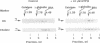Abstract
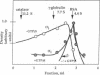
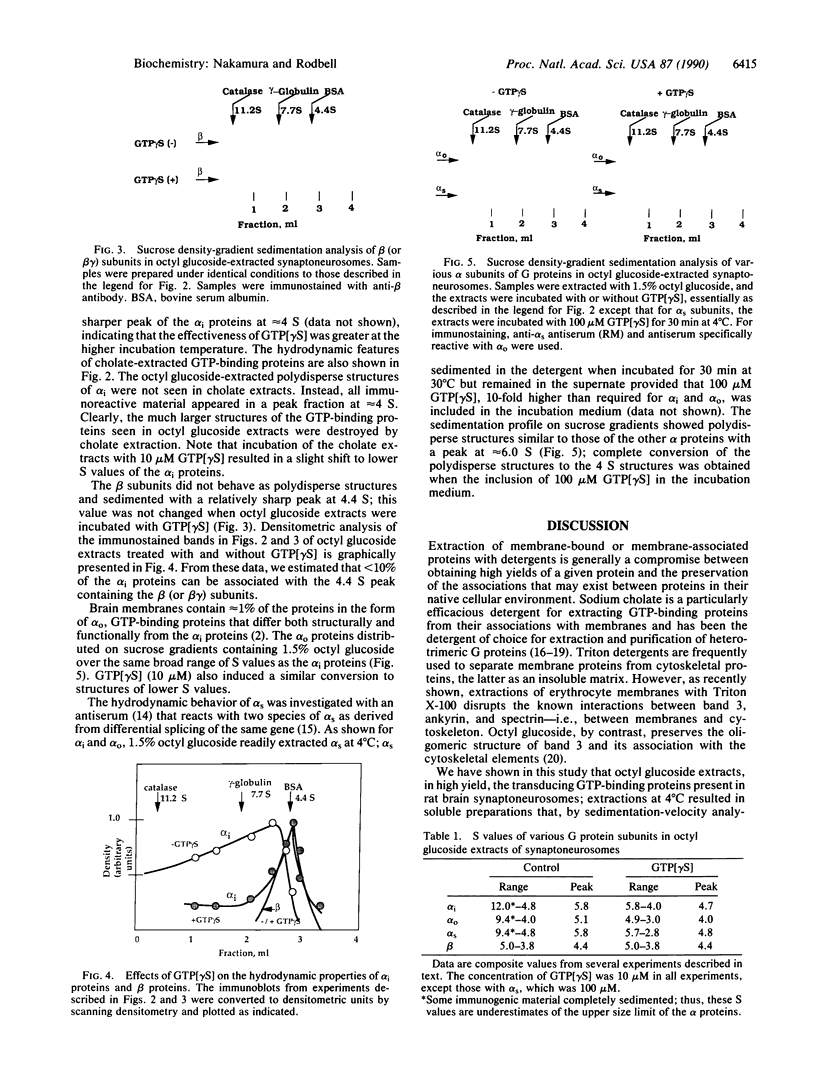
GTP-binding regulatory proteins are generally purified from cholate-extracted membranes in the form of heterotrimers (G proteins) consisting of a GTP-binding subunit (alpha protein) complexed with a tightly interacted heterodimer termed beta gamma. In this study we extracted the proteins from rat brain "synaptoneurosomes" using the neutral detergent 1-octyl beta-D-glucopyranoside (octyl glucoside). Using specific antibodies for detection by immunoblotting and sucrose gradients for analyzing hydrodynamic properties, we found that each species of alpha protein (alpha subunits of stimulatory, inhibitory, and brain GTP-binding proteins) exhibited a broad range (4 S to greater than 12 S) of polydisperse structures with peak values (5 S to 7 S) considerably greater than that of heterotrimeric G proteins. The beta subunit proteins, for example, appeared as a homogeneous peak at 4.4 S within which only a fraction of the total alpha proteins can be associated. Incubation of octyl glucose extracts at 30 degrees C rapidly sedimented the alpha proteins but not the beta proteins. Incubation at 30 degrees C with guanosine 5'[gamma-thio]triphosphate (10-100 microM) prevented rapid sedimentation. Hydrodynamic analysis revealed that all alpha proteins were converted to approximately 4 S structures by the actions of guanosine 5'-[gamma-thio]triphosphate without change in the hydrodynamic properties of the beta proteins. Extraction of the membranes with sodium cholate instead of octyl glucoside resulted in complete loss of the large, polydisperse structures of the alpha proteins; the S values were approximately 4 S, in the range for beta proteins. These findings suggest that the transducing GTP-binding proteins in synaptoneurosomes exist as polydisperse, possibly multimer, structures of various size that are stable in octyl glucoside but destroyed by cholate. The polydisperse structures are not associated with beta gamma complexes and are sensitive to the disaggregating effects of guanosine 5'-[gamma-thio]triphosphate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Morita E. A., Swanson R. J., Applebury M. L. Characterization of bovine rod outer segment G-protein. J Biol Chem. 1982 Jun 10;257(11):6452–6460. [PubMed] [Google Scholar]
- Baggiolini M., Wymann M. P. Turning on the respiratory burst. Trends Biochem Sci. 1990 Feb;15(2):69–72. doi: 10.1016/0968-0004(90)90179-f. [DOI] [PubMed] [Google Scholar]
- Bayley P., Schilstra M., Martin S. A lateral cap model of microtubule dynamic instability. FEBS Lett. 1989 Dec 18;259(1):181–184. doi: 10.1016/0014-5793(89)81523-6. [DOI] [PubMed] [Google Scholar]
- Bengtsson T., Särndahl E., Stendahl O., Andersson T. Involvement of GTP-binding proteins in actin polymerization in human neutrophils. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2921–2925. doi: 10.1073/pnas.87.8.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Bourguignon L. Y., Walker G., Huang H. S. Interactions between a lymphoma membrane-associated guanosine 5'-triphosphate-binding protein and the cytoskeleton during receptor patching and capping. J Immunol. 1990 Mar 15;144(6):2242–2252. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlson K. E., Woolkalis M. J., Newhouse M. G., Manning D. R. Fractionation of the beta subunit common to guanine nucleotide-binding regulatory proteins with the cytoskeleton. Mol Pharmacol. 1986 Nov;30(5):463–468. [PubMed] [Google Scholar]
