Abstract
Introduction
When irradiating the left breast, a small portion of the heart and left anterior descending coronary artery (LAD) are often included in the treatment field. Deep inspiration breath‐hold (DIBH) techniques reduce dose to coronary structures, but are resource intensive and may not be tolerated by all patients. The aim of this study was to evaluate a simple multi‐leaf collimator (MLC) modification technique with respect to target coverage and organ‐at‐risk sparing.
Methods
Forty nine patients with left‐sided breast cancer, planned with a simultaneous integrated boost technique were retrospectively replanned with additional shielding of the LAD. Dose to the target volumes (whole breast and boost) and organs at risk (heart, ipsilateral lung and LAD) were assessed on both plans.
Results
Significant dose reductions were observed for all organs at risk when LAD shielding was introduced, with a reduction in mean LAD dose of 7.0 Gy, mean LAD planning risk volume (PRV) dose of 5.9 Gy, maximum LAD dose of 12 Gy and mean heart dose of 0.73 Gy. Target volume coverage was clinically acceptable for 96% of patients, using the left anterior descending coronary artery shielded plan (LADSP). No difference was observed between the standard plan (SP) and LADSP in nine patients (18%).
Conclusions
For selected patients, the implementation of a simple MLC shielding technique can reduce the dose to cardiac structures, whilst maintaining breast and boost volume dosimetry. This technique is simple to implement and may be used as an alternative to DIBH for those patients who are unable to fulfill the selection criteria, or departments who are not resourced to perform DIBH.
Keywords: Breast, breast neoplasms, cardiac toxicity, organs at risk, radiotherapy
Introduction
Breast‐conserving surgery, including radiotherapy improves local control and overall survival for women with invasive breast cancer.1 When treating left breast cancer with radiotherapy, a small portion of the heart is often included in the radiation treatment field due to the anatomical relationship between the breast and heart. Studies have demonstrated a 25% increased risk of late cardiac effects including ischaemic heart disease in patients receiving radiotherapy for left‐sided breast cancer.2, 3, 4, 5, 6, 7 With respect to coronary artery disease, left‐sided breast patients have a 51% higher rate of cardiac stress test abnormalities compared to patients with right‐sided breast cancer, and 70% of those abnormalities occurred in the left anterior descending coronary artery (LAD), with stenosis rates at the mid and distal LAD significantly increased.8, 9 Therefore, techniques to reduce the dose to the heart and LAD are desirable and should be considered in these patients.
Deep inspiration breath hold (DIBH) has been shown to reduce the dose to cardiac structures by increasing the volume of lung between the breast tissue and heart, therefore removing the heart from the radiation fields.10, 11, 12, 13 Voluntary DIBH relies on the patient's ability and compliance to achieve breath‐hold, and may not be achievable for all patients. Active DIBH utilises specific equipment and therefore has associated cost and training implications for a radiotherapy department. DIBH techniques increase overall planning and treatment time, as an additional computed tomography (CT) scan is performed, and several breath‐holds are required to complete a treatment fraction.14 For these reasons, the uptake of DIBH techniques internationally has been slow, with 19% of EORTC institutions using some form of DIBH technique in 2010.15
An alternative method to reduce heart dose is the modification of tangential field beam angles,16 or the introduction of multi‐leaf collimator (MLC) shielding of the heart from the treatment fields.17 In this study, we compared standard tangential breast radiotherapy, using a simultaneous integrated boost (SIB) technique, to tangential breast radiotherapy with an LAD‐shielded technique, where the LAD is selectively shielded from the tangential fields. The aims of this study were to 1: Compare dose to the following organs at risk: LAD, heart, left lung; 2: Compare dose to the clinical target volume (CTV) and planning target volume (PTV) boost, and whole breast CTV and PTV; and 3: Investigate any factors that could affect PTV coverage when implementing an LAD‐shielded technique, compared to a standard plan (SP).
Method
This retrospective, ethical review board approved study was conducted to examine the dosimetric effect of introducing additional MLC shielding to reduce LAD dose. Forty‐nine consecutive left‐sided breast cancer patients were identified as having undergone whole breast and SIB radiotherapy between 2012 and 2014. The prescription dose was 45 Gy in 25 fractions at 1.8 Gy per fraction to the whole breast with tangential beams, and 60 Gy in 25 fractions at 2.4 Gy per fraction to the tumour bed, using a three‐dimensional, conformal photon SIB technique.
