We demonstrate that spiking can induce global increases in the intracellular calcium concentration ([Ca2+]i) that decay with a relatively long time constant. Consequently, summation of the calcium signal occurs even at low firing frequencies. As a result there is significant, persistent potentiation of synaptic transmission.
Keywords: invertebrate, Aplysia, transmitter release, calcium, synaptic plasticity
Abstract
In a type of short-term plasticity that is observed in a number of systems, synaptic transmission is potentiated by depolarizing changes in the membrane potential of the presynaptic neuron before spike initiation. This digital-analog form of plasticity is graded. The more depolarized the neuron, the greater the increase in the efficacy of synaptic transmission. In a number of systems, including the system presently under investigation, this type of modulation is calcium dependent, and its graded nature is presumably a consequence of a direct relationship between the intracellular calcium concentration ([Ca2+]i) and the effect on synaptic transmission. It is therefore of interest to identify factors that determine the magnitude of this type of calcium signal. We studied a synapse in Aplysia and demonstrate that there can be a contribution from currents activated during spiking. When neurons spike, there are localized increases in [Ca2+]i that directly trigger neurotransmitter release. Additionally, spiking can lead to global increases in [Ca2+]i that are reminiscent of those induced by subthreshold depolarization. We demonstrate that these spike-induced increases in [Ca2+]i result from the activation of a current not activated by subthreshold depolarization. Importantly, they decay with a relatively slow time constant. Consequently, with repeated spiking, even at a low frequency, they readily summate to become larger than increases in [Ca2+]i induced by subthreshold depolarization alone. When this occurs, global increases in [Ca2+]i induced by spiking play the predominant role in determining the efficacy of synaptic transmission.
NEW & NOTEWORTHY We demonstrate that spiking can induce global increases in the intracellular calcium concentration ([Ca2+]i) that decay with a relatively long time constant. Consequently, summation of the calcium signal occurs even at low firing frequencies. As a result there is significant, persistent potentiation of synaptic transmission.
in a form of short-term synaptic plasticity found in a number of systems, synaptic transmission between two neurons is potentiated by depolarizing changes in the membrane potential of the presynaptic neuron before spike initiation (Fig. 1) (e.g., Alle and Geiger 2006; Ivanov and Calabrese 2003; Nicholls and Wallace 1978; Shapiro et al. 1980; Shimahara and Peretz 1978; Shu et al. 2006). Importantly, this form of plasticity is graded; i.e., the more depolarized the neuron, the greater the potentiation observed. Induction of this plasticity therefore changes the nature of spike-mediated synaptic transmission, making it analog rather than digital. Consequently, it has been referred to as “analog modulation” (e.g., Alle and Geiger 2008; Clark and Häusser 2006; Debanne et al. 2013; Marder 2006).
Fig. 1.
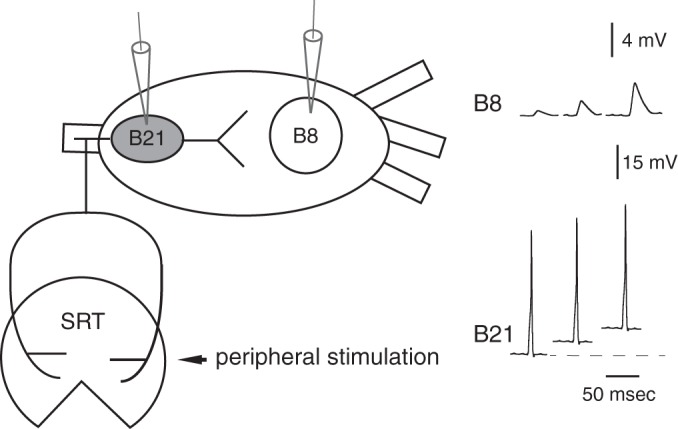
Schematic diagram of neuron B21 and its postsynaptic follower, neuron B8. B21 is a radula mechanoafferent that is peripherally activated when a mechanical stimulus is applied to the tissue it innervates, the subradula tissue (SRT). Subthreshold depolarization of the somatic region of B21 (gray) potentiates sensorimotor transmission. As shown in the schematic at right, this is a graded phenomenon. Since this is a schematic, scale bars give approximate values.
