Abstract
The mononegaviral family Filoviridae has eight members assigned to three genera and seven species. Until now, genus and species demarcation were based on arbitrarily chosen filovirus genome sequence divergence values (≈50% for genera, ≈30% for species) and arbitrarily chosen phenotypic virus or virion characteristics. Here we report filovirus genome sequence-based taxon demarcation criteria using the publicly accessible PAirwise Sequencing Comparison (PASC) tool of the US National Center for Biotechnology Information (Bethesda, MD, USA). Comparison of all available filovirus genomes in GenBank using PASC revealed optimal genus demarcation at the 55–58% sequence diversity threshold range for genera and at the 23–36% sequence diversity threshold range for species. Because these thresholds do not change the current official filovirus classification, these values are now implemented as filovirus taxon demarcation criteria that may solely be used for filovirus classification in case additional data are absent. A near-complete, coding-complete, or complete filovirus genome sequence will now be required to allow official classification of any novel “filovirus.” Classification of filoviruses into existing taxa or determining the need for novel taxa is now straightforward and could even become automated using a presented algorithm/flowchart rooted in RefSeq (type) sequences.
Keywords: cuevavirus, Ebola, ebolavirus, Filoviridae, filovirus, marburgvirus, Mononegavirales, virus taxonomy, virus classification, ICTV
1. Introduction
The family Filoviridae, one of eight families in the order Mononegavirales [1], has eight members assigned to seven species included in three genera (Table 1) [2,3,4].
Table 1.
Official filovirus taxonomy endorsed by the 2015–2017 International Committee on Taxonomy of Viruses (ICTV) Filoviridae Study Group and accepted by the ICTV.
| Current Taxonomy and Nomenclature |
|---|
| Order Mononegavirales Family Filoviridae Genus Marburgvirus Species Marburg Marburgvirus Virus 1: Marburg virus (MARV) Virus 2: Ravn virus (RAVV) Genus Ebolavirus Species Bundibugyo ebolavirus Virus: Bundibugyo virus (BDBV) Species Reston ebolavirus Virus: Reston virus (RESTV) Species Sudan ebolavirus Virus: Sudan virus (SUDV) Species Taï Forest ebolavirus Virus: Taï Forest virus (TAFV) Species Zaire ebolavirus Virus: Ebola virus (EBOV) Genus Cuevavirus Species Lloviu cuevavirus Virus: Lloviu virus (LLOV) |
Traditionally, the eight currently recognized filoviruses have been classified using phenotypic characteristics of virions and/or partial filovirus genome sequences [5,6,7]. Sequence-based filovirus taxon demarcation criteria (nucleotide and amino acid sequence identity values and/or phylogenies) were officially introduced as additional demarcation criteria in 2000 [8] and further refined thereafter [9]. Yet, true filovirus genome sequence-based taxon demarcation was only introduced in 2011. At that time, the International Committee on Taxonomy of Viruses (ICTV) Filoviridae Study Group decided arbitrarily that marburgvirus genomes differ from ebolavirus genomes by ≥50% and that ebolavirus species are differentiated on the basis of glycoprotein (GP) gene sequence differences (≥30%) or genome sequence differences (≥30%) [3]. These values were used to develop a decision algorithm/flowchart for filovirus taxon assignment that could guide filovirus classification [10]. In 2012, two pairwise sequence comparison methods, PAirwise Sequence Comparison (PASC) and DivErsity pArtitioning by hieRarchical Clustering (DEmARC), confirmed that the then official filovirus taxonomy (identical to the current one shown in Table 1) is justified, but that the 50% and 30% values ought to be adjusted objectively based on the PASC and/or DEmARC results [11,12]. Both analyses were based on the available ≈50 near-complete, coding-complete or complete filovirus genomes (see [13,14] for nomenclature) in the US National Center for Biotechnology Information (NCBI, Bethesda, MD, USA) GenBank database. Yet, at the time it was unclear whether the ICTV would accept classification of viruses based on sequence analysis alone.
In 2017, the ICTV members reached a consensus together with other experts that “the development of a robust framework for sequence-based virus taxonomy is indispensable for the comprehensive characterization of the global virome” [15]. Under proper oversight by, for instance, ICTV Study Groups, virus classification criteria can now be based on measurable objective criteria inferable only from viral genome sequence data. Thus, using automatic classification algorithms is possible.
