Abstract
Skin wound closure occurs when keratinocytes migrate from the edge of the wound and re-epithelialize the epidermis. Their migration takes place primarily before any vascularization is established, that is, under hypoxia, but relatively little is known regarding the factors that stimulate this migration. Hypoxia and an acidic environment are well-established stimuli for cancer cell migration. The carbonic anhydrases (CAs) contribute to tumor cell migration by generating an acidic environment through the conversion of carbon dioxide to bicarbonate and a proton. On this basis, we explored the possible role of CAs in tissue regeneration using mouse skin wound models. We show that the expression of mRNAs encoding CA isoforms IV and IX are increased (~25 × and 4 ×, respectively) during the wound hypoxic period (days 2–5) and that cells expressing CAs form a band-like structure beneath the migrating epidermis. RNA-Seq analysis suggested that the CA IV-specific signal in the wound is mainly derived from neutrophils. Due to the high level of induction of CA IV in the wound, we treated skin wounds locally with recombinant human CA IV enzyme. Recombinant CA IV significantly accelerated wound re-epithelialization. Thus, CA IV could contribute to wound healing by providing an acidic environment in which the migrating epidermis and neutrophils can survive and may offer novel opportunities to accelerate wound healing under compromised conditions.
Introduction
The healing of a human skin wound is a complex and highly coordinated biological process that involves diverse phenomena such as hemostasis, inflammation, re-epithelialization, angiogenesis, fibroplasia and finally tissue remodeling.1, 2 ‘Re-epithelialization' is the lateral migration of keratinocytes across a wound bed, which when successful, closes the wound.1, 2
Re-epithelialization begins within hours after injury. It is believed that a critical switch for the initiation of keratinocyte migration is the acute change in oxygen tension.1 That is, when the skin is wounded and the dermal blood vessels are clotted and no longer able to deliver oxygen to the skin, the keratinocytes experience the stress of acute hypoxia and initiate migration to close the defect. Remarkably, the re-epithelialization takes place mainly under hypoxia, and wound closure can be completed before any new re-vascularization occurs.1
Studies have shown that in addition to hypoxia, acidosis stimulates cancer cell migration.3, 4 Carbonic anhydrases (CA) are a family of zinc metalloenzymes that regulate the tissue acid–base equilibrium by catalyzing the reversible hydration of carbon dioxide to bicarbonate ions and protons (CO2+H2O<–>HCO3−+H+).3, 4, 5 Fifteen human CA isoforms have been found, of which 12 are active and 3 inactive.6, 7 These isoenzymes are expressed to some extent in all tissues and organs, but particularly in those that are metabolically highly active such as the brain and kidney.3, 5 Interestingly, the expression of CA proteins IX and XII is induced by hypoxia in different tumors.8 Through their ability to regulate pH and generate an acidic environment, these enzymes endow tumor cells with survival advantages under hypoxia/acidosis conditions and confer an increased ability to migrate.
The potency of the CAs, IX and XII, to stimulate cell migration under hypoxia prompted us to investigate the role of CAs in skin wound healing. Quite unexpectedly, nothing is known regarding CAs during skin wound healing, although the restoration of CA activity is related to duodenal ulcers. We assumed that the expression of certain CA family members could increase when a wound is exposed to hypoxia and, theoretically, could stimulate re-epithelialization by generating an acidic environment for keratinocytes. Thus, we studied the expression pattern of the enzymatically active CAs in wounds in mice, and based on the results also treated the wounds with exogenous recombinant CA IV enzyme.
Materials and methods
Generation of skin wounds
For the quantitative PCR (qPCR) and immunohistochemistry analyses, 8-week-old male BALB/c mice (weighing 23–25 g) were used. Mice were fed with standard laboratory pellets and water ad libitum. All animal experiments were performed in accordance with protocols approved by the National Animal Ethics Committee of Finland.
Six-millimeter diameter, full thickness (including panniculus carnosus muscle) excision wounds were created in the dorsal skin under sevoflurane anesthesia as previously described.9 At various time points, the animals were killed, and the wounded tissue was collected and processed for further analyses.
qPCR analysis
Total skin wound RNA collected at various time points was converted to cDNA by reverse transcription using the High Capacity cDNA Reverse Transcription Kit for RT-qPCR (Applied Biosytems, Foster City, CA, USA). Duplicate qRT-PCR reactions were performed with PowerSYBR SYBRGreen reagents (Applied Biosytems) on an ABI 7000 Real Time PCR System (Applied Biosytems). Details of the primers used are listed in Supplementary Table 1. As negative controls, no-template and no-reverse transcriptase controls were also included (which were negative herein). The data analyses were performed according to Livak and Schmittgen.10
Expression and purification of recombinant human CA IV
cDNA for the secretory form of human CA IV was cloned in pET-11d, a bacterial expression vector, as previously described.11 Briefly, the E. coli strain Rosetta(DE3)pLysS was used for enzyme production. The enzyme was purified using a CA-inhibitor affinity column.11 Affinity-purified enzyme was dialyzed against 20 mM ammonium bicarbonate for several changes of buffer. Endotoxin removal was performed using a specialized endotoxin removal column three times (Hyglos Blue Endotoxin Removal Column, Starnberg, Germany). The protein samples were then re-dialyzed with saline, filter-sterilized and stored at 4 °C. Recombinant CA IV was analyzed on an NuPAGE 4–12% gradient gel (ThermoFisher Scientific, Waltham, MA, USA). The enzymatic activity of recombinant CA IV was assayed as previously described.11
Skin wound treatment trial with recombinant CA IV
For the treatment trial, 8-week-old male littermate BALB/c mice were used. Two 6-mm, circular, full-thickness wounds were generated in each animal by biopsy punch. Donut-shaped 12-mm silicone splints (Grace Bio-Labs, Bend, OR, USA) were placed around the wounds and affixed with glue and sutures to prevent wound contraction.12 Plastic collars were used to prevent the mice from tearing off the splints.12 The mice that underwent surgery were divided into three groups. The first group consisted of mice treated with CA IV; purified CA IV was mixed with 30% Pluronic-127 (Sigma-Aldrich) gel as previously described,13 and the pH was 7.0. Fifty microliters of the mixture was added to each wound to fill up the entire wound cavity. The second group received a CA inhibitor, acetazolamide (1.0 mM, Orion, Espoo, Finland), in Pluronic-127 gel. The third group served as a control group and was treated with 50 μl of phosphate-buffered saline mixed with Pluronic-127 gel. The final concentration of Pluronic-127 in each solution was 18%. Each mouse was photographed after the silicone splints had been securely sutured in place and then killed on day 5.
