Abstract
The mammalian intestinal epithelium undergoes continuous and rapid renewal of its four principal terminally differentiated cell types. These cells arise from multipotent stem cells located at or near the base of the crypts of Lieberkühn. The differentiation process is precisely organized along two spatial dimensions (axes)--from the crypt to the villus tip and from the duodenum to the colon. The enteroendocrine cell population provides a sensitive marker of the intestine's topologic differentiation. At least 15 different regionally distributed subsets have been described based on their principal neuroendocrine products. We have used immunocytochemical methods to characterize the spatial relationships of the serotonin-, secretin-, and substance P-containing enteroendocrine cell subsets in normal adult C57BL/6J x LT/Sv mice as well as in transgenic littermates that contain rat liver fatty acid-binding protein-human growth hormone fusion genes. Our results reveal precise spatial interrelationships between these populations and suggest a differentiation pathway that may involve the sequential expression of substance P, serotonin, and secretin.
Full text
PDF
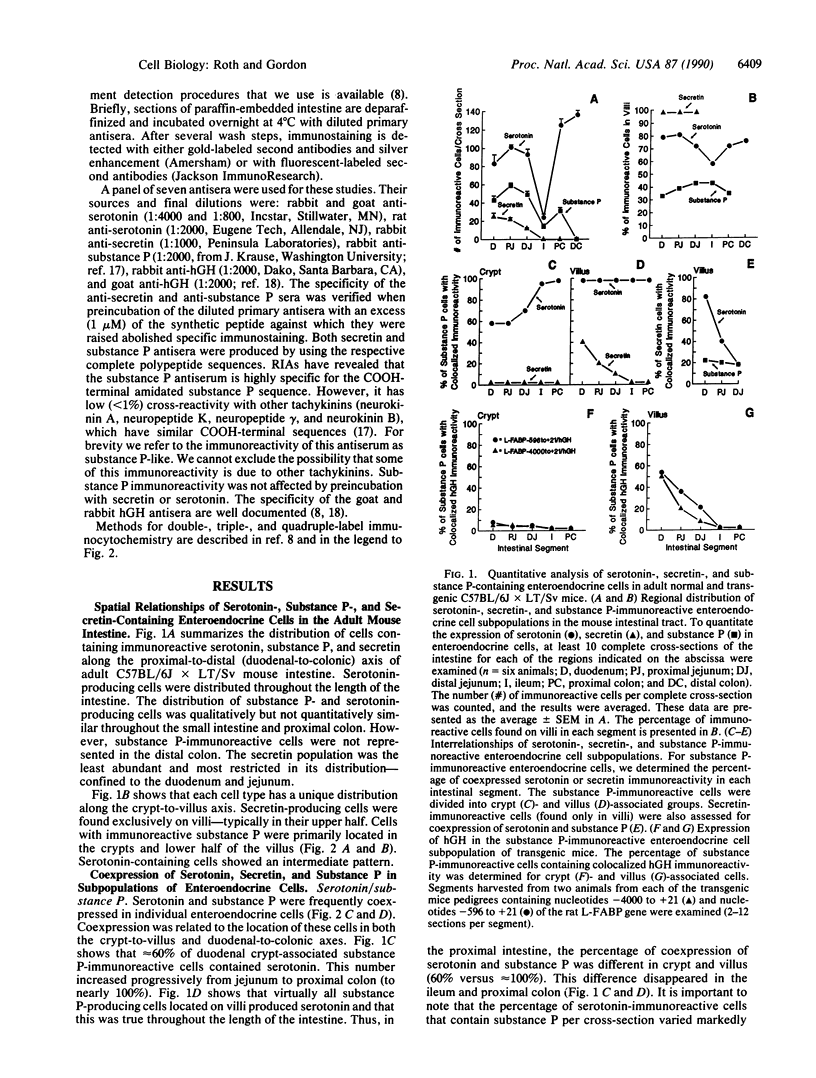
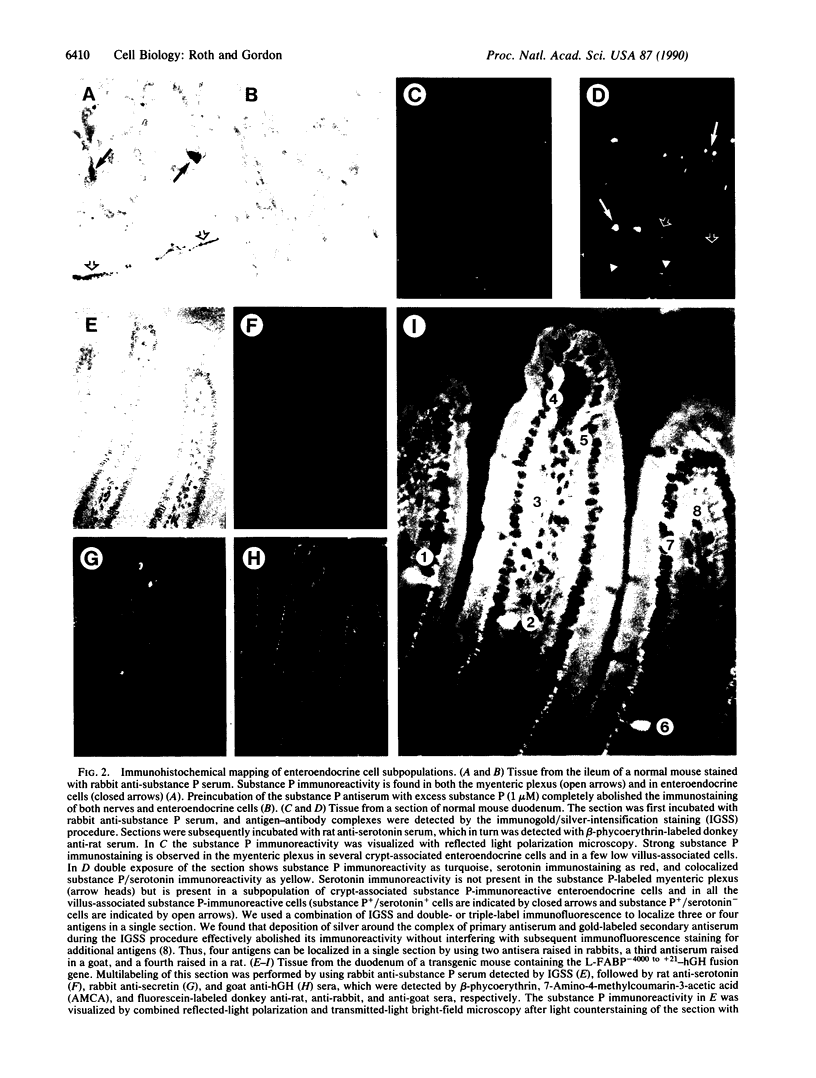


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat. 1974 Dec;141(4):503–519. doi: 10.1002/aja.1001410405. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974 Dec;141(4):537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Emson P. C., Gilbert R. F., Martensson H., Nobin A. Elevated concentrations of substance P and 5-HT in plasma in patients with carcinoid tumors. Cancer. 1984 Aug 15;54(4):715–718. doi: 10.1002/1097-0142(1984)54:4<715::aid-cncr2820540420>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Gordon J. I. Intestinal epithelial differentiation: new insights from chimeric and transgenic mice. J Cell Biol. 1989 Apr;108(4):1187–1194. doi: 10.1083/jcb.108.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz P., Polak J. M., Timson D. M., Pearse A. G. Enterochromaffin cells as the endocrine source of gastrointestinal substance P. Histochemistry. 1976 Nov 12;49(4):343–347. doi: 10.1007/BF00496138. [DOI] [PubMed] [Google Scholar]