- Codina J., Hildebrandt J. D., Birnbaumer L., Sekura R. D. Effects of guanine nucleotides and Mg on human erythrocyte Ni and Ns, the regulatory components of adenylyl cyclase. J Biol Chem. 1984 Sep 25;259(18):11408–11418. [PubMed] [Google Scholar]
- Codina J., Hildebrandt J. D., Sekura R. D., Birnbaumer M., Bryan J., Manclark C. R., Iyengar R., Birnbaumer L. Ns and Ni, the stimulatory and inhibitory regulatory components of adenylyl cyclases. Purification of the human erythrocyte proteins without the use of activating regulatory ligands. J Biol Chem. 1984 May 10;259(9):5871–5886. [PubMed] [Google Scholar]
- Garty N. B., Galiani D., Aharonheim A., Ho Y. K., Phillips D. M., Dekel N., Salomon Y. G-proteins in mammalian gametes: an immunocytochemical study. J Cell Sci. 1988 Sep;91(Pt 1):21–31. doi: 10.1242/jcs.91.1.21. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Higashi K., Ishibashi S. Specific binding of tubulin to a guanine nucleotide-binding inhibitory regulatory protein in adenylate cyclase system, Ni. Biochem Biophys Res Commun. 1985 Oct 15;132(1):193–197. doi: 10.1016/0006-291x(85)91006-x. [DOI] [PubMed] [Google Scholar]
- Hingorani V. N., Tobias D. T., Henderson J. T., Ho Y. K. Chemical cross-linking of bovine retinal transducin and cGMP phosphodiesterase. J Biol Chem. 1988 May 15;263(14):6916–6926. [PubMed] [Google Scholar]
- Hollingsworth E. B., McNeal E. T., Burton J. L., Williams R. J., Daly J. W., Creveling C. R. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: cyclic adenosine 3':5'-monophosphate-generating systems, receptors, and enzymes. J Neurosci. 1985 Aug;5(8):2240–2253. doi: 10.1523/JNEUROSCI.05-08-02240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. K., Devanney J. F. GTP gamma S-induced solubilization of actin and myosin from rabbit peritoneal neutrophil membrane. FEBS Lett. 1986 Jun 23;202(1):41–44. doi: 10.1016/0014-5793(86)80645-7. [DOI] [PubMed] [Google Scholar]
- Jesaitis A. J., Tolley J. O., Allen R. A. Receptor-cytoskeleton interactions and membrane traffic may regulate chemoattractant-induced superoxide production in human granulocytes. J Biol Chem. 1986 Oct 15;261(29):13662–13669. [PubMed] [Google Scholar]
- Kempner E. S., Schlegel W. Size determination of enzymes by radiation inactivation. Anal Biochem. 1979 Jan 1;92(1):2–10. doi: 10.1016/0003-2697(79)90617-1. [DOI] [PubMed] [Google Scholar]
- Korn E. D., Carlier M. F., Pantaloni D. Actin polymerization and ATP hydrolysis. Science. 1987 Oct 30;238(4827):638–644. doi: 10.1126/science.3672117. [DOI] [PubMed] [Google Scholar]
- Kunimoto M., Shibata K., Miura T. Comparison of the cytoskeleton fractions of rat red blood cells prepared with non-ionic detergents. J Biochem. 1989 Feb;105(2):190–195. doi: 10.1093/oxfordjournals.jbchem.a122638. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matesic D. F., Manning D. R., Wolfe B. B., Luthin G. R. Pharmacological and biochemical characterization of complexes of muscarinic acetylcholine receptor and guanine nucleotide-binding protein. J Biol Chem. 1989 Dec 25;264(36):21638–21645. [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Northup J. K., Smigel M. D., Sternweis P. C., Gilman A. G. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution of the activated 45,000-dalton (alpha) subunit. J Biol Chem. 1983 Sep 25;258(18):11369–11376. [PubMed] [Google Scholar]
