Abstract
Objective
Dimethyl fumarate (DMF) is a fumaric acid ester approved for the treatment of relapsing‐remitting multiple sclerosis (RRMS). In both the brain and periphery, DMF and its metabolite monomethyl fumarate (MMF) exert anti‐inflammatory and antioxidant effects. Our aim was to compare the effects of DMF and MMF on inflammatory and antioxidant pathways within astrocytes, a critical supporting glial cell in the central nervous system (CNS). Direct effects of fumarates on neural progenitor cell (NPC) differentiation toward the oligodendrocyte lineage were also assessed.
Methods
Primary astrocyte cultures were derived from both murine and human brains. Following pretreatment with MMF, DMF, or vehicle, astrocytes were stimulated with IL‐1β for 24 h; gene and microRNA expression were measured by qPCR. Cytokine production and reactive oxygen species (ROS) generation were also measured. NPCs were differentiated into the oligodendrocyte lineage in the presence of fumarates and immunostained using early oligodendrocyte markers.
Results
In both murine and human astrocytes, DMF, but not MMF, significantly reduced secretion of IL‐6, CXCL10, and CCL2; neither fumarate promoted a robust increase in antioxidant gene expression, although both MMF and DMF prevented intracellular ROS production. Pretreatment with fumarates reduced microRNAs ‐146a and ‐155 upon stimulation. In NPC cultures, DMF increased the number of O4+ and NG2+ cells.
Interpretation
These results suggest that DMF, and to a lesser extent MMF, mediates the anti‐inflammatory effects within astrocytes. This is supported by recent observations that in the inflamed CNS, DMF may be the active compound mediating the anti‐inflammatory effects independent from altered antioxidant gene expression.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease characterized by demyelination of the central nervous system (CNS). Dimethyl fumarate (DMF) (Tecfidera®) is an orally available drug that has emerged as an effective therapy for the treatment of relapsing‐remitting multiple sclerosis (RRMS).1 The therapeutic mechanism of action for DMF has been attributed to both immunosuppression and induction of antioxidant pathways.2, 3, 4 The induction of antioxidant response element (ARE) genes by DMF is attributed to the activation of the Nrf2 transcription factor that leads to subsequent reductions in oxidative stress and increased neuroprotection in vivo.2, 5 The induction of Nrf2 is achieved through glutathione depletion as well as direct binding of fumaric acid esters to the Nrf2 repressor KEAP1.3, 6, 7 The antioxidant effects of DMF were initially considered to be the primary mechanism of action for this disease‐modifying treatment (DMT), as mice lacking Nrf2 did not respond to treatment with DMF in the EAE model.2
In addition to its antioxidant effects, DMF has also been known to be immunosuppressive and was initially used as an effective therapy for psoriasis patients. DMF can induce apoptosis in circulating immune cells, including lymphocytes and monocytes,8, 9 and exerts anti‐inflammatory effects on both peripheral and CNS‐resident immune cells.3, 10, 11 Recent reports have demonstrated that the Nrf2‐dependent induction of AREs by DMF may be dispensable for neuroprotection, and the influence of DMF on both the innate and adaptive immune response may be the principle mechanism of action.12 Furthermore, it has been demonstrated that the anti‐inflammatory effects of fumarates may be restricted to DMF, with other fumarate metabolites having little influence on inflammation.9, 10, 13, 14 A recent report has demonstrated that DMF, but not metabolite monomethyl fumarate (MMF), is a potent inhibitor of NF‐κB signaling within immune cells through the modification in cysteine residues.15 This is consistent with findings that DMF promotes an anti‐inflammatory shift in activated microglia.9, 16 In contrast to the theory that DMF is not able to penetrate the CNS following oral administration, the presence of DMF‐glutathione conjugates have been detected in the CNS of animals dosed with DMF, suggesting the brain is directly exposed to DMF.16
Within the CNS, astrocytes are the most abundant glial cell and carry out many functions including providing metabolic support for neurons, regulating the blood–brain barrier (BBB), aiding in synapse formation, and secreting soluble factors that can promote oligodendrocyte differentiation. Astrocytes also contribute to the pathology of neuroinflammatory disorders, reacting to inflammatory stimuli and undergoing distinct changes in both morphology and gene expression17 that can either limit or promote injury. The recruitment of inflammatory cells and modulation of the local inflammatory milieu implicate the astrocyte as an important regulator of neuroinflammation.18 As such, DMTs capable of influencing astrocyte activation may be beneficial in the context of MS. DMF has previously been demonstrated to reduce the activation of astrocytes, highlighting multiple potential mechanisms through which DMF may reduce neuroinflammation.11, 13 Although these studies demonstrated an effect of DMF in vitro, differential effects of MMF and DMF, as well as any potential species effects between humans and mice have not been investigated to date. Furthermore, studies into the effects of fumarates have not taken into account the role of microRNAs, which have emerged as critical regulators of MS neuroinflammation.