The number of GenBank-deposited near-complete, coding-complete, and complete filovirus genome sequences has increased substantially in recent years (from the ≈50 in 2012 to ≈1400 at the time of writing in 2017). We analyzed these sequences using PASC, a method that can be easily used by any scientist using an open-access software platform [16,17,18]. We created inferred objective filovirus taxon demarcation criteria and updated the algorithm/flowchart for filovirus taxon assignment using the recently decided type filovirus sequences (NCBI RefSeq database sequences) [10] as starting points.
2. Materials and Methods
All 1404 near-complete, coding-complete, or complete filovirus genomes available from GenBank (NCBI, Bethesda, MD, USA) on 04/16/2017 were downloaded from the NCBI viral genomes resource [19]. Redundant filovirus genome sequences (here defined as sequences with PASC identities >99.5%) were removed, leaving 112 filovirus genome sequences for further analysis [20]. PASC analysis was performed with those 112 genome sequences as previously described [18] using the open-access PASC tool (NCBI). The new taxon demarcation algorithm/flowchart was developed based on the previously developed chart presented in [10] using type filoviruses [4] and type filovirus genome sequences (RefSeq, NCBI) [10].
3. Results
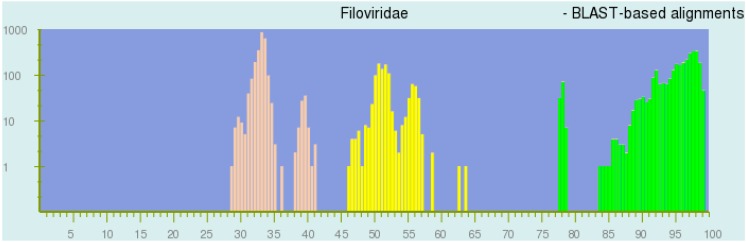
PASC analysis of 112 filovirus near-complete, coding-complete, or complete genome sequences revealed clear clustering into three higher ranks (genera), with two of those genera including single species and one genus including five species (visualized in Figure 1).
Figure 1.
Screenshot of the US National Center for Biotechnology Information (NCBI) PAirwise Sequence Comparison (PASC) tool result after comparing 112 distinct near-complete, coding-complete or complete filovirus genome sequences. Brown bars represent genome pairs assigned to (three) different genera; yellow bars represent genome pairs assigned to (seven) separate species; and green bars represent genome pairs assigned to the same species. BLAST: Basic Local Alignment Search Tool.
Unblinding of input sequences revealed the three genera and seven species to correspond to those already established and depicted in Table 1, raising confidence in PASC as a method to adequately recreate current knowledge on filovirus diversity. However, the analysis indicated an ideal genus demarcation threshold range of 55–58% sequence divergence rather than the currently used 50% threshold and an ideal species demarcation threshold range of 23–36% rather than the currently used 30% threshold.
4. Discussion
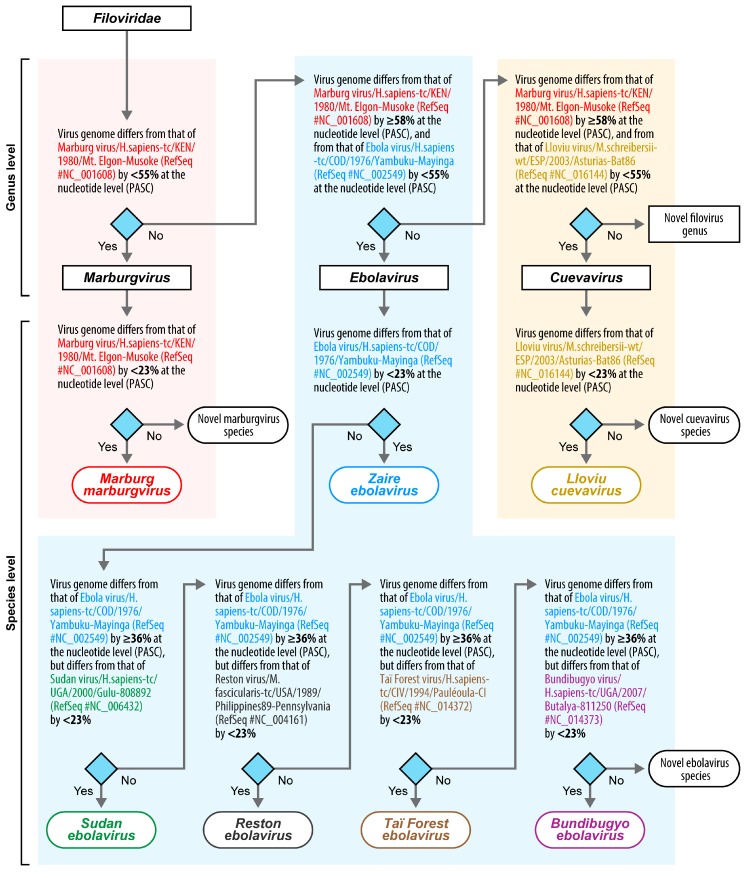
Using the new filovirus taxon demarcation criteria established here using PASC, the earliest discovered filovirus (Marburg virus; MARV) as the type virus for the family Filoviridae [4], the RefSeq MARV genome sequence as the MARV type sequence, and the remaining filovirus RefSeq genome sequences as additional anchor points, we created a filovirus classification decision matrix in form of an algorithm/flowchart (Figure 2). Using the NCBI PASC tool and Figure 2, any user can now quickly assess whether a novel filovirus sequence of interest represents a filovirus already classified in one of the established filovirus taxa or whether establishment of a new taxon/new taxa may be necessary. PASC requires at least near-complete or coding-complete genome input sequences. Therefore, the ICTV Filoviridae Study Group decided that moving forward, at least a coding-complete filovirus genome sequence will be minimally required for filovirus classification into novel filovirus taxa. Partial