Bos MA, van Vliet T. 2001. Interfacial rheological properties of adsorbed protein layers and surfactants: a review. Adv. Colloid Interface Sci. 91, 437–471. ( 10.1016/S0001-8686(00)00077-4) [DOI] [PubMed] [Google Scholar]
- 144.Cicuta P. 2007. Compression and shear surface rheology in spread layers of beta-casein and beta-lactoglobulin. J. Colloid Interface Sci. 308, 93–99. ( 10.1016/j.jcis.2006.12.056) [DOI] [PubMed] [Google Scholar]
- 145.Sengupta T, Razumovsky L, Damodaran S. 1999. Energetics of protein−interface interactions and its effect on protein adsorption. Langmuir 15, 6991–7001. ( 10.1021/la990235s) [DOI] [Google Scholar]
- 146.Perriman AW, Henderson MJ, Holt SA, White JW. 2007. Effect of the air-water interface on the stability of beta-lactoglobulin. J. Phys. Chem. B 111, 13 527–13 537. ( 10.1021/jp074777r) [DOI] [PubMed] [Google Scholar]
- 147.Ambroggio EE, Caruso B, Villarreal MA, Raussens V, Fidelio GD. 2016. Reversing the peptide sequence impacts on molecular surface behaviour. Colloids Surf. B 139, 25–32. ( 10.1016/j.colsurfb.2015.12.008) [DOI] [PubMed] [Google Scholar]
- 148.Ambroggio EE, Fidelio GD. 2013. Lipid-like behavior of signal sequence peptides at air–water interface. Biochim. Biophys. Acta, Biomembr. 1828, 708–714. ( 10.1016/j.bbamem.2012.11.004) [DOI] [PubMed] [Google Scholar]
- 149.Yokoi H, Hayashi S, Kinoshita T. 2003. Polypeptide membranes at an interface. Prog. Polym. Sci. 28, 341–357. ( 10.1016/S0079-6700(02)00081-3) [DOI] [Google Scholar]
- 150.Reiter R, Wintzenrieth F, Reiter G. 2016. Self-assembly behavior of a rod-like polypeptide at the air-water interface. Polymer 107, 379–386. ( 10.1016/j.polymer.2016.08.023) [DOI] [Google Scholar]
- 151.Shin S, Ahn S, Cheng J, Chang H, Jung D-H, Hyun J. 2016. Morphological variation of stimuli-responsive polypeptide at air–water interface. Appl. Surf. Sci. 388, 551–556. ( 10.1016/j.apsusc.2015.10.126) [DOI] [Google Scholar]
- 152.Kato N, Sasaki T, Mukai Y. 2015. Partially induced transition from horizontal to vertical orientation of helical peptides at the air–water interface and the structure of their monolayers transferred on the solid substrates. Biochim. Biophys. Acta, Biomembr. 1848, 967–975. ( 10.1016/j.bbamem.2014.12.022) [DOI] [PubMed] [Google Scholar]
- 153.Li S, Leblanc RM. 2014. Aggregation of insulin at the interface. J. Phys. Chem. B 118, 1181–1188. ( 10.1021/jp4101202) [DOI] [PubMed] [Google Scholar]
- 154.Johnson S, Liu W, Thakur G, Dadlani A, Patel R, Orbulescu J, Whyte JD, Micic M, Leblanc RM. 2012. Surface chemistry and spectroscopy of human insulin Langmuir monolayer. J. Phys. Chem. B 116, 10 205–10 212. ( 10.1021/jp3046643) [DOI] [PubMed] [Google Scholar]
- 155.Liu W, Johnson S, Micic M, Orbulescu J, Whyte J, Garcia AR, Leblanc RM. 2012. Study of the aggregation of human insulin Langmuir monolayer. Langmuir 28, 3369–3377. ( 10.1021/la204201w) [DOI] [PubMed] [Google Scholar]
- 156.Nieto-Suarez M, Vila-Romeu N, Prieto I. 2008. Behaviour of insulin Langmuir monolayers at the air-water interface under various conditions. Thin Solid Films 516, 8873–8879. ( 10.1016/j.tsf.2007.11.062) [DOI] [Google Scholar]