RNA-Seq analysis of wound-specific tissues
RNA-Seq data from human B-cells, CD4-positive cells, CD8-positive cells, monocytes, neutrophils, natural killer (NK) cells, monocytes (ArrayExpress accession E-GEOD-60424),14 dermal fibroblasts (E-GEOD-72589), undifferentiated and differentiated keratinocytes (E-MTAB-1717),15 M1 and M2 macrophages (E-GEOD-36952), and skin cells (E-MTAB-2836)16 were retrieved from the Array Express database. Reads for all samples were pooled by tissue type and mapped to CA genes in the human genome using the Bowtie17 and Tophat18 modules of the Tophat package utilizing supercomputer resources provided by CSC–IT Center for Science of the Finnish Ministry of Education and Culture. Subsequent matches were then compared to reference CA transcript structures from the Ensembl database using the Cuffcompare module of the Cufflinks package.19 Normalized fragments per kilobase of transcript per million mapped read (FPKM) values were generated for each transcript identified in each tissue using the Cuffnorm module of the Cufflinks package to compare expression values across the pooled samples. The expression levels of each CA were compared across tissue types using the Cuffmerge and Cuffdiff modules of Cufflinks. The results were visualized using the cummeRbund R library.
Statistical analysis
The data are presented as the mean±s.d. For comparisons of multiple groups, statistical analysis was conducted by two-way analysis of variance complemented by the Bonferroni post hoc test for pair wise comparisons between the test groups. P-values <0.05 were considered significant.
Supplementary Methods
Extraction of RNA, the oxygen-induced retinopathy model, immunohistochemistry, histology, quantitative analysis of histology (wound healing), promoter analysis of the primary CA4 (human gene for CA IV protein) transcript and gene ontology (GO) analysis of proteins were performed using standard methods20, 21, 22, 23, 24 and are described in detail in the Supplementary Data set.
Results
Expression of CA IV and CA IX is induced during the hypoxic phase of wound healing
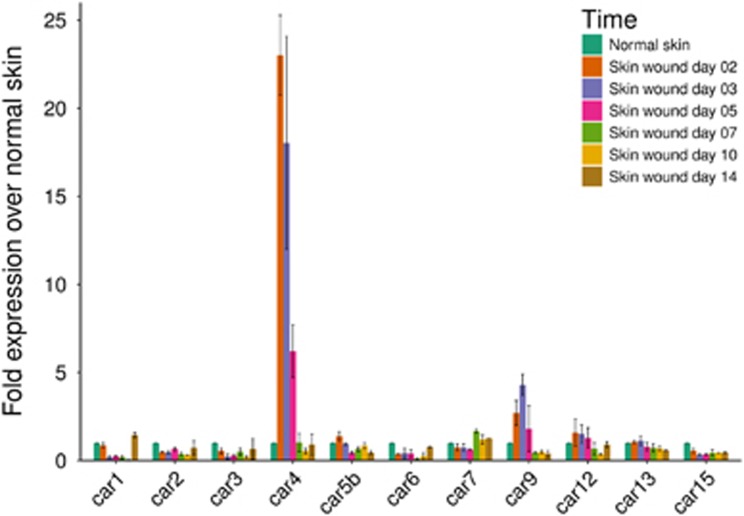
To investigate the role of CAs in the skin wound healing process, we determined the expression pattern of 11 active members of the CA family (CA I, II, III, IV, Vb, VI, VII, IX, XII, XIII and XV) by qPCR analysis of wounds at different stages of healing, and compared their expression to the levels observed in normal, unwounded skin. Hypoxia persisted in our excision wound model for 5 days after wounding, after which extensive angiogenesis vascularized the wound bed.9 Among the CA family members, the expression of CA IV mRNA was induced most strongly at almost 25-fold above normal 2 days after the wounding and remained elevated more than 5-fold at 5 days after wounding (Figure 1). CA IX was the only other enzyme that showed elevated expression during wound healing; its expression peaked at 3 days to a level more than fourfold that of normal skin and remained elevated after day 5 of healing (Figure 1). After the skin wound was vascularized (from day 5 on), none of the CAs showed any enhanced expression at the mRNA level (Figure 1).
