Abstract
Introduction
Recent studies have suggested reducing the dose submandibular glands receive when patients undergo head and neck radiotherapy can play a crucial role in preventing xerostomia. However, they are traditionally not spared due to concern that target coverage may be compromised. We investigated the possibility of sparing the contralateral submandibular gland (cSM) by utilising modern planning techniques.
Methods
10 head and neck patients previously treated with conformal therapy at our centre were retrospectively planned using intensity modulated radiation therapy (IMRT), and volumetric modulated arc therapy (VMAT). Each patient was prescribed 70 Gy in 35 fractions to the primary volume, with 56 Gy delivered to the elective nodal areas. The primary objective was to spare the cSM gland using appropriate dose constraints.
Results
Mean dose to the cSM gland was reduced to an acceptable dose level (39 Gy) for all patients replanned using an IMRT or VMAT technique, without compromising planned target volume (PTV) coverage or other critical structures. VMAT was able to reduce the mean dose to 31.5 ± 5.5 Gy compared to 34.5 ± 4.8 Gy of IMRT and offered improved plan conformity.
Conclusion
Sparing the cSM gland is possible using IMRT and VMAT planning, whilst preserving coverage on the elective PTV. This has produced a change in protocol in our department, more focus placed on sparing the SM glands. VMAT is a viable alternative method of delivering treatment and will be utilised when required.
Keywords: IMRT, radiotherapy, submandibular, VMAT, xerostomia
Introduction
Highly conformal radiotherapy often with concurrent chemotherapy is regarded as standard care for many patients presenting with locally advanced head and neck cancer. Treatment volumes are often large to facilitate coverage of all gross disease and the at risk cervical nodes, which often mandates bilateral neck irradiation. As technology has evolved, so has the potential dose reduction to adjacent critical structures. Intensity modulated radiation therapy (IMRT) planning enables high doses to be delivered in a conformal pattern to the target area. Despite these advancements, xerostomia remains a regular and morbid toxicity experienced by patients following head and neck radiotherapy. This may result in dysphagia, eating and speaking difficulties, increased risk of dental caries and osteoradionecrosis, and can have a significant impact on the quality of life.1, 2, 3, 4, 5
The occurrence and severity of xerostomia has been linked to the mean radiation dose received by the salivary glands during radiotherapy. The parotid gland produces around 65% of stimulated saliva and studies have shown that by reducing the parotid dose, the incidence of xerostomia can be decreased.6, 7 Limiting the mean dose to less than 26 Gy has become standard practice in head and neck radiotherapy. The submandibular glands have been the subject of far less research, but their importance to salivary function is beginning to be recognised. Whilst the parotid glands are the major producer of stimulated saliva, the submandibular glands are responsible for up to 90% of the unstimulated saliva. It is reasonable to assume therefore that minimising submandibular gland dose may improve background salivary function.2, 3, 4, 5, 8 Murdoch‐Kinch et al.4 examined the dose–response relationship for the submandibular gland (SM) gland and reported an exponential reduction in salivary output beyond a dose threshold of 39 Gy. Salivary recovery was seen to be higher over a 2 year period, when the mean dose was kept under this mark.
Sparing the submandibular glands however can be more difficult than sparing the parotid glands, as they frequently overlap the elective nodal volume (Fig. 1). It has been suggested that it may be possible to reduce the dose to the contralateral submandibular gland (cSM) where the overlap is often less due to the distance from the primary disease.9
Figure 1.