- 157.Kubies D, Machova L, Brynda E, Lukas J, Rypacek F. 2003. Functionalized surfaces of polylactide modified by Langmuir-Blodgett films of amphiphilic block copolymers. J. Mater. Sci.: Mater. Med. 14, 143–149. ( 10.1023/A:1022019813078) [DOI] [PubMed] [Google Scholar]
- 158.Vert M. 2015. After soft tissues, bone, drug delivery and packaging, PLA aims at blood. Eur. Polym. J. 68, 516–525. ( 10.1016/j.eurpolymj.2015.03.051) [DOI] [Google Scholar]
- 159.Ishaug SL, Yaszemski MJ, Bizios R, Mikos AG. 1994. Osteoblast function on synthetic biodegradable polymers. J. Biomed. Mater. Res. 28, 1445–1453. ( 10.1002/jbm.820281210) [DOI] [PubMed] [Google Scholar]
- 160.Kiss É, Dravetzky K, Hill K, Kutnyánszky E, Varga A. 2008. Protein interaction with a pluronic-modified poly(lactic acid) Langmuir monolayer. J. Colloid Interface Sci. 325, 337–345. ( 10.1016/j.jcis.2008.05.057) [DOI] [PubMed] [Google Scholar]
- 161.Hlady V, Jogikalmath G. 2007. Albumin binding and insertion into PS-b-PEO monolayers at air–water interface. Colloids Surf., B 54, 179–187. ( 10.1016/j.colsurfb.2006.10.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Boury F, Ivanova T, Panaieotov I, Proust JE. 1995. Dilatational properties of poly(dl-lactic acid) and bovine serum albumin monolayers spread at the air/water interface. Langmuir 11, 599–606. ( 10.1021/la00002a040) [DOI] [Google Scholar]
- 163.Schöne AC, Falkenhagen S, Travkova O, Schulz B, Kratz K, Lendlein A. 2015. Influence of intermediate degradation products on the hydrolytic degradation of poly [(rac-lactide)-co-glycolide] at the air-water interface. Polym. Adv. Technol. 26, 1402–1410. ( 10.1002/pat.3701) [DOI] [Google Scholar]
- 164.Lee WK, Nowak RW, Gardella JA. 2002. Hydrolytic degradation of polyester blend monolayers at the air/water interface: effects of a slowly degrading component. Langmuir 18, 2309–2312. ( 10.1021/La011663c) [DOI] [Google Scholar]
- 165.Kulkarni A, Reiche J, Lendlein A. 2007. Hydrolytic degradation of poly(rac-lactide) and poly[(rac-lactide)-co-glycolide] at the air-water interface. Surf. Interface Anal. 39, 740–746. ( 10.1002/Sia.2580) [DOI] [Google Scholar]
- 166.Kulkarni A, Reiche J, Kratz K, Kamusewitz H, Sokolov IM, Lendlein A. 2007. Enzymatic chain scission kinetics of Poly(epsilon-caprolactone) monolayers. Langmuir 23, 12 202–12 207. ( 10.1021/La701523e) [DOI] [PubMed] [Google Scholar]
- 167.Wang M, Smith JM, McCoy BJ. 1995. Continuous kinetics for thermal degradation of polymer in solution. AIChE J. 41, 1521–1533. ( 10.1002/aic.690410616) [DOI] [Google Scholar]
- 168.Reiche J, Kratz K, Hofmann D, Lendlein A. 2011. Current status of Langmuir monolayer degradation of polymeric biomaterials. Int. J. Artif. Organs 34, 123–128. ( 10.5301/Ijao.2011.6401) [DOI] [PubMed] [Google Scholar]
- 169.Ivanova T, Panaiotov I, Boury F, Proust JE, Benoit JP, Verger R. 1997. Hydrolysis kinetics of poly(D,L-lactide) monolayers spread on basic or acidic aqueous subphases. Colloids Surf. B 8, 217–225. ( 10.1016/S0927-7765(96)01331-8) [DOI] [Google Scholar]
- 170.van Nostrum CF, Veldhuis TFJ, Bos GW, Hennink WE. 2004. Hydrolytic degradation of oligo(lactic acid): a kinetic and mechanistic study. Polymer 45, 6779–6787. ( 10.1016/j.polymer.2004.08.001) [DOI] [Google Scholar]