Within MS lesions, there is a marked decrease in the remyelination process that is considered to be due to the failure of oligodendrocyte progenitor cells (OPCs) to differentiate into functional myelinating oligodendrocytes.19 In the cuprizone model, mice receiving DMF displayed reduced demyelination,20 a result that has been suggested to be due to reduced microglia activation. These results were similar to EAE results, which demonstrated reduced demyelination associated with reduced immune cell infiltration, astrocyte activation, and increased cytoprotection.2 In MS patients, DMF treatment significantly increased brain magnetization transfer ratio, suggesting increases in myelin density.21 Furthermore, in vitro studies have demonstrated that DMF increases NPC self‐renewal and protects both NPC and oligodendrocytes from oxidative stress.22, 23
The goal of our study was to measure and contrast the anti‐inflammatory and antioxidant effects of both DMF and MMF on human and murine astrocytes. We have demonstrated that DMF, but not MMF, reduces proinflammatory cytokine and chemokine secretion production by astrocytes and is independent of changes in antioxidant gene expression. Changes in the expression of microRNAs previously implicated in both astrocyte activation and MS were also observed in response to treatment with fumarates, particularly, miR‐155 and mir‐146a. We also demonstrated that treatment of DMF leads to increased numbers of oligodendrocyte lineage cells using both mouse and human NPCs. Taken together, these results support the hypothesis that in addition to MMF, DMF can also directly contribute to the anti‐inflammatory and regenerative effects of this DMT. These results align with recent findings that have been previously observed in peripheral immune cells9, 10, 15 and brain‐resident microglia.9, 16
Methods
Human and murine astrocyte isolation and culture
Human primary astrocytes were derived from human fetal CNS tissue (cortex, gestational age 10–20 weeks). CNS tissue was dissociated in DNase/trypsin prior to being passed through a nylon mesh to obtain a single cell suspension. Cells were then plated in tissue culture flasks in DMEM containing 5% FBS, penicillin/streptomycin and glutamine. Cells were passaged every 7–14 days or until confluent. Experiments were conducted on astrocytes between passages 3 and 5. Purity of astrocytes was confirmed by GFAP immunocytochemistry. Murine primary astrocytes were derived from the cortex of P2‐5 C57BL/6 mouse pups, dissociated in DNase/trypsin and triturated to a single cell suspension. Cells were plated in tissue culture‐treated flasks and grown in DMEM containing 10% FBS, penicillin/streptomycin, and glutamine. Murine astrocytes were maintained and utilized according to the same protocols described for human fetal astrocytes.
Human and murine NPC isolation and culture
Human primary A2B5+ cells were cultured as previously described.24 Briefly, A2B5+ progenitors were purified from human fetal CNS tissue by immunomagnetic bead isolation (Miltenyi) following DNase/Trypsin digestion and passage through a nylon mesh filter. Following isolation, 105 cells were plated into each well of a PLL/Matrigel coated 48‐well dish in DMEM‐F12 containing N1 supplement (Sigma‐Aldrich, Oakville, ON, Canada), 0.01% BSA, penicillin/streptomycin, B27 supplement (Invitrogen, Burlington, ON, Canada), PDGF‐AA (10 ng/mL), FGF2 (10 ng/mL), and T3 hormone (2 nmol/L). Medium was changed every 2–3 days for 7–10 days to expand NPCs. Cells were differentiated for 5 days in A2B5+ media without PDGF‐AA or FGF2. Murine neurospheres were derived from P0‐P2 C57BL/6 mouse pups as previously described.25 Briefly, cortical neurospheres were grown in DMEM‐F12 containing penicillin/streptomycin, B27 supplement, EGF (20 ng/mL), FGF2 (20 ng/mL), and heparin (2 μg/mL). After 1 week in culture, neurospheres were plated on 12‐mm laminin‐coated coverslips in wells of a 24‐well culture dish and differentiated for 7 days in DMEM containing 1% FBS and N1 supplement.
Mouse cortical neuron culture
Cortical neurons were cultured from E15‐E17 C57BL/6 embryos as previously described.26 Briefly, cortices were dissected from embryos before dissociation in 0.25% trypsin/EDTA. Cortical neurons were then cultured on poly‐L‐lysine 96‐well plates in Neurobasal medium containing B27 supplement, N2 supplement, penicillin/streptomycin, and glutamine. Cultures were aged for 5 days in vitro prior to usage.
In vitro drug treatments
A total volume of 20 mmol/L stock solutions of MMF and DMF (Sigma Aldrich, Oakville ON, Canada) was prepared in dimethyl sulfoxide (DMSO) and stored at −20°C. DMSO vehicle was aliquoted and stored under similar conditions. Cell cultures were grown to approximately 70% confluency prior to either drug treatment or IL‐1β stimulation. Astrocytes (human and murine) were pretreated with either MMF or DMF at concentrations of 10 μmol/L or 25 μmol/L for 8 h prior to 24‐h stimulation with recombinant human or mouse IL‐1β (10 ng/mL). For treatment of NPC cultures, DMF, MMF (25 μmol/L), or vehicle was added to the differentiation medium and remained in culture for the duration of the experiment.
Quantification of cytokine and chemokine secretion
Supernatants were collected and stored at −80°C before being used for enzyme‐linked immunosorbent assay (ELISA). ELISAs for human and murine IL‐6, CXCL10, and CCL2 were performed following manufacturer's directions (BD Biosciences, Mississauga ON, Canada). The murine CXCL10 ELISA kit was obtained from R&D Systems (R&D Systems, Inc., Minneapolis MN) and performed according to company specifications. All samples were assayed in technical duplicates with each n‐value representing biological replicates.
Quantification of Gene and microRNA expression
Cell pellets were lysed in Trizol® reagent and stored at −80°C. Total RNA was isolated by column extraction with a DNase treatment step (Qiagen, Valencia, CA). RNA was quantified using a Nanodrop 2000. For gene expression assays, RNA was reverse transcribed using MMLV reverse transcriptase (Invitrogen). Individual gene expression assays were done using specific TaqMan® probes and normalized to the endogenous control gene 18S. For microRNA expression assays, microRNA‐specific reverse transcription primers were multiplexed and RNA was reverse transcribed following the TaqMan® MicroRNA Reverse Transcription kit protocol. MicroRNA expression was normalized to RNU48, an abundant small‐nuclear RNA. Fold changes were calculated according to the ΔΔCt method.