- Inokuchi H., Fujimoto S., Kawai K. Cellular kinetics of gastrointestinal mucosa, with special reference to gut endocrine cells. Arch Histol Jpn. 1983 Apr;46(2):137–157. doi: 10.1679/aohc.46.137. [DOI] [PubMed] [Google Scholar]
- Kendall K., Jumawan J., Koldovský O. Development of jejunoileal differences of activity of lactase, sucrase and acid beta-galactosidase in isografts of fetal rat intestine. Biol Neonate. 1979;36(3-4):206–214. doi: 10.1159/000241229. [DOI] [PubMed] [Google Scholar]
- Krause J. E., MacDonald M. R., Takeda Y. The polyprotein nature of substance P precursors. Bioessays. 1989 Feb-Mar;10(2-3):62–69. doi: 10.1002/bies.950100207. [DOI] [PubMed] [Google Scholar]
- Lewin K. J. The endocrine cells of the gastrointestinal tract. The normal endocrine cells and their hyperplasias. Part I. Pathol Annu. 1986;21(Pt 1):1–27. [PubMed] [Google Scholar]
- MacDonald M. R., Takeda J., Rice C. M., Krause J. E. Multiple tachykinins are produced and secreted upon post-translational processing of the three substance P precursor proteins, alpha-, beta-, and gamma-preprotachykinin. Expression of the preprotachykinins in AtT-20 cells infected with vaccinia virus recombinants. J Biol Chem. 1989 Sep 15;264(26):15578–15592. [PubMed] [Google Scholar]
- McKeel D. W., Jr, Askin F. B. Ectopic hypophyseal hormonal cells in benign cystic teratoma of the ovary. Light microscopic histochemical dye staining and immunoperoxidase cytochemistry. Arch Pathol Lab Med. 1978 Mar;102(3):122–128. [PubMed] [Google Scholar]
- Märtensson H., Nobin A., Sundler F., Falkmer S. Endocrine tumors of the ileum. Cytochemical and clinical aspects. Pathol Res Pract. 1985 Oct;180(4):356–363. doi: 10.1016/S0344-0338(85)80106-0. [DOI] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M. Immunocytochemical localization of substance P in mammalian intestine. Histochemistry. 1975;41(4):373–375. doi: 10.1007/BF00490081. [DOI] [PubMed] [Google Scholar]