- Painter R. G., Zahler-Bentz K., Dukes R. E. Regulation of the affinity state of the N-formylated peptide receptor of neutrophils: role of guanine nucleotide-binding proteins and the cytoskeleton. J Cell Biol. 1987 Dec;105(6 Pt 2):2959–2971. doi: 10.1083/jcb.105.6.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péraldi S., Nguyen Than Dao B., Brabet P., Homburger V., Rouot B., Toutant M., Bouille C., Assenmacher I., Bockaert J., Gabrion J. Apical localization of the alpha subunit of GTP-binding protein Go in choroidal and ciliated ependymocytes. J Neurosci. 1989 Mar;9(3):806–814. doi: 10.1523/JNEUROSCI.09-03-00806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R. Abelson antigen is expressed on hematopoietic spleen colony-forming cells from mice carrying the Av-2S virus sensitivity gene. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5350–5354. doi: 10.1073/pnas.76.10.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robishaw J. D., Smigel M. D., Gilman A. G. Molecular basis for two forms of the G protein that stimulates adenylate cyclase. J Biol Chem. 1986 Jul 25;261(21):9587–9590. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Rudolph U., Schultz G., Rosenthal W. The cytosolic G-protein alpha-subunit in human neutrophils responds to treatment with guanine nucleotides and magnesium. FEBS Lett. 1989 Jul 17;251(1-2):137–142. doi: 10.1016/0014-5793(89)81443-7. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Cooper D. M., Rodbell M. Inhibition and activation of fat cell adenylate cyclase by GTP is mediated by structures of different size. Arch Biochem Biophys. 1980 May;201(2):678–682. doi: 10.1016/0003-9861(80)90559-7. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Kempner E. S., Rodbell M. Activation of adenylate cyclase in hepatic membranes involves interactions of the catalytic unit with multimeric complexes of regulatory proteins. J Biol Chem. 1979 Jun 25;254(12):5168–5176. [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurat A. V., Iurkova M. S., Kots A. Ia, Bulargina T. V. Osvobozhdenie stimuliruiushchego adenilattsikalzu GTP-sviazyvaiushchegobelka, Gs, iz membran éritrotsitov cheloveka pod deistviem ftorid- iona i guanilovykh nukleotidov. Biokhimiia. 1989 Sep;54(9):1576–1582. [PubMed] [Google Scholar]
- Sternweis P. C., Northup J. K., Smigel M. D., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties. J Biol Chem. 1981 Nov 25;256(22):11517–11526. [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Kennedy T. E., Neer E. J., Fishman M. C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990 Apr 26;344(6269):836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- Särndahl E., Lindroth M., Bengtsson T., Fällman M., Gustavsson J., Stendahl O., Andersson T. Association of ligand-receptor complexes with actin filaments in human neutrophils: a possible regulatory role for a G-protein. J Cell Biol. 1989 Dec;109(6 Pt 1):2791–2799. doi: 10.1083/jcb.109.6.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt R. R., Dhanasekaran N., Johnson G. L., Ruoho A. E. 2-Azido-[32P]NAD+, a photoactivatable probe for G-protein structure: evidence for holotransducin oligomers in which the ADP-ribosylated carboxyl terminus of alpha interacts with both alpha and gamma subunits. Proc Natl Acad Sci U S A. 1990 May;87(10):3645–3649. doi: 10.1073/pnas.87.10.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. Y., Berrios M., Malbon C. C. Indirect immunofluorescence localization of beta-adrenergic receptors and G-proteins in human A431 cells. Biochem J. 1989 Oct 15;263(2):519–532. doi: 10.1042/bj2630519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Yan K., Rasenick M. M. Tubulin binds specifically to the signal-transducing proteins, Gs alpha and Gi alpha 1. J Biol Chem. 1990 Jan 25;265(3):1239–1242. [PubMed] [Google Scholar]
- Wessling-Resnick M., Johnson G. L. Transducin interactions with rhodopsin. Evidence for positive cooperative behavior. J Biol Chem. 1987 Sep 15;262(26):12444–12447. [PubMed] [Google Scholar]