- 171.Lee JK, Ryon JH, Lee WK, Park CY, Park SB, Min SK. 2003. Study on hydrolytic kinetics of Langmuir monolayers of biodegradable polylactide derivatives. Macromol. Res. 11, 476–480. ( 10.1007/BF03218979) [DOI] [Google Scholar]
- 172.Shakesheff KM. 2011. Drug delivery systems. In Handbook of biodegradable polymers (eds Lendlein A, Sisson AL), pp. 363–378. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA. [Google Scholar]
- 173.Lee WK, Iwata T, Gardella JA. 2005. Hydrolytic behavior of enantiomeric poly(lactide) mixed monolayer films at the air/water interface: stereocomplexation effects. Langmuir 21, 11 180–11 184. ( 10.1021/La051137b) [DOI] [PubMed] [Google Scholar]
- 174.Andersson SR, Hakkarainen M, Inkinen S, Södergård A, Albertsson A-C. 2010. Polylactide stereocomplexation leads to higher hydrolytic stability but more acidic hydrolysis product pattern. Biomacromolecules 11, 1067–1073. ( 10.1021/bm100029t) [DOI] [PubMed] [Google Scholar]
- 175.Lee WK, Gardella JA. 2000. Hydrolytic kinetics of biodegradable polyester monolayers. Langmuir 16, 3401–3406. ( 10.1021/La990800r) [DOI] [Google Scholar]
- 176.Ivanova T, Malzert A, Boury F, Proust JE, Verger R, Panaiotov I. 2003. Enzymatic hydrolysis by cutinase of PEG-co PLA copolymers spread monolayers. Colloids Surf. B 32, 307–320. ( 10.1016/j.colsurfb.2003.08.005) [DOI] [Google Scholar]
- 177.Göpferich A. 1997. Polymer bulk erosion. Macromolecules 30, 2598–2604. ( 10.1021/ma961627y) [DOI] [Google Scholar]
- 178.Grozev N, Svendsen A, Verger R, Panaiotov I. 2002. Enzymatic hydrolysis by Humicola lanuginosa lipase of polycaprolactone monolayers. Colloid Polym. Sci. 280, 7–17. ( 10.1007/s003960200001) [DOI] [Google Scholar]
- 179.Li SM, Garreau H, Vert M. 1990. Structure property relationships in the case of the degradation of massive aliphatic poly-(alpha-hydroxy acids) in aqueous-media.1. Poly(dl-lactic acid). J. Mater. Sci: Mater. Med. 1, 123–130. ( 10.1007/Bf00700871) [DOI] [Google Scholar]
- 180.Jo NJ, Iwata T, Lim KT, Jung SH, Lee WK. 2007. Degradation behaviors of polyester monolayers at the air/water interface: alkaline and enzymatic degradations. Polym. Degrad. Stab. 92, 1199–1203. ( 10.1016/j.polymdegradstab.2007.04.002) [DOI] [Google Scholar]
- 181.Lambeek G, Vorenkamp EJ, Schouten AJ. 1995. Structural study of Langmuir-Blodgett monolayers and multilayers of poly(beta-hydroxybutyrate). Macromolecules 28, 2023–2032. ( 10.1021/ma00110a041) [DOI] [Google Scholar]
- 182.Lendlein A. 1999. Polymere als Implantatwerkstoffe. Chem. Unserer Zeit 33, 279–295. ( 10.1002/ciuz.19990330505) [DOI] [Google Scholar]
- 183.Peng H, Ling J, Liu J, Zhu N, Ni X, Shen Z. 2010. Controlled enzymatic degradation of poly(ɛ-caprolactone)-based copolymers in the presence of porcine pancreatic lipase. Polym. Degrad. Stab. 95, 643–650. ( 10.1016/j.polymdegradstab.2009.12.005) [DOI] [Google Scholar]
- 184.Vidaurre A, Dueñas JMM, Estellés JM, Cortázar IC. 2008. Influence of enzymatic degradation on physical properties of poly(ɛ-caprolactone) films and sponges. Macromol. Symp. 269, 38–46. ( 10.1002/masy.200850907) [DOI] [Google Scholar]