Figure 1.
mRNA expression of CA4 and CA9 during wound healing. Skin excision wounds were generated in WT mice as described in the Methods section. Normal skin and skin wound samples were collected from unwounded mice and from mice killed at various time points after wounding. The skin samples were processed for qPCR analysis as described in the Methods. The results for all enzymatically active CAs are shown as the mean±s.d. Animal numbers: unwounded: n=2; day 2: n=2; day 3: n=2; day 5: n=3; day 7: n=2; day 10: n=2; day 14: n=2.
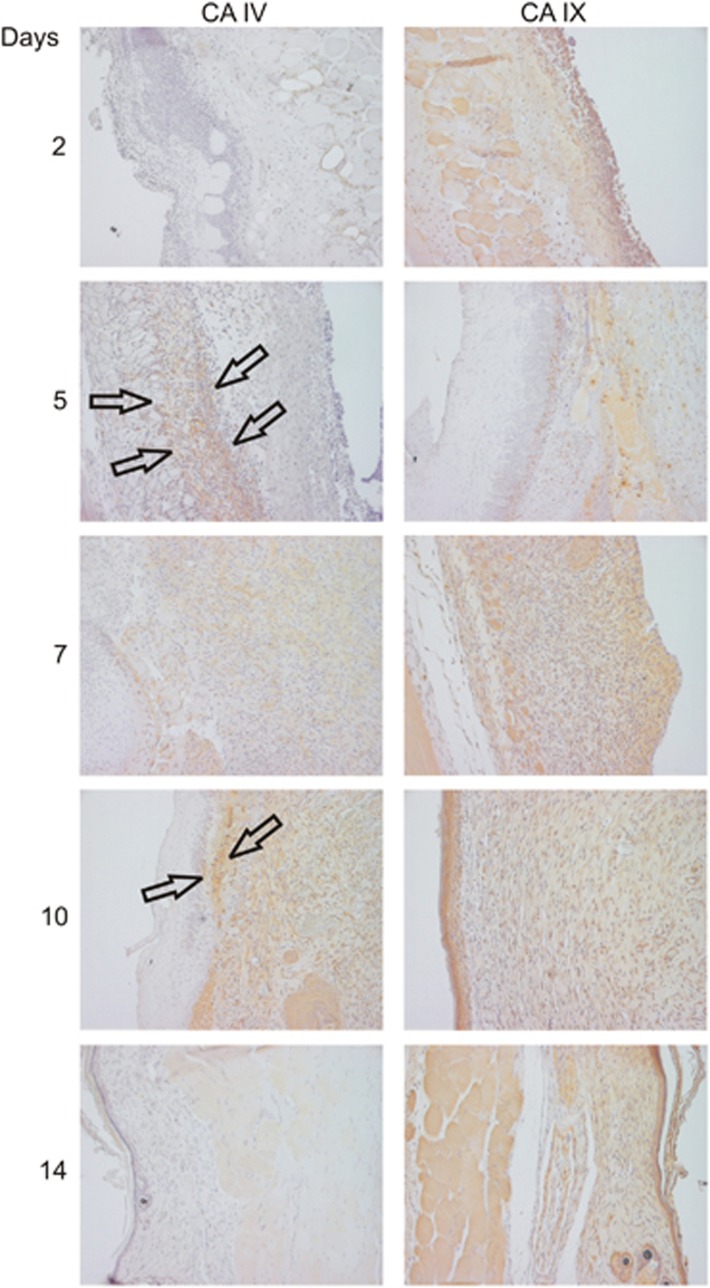
As CAs IV and IX showed increased mRNA expression during the hypoxic phase of wound healing, we decided to explore their expression in detail by immunohistochemistry. CA IV protein expression started to accumulate in early granulation tissue at day 5 after healing and remained positive in the wound until day 10, after which the expression disappeared (Figure 2). CA IV protein was expressed mainly by cells in granulation tissue with especially strong, band-like, expression just beneath the migrating epidermis (Figure 2). CA IX protein expression, in turn, was already observed in the skin wound at day 2 and remained elevated throughout the wound healing process, that is, after CA IV had disappeared from the wound tissue (Figure 2). Both the epidermis and underlying granulation tissue expressed CA IX, but the strongest CA IX protein expression was also observed in the migrating epidermis and just beneath the migrating epidermis in the top layer of the dermis (Figure 2).
Figure 2.
Protein expression of Car4 and Car9 during wound healing. Skin excision wounds were generated in WT mice as described in the Methods section. Normal skin and skin wound samples were collected from unwounded mice and from mice killed at various time points after wounding. The skin samples were processed for IHC analysis, and CA IV and CA IX were detected using specific antibodies as described in the Supplementary Methods. The results obtained at all studied time points are shown. CA IV protein expression started to accumulate in the early granulation tissue at day 5 of healing, and positive expression remained in the wound until day 10, after which the expression disappeared. CA IV protein was expressed mainly by the cells in granulation tissue, with especially strong expression just beneath the migrating epidermis (arrows). CA IX protein expression, in turn, was already detected in the skin wound at day 2 and remained elevated throughout the wound healing process. Animal numbers: all time points: n=6 mice with two wounds; total n=12.