Target and organ at risk delineation
All patients were simulated supine, on an inclined breast stabilisation board, with arms above head. Patients underwent a free‐breathing, non‐contrast CT scan of the thorax. Target volumes were delineated according to previously published methods18 and included the whole breast CTV (CTVbreast), including the glandular tissue of the left breast, excluding the ribs and constrained to 5 mm within the skin surface; the SIB CTV (CTVboost), including the seroma or other surgical‐induced tissue changes and surgical clips. Whole breast (PTVbreast) and SIB PTV (PTVboost) were created as 5 mm expansions of the CTVbreast and CTVboost respectively. For dose‐volume histogram (DVH) analysis, evaluation (Eval) structures (PTVbreast Eval and PTVboost Eval) were created, constrained to 5 mm within the skin to account for the build‐up of dose in this region. The heart and LAD were delineated according to guidelines by Feng et al.19 Due to the known uncertainty in delineation and movement of the LAD,20 an LAD planning risk volume (PRV) was created with a 5 mm expansion of the LAD contour. Target volumes and the LAD were contoured by a breast radiation oncologist, and heart and lung contours were completed by a radiation therapist to eliminate inter‐observer variability in delineation.
Planning process
Two treatment plans were created for each patient, the SP and the LAD shielded plan (LADSP), with additional MLC shielding of the LAD. To create the SP, the PTVbreast was planned with tangential fields, angled to maximise coverage of PTVbreast, while minimising dose to ipsilateral lung and heart. A 1 cm margin of MLC field shaping on PTVbreast was used, except where it was necessary to reduce the maximum depth of the lungs in the field to a maximum of 2 cm (Fig. 1). Wedges, weightings and sub‐fields were used to optimise dosimetry to PTVbreast. The PTVboost was planned with a 3D conformal photon technique utilising 2–4 beam angles, optimised for PTVboost coverage, and minimisation of dose to ipsilateral lung and heart where possible, using MLC field shaping. To create the LADSP, additional MLC shielding was introduced to the tangential fields to selectively shield LAD (Fig. 2). No other changes were made to the plan.
Figure 1.
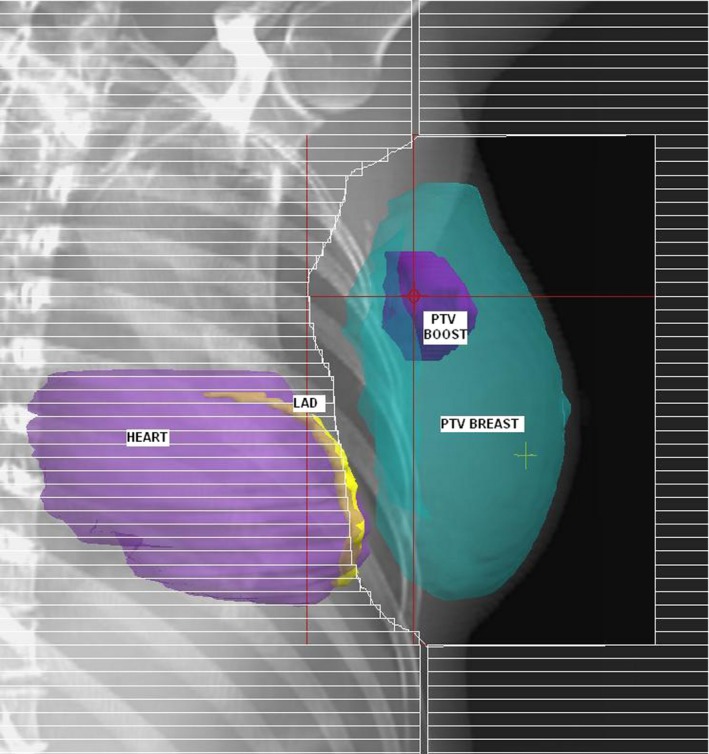
Beam's Eye View demonstrating tangential field shaping using multi‐leaf collimators with a 1 cm margin on the breast planning target volume.