filovirus-like nucleic acids, for instance, those recently discovered in Chinese bats [21,22], may point towards the existence of novel filoviruses but will not suffice for official recognition of novel filoviruses or establishment of novel filovirus taxa. The Study Group recommends that such sequences be referred to as “filovirus-like sequences” and not as “filoviruses.” Likewise, a virus for which a partial filovirus-like sequence information exists ought to be referred to as a “putative filovirus” until at least coding-complete genome sequence information is available.
Figure 2.
Algorithm/flow chart for filovirus classification based on genomics sequence information (modified from [10]) and PASC-derived sequence demarcation criteria. A putative filovirus genome of interest is compared to the type filovirus RefSeq genome sequence (i.e., that of Marburg virus/H.sapiens-tc/KEN/1980/Mt. Elgon-Musoke [10]) and then sequentially moved through the process until its proper placement in a species is revealed. If the sequence comparison reveals the need for the creation of a novel genus and/or species, official taxonomic proposals ought to be submitted to the ICTV.
Importantly, PASC analysis followed by use of the algorithm/flowchart (Figure 2) alone does not constitute official classification, and the Study Group sees PASC results as highly informative, but not binding. Thus, if the PASC algorithm/flowchart indicates the need for a novel filovirus genus and/or species to a user analyzing a particular sequence, the user should follow the official pathway for ICTV classification starting with submission of an official taxonomic proposal (TaxoProp [23]). The user is recommended to engage with the ICTV Filoviridae Study Group as early as possible during that process. The Study Group and ICTV will evaluate all available data on a particular putative filovirus (e.g., host information, disease phenotype, biophysical properties of virions) and make their decisions accordingly. Phylogenetic results obtained with methods more sophisticated than PASC are always desired and may ultimately overrule PASC results.
Acknowledgments
We thank Laura Bollinger and Jiro Wada (U.S. National Institutes of Health and National Institute of Allergy and Infectious Diseases (NIH/NIAID) Integrated Research Facility at Fort Detrick, Frederick, MD, USA) for critically editing the manuscript and figure creation, respectively. The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the US Department of the Army, the U.S. Department of Defense, the U.S. Department of Health and Human Services, the Department of Homeland Security (DHS) Science and Technology Directorate (S&T), or of the institutions and companies affiliated with the authors. In no event shall any of these entities have any responsibility or liability for any use, misuse, inability to use, or reliance upon the information contained herein. The U.S. departments do not endorse any products or commercial services mentioned in this publication. This work was supported in part through Battelle Memorial Institute’s prime contract with the U.S. NIAID under Contract No. HHSN272200700016I. A subcontractor to Battelle Memorial Institute who performed this work is: J.H.K., an employee of Tunnell Government Services, Inc. This work was also supported in part by the 100 Talent Program of the Chinese Academy of Sciences (Y.B.). This work was also funded in part under Contract No. HSHQDC-15-C-00064 awarded by DHS S&T for the management and operation of the National Biodefense Analysis and Countermeasures Center (NBACC), a Federally Funded Research and Development Center (V.W.-J.).