Viability and proliferation
Astrocyte viability was determined following pretreatment with drugs or vehicle using the XTT assay (Thermo Scientific). Following a 2‐h incubation with XTT, absorbance was read at 450 nm using a microplate reader (Cytation5®, BioTek). Neuronal viability was assayed following 48‐h incubation with resting or activated astrocyte‐conditioned media. For generation of astrocyte‐conditioned media, astrocytes were grown and treated in serum‐free ultraculture medium (Lonza). Proliferation was assessed by CFSE staining of astrocyte cultures. Briefly, astrocytes were serum starved for 24 h to induce synchronization of cell cycle. Astrocytes were then labeled with 5 μmol/L CFSE (Molecular Probes) for 15 min prior to being returned to serum‐containing medium for 72 h in the presence of treatment conditions. Cells were harvested and fluorescence intensity was measured by flow cytometry using the MoFlo® Astrios™ flow cytometer (Beckman Coulter, Inc.). Proliferation was quantified by loss of CFSE fluorescence.
ROS production assay
Murine astrocytes were cultured to 70% confluence in 96‐well plates and pretreated for 8 h with drug treatments. Following pretreatment, cells were loaded with 10 μmol/L Carboxy‐H2DCFDA (Molecular Probes), a cell permeable dye that becomes fluorescent after cleavage to dichloroflourescin (DCF) by reactive oxygen species (ROS). Following a 1 h incubation with dye, cells were cultured in the Cytation5® live cell imager/plate reader (BioTek) in the presence of IL‐1β (10 ng/mL) and IFNγ (10 ng/mL) to promote inflammatory ROS production.27 Fluorescence intensity was read hourly over a 24‐h period. For measurement of ROS production in human fetal astrocytes, pretreated cells were activated with IL‐1β (20 ng/mL) and IFNγ (20 ng/mL) for 24 h prior to incubation with CellROX® Deep Red reagent (5 μmol/L; 30 min). Cells were washed and fluorescence intensity was measured using a plate reader (Cytation5®; Biotek).
Immunocytochemistry and image analysis
A2B5+ cultures were stained with anti‐O4 (1:50; IgM purified from hybridoma culture) for 30 min prior to fixation with 4% PFA for 20 min at room temperature followed by goat antimouse IgM Cy3 (Millipore; 1:200). Murine neurospheres were fixed in 4% PFA for 20 min at room temperature followed by rabbit anti‐NG2 (Millipore; 1:500) and mouse anti‐GFAP (Sigma; 1:1000) for 1 h at room temperature. Cells were incubated with appropriate secondary antibodies (Sigma; 1:1000) for 1 h at room temperature. DAPI (1:1000) was used as a nuclear counterstain for 5 min. All slides were imaged by fluorescence microscopy and quantified by a blinded observer. Cells for each donor/biological replicate were plated in duplicate, and multiple fields of view (6–8 per biological replicate) were imaged. Quantification was performed using manual (NG2+ and O4+ cells) and automatic (DAPI+ Nuclei) counter plugins within the ImageJ (imagej.nih.gov/ij/) software.
Statistical analysis
Statistics were performed using Prism 6 (GraphPad Software). All values reported are the mean ± sem. All analyzes utilized an alpha = 0.05. One‐way or two‐way ANOVAs with either Dunnett's or Tukey's post hoc comparisons were used to compare samples where appropriate, as indicated in the figure legends.
Results
DMF, but not MMF, significantly decreased proinflammatory cytokines and chemokines in murine and human astrocytes
Following pretreatment with DMF, a significant reduction in IL‐6, CXCL10, and CCL2 secretion by human fetal astrocytes was observed following stimulation with IL‐1β compared to vehicle (Fig. 1A–C). Reduced chemokine/cytokine secretion was also observed in mouse astrocytes following pretreatment with DMF (Fig. 1D–F). The observed reductions in cytokine secretion were dose dependent and restricted to DMF; pretreatment with MMF did not significantly reduce cytokine/chemokine production. As fumarates have been previously shown to alter viability and proliferation of peripheral and CNS‐resident cells,8, 28 we assessed whether the changes in cytokine and chemokine levels were due to changes in viability or proliferation. Following pretreatment with DMF, MMF, or vehicle no reductions in viability or proliferation were observed (Fig. S1A–C). Based on reduced secretion of inflammatory factors, we performed experiments that investigated whether a pretreatment of astrocytes with fumarates (both MMF and DMF) would reduce neurotoxicity by the activated astrocytes. Following culture of cortical neurons for 48 h with either “resting” or “activated” astrocyte‐conditioned media (ACM), we observed that activated ACM significantly reduced viability of neuronal cultures, which was partially rescued by pretreatment with DMF (Fig. S2).
Figure 1.