Figure 2.
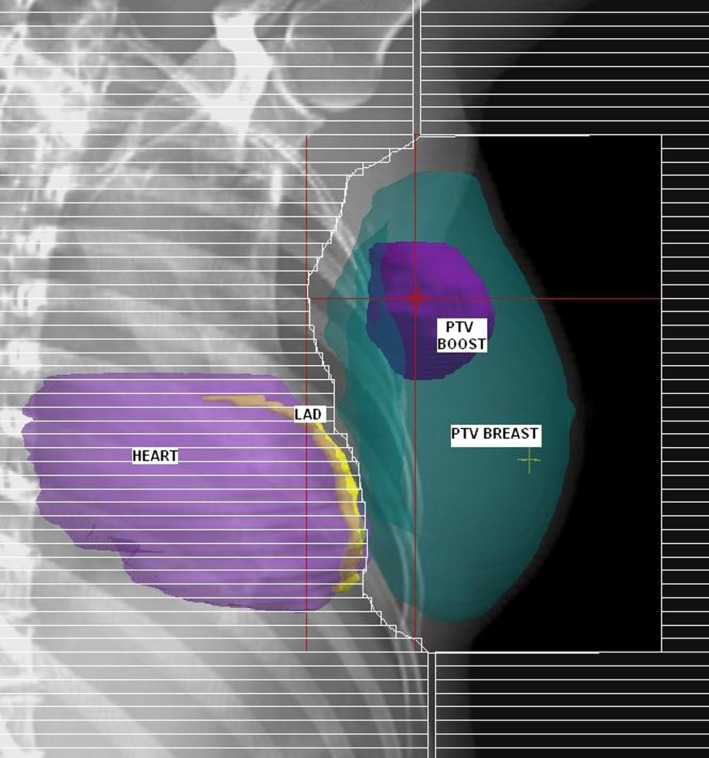
Beam's Eye View demonstrating selective shielding of the left anterior descending coronary artery (yellow) from the tangential field.
Data analysis
A comparison of the dosimetric values was performed between the SP and LADSP. From the DVHs of the SP and LADSP, comparisons were made for the following organs at risk: heart, LAD, LAD PRV and left lung. Comparison of dose for each of the following target structures was also performed: CTVbreast, CTVboost, PTVbreast Eval and PTVboost Eval. For this analysis, at least 95% of the PTVbreast Eval receiving 95% of the prescribed dose was considered an ideal plan, with coverage of greater than 90% considered acceptable. Investigation was undertaken to determine any factors that could affect PTV coverage when using the LAD‐shielded technique. Statistical analysis included a paired t test, and linear regression, with a P < 0.05 considered statistically significant, using GraphPad (GraphPad Software Inc, La Jolla, CA).
Results
Of the 49 patients, the SP and LADSP were identical for nine patients (18%) as the LAD was outside of the tangential fields in the SP.
Organs at risk
As shown in Table 1, statistically significant reductions were seen across all measured dose outcomes for heart, LAD, LAD PRV and left lung, for the LADSP compared to SP.
Table 1.