In a number of systems, analog modulation is calcium dependent (Awatramani et al. 2005; Bouhours et al. 2011; Christie et al. 2011; Fekete et al. 2014; Ivanov and Calabrese 2003; Ludwar et al. 2009; Shu et al. 2006). Subthreshold depolarization activates calcium currents that are distinct from those that directly trigger transmitter release (Bouhours et al. 2011; Fekete et al. 2014; Ivanov and Calabrese 2003; Yu et al. 2010). Resulting increases in the intracellular calcium concentration ([Ca2+]i) are global (compared with highly localized calcium hotspots; Bouhours et al. 2011; Ivanov and Calabrese 2003; Ludwar et al. 2009) and act via mechanisms that are not completely understood to modify synaptic transmission. We study a preparation in which this form of plasticity is observed (Ludwar et al. 2009, 2012). It consists of two circuit elements activated when the mollusk Aplysia feeds: a presynaptic sensory neuron (the cell B21) and a follower motor neuron (B8) (Fig. 1).
The graded nature of this calcium-dependent form of analog modulation is often presumed to be the consequence of a direct relationship between an increase in [Ca2+]i and the potentiation of synaptic transmission (e.g., Awatramani et al. 2005; Ludwar et al. 2012). It is therefore of interest to fully characterize factors that determine the magnitude of this type of calcium signal. A possibility that we explore in this study is that it is not solely determined by currents induced by subthreshold depolarization. More specifically, we ask whether currents activated during spiking make an additional contribution. When neurons spike, there are localized increases in the intracellular calcium concentration that trigger neurotransmitter release (Calocal) (e.g., Fogelson and Zucker 1985; Simon and Llinás 1985). Although relatively high (i.e., micromolar) concentrations are only observed briefly, smaller amounts of Calocal can remain in the neuron after it has spiked (Cares) (Katz and Miledi 1968). The role of this Cares in modifying subsequent synaptic transmission has been extensively studied (e.g., Delaney and Tank 1994; Regehr et al. 1994; Swandulla et al. 1991).
Additionally, previous investigators working in a number of systems have noted that spiking can lead to more diffuse increases in [Ca2+]i that are reminiscent of those observed after subthreshold depolarization (e.g., Andjelic and Torre 2005; Callewaert et al. 1996). For example, this has been noted in preparations in which calcium imaging is used as a monitor of neural activity (e.g., Wallace et al. 2008). An important question that we address in this study is whether this type of relatively global spike-induced increase in [Ca2+]i can impact synaptic transmission.
MATERIALS AND METHODS
Preparation.
Experiments were conducted on Aplysia californica (100–400 g) obtained from Marinus Scientific (Long Beach, CA) and held in tanks at 14–16°C for up to 2 wk. Aplysia are hermaphrodites and are therefore both male and female. Animals were anesthetized by injection of an isotonic MgCl2 solution. The amount injected was ~50% of body weight. Buccal ganglia were removed and desheathed as has been described (Ludwar et al. 2012). During experiments ganglia were continuously superfused with artificial seawater (ASW) cooled to 14–16°C.
Reagents.
Most experiments were conducted in ASW with the following composition (in mM): 460 NaCl, 10 KCl, 55 MgCl2, 11 CaCl2, and 10 HEPES buffer, pH 7.6. However, experiments involving the application of cyclopiazonic acid (CPA) or carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) were conducted in a high-divalent saline (HiDi) with a 2× magnesium concentration and a 1.25× calcium concentration.
Stock solutions of nifedipine (Sigma-Aldrich, St. Louis, MO), CPA (Sigma-Aldrich), FCCP (Sigma-Aldrich), and A23187 (Sigma-Aldrich or Fisher Scientific, Hampton, NH) were prepared with DMSO. For all chemicals except A23187, the final DMSO concentration was <1%; for A231871, it was 1.7%. EGTA (Sigma-Aldrich) was intracellularly injected into the somatic region of B21 via a pipette filled with a 250 mM solution.