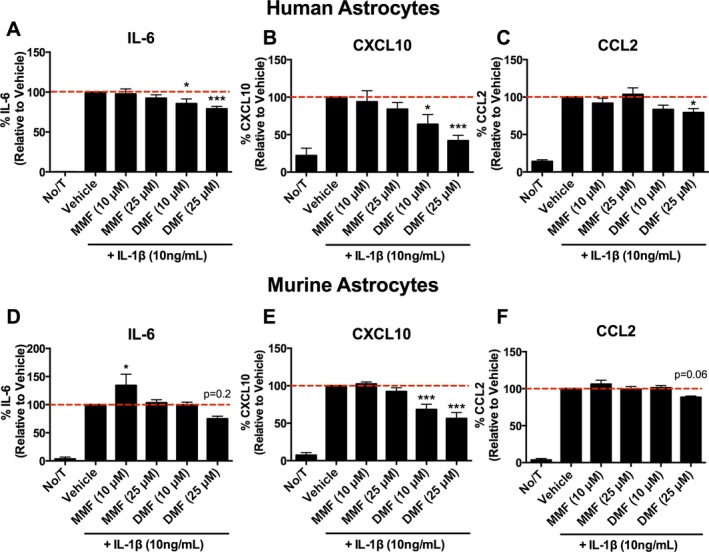
Effects of DMF and metabolite monomethyl fumarate (MMF) on cytokine and chemokine secretion by astrocytes. (A–C) DMF, not MMF, significantly reduces IL‐6 (A; n = 7 for 10 μmol/L DMF & MMF; n = 9 for all other conditions), CXCL10 (B; n = 7 for 10 μmol/L DMF & MMF; n = 9 for all other conditions) and CCL2 (C; n = 5 for 10 μmol/L DMF & MMF; n = 7 for all other conditions) by IL‐1β‐stimulated human fetal astrocytes in vitro (one‐way ANOVA; Dunnet post hoc comparison). (D–F) DMF also reduces IL‐6 (D; n = 5), CXCL10 (E; n = 5), and CCL2 (F; n = 4) secretion by IL‐1β‐stimulated murine astrocytes in vitro (one‐way ANOVA; Dunnet post hoc comparison). Error bars represent mean ± SEM; *P < 0.05, ***P < 0.001 compared to vehicle treatment alone.
Fumarates did not significantly alter antioxidant gene expression in murine and human astrocytes
Next, we measured the expression of ARE gene expression in IL‐1β‐stimulated astrocytes treated with DMF or MMF. In human fetal astrocyte treated with DMF, there appeared to be a trend toward increased expression of Nrf2‐controlled genes, particularly HMOX1 and NQO1, however, these results were not statistically significant from the vehicle control (Fig. 2A–C). Additionally, no significant changes in ARE gene expression (Hmox1, Osgin1, Nqo1) were detected in mouse astrocytes pretreated with DMF or MMF (Fig. 2D–F).
Figure 2.
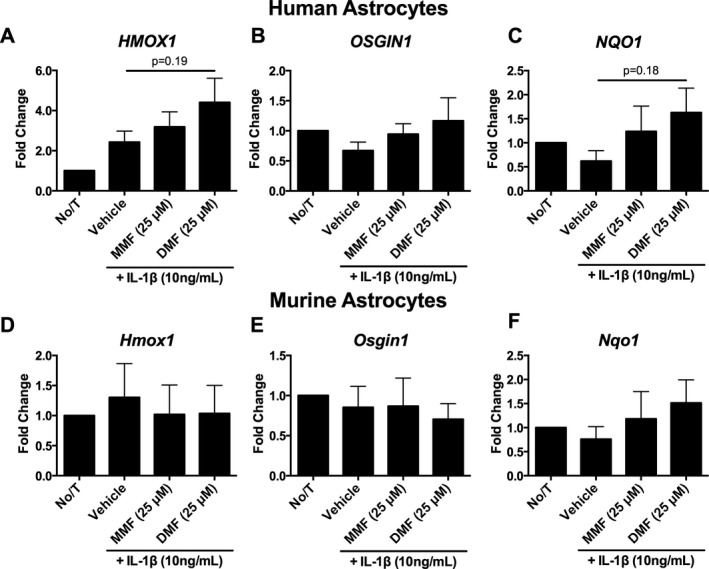
Fumarate treatment induces HMOX1 antioxidant gene expression in human fetal astrocytes. (A–C) DMF significantly increased HMOX1 expression from no treatment (A) while expression levels of OSGIN1 (B) and NQO1 (C) were not statistically significant (n = 5 per group; one‐way ANOVA; Dunnett's post hoc comparison). (D–F) No statistically significant changes in Hmox1 (D), Osgin1 (E), or Nqo1 (F) expression were observed in mouse astrocytes (n = 5 per group; one‐way ANOVA; Dunnett's post hoc comparison). Error bars represent mean ± SEM.
DMF significantly decreased proinflammatory microRNA expression in mouse and human astrocytes
To further assess fumarate mechanisms of action, we measured microRNA expression in IL‐1β‐stimulated astrocytes pretreated with either DMF or MMF. Pretreatment with either DMF or MMF significantly reduced miR‐146a expression in mouse astrocytes; a similar trend was also observed in human astrocytes (Fig. 3A and D). The expression of miR‐155 was significantly reduced by pretreatment with DMF in both human and murine astrocytes (Fig. 3B and E). No consistent effect was observed for the anti‐inflammatory miR‐223 microRNA (Fig. 3C and F), although a trend for increased mir‐223 expression was observed in human astrocytes.
Figure 3.