Comparison of dosimetric data of organs at risk between the standard plan (SP) and left anterior descending coronary artery – shielded plan (LADSP)
| SP | LADSP | Difference (Range) | P value | ||
|---|---|---|---|---|---|
| Heart | Mean (Gy) | 3.23 | 2.50 | 0.73 (0.0–2.5) | <0.0001 |
| Dmax (Gy) | 43.7 | 39.7 | 4.0 (0.0–13.28) | <0.0001 | |
| V5Gy (%) | 12.4 | 10.0 | 2.4 (0.0–8.83) | <0.0001 | |
| V20Gy (%) | 3.4 | 1.5 | 1.9 (0.0–7.14) | <0.0001 | |
| V30Gy (%) | 2.2 | 0.7 | 1.5 (0.0–4.81) | <0.0001 | |
| LAD | Mean (Gy) | 15.5 | 8.5 | 7.0 (0.0–20.5) | <0.0001 |
| Dmax (Gy) | 34.8 | 22.5 | 12.3 (0.0–24.1) | <0.0001 | |
| V5Gy (%) | 71.2 | 68.3 | 2.7 (‐0.14–16.15) | <0.0001 | |
| V10Gy (%) | 47.0 | 32.6 | 14.4 (0.0–78.03) | <0.0001 | |
| V30Gy (%) | 21.5 | 0.0 | 21.5 (0.0–72.04) | <0.0001 | |
| LAD PRV | Mean (Gy) | 15.1 | 9.2 | 5.9 (0.0–17.8) | <0.0001 |
| Dmax (Gy) | 41.1 | 35.5 | 5.6 (0.0–12.5) | <0.0001 | |
| V5Gy (%) | 67.1 | 62.1 | 5.0 (0.0–14.12) | <0.0001 | |
| V10Gy (%) | 45.4 | 32.0 | 13.4 (0.0–33.43) | <0.0001 | |
| V30Gy (%) | 20.7 | 2.3 | 18.4 (0.0–58.77) | <0.0001 | |
| Left lung | Mean (Gy) | 6.5 | 6.2 | 0.3 (0.0–0.94) | <0.0001 |
| Dmax (Gy) | 54.6 | 54.1 | 0.5 (−0.32–3.56) | <0.0001 | |
| V5Gy (%) | 24.9 | 24.4 | 0.5 (−0.49–2.02) | <0.0001 | |
| V20Gy (%) | 11.3 | 10.6 | 0.7 (−0.10–2.28) | <0.0001 | |
SP, standard plan; LADSP, left anterior descending coronary artery – shielded plan; PRV, planning risk volume.
Target volumes
The target volume isodose coverage (90%, 95% and 100%) for all targets (CTVbreast, CTVboost, PTVbreast Eval and PTVboost Eval) was also evaluated (Table 2).
Table 2.
Comparison of target volumes isodose coverage (%) between the standard plan (SP) and left anterior descending coronary artery – shielded plan (LADSP)
| SP (% volume) | LADSP (% volume) | P value | ||
|---|---|---|---|---|
| CTVbreast | 90% isodose coverage | 99.37 | 99.07 | 0.0032 |
| 95% isodose coverage | 97.61 | 97.22 | 0.0005 | |
| 100% isodose coverage | 84.32 | 83.56 | 0.0001 | |
| CTVboost | 90% isodose coverage | 99.97 | 99.97 | 0.3223 |
| 95% isodose coverage | 99.34 | 99.32 | 0.4890 | |
| 100% isodose coverage | 59.13 | 59.13 | 0.9851 | |
| Dmax (Gy) | 61.89 | 61.93 | 0.0753 | |
| PTVbreast Eval | 90% isodose coverage | 98.7 | 97.69 | <0.0001 |
| 95% isodose coverage | 95.91 | 94.58 | <0.0001 | |
| 100% isodose coverage | 79.1 | 77.41 | <0.0001 | |
| PTVboost Eval | 90% isodose coverage | 99.85 | 99.82 | 0.0952 |
| 95% isodose coverage | 95.56 | 95.26 | 0.0096 | |
| 100% isodose coverage | 43.46 | 43.27 | 0.4308 | |
| Dmax (Gy) | 61.96 | 61.98 | 0.0441 | |
SP, standard plan; LADSP, left anterior descending coronary artery – shielded plan; CTV, clinical target volume; PTV, planning target volume.
As expected, introducing LAD shielding resulted in a significant reduction in CTVbreast and PTVbreast Eval isodose coverage between the two plans across all isodoses evaluated. The 95% isodose coverage of CTVbreast was reduced by 0.39% (range −0.2–3.7%), PTVbreast Eval was reduced by 1.33% (range 0–5.66%), and PTVboost Eval by 0.3% (range −2.0–2.6%). No significant difference was observed in coverage of CTVboost.