Imaging.
For most calcium imaging experiments B21 was injected with Calcium Orange (Thermo Fisher Scientific, Waltham, MA) or the low-affinity dye Calcium Green 5N (Thermo Fisher Scientific). Calcium fluorescence was measured using methods that have been described previously in detail (Ludwar et al. 2009, 2012). Briefly, we utilized a Nikon FN-1 fixed-stage microscope with a ×16/NA0.8 CFI75 or ×10/0.30w Plan Fluor water-immersion lens and ET-Cy3 or ET-EYFP filter cubes (Chroma Technology, Bellows Falls, VT). Illumination was provided by an X-Cite 120 PC metal halide lamp, and images were acquired with a CoolSNAP HQ2 charge-coupled device camera (Photometrics, Tucson, AZ) and Nikon NIS Elements AR software (version 3.10), typically at 30 images/s. All fluorescence signals were background subtracted and are given as percent relative change [(F − F0)/F0] (Ludwar et al. 2012). One experiment used the ratiometric dye Fura-2 (Sigma), which was detected with an ET-ECFP/EYFP filter block (Chroma Technology) and an additional Ludl motorized filter wheel controlled by MetaFluor version 7.65 imaging software (Molecular Devices, Sunnyvale, CA).
Electrophysiology.
Electrophysiological recordings were obtained with sharp glass electrodes filled with 3 M K-acetate and 30 mM KCl (typical impedance 7 MΩ). Signals were amplified with an AxoClamp 2B (Molecular Devices) or an SEC-10LX amplifier (×10 headstage; npi electronic, Tamm, Germany), filtered with a model 410 instrumentation amplifier (Brownlee Precision, San Jose, CA) and digitized with a Power 1401 analog-to-digital converter [Cambridge Electronic Design (CED), Cambridge, UK] and Spike II software (CED; version 7.01–7.08).
In experiments in which we studied synaptic transmission, excitatory postsynaptic currents (EPSCs) and potentials (EPSPs) were normalized. In each experiment, normalization was to the same value (the peak amplitude of the last EPSP or EPSC before the experimental manipulation). Amplitude was expressed as a percentage of this value. “Control” (no manipulation) amplitudes are the average of the three EPSPs or EPSCs that immediately preceded the experimental manipulation.
Statistics.
In some experiments statistical significance was evaluated using an appropriate t-test (for 2 group comparisons) or ANOVA (for multiple group analyses) with subsequent comparisons performed using a Bonferroni correction. Data are means ± SE. Data from EGTA experiments were subjected to regression analyses. All statistics were performed using Prism (GraphPad Software, San Diego, CA). Effects were considered significant at P < 0.05.
RESULTS
Single spike-induced increases in calcium fluorescence.
Previous experiments have established that global increases in [Ca2+]i can be produced by subthreshold changes in the somatic membrane potential of B21 (Ludwar et al. 2009, 2012). With spiking, similar alterations in [Ca2+]i have been reported in other preparations (e.g., Andjelic and Torre 2005; Callewaert et al. 1996; Wallace et al. 2008). To determine whether they are also observed at the B21-B8 synapse, we performed experiments in which we took advantage of the fact that B21 is a sensory neuron (Cropper et al. 1996). Consequently, it can be peripherally activated by applying a mechanical stimulus to the tissue it innervates, the subradula tissue (SRT; Fig. 1). In these experiments central depolarization of B21 was necessary to ensure that spikes propagated to the process that contacts B8 (i.e., the lateral process; Evans et al. 2003). However, with our paradigm there was a significant (~1 s) delay between the onset of current injection and peripheral activation (bottom trace in Fig. 2A). Current injection results in changes in [Ca2+]i that are observed relatively quickly, i.e., within milliseconds (Ludwar et al. 2009). Consequently, the delay made it possible to distinguish increases in [Ca2+]i induced by the subthreshold current injection from those induced by spiking.