Author Contributions
Y.B. and J.H.K. conceived and designed the experiments; Y.B. performed the experiments; all authors analyzed the data; J.H.K. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Amarasinghe G.K., Bào Y., Basler C.F., Bavari S., Beer M., Bejerman N., Blasdell K.R., Bochnowski A., Briese T., Bukreyev A., et al. Taxonomy of the order Mononegavirales: Update 2017. Arch. Virol. 2017;162 doi: 10.1007/s00705-017-3311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bukreyev A.A., Chandran K., Dolnik O., Dye J.M., Ebihara H., Leroy E.M., Mühlberger E., Netesov S.V., Patterson J.L., Paweska J.T., et al. Discussions and decisions of the 2012–2014 International Committee on Taxonomy of Viruses (ICTV) Filoviridae Study Group, January 2012–June 2013. Arch. Virol. 2014;159:821–830. doi: 10.1007/s00705-013-1846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn J.H., Becker S., Ebihara H., Geisbert T.W., Jahrling P.B., Kawaoka Y., Netesov S.V., Nichol S.T., Peters C.J., Volchkov V.E., et al. Family Filoviridae. In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Virus Taxonomy—Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press; London, UK: 2011. pp. 665–671. [Google Scholar]
- 4.Kuhn J.H., Becker S., Ebihara H., Geisbert T.W., Johnson K.M., Kawaoka Y., Lipkin W.I., Negredo A.I., Netesov S.V., Nichol S.T., et al. Proposal for a revised taxonomy of the family Filoviridae: Classification, names of taxa and viruses, and virus abbreviations. Arch. Virol. 2010;155:2083–2103. doi: 10.1007/s00705-010-0814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiley M.P., Bowen E.T.W., Eddy G.A., Isaäcson M., Johnson K.M., McCormick J.B., Murphy F.A., Pattyn S.R., Peters D., Prozesky O.W., et al. Filoviridae: A taxonomic home for Marburg and Ebola viruses? Intervirology. 1982;18:24–32. doi: 10.1159/000149300. [DOI] [PubMed] [Google Scholar]
- 6.Francki R.I.B., Fauquet C.M., Knudson D.L., Brown F. Classification and Nomenclature of Viruses—Fifth Report of the International Committee on Taxonomy of Viruses. Volume 2 Springer-Verlag; Vienna, Austria: 1991. [Google Scholar]
- 7.Jahrling P.B., Kiley M.P., Klenk H.-D., Peters C.J., Sanchez A., Swanepoel R. Family Filoviridae. In: Murphy F.A., Fauquet C.M., Bishop D.H.L., Ghabrial S.A., Jarvis A.W., Martelli G.P., Mayo M.A., Summers M.D., editors. Virus Taxonomy—Sixth Report of the International Committee on Taxonomy of Viruses. Volume 10. Springer-Verlag; Vienna, Austria: 1995. pp. 289–292. [Google Scholar]
- 8.Netesov S.V., Feldmann H., Jahrling P.B., Klenk H.-D., Sanchez A. Family Filoviridae. In: van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens E.B., Estes M.K., Lemon S.M., Maniloff J., Mayo M.A., McGeoch D.J., Pringle C.R., editors. Virus Taxonomy—Seventh Report of the International Committee on Taxonomy of Viruses. Academic Press; San Diego, CA, USA: 2000. pp. 539–548. [Google Scholar]
- 9.Feldmann H., Geisbert T.W., Jahrling P.B., Klenk H.-D., Netesov S.V., Peters C.J., Sanchez A., Swanepoel R., Volchkov V.E. Family Filoviridae. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. Virus Taxonomy—Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press; San Diego, CA, USA: 2005. pp. 645–653. [Google Scholar]