Given that a reduction in PTV isodose coverage is an unwanted consequence of this technique, further analysis was undertaken to examine this effect. Of the 49 patients in this study, the SP produced isodose coverage of <95% of the PTVbreast Eval receiving 95% of the prescribed dose in 15 cases (30%) with 1 case (2%) <90% coverage, due to the limitation of no more than 2 cm of lung within the tangential fields. Introduction of the LAD shielding produced plans with <95% coverage of the PTVbreast Eval by the 95% isodose line in further 11 cases (22%), including a reduction to <90% coverage in one additional case. Therefore, maintenance of an ideal plan in which at least 95% of PTVbreast Eval is covered by 95% of the prescribed dose was observed for 23 cases (47%), and an acceptable plan in which at least 90% of PTVbreast Eval covered by 95% of the prescribed dose was observed in 47 cases (96%), using this technique. The dose to the CTVboost was maintained with the introduction of LAD shielding.
Regression analysis was undertaken to examine whether any patient variables were related to PTVbreast Eval coverage, using the LADSP. The variables tested were whole breast volume (CTVbreast), boost volume (CTVboost), maximum heart depth in field, volume of heart in field, percentage of heart in field and whether the heart abuts the chest wall (Yes or No). None had a significant effect (P > 0.05) on PTVbreast Eval coverage.
Discussion
Irradiation of the left breast has been demonstrated to increase the risk of late cardiac complications 15–20 years following treatment.2, 3, 6 When compared with right‐sided breast irradiation, cardiovascular mortality is increased by 25%.2 A history of smoking,7 and prior diagnosis of ischaemic heart disease have been shown to further increase this risk.5 In a population‐based study by Darby et al.3 a mean heart dose (MHD) of 6.6 Gy was reported in left‐sided breast irradiation, with 7.4% increased risk of major coronary events with every 1 Gy increase in MHD with no apparent threshold for cardiac complications. This increased risk was shown to begin at 5 years after irradiation and continue for at least 20 years.3 Cardiac angiography and cardiac stress testing in patients with previous radiotherapy treatment for breast cancer demonstrate a higher rate of abnormalities in the LAD coronary artery.8, 9 In left‐sided patients, 85% of stress test abnormalities occurred in the LAD.8
Following implementation of this simple LAD shielding technique, a statistically significant reduction in MHD, mean LAD dose and max LAD dose was observed. Thirty‐three percent of patients showed a reduction of >1 Gy in MHD and >10 Gy reduction in mean LAD dose, using this technique. It has been reported that the use of DIBH results in 1.5–3 Gy reduction in MHD10, 11 and a reduction in mean, median and maximum LAD and LAD PRV dose.11, 12 Mast et al. reported a reduction in mean and max LAD dose to 9.6 Gy and 25.2 Gy, respectively, using DIBH, which is comparable to our LAD shielding technique giving 8.5 Gy and 22.5 Gy, respectively. Previous studies have demonstrated a potential benefit from cardiac‐sparing techniques with Bartlett et al. reporting MHD of 0.8 Gy and mean LAD dose of 6.7 Gy when selectively shielding the heart, using MLCs and Vivekanandan et al. reporting a 42% reduction in LAD V5 Gy and a 37% reduction in LAD V30 Gy when the tangential beam angles were optimised to spare the LAD.16, 17
However, we note that caution must be used in comparison of heart and LAD doses due to contouring variability. A non‐contrast CT is standard practice in our department for breast irradiation. Non‐contrast CT results in poorer visualisation of the atria, ventricles and coronary arteries, particularly when cardiac and respiratory motion are considered.20 We employed several methods in this study to maintain delineation accuracy, including the use of a heart contouring atlas,19 delineation by one specialist breast radiation oncologist and one radiation therapist to eliminate inter‐observer variability, and the creation of a PRV on the LAD. However some delineation uncertainty remains.
DIBH techniques, when implemented, have generally been used for all patients with left‐sided breast cancer who are able to maintain an adequate breath hold, however in doing so, a substantial increase in planning and treatment time and workload is required.11, 12, 14 Results of this study indicate that DIBH techniques would have no benefit in 18% of cases, as the heart lies outside the radiation fields and similar cardiac sparing can be achieved for some patients with a simple modification of the tangential field shape. However, introduction of this LAD‐sparing technique reduced the 95% isodose coverage of PTVbreast Eval, due to the introduction of additional MLC shielding over the PTVbreast Eval. Due to the tangential field arrangement, the reduction in coverage is primarily in the medial and lateral portions of the PTV (Fig. 3). A small reduction in PTVbreast Eval coverage, with excellent PTVboost Eval coverage, using the SIB technique, may be an acceptable alternative to DIBH to achieve reductions in cardiac doses in some cases. However, a limitation of this study was the use of a free‐breathing CT scan, which may under‐ or over‐estimate the dose to targets and organs at risk as motion due to respiration is not accounted for.
