Abstract
Super‐refractory status epilepticus (SRSE) is associated with high morbidity and mortality. Treatment of SRSE is complicated by progressive cortical hyperexcitability believed to result in part from synaptic GABA receptor internalization and desensitization. Allopregnanolone, a neurosteroid that positively modulates synaptic and extrasynaptic GABAA receptors, has been proposed as a novel treatment. We describe the first two patients with SRSE who were each successfully treated with a 120‐h continuous infusion of allopregnanolone. Both patients recovered from prolonged SRSE with good cognitive outcomes.
Introduction
Super‐refractory status epilepticus occurs when therapeutic coma fails to control seizures in status epilepticus.1, 2 The efficacy of therapeutic coma has not been well established and it may be associated with increased hospitalization length, cost of care, and in some cases even mortality.3, 4, 5 When prolonged therapeutic coma is used, tachyphylaxis to anesthetics that act via GABAA receptors, such as barbiturates and benzodiazepines, is frequently observed. Both continuous seizure activity and GABAergic drugs cause synaptic GABAA receptor internalization and desensitization leading to cortical hyperexcitability.6, 7, 8 Extrasynaptic GABAA receptors are not internalized and could be targeted during treatment of SRSE.9 Development of treatments for status epilepticus has largely relied on application of conventional anticonvulsant and anesthetic agents without regard to mechanistic considerations. Allopregnanolone, an endogenous metabolite of progesterone, is a positive allosteric modulator of synaptic and extrasynaptic GABAA receptors making it an attractive agent for use in the treatment of SRSE.10 Studies in animal models support the potential of allopregnanolone in the treatment of refractory status epilepticus.9 Here, we describe the first‐in‐man experience with allopregnanolone in the treatment of SRSE, providing key translational proof of principle to support its clinical development as a novel therapeutic agent. Two adult patients with SRSE in therapeutic coma who had persistent seizures with repeated attempts to wean anesthetic agents were successfully weaned when allopregnanolone was added to their treatment regimen. Dose selection was based on pharmacokinetic modeling,11 with a target plasma allopregnanolone concentration of 150 nmol/L, the physiological maximum concentration seen in pregnant women and the maximum concentration permitted at the time by the Food and Drug Administration (FDA). Subsequent successful use of allopregnanolone in pediatric patients has been reported.12
Patients and Methods
Patient 1 is a 23‐year‐old man who presented with generalized convulsions and myoclonus, preceded by a week of malaise and headache. The seizures were medically refractory and intravenous general anesthesia was instituted. The patient underwent eight unsuccessful attempts to discontinue pentobarbital while multiple antiepileptic and immunologic interventions were administered (see Fig. 1). No clear cause for SRSE was identified. The FDA approved an emergency IND (116214) permitting allopregnanolone infusion as a bridge therapy to wean the patient off pentobarbital. Allopregnanolone (3α‐hydroxy‐5α‐pregnan‐20‐one) intravenous solution, containing 6% hydroxypropyl‐β‐cyclodextrin (Trappsol; Cyclodextrin Technologies Development, Alachua, FL) in 0.9% sodium chloride injection, USP, was manufactured by the Good Manufacturing Products Laboratory at the University of California, Davis. The patient received 670.8 mg of allopregnanolone at 86 μg/kg/h (5.6 mg/h) by continuous infusion. During this 5‐day infusion, the patient was successfully weaned from pentobarbital without recurrence of SE (Fig. 2). He remains cognitively intact and seizure free at 3‐year follow up. Preliminary description of this case was reported in abstract form.13
Figure 1.
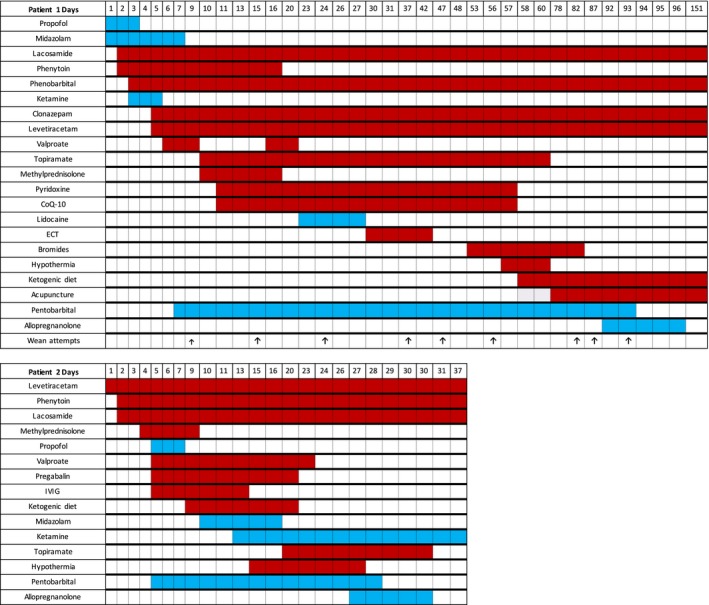
Graphical representation of most significant interventions during hospitalization. The color shadings correspond to the days of exposure. The blue shading represents continuous infusion and the red shading represents interval dosing. Arrows signify formal wean initiations.