- 10.Kuhn J.H., Andersen K.G., Bào Y., Bavari S., Becker S., Bennett R.S., Bergman N.H., Blinkova O., Bradfute S., Brister J.R., et al. Filovirus RefSeq entries: Evaluation and selection of filovirus type variants, type sequences, and names. Viruses. 2014;6:3663–3682. doi: 10.3390/v6093663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao Y., Chetvernin V., Tatusova T. PAirwise Sequence Comparison (PASC) and its application in the classification of filoviruses. Viruses. 2012;4:1318–1327. doi: 10.3390/v4081318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauber C., Gorbalenya A.E. Genetics-based classification of filoviruses calls for expanded sampling of genomic sequences. Viruses. 2012;4:1425–1437. doi: 10.3390/v4091425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladner J.T., Beitzel B., Chain P.S.G., Davenport M.G., Donaldson E.F., Frieman M., Kugelman J.R., Kuhn J.H., O’Rear J., Sabeti P.C., et al. Standards for sequencing viral genomes in the era of high-throughput sequencing. MBio. 2014;5:e01360-14. doi: 10.1128/mBio.01360-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladner J.T., Kuhn J.H., Palacios G. Standard finishing categories for high-throughput sequencing of viral genomes. Rev. Sci. Tech. 2016;35:43–52. doi: 10.20506/rst.35.1.2416. [DOI] [PubMed] [Google Scholar]
- 15.Simmonds P., Adams M.J., Benkő M., Breitbart M., Brister J.R., Carstens E.B., Davison A.J., Delwart E., Gorbalenya A.E., Harrach B.Z., et al. Consensus statement: Virus taxonomy in the age of metagenomics. Nat. Rev. Microbiol. 2017;15:161–168. doi: 10.1038/nrmicro.2016.177. [DOI] [PubMed] [Google Scholar]
- 16.Bao Y., Chetvernin V., Tatusova T. Improvements to pairwise sequence comparison (PASC): A genome-based web tool for virus classification. Arch. Virol. 2014;159:3293–3304. doi: 10.1007/s00705-014-2197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao Y., Kapustin Y., Tatusova T. Virus classification by PAirwise Sequence Comparison (PASC) In: Mahy B.W.J., van Regenmortel M.H.V., editors. Encyclopedia of Virology. 3rd ed. Volume 5. Elsevier; Oxford, UK: 2008. pp. 342–348. [Google Scholar]
- 18.Bào Y., Kuhn J.H. Preliminary classification of novel hemorrhagic fever-causing viruses using sequence-based PAirwise Sequence Comparison (PASC) analysis. In: Salvato M.S., editor. Hemorrhagic Fever Viruses: Methods and Protocols. Humana Press; Totowa, NJ, USA: 2017. in press. [DOI] [PubMed] [Google Scholar]
- 19.Brister J.R., Ako-Adjei D., Bao Y., Blinkova O. NCBI viral genomes resource. Nucleic Acids Res. 2015;43:D571–D577. doi: 10.1093/nar/gku1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Biotechnology Information PASC—Filoviridae. List of Non-Redundant Sequences (Using BLAST-Based Alignments) [(accessed on 9 May 2017)];2017 Available online: https://www.ncbi.nlm.nih.gov/sutils/pasc/viridty.cgi?textpage=main&action=gilist&id=333.
- 21.He B., Feng Y., Zhang H., Xu L., Yang W., Zhang Y., Li X., Tu C. Filovirus RNA in fruit bats, China. Emerg. Infect. Dis. 2015;21:1675–1677. doi: 10.3201/eid2109.150260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X.-L., Zhang Y.-Z., Jiang R.-D., Guo H., Zhang W., Li B., Wang N., Wang L., Waruhiu C., Zhou J.-H., et al. Genetically diverse filoviruses in Rousettus and Eonycteris spp. bats, China, 2009 and 2015. Emerg. Infect. Dis. 2017;23:482–486. doi: 10.3201/eid2303.161119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Committee on Taxonomy of Viruses. [(accessed on 9 May 2017)];Taxonomy Proposal Templates. Available online: https://talk.ictvonline.org/files/taxonomy-proposal-templates/