Figure 3.
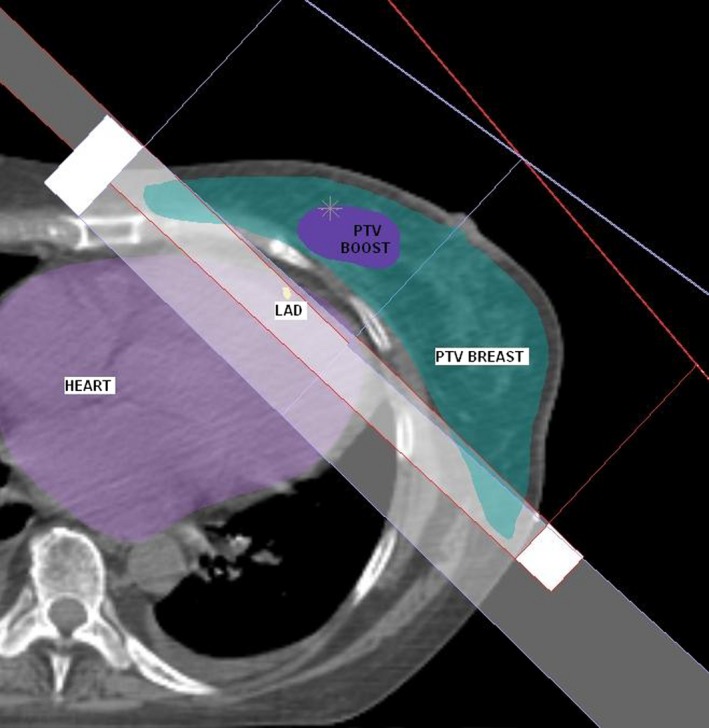
Medial and lateral portions of PTV breast Eval shielded in LAD Shielded Plan.
One study has implemented selection criteria of >10 cc of heart within the tangential fields as a minimum for use of DIBH.21 Our results did not demonstrate a correlation between irradiated heart volume and a reduction in PTVbreast Eval coverage with the introduction of LAD shielding. The difference in our results compared to that of Wang et al. may be attributable to our technique specifically shielding LAD, which although is generally located in close proximity to the breast target volume, may not correlate well with the irradiated heart volume in all cases. Indeed, it appears that a complex relationship exists between irradiated heart volume, LAD position and breast tissue.
Conclusion
This study has demonstrated that in some cases, implementation of a simple MLC shielding technique can produce ideal dosimetric outcomes for patients with left‐sided breast cancer, whilst reducing cardiac doses. This technique requires no additional specialised equipment and is suitable for those patients who are unable to achieve breath hold or where such techniques are unavailable.
Acknowledgements
We thank Dr Jennifer G Smith for assistance with the statistical analysis.