Figure 2.
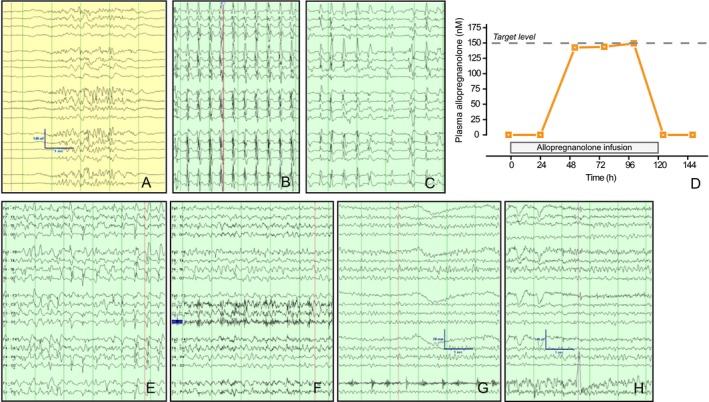
EEG recordings from Patient 1 illustrate cortical hyperexcitability after developing tolerance to barbiturates. Panel A, burst suppression pattern achieved with 1.5 mg/kg/h of pentobarbital. Panel B, EEG pattern 30 days later while on 1.5 mg/kg/h pentobarbital infusion. Panel C, EEG pattern 80 days later on 6 mg/kg/h of pentobarbital. Panel D, arterial plasma allopregnanolone levels. Plasma was analyzed 2 h prior to the start of the infusion and then at 24, 52, 76, 100, 124, and 148 h. Panels E‐H demonstrate progressive normalization of the EEG at 12, 24, 36, and 48 h after discontinuation of pentobarbital while on allopregnanolone infusion. Scale bars, 140 μV, 1 sec.
Patient 2 is a 28 year‐old man who was admitted for confusion. One week later, he developed SRSE of unknown cause. Status epilepticus recurred with each of three attempts to wean the patient from pentobarbital. An emergency IND (120079) was obtained and allopregnanolone was administered over 5 days as bridge therapy during the successful wean from pentobarbital. The formulation in this case contained sulfobutylether‐β‐cyclodextrin (Captisol; CyDex Pharmaceuticals, Division of Ligand Pharmaceuticals, Lenexa, KS). At 3‐year follow up, the patient demonstrated good cognitive function with occasional breakthrough seizures despite five antiseizure medications.
Discussion
These cases demonstrate that allopregnanolone can be safely administered in critically ill patients with SRSE and may be an effective approach to wean patients from general anesthetics. Barbiturate‐induced therapeutic coma is recognized to have high morbidity due to systemic side effects and may be associated with increased mortality.3, 14 Tolerance and physical dependence after prolonged exposure to barbiturates and benzodiazepines results in a need for increased doses of these toxic drugs and there is a high propensity for seizures recurrence.15 Allopregnanolone has limited systemic side effects,16 and is highly effective in terminating ongoing status epilepticus in animal models.9, 17 These cases provided critical translational evidence of the activity of allopregnanolone in patients with SRSE, that led directly to the treatment of additional patients, including two children.12 Cumulatively, these patient experiences guided the development of the first placebo‐controlled Phase III study of a novel drug for SRSE that is currently ongoing.18, 19
Author Contributions
HV: Conceived the project, obtained IND and IRB approvals, collected data, wrote the manuscript. WB: Conceived the project, obtained IND and IRB approvals, collected data, edited the manuscript. ESR: Conceived the project, analyzed and interpreted data, edited the manuscript. JR: Contributed to the project, sponsored the IRB approval, edited the manuscript. AH: Conceived the project, obtained IND and IRB approvals, collected data, edited the manuscript. KR: Collected and analyzed data, edited the manuscript. MAR: Conceived the project, provided access to IND and contributed intravenous allopregnanolone solution, wrote the manuscript. AJC: Conceived, coordinated and facilitated the project, edited and revised the manuscript.
Conflicts of Interest
HV: Served as a consultant to Sage Therapeutics. WB: None. ESR: Employed by Massachusetts General Hospital, which has an Institutional Clinical Research Sponsor Agreement with Sage Therapeutics, and receives consulting fees from GLG Consulting and Guidepoint Global Consultants on the topic of status epilepticus. JR: None. AH: Serves as a consultant to UCB Pharmaceuticals, Jazz pharmaceuticals, Biogen Idec, Sage Therapeutics, Marinus Pharmaceuticals, and Turing Pharmaceuticals. Receives royalties from Demos Medical Publishing. Editor at Wolters Kluwer. KR: Was employed by Sage Therapeutics during the time the project was conceived. MAR: Previously served as a consultant to Sage Therapeutics, which has obtained a commercialization license from the Regents of the University of California and the University of California, Davis. AJC: Serves as a consultant to Sage Therapeutics.