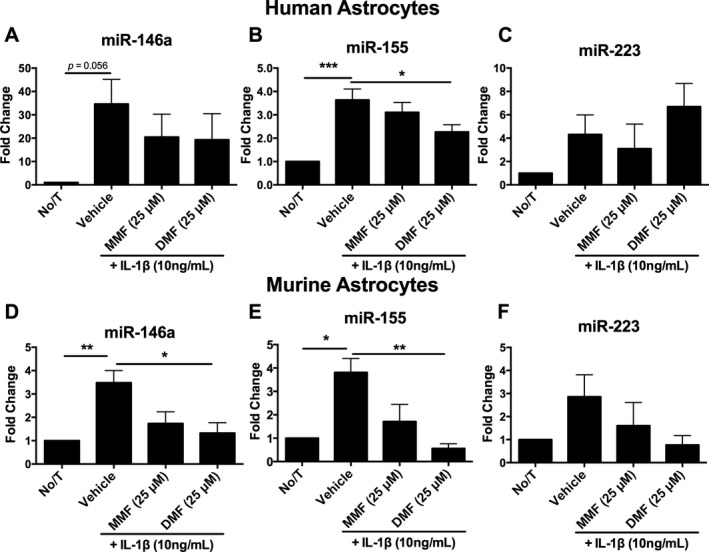
Treatment of human fetal astrocytes with fumarates alters microRNA expression in vitro. miR‐146a (A, D) and miR‐155 (B, E) were induced by IL‐1β activation of human and murine astrocytes in vitro and were reduced by both DMF and metabolite monomethyl fumarate (MMF) in vitro (n = 3–4 per group; one‐way ANOVA; Dunnet post hoc comparison). Expression of miR‐223 was unaltered by either IL‐1β or fumarate treatment (C, F) (n = 4–5 per group; one‐way ANOVA; Dunnet post hoc comparison). Error bars represent mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 compared to vehicle treatment alone.
Fumarates reduced inflammatory ROS production in astrocytes
As fumarates have been shown to protect against oxidative stress, we assessed if pretreatment with fumarates could reduce ROS production in activated astrocytes. We observed significantly reduced ROS production by fumarate‐treated mouse astrocytes over a 24‐h time course of activation with IL‐1β and IFNγ (Fig. 4A and B). We further demonstrated a similar effect in activated human astrocytes, with DMF significantly reducing inflammation‐associated ROS production (Fig. 4C).
Figure 4.
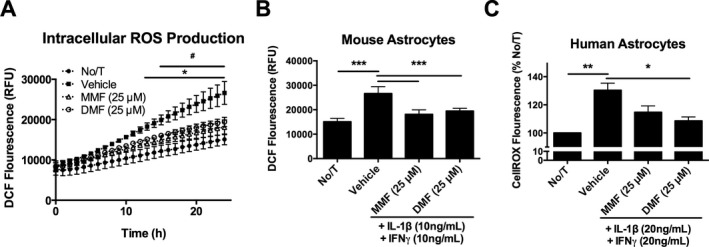
Both DMF and metabolite monomethyl fumarate (MMF) reduce inflammatory reactive oxygen species (ROS) production by astrocytes in vitro. Time course of ROS production by murine astrocytes activated with IL‐1β and IFN γ (A) and 24‐h time point (B) (n = 3 per group; two‐way ANOVA; Tukey post hoc comparison). ROS production by human fetal astrocytes following 24 h activation with IL‐1β and IFN γ (C) (n = 3 per group; one‐way ANOVA; Tukey post hoc comparison). Error bars represent mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 compared to vehicle treatment alone. In (A), # P < 0.05 between MMF and Vehicle control; *P < 0.05 between DMF and Vehicle control.
DMF increased differentiation of murine and human oligodendrocyte progenitor cells
To determine whether fumarates could directly influence the differentiation of OPCs, mouse neurospheres and expanded human A2B5+ progenitors were differentiated in the presence of vehicle, MMF, or DMF. After 7 days of treatment, DMF‐treated mouse neurospheres (Fig. 5A–D) had a significant increase in the numbers of NG2+ cells compared to vehicle control (Fig. 5I). Additionally, human A2B5+ cells (Fig. 5E–H) cultured with DMF showed a trend toward increased numbers of O4+ cells (Fig. 5J). Consistent with our astrocyte data, this effect was restricted to DMF; MMF had little effect on differentiation. This effect was not due to increased cell number, as the average number of cells per field did not change (Fig. S3 A & B).
Figure 5.
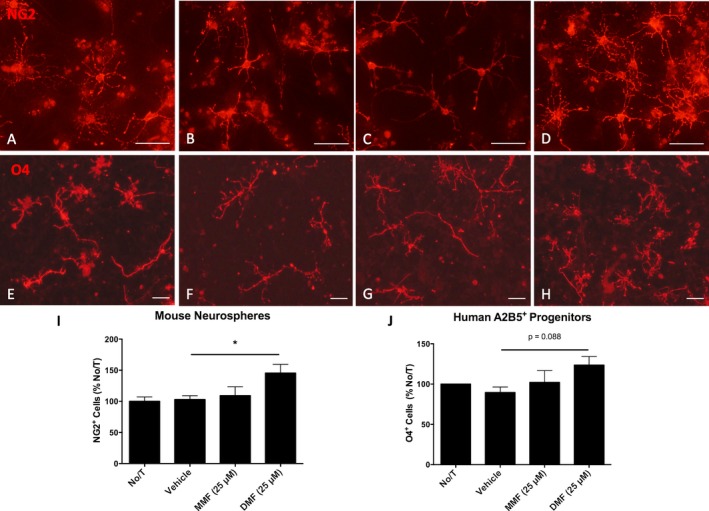
Treatment of human and murine NPCs with DMF promotes differentiation of immature oligodendrocytes. Representative images of murine NG2+ OPCs differentiated from cultured neurospheres following 7 days of treatment (A‐D) and human O4 + immature oligodendrocytes following differentiation from cultured A2B5+ progenitors for 5 days (E‐H). Graphs illustrate increases in NG2 (I) (n = 6–7 per group; one‐way ANOVA; Dunnet post hoc comparison) and O4 (J) (n = 3 per group) cell number. Scale bars, 50 microns. Error bars represent mean ± SEM; *P < 0.05 compared to vehicle treatment alone.