Fig. 2.
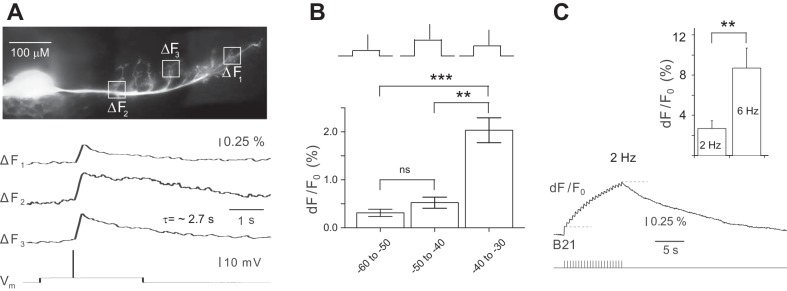
Spike-induced increases in calcium fluorescence in B21. A, top: low-power (×10) image of the soma and lateral process of a B21 injected with Calcium Orange dye. The boxes on the lateral process indicate where the optical measurements shown below were made. Bottom, B21was centrally depolarized via current injection into the soma and a spike was peripherally triggered (Vm trace). This induced an increase in calcium fluorescence in all regions imaged (ΔF1–ΔF3 traces) (n = 12). Note the relatively long time constant (n = 6 for the time constant measurements). B: experiment as in A, except that spikes were induced at different membrane potentials using a randomized design (as shown in the inset). Data were binned and divided into 3 different groups (spikes induced between −60 and −50 mV, −50 and −40 mV, and −40 and −30 mV; 54 measurements from 4 preparations). C, left: summation of spike-induced increases in calcium fluorescence when B21 was stimulated at 2 Hz for 10 s. Action potentials triggered in B21 are shown in the bottom trace. The top trace shows the change in calcium fluorescence imaged in the lateral process of B21. The dashed lines mark the peak amplitude of the change in fluorescence induced by the first spike and the peak amplitude of the summated signal (n = 4). Right, effect of frequency on the peak amplitude of the summated signal. Note that the signal was larger when B21 was stimulated at a higher frequency (n = 6). **P ≤ 0.01; ***P ≤ 0.001.
We imaged the lateral process of B21 and observed a spike-induced increase in calcium fluorescence in all preparations (n = 12; Fig. 2A). Imaged changes in fluorescence were clearly not traditional calcium “hot spots,” because they could be visualized throughout the entire lateral branch of B21 (Fig. 2A). Changes in fluorescence peaked relatively rapidly (i.e., within ~30 ms) but decayed relatively slowly (Fig. 2A). The average time constant was 2.72 ± 0.18 s (n = 6). These data, taken together with previous results (Ludwar et al. 2009, 2012), indicate that global increases in the [Ca2+]i of B21 will occur under at least two conditions: when there is a depolarizing change in membrane potential that is subthreshold for spike initiation, and when spikes are triggered.
The spike-induced increases in [Ca2+]i that we imaged in the experiments shown in Fig. 2A were relatively small. B21 was centrally depolarized in these experiments, but only enough to ensure spike propagation (Fig. 2A). Studies in B21 and other preparations have noted that increases in [Ca2+]i induced by subthreshold depolarization are membrane potential dependent (e.g., Ludwar et al. 2009). To determine whether spike-induced increases in calcium fluorescence would also be larger at more depolarized potentials, we peripherally triggered single spikes and varied the holding potential using a randomized design (as shown in the inset in Fig. 2B). Experiments were conducted in the range in which it is possible to somatically depolarize B21 without triggering a spike centrally, i.e., from −60 mV (normal resting membrane potential) to approximately −30 mV. We obtained 54 measurements of changes in calcium fluorescence (dF/F0) from 4 preparations and grouped them into three ~10-mV bins (e.g., the first bin consisted of measurements made from −60 to −50 mV).