Acknowledgments
We thank G. Bauer and C.‐Y. Wu for GMP intravenous formulation manufacturing, and Sage Therapeutics for facilitating discussions.
References
- 1. Hocker S, Tatum WO, LaRoche S, et al. Refractory and super‐refractory status epilepticus–an update. Curr Neurol Neurosci Rep 2014;14:452. [DOI] [PubMed] [Google Scholar]
- 2. Shorvon S. Super‐refractory status epilepticus: an approach to therapy in this difficult clinical situation. Epilepsia 2011;52(Suppl 8):53–56. [DOI] [PubMed] [Google Scholar]
- 3. Marchi NA, Novy J, Rossetti AO, et al. Status epilepticus: impact of therapeutic coma on outcome. Crit Care Med 2015;43:1003–1009. [DOI] [PubMed] [Google Scholar]
- 4. Sutter R, Marsch S, Rüegg S, et al. Anesthetic drugs in status epilepticus: risk or rescue? A 6‐year cohort study. Neurology 2014;82:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alvarez V, Lee JW, Westover MB, et al. Therapeutic coma for status epilepticus: Differing practices in a prospective multicenter study. Neurology 2016;87:1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naylor DE, Liu H, Wasterlain CG. J, Neurosci. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 2005;25:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friedman LK, Gibbs TT, Farb DH. γ‐Aminobutyric acidA receptor regulation: heterologous uncoupling of modulatory site interactions induced by chronic steroid, barbiturate, benzodiazepine, or GABA treatment in culture. Brain Res 1996;707:100–109. [DOI] [PubMed] [Google Scholar]
- 8. Yu R, Ticku MK. Effects of chronic pentobarbital treatment on the GABAA receptor complex in mammalian cortical neurons. J Pharmacol Exp Ther 1995;275:1442–1446. [PubMed] [Google Scholar]
- 9. Rogawski MA, Loya CM, Reddy K, et al. Neuroactive steroids for the treatment of status epilepticus. Epilepsia 2013;54(Suppl 6):93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reddy DS, Rogawski MA. Neurosteroids — endogenous regulators of seizure susceptibility and role in the treatment of epilepsy In: Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th ed. National Center for Biotechnology Information (US): Bethesda (MD), 2012. [PubMed] [Google Scholar]
- 11. Timby E, Balgård M, Nyberg S, et al. Pharmacokinetic and behavioral effects of allopregnanolone in healthy women. Psychopharmacology 2006;186:414–424. [DOI] [PubMed] [Google Scholar]
- 12. Broomall E, Natale JE, Grimason M, et al. Pediatric super‐refractory status epilepticus treated with allopregnanolone. Ann Neurol 2014;76:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vaitkevicius H, Ng M, Moura L, et al. Successful allopregnanolone treatment of new onset refractory status epilepticus (NORSE) syndrome: first in man experience. The 4th London‐Innsbruck Colloquium on Status Epilepticus and Acute Seizures. Epilepsia 2013;54 (Suppl 6):19. [Google Scholar]
- 14. Priano LL, Traber DL, Wilson RD. Barbiturate anesthesia: an abnormal physiologic stimulation. J Pharmacol Exp Ther 1969;165:126–135. [PubMed] [Google Scholar]
- 15. Saunders PA, Ito Y, Baker ML, et al. Pentobarbital tolerance and withdrawal: correlation with effects on the GABAA receptor. Pharmacol Biochem Behav 1990;37:343–348. [DOI] [PubMed] [Google Scholar]
- 16. Bruun DA, Cao Z, Inceoglu B, et al. Combined treatment with diazepam and allopregnanolone reverses tetramethylenedisulfotetramine (TETS)‐induced calcium dysregulation in cultured neurons and protects TETS‐intoxicated mice against lethal seizures. Neuropharmacology 2015;95:332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine‐ and kainic acid‐induced limbic seizures and status epilepticus in mice. Neuropharmacology 1996;35:1049–1056. [DOI] [PubMed] [Google Scholar]
- 18. Reddy K, Reife R, Cole AJ. SGE‐102: a novel therapy for refractory status epilepticus. The 4th London‐Innsbruck Colloquium on Status Epilepticus and Acute Seizures. Epilepsia 2013;54(Suppl 6):81–83. [DOI] [PubMed] [Google Scholar]
- 19. A Randomized, Double‐Blind , Placebo‐Controlled Study to Evaluate the Efficacy and Safety of SAGE‐547 Injection in the Treatment of Subjects With Super‐Refractory Status Epilepticus. ClinicalTrials.gov Identifier: NCT02477618