- 185.Miao ZM, Cheng SX, Zhang XZ, Wang QR, Zhuo RX. 2007. Degradation and drug release property of star poly(epsilon-caprolactone)s with dendritic cores. J. Biomed. Mater. Res. B 81b, 40–49. ( 10.1002/jbm.b.30634) [DOI] [PubMed] [Google Scholar]
- 186.Kulkarni A, Reiche J, Hartmann J, Kratz K, Lendlein A. 2008. Selective enzymatic degradation of poly(epsilon-caprolactone) containing multiblock copolymers. Eur. J. Pharm. Biopharm. 68, 46–56. ( 10.1016/j.ejpb.2007.05.021) [DOI] [PubMed] [Google Scholar]
- 187.Schöne A-C, Kratz K, Schulz B, Lendlein A. 2016. The relevance of hydrophobic segments in multiblock copolyesterurethanes for their enzymatic degradation at the air-water interface. Polymer 102, 92–98. ( 10.1016/j.polymer.2016.09.001) [DOI] [Google Scholar]
- 188.Roßberg J, Rottke FO, Schulz B, Lendlein A. 2016. Enzymatic degradation of oligo(ɛ-caprolactone)s end-capped with phenylboronic acid derivatives at the air–water interface. Macromol. Rapid Commun. 37, 1966–1971. ( 10.1002/marc.201600471) [DOI] [PubMed] [Google Scholar]
- 189.Schöne A-C, Kratz K, Schulz B, Lendlein A. 2016. Polymer architecture versus chemical structure as adjusting tools for the enzymatic degradation of oligo(ɛ-caprolactone) based films at the air-water interface. Polym. Degrad. Stab. 131, 114–121. ( 10.1016/j.polymdegradstab.2016.07.010) [DOI] [Google Scholar]
- 190.Miller R, Ferri JK, Javadi A, Krägel J, Mucic N, Wüstneck R. 2010. Rheology of interfacial layers. Colloid Polym. Sci. 288, 937–950. ( 10.1007/s00396-010-2227-5) [DOI] [Google Scholar]
- 191.Li Z, Ma X, Zang D, Guan X, Zhu L, Liu J, Chen F. 2015. Interfacial rheology and aggregation behaviour of amphiphilic CBABC-type pentablock copolymers at the air-water interface: effects of block ratio and chain length. RSC Adv. 5, 82 869–82 878. ( 10.1039/C5RA08109B) [DOI] [Google Scholar]
- 192.Noskov BA, Loglio G, Miller R. 2011. Dilational surface visco-elasticity of polyelectrolyte/surfactant solutions: formation of heterogeneous adsorption layers. Adv. Colloid Interface Sci. 168, 179–197. ( 10.1016/j.cis.2011.02.010) [DOI] [PubMed] [Google Scholar]
- 193.Kotsmar C, et al. 2009. Thermodynamics, adsorption kinetics and rheology of mixed protein–surfactant interfacial layers. Adv. Colloid Interface Sci. 150, 41–54. ( 10.1016/j.cis.2009.05.002) [DOI] [PubMed] [Google Scholar]
- 194.Maheshkumar J, Dhathathreyan A. 2013. Langmuir and Langmuir–Blodgett films of capsules of haemoglobin at air/water and solid/air interfaces. J. Chem. Sci. 125, 219–227. ( 10.1007/s12039-013-0370-5) [DOI] [Google Scholar]
- 195.Grasso EJ, Oliveira RG, Maggio B. 2014. Rheological properties of regular insulin and aspart insulin Langmuir monolayers at the air/water interface: Condensing effect of Zn2+ in the subphase. Colloids Surf. B 115, 219–228. ( 10.1016/j.colsurfb.2013.11.031) [DOI] [PubMed] [Google Scholar]
- 196.Theodoratou A, et al. 2016. Semifluorinated alkanes at the air–water interface: tailoring structure and rheology at the molecular scale. Langmuir 32, 3139–3151. ( 10.1021/acs.langmuir.5b04744) [DOI] [PubMed] [Google Scholar]
- 197.Hofmann D, Entrialgo-Castaño M, Kratz K, Lendlein A. 2009. Knowledge-based approach towards hydrolytic degradation of polymer-based biomaterials. Adv. Mater. 21, 3237–3245. ( 10.1002/adma.200802213) [DOI] [PubMed] [Google Scholar]