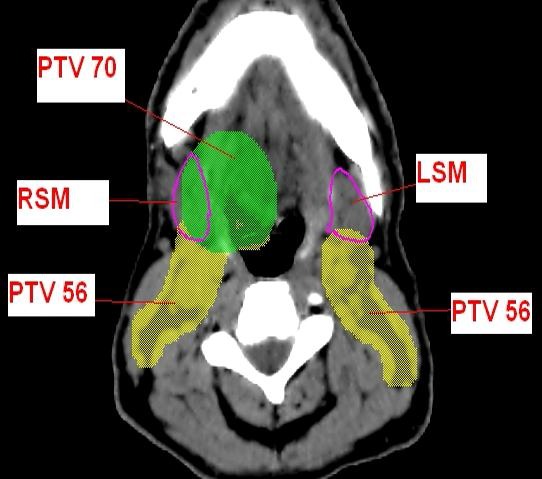
Delineated anatomy on sample Axial cross‐section. PTV, planning target volume; RSM, right submandibular gland; LSM, left submandibular gland
Our institution currently uses IMRT for the majority of our radical H&N patients and volumetric modulated arc therapy (VMAT) has also recently been commissioned for clinical use. An IMRT/VMAT program is only acceptable with a robust image guided radiation therapy program with respect to issues at planning of immobilisation and at treatment with image guidance techniques.10 The advantage of IMRT over conventional radiotherapy for parotid sparing has been extensively reported with clinical reduction in xerostomia demonstrated.11 IMRT is however associated with increased treatment delivery time which can impact on both patient compliance and departmental workflow. VMAT, which delivers IMRT through the use of arcs, can achieve shorter treatment times, potentially improving overall accuracy via increased patient compliance and reduced intrafraction movement.4, 12, 13, 14, 15 This planning study aimed to assess and compare the ability of IMRT and VMAT to reduce the contralateral submandibular dose without compromising target coverage. A secondary objective of the study was to observe overall treatment time and monitor units (MUs) delivered, considering the benefit to patient and departmental workflow.
Method
Ten patients treated with conformal radiation for locally advanced head and neck carcinoma between 2010 and 2012 at our centre were replanned using IMRT and VMAT, with a specific planning goal to spare the cSM. The 10 patients were selected sequentially from commencement of IMRT program at the centre. This study has been undertaken as originally approved by the Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research, and conducted in compliance with the NHMRC National Statement on Ethical Conduct in Human Research (NHMRC, 2007). Informed Consent was not required as all data were accumulated retrospectively and de‐identified. Each patient presented with Stage III or IVa/b disease with oral or oropharyngeal primaries. Selection criteria for inclusion in the study were treatment to the primary disease and involved nodal regions of 70 Gy and bilateral uninvolved nodal regions of 56 Gy in 35 fractions with no primary disease crossing the mid‐line. A planning computed tomography (CT) scan was acquired on a Toshiba Aquilion Wide Bore scanner for each patient with a slice thickness of 2 mm. Patients were positioned using a thermoplastic immobilisation mask and vaclok support under head and shoulders. The datasets were then exported for target delineation.
Two radiation oncologists reviewed and edited the target and organ at risk volumes for each of the plans to reduce variables in contouring. They were planned to two dose levels using a simultaneous integrated boost with 70 Gy delivered to the primary volume (PTV boost) and 56 Gy to the elective nodal areas (PTV elect). The primary volume included all gross tumour volume and involved lymph nodes with an anatomically modified 5 mm margin applied for the clinical target volume and a further 5 mm to achieve our planned target volume (PTV). The elective volume consisted of at risk nodal areas with a 5 mm margin applied for setup error. The PTVs were clipped at 5 mm from the patient surface to prevent optimisation problems in the build‐up region. The spinal canal, brainstem, parotid glands, oral cavity and submandibular glands were also delineated or adjusted as required, with a 3 mm margin applied to the spinal cord and brainstem to produce a planning risk volume (PRV), accounting for any daily variation in treatment position. The primary endpoint of this study was to compare IMRT and VMAT planning techniques in reducing the mean dose to the contralateral submandibular gland, without impacting on target volume coverage. Planning parameters included limiting the dose to the spinal canal and brainstem as the highest priority with a maximum dose of 48 and 54 Gy assigned to the respective structure. The objective for the primary and elective PTVs was to deliver 95% (V95) of the prescribed dose to 99% of the volume. Dose exceeding 110% was assessed via a conformity index (CI 95%) for the primary volume to assess the homogeneity of the plan. The CI 95% was calculated by dividing the volumetric area (cc) covered by the 66.5 Gy isodose by the volume of the primary PTV. Other dose objectives included a mean dose <26 Gy to both parotid glands where possible, and a mean dose of <45 Gy to the oral cavity for involved volumes. Assuming these goals were met, an attempt was made to reduce the mean dose of the cSM (and ipsilateral submandibular gland (iSM) where possible) to <39 Gy.