Overall, there was a significant effect of membrane potential [1-way ANOVA, F(2,51) = 12.85, P < 0.0001]. Changes in fluorescence were, on average, 0.31 ± 0.08 when spikes were triggered between −60 and −50 mV, 0.52 ± 0.12 when spikes were triggered between −50 and −40 mV, and 2.03 ± 0.26 when spikes were triggered between −40 and −30 mV (Fig. 2B). However, post hoc analyses conducted with a Bonferroni correction for repeated measures indicated that differences were only significant when comparisons included the data that were obtained in the most depolarized range [−40 to −30 mV; Fig. 2B; comparison for −60 to −50 mV vs. −40 to −30 mV: t(3) = 4.38, P ≤ 0.001; comparison for −50 to −40 mV vs. −40 to −30 mV: t(3) = 3.59, P ≤ 0.01]. When the data obtained between −60 and −50 mV were compared with the data obtained between −50 and −40 mV, there was no significant difference [t(3) = 0.43, P > 0.05]. During motor programs, centrally induced depolarization that have been described in previous work are generally ~15–20 mV, bringing the membrane potential to approximately −45 to −40 mV (Ludwar et al. 2009). This suggests that under physiological conditions, membrane potential will not have a graded effect on the magnitude of the spike-induced change in [Ca2+]i. However, the slow time constant suggested that spike-induced calcium signals would summate even when B21 fires at relatively low frequencies. To determine whether this is the case, we triggered a fixed-duration burst of spikes at 2 Hz (the low end of the range of frequencies that is likely to be physiologically relevant; Borovikov et al. 2000; Evans et al. 2011b). We found that the peak amplitude of the calcium signal induced by the burst was approximately eight times larger than the peak amplitude of the increase in calcium fluorescence induced by a single spike (i.e., the dF/F0 was 0.46 ± 0.28% for the single spike vs. 3.88 ± 1.26% for the burst) [Fig. 2C; paired t-test, t(3) = −3.39, P < 0.05; n = 4]. Thus summation can occur. As might be expected, we found that the magnitude of the summated signal depended on the firing frequency of B21. For example, if B21 was stimulated at 6 Hz, it was larger than if B21 was stimulated at 2 Hz [Fig. 2C; paired t-test, t(5) = −4.95, P ≤ 0.01; n = 6].
To determine how the magnitude of the summated signal compares to the changes in calcium fluorescence induced by subthreshold depolarization, we performed experiments in which changes in calcium fluorescence were measured ratiometrically via the fura-2 calcium indicator. We found that a subthreshold depolarization of 20 mV (which is in the upper range for the depolarization observed during a motor program; Ludwar et al. 2009) produced a 0.4 ± 0.1% increase in the fura-2 signal. In contrast, a burst of action potentials generated with physiologically relevant parameters (i.e., 6 Hz, 3 s; Borovikov et al. 2000; Evans et al. 2011b) produced a summated signal that was significantly larger (i.e., 11.01 ± 2.9%; paired t-test, t(4) = −3.7, P ≤ 0.05; n = 5; Fig. 3, A1 and A2). Thus, although increases in calcium fluorescence triggered by a single action potential are comparable in magnitude to increases in fluorescence that result from subthreshold depolarization, spike-induced changes in fluorescence have a long time constant. Consequently, they summate when B21 fires at physiologically relevant frequencies and can become significantly larger than increases in fluorescence observed in the absence of spiking.
Fig. 3.