CA transcripts in wound-related cells
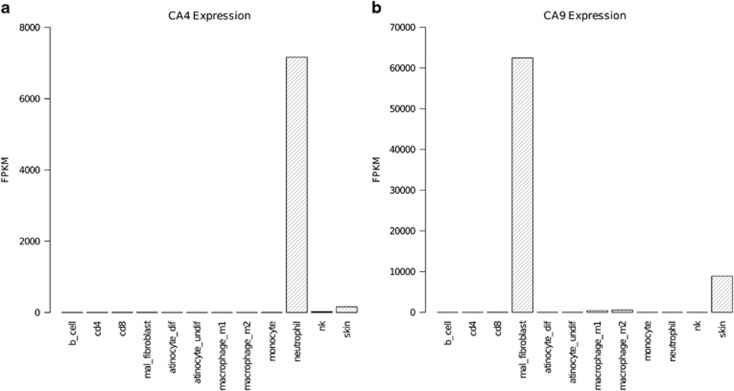
To elucidate the cell sources that produce CAs during wound healing, a thorough analysis of human RNA-Seq data was performed. RNA-Seq analysis of 12 cell types and tissues revealed that a CA4 transcript was significantly expressed only in skin and neutrophils (Figure 3). In both tissues, only the 312-amino-acid ENST00000300900 CA4 transcript was expressed. In the skin, 12 CA genes were expressed (Supplementary Table 2). Of these, CA4 had one of the lowest levels of expression, with a normalized FPKM value of 160.18. In neutrophils, the CA4 expression level was significantly higher than that in skin, with a FPKM of 7162.48. CA9 was significantly expressed in fibroblasts, skin, and macrophages (Figure 3), with significantly higher expression in fibroblasts compared with the other tissues.
Figure 3.
Expression of CA4 and CA9 in wound-related cells. RNA-Seq data for multiple immune and wound-healing-related cells was retrieved from experiments performed using the ArrayExpress database and mapped to the genomic locations of all CA genes using Tophat. The reads were then merged using Cuffmerge, and differential expression was determined using Cuffdiff. Finally, the results were manipulated using the R package cummeRbund and visualized in R. Expression abundance measurements are represented on the y axis as normalized fragments per kilobase of transcript per million mapped reads (FPKM) for CA4 (a) and CA9 (b).
Characterization of the CA4 promoter
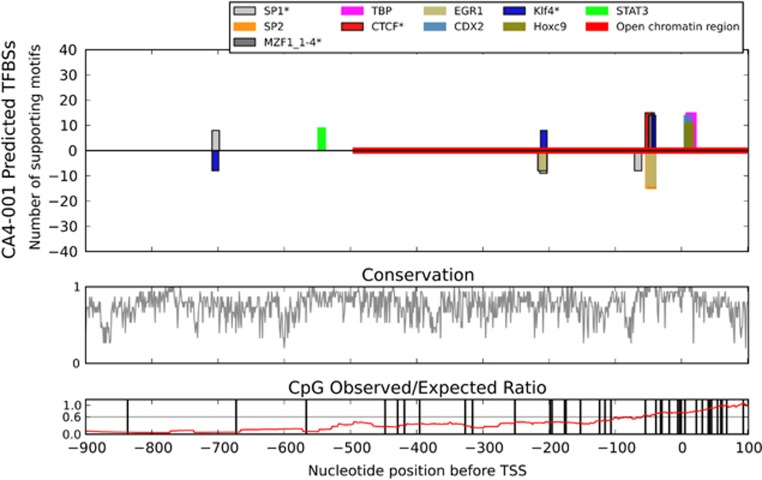
The unexpected and high-level induction of CA4 mRNA in the skin wound prompted us to characterize the CA4 promoter in detail to provide clues regarding the potential regulation of its transcription. Our comparative genomics analysis of the aligned CA4 promoter regions of 15 mammal species revealed a distinct cluster of high scoring and well-conserved potential transcription factor-binding sites immediately upstream of a similarly predicted TATA-binding protein-binding site (Figure 4). All 15 species presented a strong signal for a CTCF-binding site located from −55 to −44 bp upstream of the TSS (Figure 4). In 13 of the species, including humans, this region was an exact match for previously experimentally determined CTCF-binding sites. Overlapping the probable CTCF site were predicted binding sites for SP1, KLF4 and MZF1. The SP1 and MZF1 sites matched previously experimentally determined sites in all 15 species, while KLF4 had a match in 14 species. The binding locations were −55 to −44 on the sense-strand (CTCF), −50 to −39 on the anti-sense strand (SP1), −47 to −41 on the sense-strand (MZF1), and −49 to −39 on the sense-strand (KLF4) (Figure 4). Importantly, our results did not reveal any HIF-1α-binding sites in the CA4 promoter.
Figure 4.
Comparative genomics analysis of the CA4 promoter. An alignment of 15 mammalian sequences corresponding to the promoter of the full-length human CA4 transcript ENST00000300900 was analyzed for putative transcription factor-binding sites by comparative genomics. The 10 best scoring transcription factors were included in the figure, where height indicates the number of species supporting that prediction. A positive y axis result indicates a TFBS predicted on the sense strand, while the negative y axis indicates the anti-sense strand.
GO analysis of biological processes associated with CA4 in skin
Next, we wanted to understand the potential function of CA4 in skin wound healing and thus performed GO enrichment analysis of biological processes associated with CA4 expression. GO enrichment analysis of genes with a strong expression correlation (⩾0.50) with CA4 in skin resulted in 40 terms that were represented two-fold or higher in our set versus the expected levels (Supplementary Table 3). The terms with the highest over-representation in our set were strongly related to immune cell recruitment. In addition, terms related to angiogenesis, endocytosis, inflammation and ion homeostasis were also over-represented.