The same radiation therapist specialised in head and neck planning optimised each plan, to limit any variability posed by planning experience. The IMRT plans were optimised on the Pinnacle3® planning system version 9.0 (Phillips Medical Systems, Madison, WI) using seven coplanar fields of 6 MV. Direct machine parameter optimisation functionality was utilised in conjunction with the collapsed‐cone dose‐calculation algorithm with a maximum of 70 segments per plan with Step‐and‐Shoot delivery. The dose grid was set to 3 mm in all directions. Optimisation for the two plans followed a similar process, with minimum and maximum dose constraints used for the PTVs, spinal cord and brainstem. The dose to the remaining critical structures (parotids, SM gland and oral cavity) were generally controlled using an equivalent uniform dose constraint set to the uninvolved region of the structure. This allowed the dose to the structure to be minimised, without impacting on PTV coverage. A standard conformal dose ring (1 cm outside planning volumes) and normal tissue structure were used to improve plan conformity and manage dose to other adjacent critical structures.
VMAT planning utilised the SmartArc functionality and employed a single 360 degree arc, consisting of 91 control points and 6 MV energy. Varying gantry speed and dose rate were available for treatment delivery. Both IMRT and VMAT plans were able to be successfully delivered on an Elekta Synergy Linear Accelerator with 1 cm multi‐leaf collimator leaves. Each plan was timed from the commencement of first beam to the completion of the last MU to establish an overall beam‐on time.
The data were collated and compared using Microsoft Excel (2013), with a paired sample t‐test utilised to determine which elements were of statistical significance.
Results
Clinically acceptable plans for IMRT and VMAT were achieved for all 10 patients included in the study, with each plan deliverable on an Elekta Linac. All plans were quality approved by our physics department and clinically approved by the radiation oncologist in accordance with the standard protocols outlined for IMRT planning. The planning data were compared for each technique and reached significance for the submandibular glands, conformity and number of MUs (Table 1).
Table 1.
Mean results of the 10 patients included in study
| IMRT | VMAT | P‐value | |
|---|---|---|---|
| PTV boost (V95(%)) | 99.2 ± 0.2 | 99.2 ± 0.3 | 0.702 |
| PTV elect (V95(%)) | 99.1 ± 0.1 | 99.1 ± 0.1 | 0.226 |
| Submandibular gland mean dose | |||
| Contralateral (Gy) | 34.7 ± 4.8 | 31.5 ± 5.5 | 0.004a |
| Ipsilateral (Gy) | 59.4 ± 13.0 | 57.8 ± 13.1 | 0.011a |
| CI PTV boost | 1.54 ± 0.2 | 1.41 ± 0.1 | 0.003a |
| MU | 725 | 530 | 3.56E−5 a |
V95, The volume of the structure (%) receiving 95% of the prescribed dose; PTV, planned target volume; CI, conformity index; MU, monitor unit; IMRT, intensity modulated radiation therapy; VMAT, volumetric modulated radiation therapy; P‐value, Paired sample t‐test (P < 0.05).
Statistically significant.
Submandibular glands
The dose to the cSM gland met the <39 Gy threshold for 100% (n = 10) of patients using IMRT or VMAT. The VMAT plan produced a mean dose of 31.5 ± 5.5 Gy, compared to 34.5 ± 4.8 Gy for the IMRT plan. The dose to the iSM gland also provided improved sparing on the ipsilateral gland with the VMAT plan, with a mean dose of 57.8 ± 13.1 Gy compared to 59.4 ± 13.0 Gy on the IMRT plan. The difference in dose for the cSM and iSM glands reached statistical significance with a P‐value of 0.004 and 0.011 respectively. Only one dataset however were able to achieve the target constraint of 39 Gy mean dose for iSM gland, achieved by both the VMAT and IMRT plans.
Target coverage
All plans received acceptable levels of coverage for the PTV boost and PTV elect.