Discussion
In this report, we directly measured and compared the anti‐inflammatory effects of DMF and MMF on both human and murine astrocytes and OPCs. To date, direct comparisons of fumarates on human and murine glial cells have not been performed. In addition, the biological role(s) of the active metabolite of DMF treatment has been controversial, with evidence supporting that both MMF2, 29 and DMF9, 16 modulate anti‐inflammatory and antioxidant effects. Although the debate against DMF has been widely attributed to previous reports citing the inability to measure biologically significant concentrations in the brain and periphery, a recent report has shown that DMF is indeed present in the CNS following oral administration.16
Consistent with previously published reports,6, 11, 13, 30 our data demonstrate that fumarates significantly reduce inflammatory cytokine and chemokine secretion in response to proinflammatory stimuli. We further demonstrate that the reduction in cytokine and chemokine production by fumarates within astrocytes is limited to DMF, as MMF had no significant effect on cytokine and chemokine secretion. This is in agreement with recently published reports suggesting that DMF, not MMF, is anti‐inflammatory in human myeloid cells.9, 13, 14 In response to inflammation, astrocytes secrete chemokines to recruit immune cells, a process further facilitated by their position at the interface of the blood–brain barrier.18 Here, we demonstrated for the first time in astrocytes that CCL2 and CXCL10, two chemokines secreted by astrocytes that influence MS pathology and oligodendrocyte differentiation,31, 32 are reduced in the presence of DMF. Additionally, we demonstrate that DMF also reduces IL‐6, a proinflammatory cytokine during acute inflammation. The finding that DMF reduces astrocyte cytokine and chemokine secretion is consistent between species and highlights further mechanisms through which DMF may limit CNS inflammation. Furthermore, these similarities between species suggest conserved mechanisms through which mouse and human astrocytes respond to DMF. Our results therefore support the validity of studying the mechanisms of action for DMF within murine systems and are likely to translate to humans. Interestingly, MMF (10 μmol/L) also increased IL‐6 production by murine astrocytes, an observation that has also been previously observed in human fetal microglia9; this effect was not observed for the other chemokines that were measured, suggesting that this effect was restricted to IL‐6, a highly pleiotropic cytokine, but not reflective of an astrocyte in a heightened proinflammatory state. Reductions in astrocyte chemokine and cytokine secretion may contribute to the reduced leukocyte infiltration and activation seen in vivo in animals treated with DMF.2, 20, 33 Furthermore, since CXCL10 is known to inhibit of OPC differentiation,24 DMF may impact CNS remyelination indirectly through the astrocyte.
We also provide evidence that the induction of ARE genes in astrocytes is independent of the immunosuppressive effects of DMF. While HMOX1 and NQO1 expression was increased in human astrocytes in the presence of DMF, these results were not statistically significant from the vehicle control and suggest that in astrocytes, DMF may not be able to substantially induce ARE genes in the presence of inflammation. Similar results were observed in murine astrocytes. These results differ from a previous report that has shown an increase in the Nrf2‐responsive genes in astrocytes following DMF treatment6; however, it should be noted that in this previous report, the expression of ARE genes was not measured in the presence of a proinflammatory stimulus, which is not representative of the acute inflammatory milieu observed in MS lesions. While reports have demonstrated that fumarates can activate the Nrf2 pathway in vitro2 and in vivo within the circulating immune cells of MS patients treated with DMF,9, 34 our results support the emerging view that DMF exerts potent, MS‐relevant anti‐inflammatory effects that may be cell specific and/or Nrf2 independent.
In the brains of MS patients, microRNA expression is significantly altered and has been suggested to significantly influence inflammation and repair capacity.35, 36 In astrocytes, both miR‐155 and miR‐146a are significantly upregulated in active MS lesions; laser‐captured astrocytes from MS lesions express miR‐155 in situ.35 Furthermore, activation of astrocytes with various proinflammatory stimuli upregulates the expression of miR‐146a and miR‐155 in vitro.35, 37 The increase in miR‐155 expression by IL‐1β was confirmed in our study, however, to further elucidate on the mechanism of action for DMF, we demonstrated that fumarates reduced miR‐155 expression. This finding has important clinical implications given mir‐155 expression is increased in active MS lesions. Additionally, the validated miR‐155 target SOCS138 is a potent regulator of astrocyte cytokine/chemokine secretion,39 suggesting that a miR‐155/SOCS1 axis could mediate the anti‐inflammatory effects of DMF. miR‐146a is an inflammation resolving microRNA whose expression is upregulated in CNS lesions and peripheral immune cells of MS patients.35, 36 As such, the reduced expression of miR‐146a in fumarate‐treated astrocytes suggests an MS‐relevant resolution of inflammation. miR‐223 is an anti‐inflammatory microRNA that targets a myriad of proinflammatory genes,40 was also measured in fumarate‐treated astrocytes, and demonstrated an increase in human but not murine astrocytes treated with DMF.
In addition to the effects of fumarates on astrocytes, we also demonstrate that treatment of NPCs with DMF significantly increases the number of immature oligodendrocytes in vitro in both murine and human cell cultures. Previously, it has been demonstrated that DMF did not increase remyelination within the cuprizone model or increase the survival of the CG4 oligodendrocyte lineage cell line in vitro.20 However, within mouse neurospheres, DMF has been previously shown to increase NPC self‐renewal and survival,23 a result that supports our findings. We further utilized human A2B5+ cell cultures to demonstrate increased OPC differentiation in vitro; similar effects were observed between both murine and human progenitors. Multiple potential mechanisms could lead to increased OPC differentiation, such as the altered metabolism that has been reported in oligodendrocytes exposed to fumarates,22, 41 or perhaps the indirect effects of astrocyte‐derived growth factors that could be released from astrocytes treated with DMF. Regardless, our results suggest that DMF exerts beneficial regenerative effects in CNS glia, in addition to the well‐described anti‐inflammatory mechanisms.