J Med Radiat Sci 64 (2017) 114–119
References
- 1. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15‐year survival: An overview of the randomised trials. Lancet 2005; 366: 2087–106. [DOI] [PubMed] [Google Scholar]
- 2. Roychoudhuri R, Robinson D, Putcha V, Cuzick J, Darby S, Moller H. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: A population‐based study. BMC Cancer 2007; 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Darby S, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368: 987–98. [DOI] [PubMed] [Google Scholar]
- 4. Borger J, Hooning M, Boersma L, et al. Cardiotoxic effects of tangential breast irradiation in early breast cancer patients: The role of irradiated heart volume. Int J Radiat Oncol Biol Phys 2007; 69: 1131–8. [DOI] [PubMed] [Google Scholar]
- 5. McGale P, Darby S, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 2011; 100: 167–75. [DOI] [PubMed] [Google Scholar]
- 6. Harris E, Correa C, Hwang W, et al. Late cardiac mortality and morbidity in early‐stage breast cancer patients after breast‐conservation treatment. J Clin Oncol 2006; 24: 4100–6. [DOI] [PubMed] [Google Scholar]
- 7. Hooning M, Botma A, Aleman B, et al. Long‐term risk of cardiovascular disease in 10‐year survivors of breast cancer. J Natl Cancer Inst 2007; 99: 365–75. [DOI] [PubMed] [Google Scholar]
- 8. Correa C, Litt H, Hwang W, Ferrari V, Solin L, Harris E. Coronary artery findings after left‐sided compared with right‐sided radiation treatment for early‐stage breast cancer. J Clin Oncol 2007; 21: 3031–7. [DOI] [PubMed] [Google Scholar]
- 9. Nilsson G, Holmberg L, Garmo H, et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol 2012; 30: 380–6. [DOI] [PubMed] [Google Scholar]
- 10. Hayden A, Rains M, Tiver K. Deep inspiration breath hold technique reduces heart dose from radiotherapy for left‐sided breast cancer. J Med Imaging Radiat Oncol 2012; 56: 464–72. [DOI] [PubMed] [Google Scholar]
- 11. Mast M, Kempen‐Harteveld L, Heijenbrok M, et al. Left‐sided breast cancer radiotherapy with and without breath‐hold: Does IMRT reduce the cardiac dose even further? Radiother Oncol 2013; 108: 248–53. [DOI] [PubMed] [Google Scholar]
- 12. Pedersen A, Korreman S, Nystrom H, Specht L. Breathing adapted radiotherapy of breast cancer: Reduction of cardiac and pulmonary doses using voluntary inspiration breath‐hold. Radiother Oncol 2004; 72: 53–60. [DOI] [PubMed] [Google Scholar]
- 13. Korreman S, Pedersen A, Aarup L, Nottrup T, Specht L, Nystrom H. Reduction of cardiac and pulmonary complication probabilities after breathing adapted radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys 2006; 65: 1375–80. [DOI] [PubMed] [Google Scholar]
- 14. Bartlett F, Colgan R, Carr K, et al. The UK HeartSpare Study: Randomised evaluation of voluntary deep‐inspiratory breath‐hold in women undergoing breast radiotherapy. Radiother Oncol 2013; 108: 242–7. [DOI] [PubMed] [Google Scholar]
- 15. Van der Laan H, Hurkmans C, Kuten A, Westenberg H. Current technological clinical practice in breast radiotherapy; results of a survey in EORTC‐Radiation Oncology Group affiliated institutions. Radiother Oncol 2010; 94: 280–5. [DOI] [PubMed] [Google Scholar]
- 16. Vivekanandan S, Mhlanga J, Launders D, Przeslak A, Morgan D. Beam angle manipulation to reduce cardiac dose during breast radiotherapy. Br J Radiol 2012; 85: 265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bartlett F, Yarnold J, Donovan E, Evans P, Locke I, Kirby A. Multileaf collimation cardiac shielding in breast radiotherapy: Cardiac doses are reduced, but at what cost? Clin Oncol 2013; 25: 690–6. [DOI] [PubMed] [Google Scholar]
- 18. Alford S, Prassas G, Vogelesang C, Leggett H, Hamilton C. Adjuvent breast radiotherapy using a simultaneous integrated boost: Clinical and dosimetric perspectives. J Med Imaging Radiat Oncol 2013; 57: 222–9. [DOI] [PubMed] [Google Scholar]
- 19. Feng M, Moran J, Koelling T, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 2011; 79: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lorenzen E, Taylor C, Maraldo M, et al. Inter‐observer variation in delineation of the heart and left anterior descending coronary artery in radiotherapy for breast cancer: A multi‐centre study from Denmark and the UK. Radiother Oncol 2013; 108: 254–8. [DOI] [PubMed] [Google Scholar]
- 21. Wang W, Purdie T, Rahman M, Marshall A, Liu F, Fyles A. Rapid automated treatment planning process to select breast cancer patients for active breathing control to achieve cardiac dose reduction. Int J Radiat Oncol Biol Phys 2012; 82: 386–93. [DOI] [PubMed] [Google Scholar]