Spinal cord and brainstem
All plans achieved the target objective to the structure and the respective PRV's for both spinal cord and brainstem.
Parotid glands
Dose received by the parotid glands was similar between the two techniques, with a mean dose of <26 Gy achieved in 80% (n = 8) for the treatment plans within the cohort. In the remaining two plans, only the objective for the contralateral parotid gland could be met, with the ipsilateral side overlapping with the PTV boost.
Oral cavity, normal tissue and conformity
The oral cavity objective (mean 45 Gy) was achieved for 80% (n = 8) of the patient cohort using each technique. There was no specific tolerance stated for healthy tissue but a ring and normal tissue structure was used to obtain plan conformity and hence no regions of greater than 50% were observed (35 Gy) at distance from the PTV volume (Fig. 2). VMAT proved to be more effective in achieving conformity, on average producing a CI 95% index of 1.41 compared to 1.54 for IMRT. The global max point of the plan was also generally seen to be 2–3 Gy lower on the VMAT plan.
Figure 2.
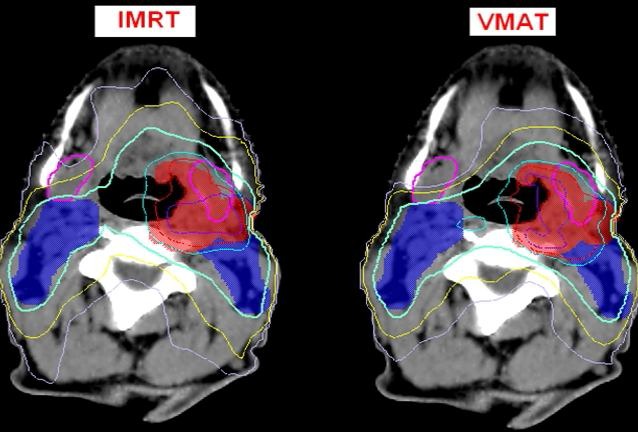
Typical dose distribution for intensity modulated radiation therapy and VMAT plans aimed at sparing the cSM gland on sample axial cross‐section. The planned target volume (PTV) boost volume is delineated in red, the PTV elect is delineated in blue. The submandibular glands are outlined in pink. The dose to the cSM was reduced to a mean dose of under 39 Gy whilst preserving target coverage. The isodose lines highlight the improved conformity on the VMAT plan, with the yellow 45 Gy and light purple 35 Gy isodose closely following target volume, also lowering the mean dose to the structure. Purple – 73.5 Gy, Red – 70 Gy, Cyan – 66.5 Gy, Light Green – 53.2 Gy, Yellow – 45 Gy, Light Purple – 35 Gy.
Treatment times and monitor units (MUs)
The VMAT plans reduced both the MU's delivered and treatment delivery time. Treatment times were on average 68% quicker, with an average VMAT treatment completed in 170 sec compared to an average IMRT beam‐on time of almost 9 min.
Discussion
Xerostomia remains a morbid side effect for patients receiving radiotherapy to their head and neck region, which can have a significant impact on quality of life. Traditionally, research has focused on the role of the parotid gland in producing saliva, however recently reports have highlighted the importance of unstimulated saliva from the submandibular glands. IMRT has been shown to reduce xerostomia following radiotherapy through parotid sparing. This study assessed the ability to also spare contralateral submandibular dose with both IMRT and VMAT planning techniques.
The number of participants in the comparison was restricted due to the limiting nature of the inclusion criteria and being a single institution study. Results were similar between the techniques for many parameters but clinically and statistically significant differences were seen in three key areas, despite the small sample size.