Conclusion
Our results demonstrate that fumarates reduce inflammatory cytokine and chemokine secretion by activated astrocytes in vitro, which is coupled with changes in microRNA expression. These effects were primarily mediated by DMF with MMF exerting little effect, were consistent between both human and mouse astrocytes, and provide additional support for a direct mechanism of action for DMF in the inflamed CNS.
Conflict of Interest
CSM has received speaker honoraria from Biogen, EMD Serono, and Roche/Genentech. DAG and JBW have nothing to disclose.
Supporting information
Figure S1. Pretreatment of astrocytes with DMF, MMF, or vehicle produced no significant changes in viability as measured by the XTT assay (Fig. S1A) (n = 2 per group; one‐way ANOVA) or proliferation measured by CFSE staining (Fig. S1B, C) (n = 2 per group; one‐way ANOVA).
Figure S2. Conditioned media from mouse astrocytes pretreated with DMF, MMF, or vehicle and subsequently stimulated with IL‐1β were applied to neuronal cultures for 48 h. IL‐1β‐activated astrocytes significantly reduced neuronal viability compared to resting ACM, while pretreatment with DMF partially rescued this effect (n = 3 per group; one‐way ANOVA, Dunnett's post hoc, Error bars represent mean ± SEM; *P < 0.05.
Figure S3. Treatment of human and murine NPCs with fumarates or vehicle did not alter total cell numbers. DAPI+ nuclei were quantified from both mouse neurospheres (A, n = 7) and human cultured A2B5+ progenitors (B, n = 3). Error bars represent mean ± SEM.
Acknowledgments
The authors would like to acknowledge the technical support of Tangyne Berry. In addition, authors would like to acknowledge Dr. Atamjit Gill (Chair of Obstetrics/Gynecology at Memorial University) and the nursing staff of Floor 5BN at the Health Sciences Centre at Eastern Health in St. John's, Newfoundland, Ms. Kathy Murphy‐Peddle (research coordinator), Dr. Jane Barron, Ms Denise Maher, and the pathology staff at Eastern Health.
References
- 1. Gold R, Kappos L, Arnold DL, et al. Placebo‐controlled phase 3 study of oral BG‐12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098–1107. [DOI] [PubMed] [Google Scholar]
- 2. Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011;134(Pt 3):678–692. [DOI] [PubMed] [Google Scholar]
- 3. Lehmann JC, Listopad JJ, Rentzsch CU, et al. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J Invest Dermatol 2007;127:835–845. [DOI] [PubMed] [Google Scholar]
- 4. Gold R, Linker RA, Stangel M. Fumaric acid and its esters: an emerging treatment for multiple sclerosis with antioxidative mechanism of action. Clin Immunol 2012;142:44–48. [DOI] [PubMed] [Google Scholar]
- 5. Scannevin RH, Chollate S, Jung MY, et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid‐derived 2)‐like 2 pathway. J Pharmacol Exp Ther 2012;341:274–284. [DOI] [PubMed] [Google Scholar]
- 6. Brennan MS, Matos MF, Li B, et al. Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS ONE 2015;10:e0120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Itoh K, Wakabayashi N, Katoh Y, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino‐terminal Neh2 domain. Genes Dev 1999;13:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Treumer F, Zhu K, Glaser R, Mrowietz U. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol 2003;121:1383–1388. [DOI] [PubMed] [Google Scholar]
- 9. Michell‐Robinson MA, Moore CS, Healy LM, et al. Effects of fumarates on circulating and CNS myeloid cells in multiple sclerosis. Ann Clin Transl Neurol 2016;3:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li R, Rezk A, Ghadiri M, et al. Dimethyl Fumarate Treatment Mediates an Anti‐Inflammatory Shift in B Cell Subsets of Patients with Multiple Sclerosis. J Immunol 2017;198:691–698. [DOI] [PubMed] [Google Scholar]
- 11. Wilms H, Sievers J, Rickert U, et al. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL‐1beta, TNF‐alpha and IL‐6 in an in‐vitro model of brain inflammation. J Neuroinflammation 2010;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schulze‐Topphoff U, Varrin‐Doyer M, Pekarek K, et al. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci U S A 2016;113:4777–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miljkovic D, Blazevski J, Petkovic F, et al. A comparative analysis of multiple sclerosis‐relevant anti‐inflammatory properties of ethyl pyruvate and dimethyl fumarate. J Immunol 2015;194:2493–2503. [DOI] [PubMed] [Google Scholar]
- 14. Gillard GO, Collette B, Anderson J, et al. DMF, but not other fumarates, inhibits NF‐kappaB activity in vitro in an Nrf2‐independent manner. J Neuroimmunol 2015;15:74–85. [DOI] [PubMed] [Google Scholar]
- 15. Blewett MM, Xie J, Zaro BW, et al. Chemical proteomic map of dimethyl fumarate‐sensitive cysteines in primary human T cells. Sci Signal 2016;9:rs10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peng H, Li H, Sheehy A, et al. Dimethyl fumarate alters microglia phenotype and protects neurons against proinflammatory toxic microenvironments. J Neuroimmunol 2016;15:35–44. [DOI] [PubMed] [Google Scholar]
- 17. Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia 2005;50:427–434. [DOI] [PubMed] [Google Scholar]
- 18. Rothhammer V, Quintana FJ. Control of autoimmune CNS inflammation by astrocytes. Semin Immunopathol 2015;37:625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kotter MR, Stadelmann C, Hartung HP. Enhancing remyelination in disease–can we wrap it up? Brain 2011;134(Pt 7):1882–1900. [DOI] [PubMed] [Google Scholar]