- Peck J. J., Shields A. B., Boyden A. M., Dworkin L. A., Nadal J. W. Carcinoid tumors of the ileum. Am J Surg. 1983 Jul;146(1):124–132. doi: 10.1016/0002-9610(83)90272-6. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Schmidt G. H., Wilkinson M. M., Wood M. J., Monk M., Reid A. Derivation of mouse intestinal crypts from single progenitor cells. Nature. 1985 Feb 21;313(6004):689–691. doi: 10.1038/313689a0. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Hertz J. M., Gordon J. I. Mapping enteroendocrine cell populations in transgenic mice reveals an unexpected degree of complexity in cellular differentiation within the gastrointestinal tract. J Cell Biol. 1990 May;110(5):1791–1801. doi: 10.1083/jcb.110.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth K. A., Makk G., Beck O., Faull K., Tatemoto K., Evans C. J., Barchas J. D. Isolation and characterization of substance P, substance P 5-11, and substance K from two metastatic ileal carcinoids. Regul Pept. 1985 Nov 7;12(3):185–199. doi: 10.1016/0167-0115(85)90060-6. [DOI] [PubMed] [Google Scholar]
- Schmidt G. H., Wilkinson M. M., Ponder B. A. Cell migration pathway in the intestinal epithelium: an in situ marker system using mouse aggregation chimeras. Cell. 1985 Feb;40(2):425–429. doi: 10.1016/0092-8674(85)90156-4. [DOI] [PubMed] [Google Scholar]
- Sjölund K., Sandén G., Håkanson R., Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983 Nov;85(5):1120–1130. [PubMed] [Google Scholar]
- Skrabanek P., Cannon D., Kirrane J., Powell D. Substance P secretion by carcinoid tumours. Ir J Med Sci. 1978 Feb;147(2):47–49. doi: 10.1007/BF02939369. [DOI] [PubMed] [Google Scholar]
- Sokolski K. N., Lechago J. Human colonic substance P-producing cells are a separate population from the serotonin-producing enterochromaffin cells. J Histochem Cytochem. 1984 Oct;32(10):1066–1074. doi: 10.1177/32.10.6207221. [DOI] [PubMed] [Google Scholar]
- Sternini C., Anderson K., Frantz G., Krause J. E., Brecha N. Expression of substance P/neurokinin A-encoding preprotachykinin messenger ribonucleic acids in the rat enteric nervous system. Gastroenterology. 1989 Aug;97(2):348–356. doi: 10.1016/0016-5085(89)90070-x. [DOI] [PubMed] [Google Scholar]
- Sweetser D. A., Birkenmeier E. H., Hoppe P. C., McKeel D. W., Gordon J. I. Mechanisms underlying generation of gradients in gene expression within the intestine: an analysis using transgenic mice containing fatty acid binding protein-human growth hormone fusion genes. Genes Dev. 1988 Oct;2(10):1318–1332. doi: 10.1101/gad.2.10.1318. [DOI] [PubMed] [Google Scholar]