Expression of Car4 is not induced by hypoxia in the pure hypoxia-driven angiogenesis model
To explore whether hypoxia induced the expression of Car4 (rodent gene for CA IV protein) during wound healing, we next employed a pure hypoxia-driven angiogenesis of oxygen-induced retinopathy model. We could not detect any induction of Car4 mRNA either by hypoxia at P12 or by revascularization at P17, thus eliminating the possibility that CA4 is a hypoxia inducible gene (Supplementary Figure 1).
Expression and characterization of recombinant CA IV
To further explore the function of CA IV in skin wound healing, we produced and purified recombinant CA IV enzyme. CA IV is especially suitable for therapeutic applications because it is an extracellular enzyme and has one of the highest enzymatic activity levels among the CAs. In SDS gel electrophoresis, a major band was identified at 30 kDa that represented full-length recombinant CA IV (Supplementary Figure 2). The affinity-purified CA IV enzyme had a specific activity of 3000–4000 units per mg of pure enzyme.
Wound treatment with CA IV and acetazolamide
Next, we examined the effects of recombinant CA IV and endogenously expressed CAs on wound healing, focusing on the re-epithelialization obtained by keratinocyte migration during the hypoxic phase of healing. Rodents wound closure can occur via two different means: wound contraction or true re-epithelialization.12 To alleviate wound contraction and to explore re-epithelialization specifically, a special skin excision wound model was employed, in which a round silicone splint is sutured into the skin to firmly attach the underlying dermis and subcutis.12
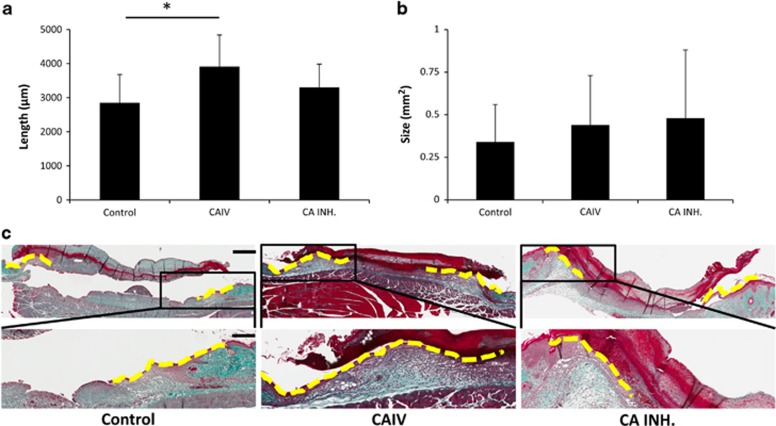
The wounded animals were divided into three groups that received topically applied saline (control), recombinant CA IV enzyme or CA inhibitor (azetazolamide) in Pluronic-127 gels.13 There were no differences in the wound size immediately after wounding or in the size of the scab covering the wound at the end of the treatment trial (Supplementary Figure 3). The recombinant CA IV enhanced the wound re-epithelialization (Figure 5). The epithelial tongues were significantly longer in the recombinant CA IV-treated wounds than in the control wounds (P<0.0001) (Figure 5). We could not detect any differences in the amount of granulation tissue produced in the wounds among the three treatment groups (Figure 5).
Figure 5.
Accelerated re-epithelialization during wound healing in mice treated with recombinant Car4 enzyme. Mice with full-thickness skin excision wounds were treated with either recombinant Car4 enzyme or CA inhibitor applied topically to the wound immediately after wounding. Scars were harvested on day 5, and the re-epithelialization (a) and cross-sectional area of the granulation tissue (b) of the wounds were quantified by examining two microscopic sections from each wound. The results are expressed as the average of the two values. There were five animals, each with two wounds, in every treatment group. *P<0.05; analysis of variance. The results are expressed as the mean±s.d., n=10 wounds. (c) Representative sections from wounds treated with recombinant CAIV or CA inhibitor and collected on day 5 after wounding are shown for re-epithelialization. Scale bars, 600 μm low magnification, 240 μm high magnification.
Discussion
Skin wound closure is achieved by re-epithelialization, that is, the lateral migration of keratinocytes. This process occurs mainly under hypoxia, but its stimuli are poorly understood. The present study shows that mRNA expression of two members of the CA family, CA IV and IX, are increased during the early phase of wound healing, and the cells expressing the CAs form a band-like structure just underneath the migrating epidermis in the healing skin wound. Furthermore, we demonstrate that exogenous application of recombinant Car4 enzyme accelerates wound re-epithelialization during the hypoxic phase of wound healing.
In hypoxic tissues, such as fast-growing cancers and wounds, metabolic processes produce large amounts of both lactic acid and carbon dioxide. CAs are enzymes that convert carbon dioxide and water to bicarbonate and protons.3, 4, 5 Because the intracellular pH must remain stable, all excess acidity is efficiently exported from the cells, which in turn acidifies the extracellular environment to provide a favorable environment for cancer cell migration and invasion.25 CAs have been linked to this process in reports showing a CA inhibitor-induced reduction of cancer cell invasion in contrast to a facilitation of cancer cell migration and invasion by CA expression.25 CA IX, in turn, is unique in the sense that it can stimulate cell migration independent of extracellular acidification.26, 27 Namely, it can enhance cell migration by weakening cell adhesions26, 27 as well as by stimulating Rho-GTPase dependent cell motility.26 In our study, CA IX expression persisted in the migrating epidermis throughout the healing process, whereas the expression of CA IV disappeared substantially earlier. The strong expression of CA IX observed in the migrating epidermis long beyond the hypoxic phase could be related to its capability to stimulate cell migration independently of acidification of the hypoxic environment, and may be stimulated by hepatocyte growth factor under normoxic conditions.27
Of the two CAs that showed enhanced expression during wound healing, CA IX is known to be induced by hypoxia. It has a hypoxia-response element in its promotor region and is one of the known target genes for hypoxia inducible factor-1α (HIF-1α).3, 4 Strikingly, the expression of CA IV was substantially higher than CA IX at the mRNA level during hypoxia in the skin wound. The expression of CA IV is usually reduced in tumors,28 and the present results show that it does not possess a classical hypoxia-response element in its promoter region for HIF-1α binding. We further employed a pure hypoxia-driven angiogenesis model in the retina and could not detect any changes in the expression of Car4 by varying the oxygen levels. Our results, in essence, eliminate the possibility that hypoxia is responsible for the induction of Car4 expression.