- 20. Moharregh‐Khiabani D, Blank A, Skripuletz T, et al. Effects of fumaric acids on cuprizone induced central nervous system de‐ and remyelination in the mouse. PLoS ONE 2010;5:e11769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arnold DL, Gold R, Kappos L, et al. Magnetization transfer ratio in the delayed‐release dimethyl fumarate DEFINE study. J Neurol 2014;261:2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang H, Taraboletti A, Shriver LP. Dimethyl fumarate modulates antioxidant and lipid metabolism in oligodendrocytes. Redox Biol 2015;5:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Q, Chuikov S, Taitano S, et al. Dimethyl Fumarate Protects Neural Stem/Progenitor Cells and Neurons from Oxidative Damage through Nrf2‐ERK1/2 MAPK Pathway. Int J Mol Sci 2015;16:13885–13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moore CS, Cui QL, Warsi NM, et al. Direct and indirect effects of immune and central nervous system‐resident cells on human oligodendrocyte progenitor cell differentiation. J Immunol 2015;194:761–772. [DOI] [PubMed] [Google Scholar]
- 25. Moore CS, Milner R, Nishiyama A, et al. Astrocytic tissue inhibitor of metalloproteinase‐1 (TIMP‐1) promotes oligodendrocyte differentiation and enhances CNS myelination. J Neurosci 2011;31:6247–6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sanz R, Ferraro GB, Fournier AE. IgLON cell adhesion molecules are shed from the cell surface of cortical neurons to promote neuronal growth. J Biol Chem 2015;290:4330–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sheng WS, Hu S, Feng A, Rock RB. Reactive oxygen species from human astrocytes induced functional impairment and oxidative damage. Neurochem Res 2013;38:2148–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghods AJ, Glick R, Braun D, Feinstein D. Beneficial actions of the anti‐inflammatory dimethyl fumarate in glioblastomas. Surg Neurol Int 2013;4:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parodi B, Rossi S, Morando S, et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol 2015;130(2):279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin SX, Lisi L, Dello Russo C, et al. The anti‐inflammatory effects of dimethyl fumarate in astrocytes involve glutathione and haem oxygenase‐1. ASN Neuro 2011;3(2):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Der Voorn P, Tekstra J, Beelen RH, et al. Expression of MCP‐1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol 1999;154:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sorensen TL, Trebst C, Kivisakk P, et al. Multiple sclerosis: a study of CXCL10 and CXCR3 co‐localization in the inflamed central nervous system. J Neuroimmunol 2002;127:59–68. [DOI] [PubMed] [Google Scholar]
- 33. Schilling S, Goelz S, Linker R, et al. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin Exp Immunol 2006;145:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gopal S, Mikulskis A, Gold R, et al. Evidence of activation of the Nrf2 pathway in multiple sclerosis patients treated with delayed‐release dimethyl fumarate in the Phase 3 DEFINE and CONFIRM studies. Mult Scler 2017;01:1352458517690617. [DOI] [PubMed] [Google Scholar]
- 35. Junker A, Krumbholz M, Eisele S, et al. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 2009;132(Pt 12):3342–3352. [DOI] [PubMed] [Google Scholar]
- 36. Moore CS, Rao VT, Durafourt BA, et al. miR‐155 as a multiple sclerosis‐relevant regulator of myeloid cell polarization. Ann Neurol 2013;74:709–720. [DOI] [PubMed] [Google Scholar]
- 37. Tarassishin L, Loudig O, Bauman A, et al. Interferon regulatory factor 3 inhibits astrocyte inflammatory gene expression through suppression of the proinflammatory miR‐155 and miR‐155*. Glia 2011;59:1911–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu LF, Gasteiger G, Yu IS, et al. A Single miRNA‐mRNA Interaction Affects the Immune Response in a Context‐ and Cell‐Type‐Specific Manner. Immunity 2015;43:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qin H, Niyongere SA, Lee SJ, et al. Expression and functional significance of SOCS‐1 and SOCS‐3 in astrocytes. J Immunol 2008;181:3167–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haneklaus M, Gerlic M, O'Neill LA, Masters SL. miR‐223: infection, inflammation and cancer. J Intern Med 2013;274:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zheng L, Cardaci S, Jerby L, et al. Fumarate induces redox‐dependent senescence by modifying glutathione metabolism. Nat Commun 2015;23:6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Pretreatment of astrocytes with DMF, MMF, or vehicle produced no significant changes in viability as measured by the XTT assay (Fig. S1A) (n = 2 per group; one‐way ANOVA) or proliferation measured by CFSE staining (Fig. S1B, C) (n = 2 per group; one‐way ANOVA).
Figure S2. Conditioned media from mouse astrocytes pretreated with DMF, MMF, or vehicle and subsequently stimulated with IL‐1β were applied to neuronal cultures for 48 h. IL‐1β‐activated astrocytes significantly reduced neuronal viability compared to resting ACM, while pretreatment with DMF partially rescued this effect (n = 3 per group; one‐way ANOVA, Dunnett's post hoc, Error bars represent mean ± SEM; *P < 0.05.
Figure S3. Treatment of human and murine NPCs with fumarates or vehicle did not alter total cell numbers. DAPI+ nuclei were quantified from both mouse neurospheres (A, n = 7) and human cultured A2B5+ progenitors (B, n = 3). Error bars represent mean ± SEM.