The VMAT plan obtained an average mean dose of 31.5 Gy, compared to 34.5 Gy on the IMRT plan. This relates directly back to our primary objective and demonstrates that sparing the cSM is possible using either IMRT or VMAT, however the latter shows a significantly better result. A mean dose constraint <39 Gy to the submandibular gland was selected based on the work of Murdock‐Kinch et al.4 that suggested that mean doses above 39 Gy resulted in negligible unstimulated salivary flow. This suggests that the submandibular gland is less radiosensitive than the parotid gland, and enables a constraint that is clinically achievable without impacting on target coverage. Alternatively Deasy et al.16 suggest when possible, the mean submandibular gland dose should be kept to <35 Gy. For this report, the benchmark was set at 39 Gy with intent to reduce the dose to a low as possible without impacting target coverage. To be clinically approved, the V95 for each PTV in this series was required to be >99%. The PTV coverage was similar between plans for both dose levels. This was expected as identical dose level constraints were utilised for each technique. On average the PTV70 retained an increased numeric value compared to the elective volume (PTV56). This can be explained by the intent of the study to minimise dose to the cSM which frequently overlaps with the elective nodal volume. This resulted in an occasional underdosage in a small volume of the PTV elect (0–0.5 cm³), more commonly with the IMRT plans, however the D99 for the elective PTV remained above 53.2 Gy (95%). It was covered by 90% of the prescribed dose in all cases and it occurred where the PTV elect overlapped with the contralateral SM volume. This “underdosing” was more significant in the IMRT plans. Dooenart et al.3 observed similar dosimetric findings but reported no local recurrences in this region. To negate this effect, Dooenart et al.3 extended the planning PTV in this region by 2 mm and reported an improved V95 with negligible impact on the cSM dose, whilst Houwelling et al.9 evaluated the minimum dose to 1 cc of the PTV elect, as an alternative to assessing the D99. This ensured the entire PTV received an adequate dose. Neither method was applied in this study, but may merit discussion in determining future planning guidelines.
Alternatively, once the required objectives were achieved for the remaining organs at risk, they were not optimised further, leading to a greater level of dose coverage for the primary volume.
PTV conformity was also significant with the plan conformity (CI 95%) superior in the VMAT plan. The plan hotspots were reduced in the VMAT plans, with a reduction seen in both the size and intensity of the high dose regions. It is intuitive that VMAT produced a more conformal result. The increased number of beam angles allows the dose to be conformed more tightly around the PTV producing greater target homogeneity and improved sparing of organs at risk (Fig. 2). Single arc VMAT was used in this case to test the capability of the optimiser. This technique was more than sufficient at providing a homogenous treatment plan with improved conformity and organ sparing, compared to a 7field IMRT plan.
The beam time for the VMAT plans was reduced by 6–7 min when delivered on an Elekta Synergy linac, encouraging increased patient compliance, reduced risk of intrafraction movement and improved workflow. Monitor units were also significantly reduced based on results of the study. Other publications have reported higher reductions in MU's delivered which could be explained by these studies utilising different optimisation and/or delivery techniques for IMRT.4, 12, 13, 14, 15 It is noted that some studies have suggested that a single arc is not adequate for more complex head and neck plans, however that is beyond the scope of this report, and would need to be evaluated on a case by case scenario.13, 14
The results of this series have altered planning practices in our department. The cSM is now routinely volumed for dose sparing where clinically appropriate. Greater investigation into the clinical effect on unstimulated saliva flow, xerostomia and quality of life scores is warranted given that such dose sparing is technically achievable with both IMRT and VMAT techniques. VMAT will be further investigated as a department standard due to its potential to reduce treatment times, improving compliance and accuracy, whilst preserving plan quality.
Conclusion
Sparing the cSM gland is feasible using IMRT and VMAT planning, whilst preserving required coverage on the elective PTV. VMAT offered improved tissue sparing plan conformity and potential improved throughput compared to IMRT. Collaboration with other disciplines will be investigated to determine the impact on long‐term salivary function and quality of life.
Acknowledgements
I would like to thank the staff at the Alan Walker Cancer Care Centre, Darwin for their support and guidance in completing this report.