Comparative genomics prediction of potential transcription factor-binding sites in the promoter of the primary human CA4 transcript ENST00000300900 revealed some high-scoring candidates that may alter the expression of this protein. In particular, there is a cluster of well-conserved binding sites for KLF4, SP1 and MZF1, all of which were positioned near a similarly conserved CTCF-binding site. CTCF is a well-established contributor to the chromatin configuration and gene transcription across the genome,29 and thus its presence and proximity provide greater weight to the other proximal predictions. Furthermore, CTCF also promotes wound healing.30 MZF1 is involved in myeloid cell differentiation31 and is a strong enhancer of cell migration and invasion,32 thus potentially explaining the origin of the strong CA4 expression in neutrophils. KLF4, in turn, has been shown to be expressed during inflammation33 and wound healing34, 35, 36, 37, and to facilitate cutaneous wound healing.36
Our RNA-Seq analysis revealed low levels of CA4 RNA in normal skin and high levels in neutrophils. This finding implies that the observed elevation of CA4 mRNA and protein in our experiments may be due to the recruitment of neutrophils to the wound site, which is consistent with the finding that abundant neutrophil extravasation occurs in skin wounds rapidly after wounding.
The few neutrophils that persist in the wound are known to form a band-like structure immediately beneath the migrating epidermis,38 a pattern that is strikingly similar to the expression of CA IV. Despite long being considered detrimental for tissue regeneration in general, especially during the early phase of healing, neutrophils are currently considered crucial for skin wound healing and tissue regeneration.39 Specifically, neutrophils that survive in the injured tissue for a long period of time are crucial for tissue regeneration.40, 41 Interestingly, neutrophils require an acidic extracellular environment to be properly activated and to prolong their functional life span, that is, avoid apoptosis.42, 43 Furthermore, excessive, early neutrophil apoptosis (NETosis) has, in turn, been shown to retard diabetic wound healing in both mice and humans,40 while proper wound healing can be rescued in diabetes by the inhibition of early NETosis, that is, keeping neutrophils alive and prolonging their lifespan.40 This phenomenon could specifically explain why neutrophils express an extracellular member of the CAs, CA IV, which is crucial for extracellular acidification.5 Especially strong production of CA IV and IX just beneath the migrating epidermis suggests that these enzymes could generate an acidic ‘micro-environment' within healing wound tissue to selectively aid not just neutrophil survival and function but also keratinocyte migration, that is, closure of the wound.
Concerning CA IX expression in the skin wound, RNA-Seq analysis confirmed the expression of CA9 mRNA only in fibroblasts and skin, with significantly higher expression in fibroblasts. The CA IX protein has been shown to be upregulated in tumor fibroblasts undergoing hypoxia,44, 45 while fibroblast-derived HIF-1α has been shown to be crucial for wound healing.46
Based on the expression pattern of CA family members, we conclude that CAs IV and IX are responsible for maintaining the pH balance during skin wound healing. We were able to observe the induction of CA IV and CA IX mRNAs during the early hypoxic phases of wound healing, and both mRNAs returned to the same level as the normal skin in 7 days. This finding implies that there is a rapid induction of these proteins in response to hypoxia (HIF1α-mediated for CA IX) and inflammation (CA IV).
To understand the function of CAs in tissue regeneration in general, we treated skin wounds with recombinant human CA IV enzyme and with a clinically used CA inhibitor to block the endogenous CA activity. The CA IV enzyme is especially suitable for pharmaceutical studies because it is an extracellular enzyme with one of the highest enzymatic activity levels among the CAs.5, 47 We could detect accelerated wound re-epithelialization with recombinant CA IV, but blocking the activity of endogenously expressed CA had no significant effect on wound re-epithelialization. This discrepancy is most probably explained by the fact that endogenous CA accumulation in the skin wound starts at least a few days after the wounding. Thus, the CA inhibitor was unlikely to encounter any enhanced CA protein expression during the majority of our study period (days 0–5), whereas the addition of exogenous recombinant CA enzyme provided a clear benefit over endogenous enzyme because it was present in the wound immediately after wounding. This result is highly relevant for CA4 because our data indicate that its primary source in the wound bed is neutrophils, which begin to extravasate to the wound within minutes after injury and are crucial for wound healing but also require extracellular acidification to prolong their survival, which in turn is crucial for the proper progression of wound healing. Furthermore, closure of the wound by keratinocyte migration starts immediately after wounding. Immediate acidification of the extracellular milieu by exogenous CA4 should provide a boost for keratinocyte migration during the early stages of healing.
Future studies are warranted to address whether CAs can be used to stimulate tissue regeneration during injury-induced hypoxia. CA IV may participate in promoting tissue regeneration by generating an acidic ‘micro-environment' for neutrophils to survive and for the migrating epidermis to close the wound during the hypoxic phase of wound healing.
Acknowledgments
We thank Marianne Karlsberg and Marja-Leena Koskinen for practical support and for performing the histochemical work and immunohistological staining and Mrs Guillermina Garcia (Sanford Burnham Prebys Medical Discovery Institute, La Jolla, CA, USA) is thanked for her technical expertise and help with quantitative microscopy. This work was funded by the Sigrid Juselius Foundation, the Academy of Finland, Päivikki and Sakari Sohlberg Foundation, Instrumentarium Research Foundation, Finnish Medical Foundation, Pirkanmaa Hospital District Research Foundation and the Finnish Cultural Foundation. We also thank CSC – IT Center for Science of the Finnish Ministry of Education and Culture for providing the high-performance computing resources needed to perform the computational analyses.
Author contributions
TJ, MA, HU-J and SPar designed the research. SPas raised the CA IX antibody. AW and WSS produced the recombinant CA IV enzyme and raised the CA IV antibody. HB performed the comparative genomics, RNA-Seq and GO enrichment computational analyses. MA, HB, PK, PP, MV, UM and SPar performed the research. MA, HB, PP, SPar and TJ analyzed the data. MA, HB, SPar and TJ wrote the manuscript. MA, HB, PP, SPar and TJ generated the figures. All authors reviewed and accepted the text of the manuscript.
Footnotes
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website (http://www.nature.com/emm)
The authors declare no conflict of interest.
Supplementary Material
References
- Woodley DT, Wysong A, DeClerck B, Chen M, Li W. Keratinocyte migration and a hypothetical new role for extracellular heat shock protein 90 alpha in orchestrating skin wound healing. Adv Wound Care 2015; 4: 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol 2015; 173: 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benej M, Pastorekova S, Pastorek J. Carbonic anhydrase IX: regulation and role in cancer. Subcell Biochem 2014; 75: 199–219. [DOI] [PubMed] [Google Scholar]
- Pastorekova S, Parkkila S, Parkkila AK, Opavsky R, Zelnik V, Saarnio J et al. Carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology 1997; 112: 398–408. [DOI] [PubMed] [Google Scholar]
- Waheed A, Sly WS. Membrane associated carbonic anhydrase IV (CA IV): a personal and historical perspective. Subcell Biochem 2014; 75: 157–179. [DOI] [PubMed] [Google Scholar]
- Hilvo M, Innocenti A, Monti SM, De Simone G, Supuran CT, Parkkila S. Recent advances in research on the most novel carbonic anhydrases, CA XIII and XV. Curr Pharm Des 2008; 14: 672–678. [DOI] [PubMed] [Google Scholar]
- Aspatwar A, Tolvanen ME, Parkkila S. An update on carbonic anhydrase-related proteins VIII, X and XI. J Enzyme Inhib Med Chem 2013; 28: 1129–1142. [DOI] [PubMed] [Google Scholar]
- Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 2001; 158: 905–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen TA, Ruoslahti E. Molecular changes in the vasculature of injured tissues. Am J Pathol 2007; 171: 702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Waheed A, Pham T, Won M, Okuyama T, Sly WS. Human carbonic anhydrase IV: in vitro activation and purification of disulfide-bonded enzyme following expression in Escherichia coli. Protein Expr Purif 1997; 9: 279–287. [DOI] [PubMed] [Google Scholar]
- Davidson JM, Yu F, Opalenik SR. Splinting strategies to overcome confounding wound contraction in experimental animal models. Adv Wound Care 2013; 2: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc-Brude OP, Yu J, Simosa H, Conte MS, Sessa WC, Altieri DC. Inhibitor of apoptosis protein survivin regulates vascular injury. Nat Med 2002; 8: 987–994. [DOI] [PubMed] [Google Scholar]
- Linsley PS, Speake C, Whalen E, Chaussabel D. Copy number loss of the interferon gene cluster in melanomas is linked to reduced T cell infiltrate and poor patient prognosis. PLoS ONE 2014; 9: e109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M, Dry IR, Frampton D, Singh M, Kanda RK, Yee MB et al. RNA-seq analysis of host and viral gene expression highlights interaction between varicella zoster virus and keratinocyte differentiation. PLoS Pathog 2014; 10: e1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A et al. Proteomics. Tissue-based map of the human proteome. Science 2015; 347: 1260419. [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 2009; 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009; 25: 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 2010; 28: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May U, Prince S, Vähätupa M, Laitinen AM, Nieminen K, Uusitalo-Järvinen H et al. Resistance of R-Ras knockout mice to skin tumour induction. Sci Rep 2015; 5: 11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo-Jarvinen H, Kurokawa T, Mueller BM, Andrade-Gordon P, Friedlander M, Ruf W. Role of protease activated receptor 1 and 2 signaling in hypoxia-induced angiogenesis. Arterioscler Thromb Vasc Biol 2007; 27: 1456–1462. [DOI] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc 2013; 8: 1551–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vähätupa M, Prince S, Vataja S, Mertimo T, Kataja M, Kinnunen K et al. Lack of R-Ras leads to increased vascular permeability in ischemic retinopathy. Invest Ophthalmol Vis Sci 2016; 57: 4898–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen TA, Ruoslahti E. Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proc Natl Acad Sci USA 2010; 107: 21671–21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorekova S, Parkkila S, Zavada J. Tumor-associated carbonic anhydrases and their clinical significance. Adv Clin Chem 2006; 42: 167–216. [PubMed] [Google Scholar]