J Med Radiat Sci 64 (2017) 125–130
References
- 1. Bjordal K, Kaasa S, Mastekaasa A. Quality of life in patients treated for head and neck cancer: A follow‐up study 7–11 years after radiotherapy. Int J Radiat Oncol Biol Phys 1994; 28: 847–56. [DOI] [PubMed] [Google Scholar]
- 2. Collan J, Kapanen M, Makitie A. Submandibular gland‐sparing intensity modulated radiotherapy in the treatment of head and neck cancer: Sites of locoregional relapse and survival. Acta Oncol 2012; 51: 735–42. [DOI] [PubMed] [Google Scholar]
- 3. Doornaert P, Verbakel W, Rietveld D, Slotman BJ, Senan S. Sparing the contralateral submandibular without compromising PTV coverage by using volumetric modulated arc therapy. Radiat Oncol 2011; 6: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murdoch‐Kinch A, Kim HM, Vineberg KA, Ship JA, Eisbruch A. Dose‐effect relationships for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys 2008; 72: 373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strigari L, Benassi M, Arcangeli G, Bruzzaniti V, Giovinazzo G, Marucci L. A novel dose constraint to reduce xerostomia in head‐and‐neck cancer patients treated with intensity‐modulated radiotherapy. Int J Radiat Oncol Biol Phys 2010; 77: 269–76. [DOI] [PubMed] [Google Scholar]
- 6. Braam PM, Roesink JM, Raaijmakers CP, Busschers WB, Terhaard CH. Quality of life and salivary output in patients with head‐and‐neck cancer 5 years after radiotherapy. Radiat Oncol 2007; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Kruijf WJ, Heijmen BJ, Levendag PC. Quantification of trade‐off between parotid gland sparing and planning target volume underdosages in clinically node‐negative head‐and‐neck intensity‐modulated radiotherapy. Int J Radiat Oncol Biol Phys 2007; 68: 136–43. [DOI] [PubMed] [Google Scholar]
- 8. Wang Z, Yan C, Zhang Z, et al. Impact of salivary gland dosimetry on post‐IMRT recovery of saliva output and xerostomia grade for head‐and‐neck cancer patients treated with or without contralateral submandibular gland sparing: A longitudinal study. Int J Radiat Oncol Biol Phys 2011; 81: 1479–87. [DOI] [PubMed] [Google Scholar]
- 9. Houweling AC, Dijkema T, Roesnik JM, Terhaard CH, Raaijmakers CP. Sparing the contralateral submandibular gland in oropharyngeal cancer patients: A planning study. Radiother Oncol 2008; 89: 64–70. [DOI] [PubMed] [Google Scholar]
- 10. Webster GJ, Rowbottom CG, Mackay RI. Accuracy and precision of an IGRT solution. Med Dosim 2009; 34: 99–106. [DOI] [PubMed] [Google Scholar]
- 11. Braam PM, Terhaard CH, Roesnik JM, Raaijmakers CP. Intensity‐modulated radiotherapy significantly reduces xerostomia compared with conventional radiotherapy. Int J Radiat Oncol Biol Phys 2006; 66: 975–80. [DOI] [PubMed] [Google Scholar]
- 12. Bertelsen A, Hansen CR, Johansen J, Brink C. Single arc volumetric modulated arc therapy of head and neck cancer. Radiother Oncol 2010; 95: 142–8. [DOI] [PubMed] [Google Scholar]
- 13. Guckenberger M, Richter A, Krieger T, Wilbert J, Baier K, Flentje M. Is a single arc sufficient in volumetric‐modulated arc therapy (VMAT) for complex‐shaped target volumes. Radiother Oncol 2009; 93: 259–65. [DOI] [PubMed] [Google Scholar]
- 14. Vanetti E, Clivio A, Nicolini G, et al. Volumetric modulated arc radiotherapy for carcinomas of the oro‐pharynx, hypo‐pharynx and larynx: A treatment planning comparison with fixed field IMRT. Radiother Oncol 2009; 92: 111–7. [DOI] [PubMed] [Google Scholar]
- 15. Verbakel WF, Cuijpers JP, Hoffmans D, Bieker M, Slotman BJ, Senan S. Volumetric intensity‐modulated arc therapy vs. conventional IMRT in head‐and‐neck cancer: A comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys 2009; 74:252–9. [DOI] [PubMed] [Google Scholar]
- 16. Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose‐volume effects on salivary gland function. Int J Radiat Oncol Biol Phys 2010; 76: S58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]