- Shin HJ, Rho SB, Jung DC, Han IO, Oh ES, Kim JY. Carbonic anhydrase IX (CA9) modulates tumor-associated cell migration and invasion. J Cell Sci 2011; 124: 1077–1087. [DOI] [PubMed] [Google Scholar]
- Svastova E, Witarski W, Csaderova L, Kosik I, Skvarkova L, Hulikova A et al. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J Biol Chem 2012; 287: 3392–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemela AM, Hynninen P, Mecklin JP, Kuopio T, Kokko A, Aaltonen L et al. Carbonic anhydrase IX is highly expressed in hereditary nonpolyposis colorectal cancer. Cancer Epidemiol Biomarkers Prev 2007; 16: 1760–1766. [DOI] [PubMed] [Google Scholar]
- Vietri Rudan M, Hadjur S. Genetic tailors: CTCF and cohesin shape the genome during evolution. Trends Genet 2015; 31: 651–660. [DOI] [PubMed] [Google Scholar]
- Wang L, Wu X, Shi T, Lu L. Epidermal growth factor (EGF)-induced corneal epithelial wound healing through nuclear factor kappaB subtype-regulated CCCTC binding factor (CTCF) activation. J Biol Chem 2013; 288: 24363–24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui P, Guo X, Bradford PG. Isolation and functional characterization of the human gene encoding the myeloid zinc finger protein MZF-1. Biochemistry 1995; 34: 16493–16502. [DOI] [PubMed] [Google Scholar]
- Eguchi T, Prince T, Wegiel B, Calderwood SK. Role and regulation of myeloid zinc finger protein 1 in cancer. J Cell Biochem 2015; 116: 2146–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla LM, Latorre V, Carceller E, Boix J, Vodak D, Mills IG et al. Glucocorticoid receptor and Klf4 co-regulate anti-inflammatory genes in keratinocytes. Mol Cell Endocrinol 2015; 412: 281–289. [DOI] [PubMed] [Google Scholar]
- Kaushik DK, Gupta M, Das S, Basu A. Kruppel-like factor 4, a novel transcription factor regulates microglial activation and subsequent neuroinflammation. J Neuroinflammation 2010; 7: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang C, Liu Z, Zhang J, Xiang Z, Sun T. Honokiol downregulates Kruppel-like factor 4 expression, attenuates inflammation, and reduces histopathology after spinal cord injury in rats. Spine 2015; 40: 363–368. [DOI] [PubMed] [Google Scholar]
- Ou L, Shi Y, Dong W, Liu C, Schmidt TJ, Nagarkatti P et al. Kruppel-like factor KLF4 facilitates cutaneous wound healing by promoting fibrocyte generation from myeloid-derived suppressor cells. J Invest Dermatol 2015; 135: 1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zheng H, Wang J, Yu F, Morris RJ, Wang TC et al. Expression of Kruppel-like factor KLF4 in mouse hair follicle stem cells contributes to cutaneous wound healing. PLoS ONE 2012; 7: e39663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grguric-Smith LM, Lee HH, Gandhi JA, Brennan MB, DeLeon-Rodriguez CM, Coelho C et al. Neutropenia exacerbates infection by Acinetobacter baumannii clinical isolates in a murine wound model. Front Microbiol 2015; 6: 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos CA. Neutrophil-epithelial interactions: a double-edged sword. Am J Pathol 2016; 186: 1404–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini GP, Menegazzo L, Rigato M, Scattolini V, Poncina N, Bruttocao A et al. NETosis delays diabetic wound healing in mice and humans. Diabetes 2016; 65: 1061–1071. [DOI] [PubMed] [Google Scholar]
- Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J 2017; 38: 187–197. [DOI] [PubMed] [Google Scholar]
- Martinez D, Vermeulen M, Trevani A, Ceballos A, Sabatte J, Gamberale R et al. Extracellular acidosis induces neutrophil activation by a mechanism dependent on activation of phosphatidylinositol 3-kinase/Akt and ERK pathways. J Immunol 2006; 176: 1163–1171. [DOI] [PubMed] [Google Scholar]
- Trevani AS, Andonegui G, Giordano M, Lopez DH, Gamberale R, Minucci F et al. Extracellular acidification induces human neutrophil activation. J Immunol 1999; 162: 4849–4857. [PubMed] [Google Scholar]
- Santi A, Caselli A, Paoli P, Corti D, Camici G, Pieraccini G et al. The effects of CA IX catalysis products within tumor microenvironment. Cell Commun Signal 2013; 11: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii G, Sangai T, Ito T, Hasebe T, Endoh Y, Sasaki H et al. In vivo and in vitro characterization of human fibroblasts recruited selectively into human cancer stroma. Int J Cancer 2005; 117: 212–220. [DOI] [PubMed] [Google Scholar]
- Duscher D, Maan ZN, Whittam AJ, Sorkin M, Hu MS, Walmsley GG et al. Fibroblast-specific deletion of hypoxia inducible factor-1 critically impairs murine cutaneous neovascularization and wound healing. Plast Reconstr Surg 2015; 136: 1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandernoth PM, Mannowetz N, Szczyrba J, Grannemann L, Wolf A, Becker HM et al. Normal fertility requires the expression of carbonic anhydrases II and IV in sperm. J Biol Chem 2015; 290: 29202–29216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.