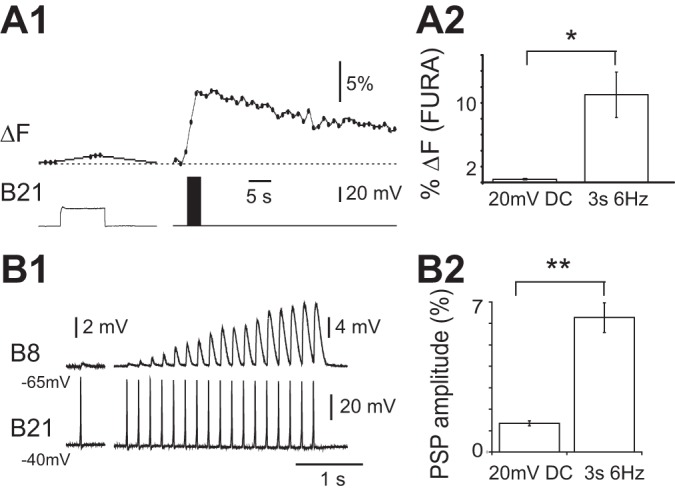
A1: spike-induced increases in calcium fluorescence in B21 vs. increases in calcium fluorescence (ΔF) induced by subthreshold depolarization. Ratiometric (fura) imaging was from the lateral process when B21 was maximally depolarized (i.e., depolarized by 20 mV; left) and when it was stimulated at 6 Hz for 3 s and minimally depolarized (i.e., depolarized by <10 mV; right). A2: group data for the experiment shown in A1. Note that spiking induced the larger signal (n = 5). B1: synaptic transmission between B21 and B8 under conditions where spike-induced increases in calcium fluorescence cannot summate (only one spike is triggered) vs. situation where B21 is repeatedly activated at a frequency where summation can occur. Left, B21 was centrally depolarized by 20 mV (i.e., was held at −40 mV) and a single spike induced. The PSP evoked in B8 was recorded with B8 at its normal resting potential (−65 mV). Right, repetitive stimulation of B21 at 6 Hz for 3 s with B21 and B8 at −40 and −65 mV, respectively. B2: group data for the experiment shown in B1. Note that with repetitive stimulation, B21-induced PSPs in B8 became progressively larger than the PSP potentiated by depolarization alone (n = 4). *P < 0.05; **P ≤ 0.01.
Source of spike-induced increase in [Ca2+]i.
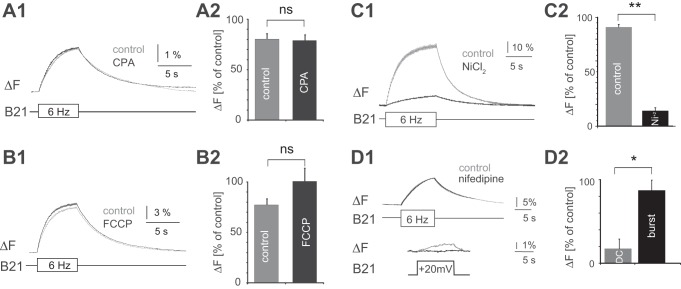
In principle, spike-induced increases in [Ca2+]i could result from the activation of a calcium current and subsequent calcium influx and/or release of calcium from intracellular stores. We found that peak increases in calcium fluorescence were not significantly decreased by 20 μM CPA, which depletes the Ca2+ stored in the endoplasmic reticulum (Geiger and Magoski 2008) (Fig. 4, A1 and A2). Signals were 78.8 ± 5.8% of control values after 30 min in CPA, and were 80.1 ± 5.4% of control values after 30 min in vehicle [paired t-test, t(4) = 0.20, P = 0.85; n = 5]. Furthermore, signals were not significantly decreased by 20 μM FCCP, which depletes mitochondrial Ca2+ stores (Geiger and Magoski 2008) (Fig. 4, B1 and B2). Signals were 100.5 ± 20.5% after 30 min in FCCP and were 77.9 ± 8.1% after 30 min in vehicle [paired t-test, t(4) = −1.43, P = 0.22; n = 5]. Finally, signals were decreased by 10 μM Ni+, which is a nonspecific calcium channel blocker in mollusks (e.g., Byerly et al. 1985; McFarlane and Gilly 1998) (Fig. 4, C1 and C2). After 10 min in 10 μM Ni+, signals were 14.1 ± 2.9% of control values, whereas signals imaged after 10 min in seawater were 91.1 ± 2.7% of control [paired t-test, t(2) = 24.258, P ≤ 0.01; n = 3]. Taken together, these data suggest a major contribution from calcium influx.
Fig. 4.
Effects of pharmacological manipulations on spike-induced increases in calcium fluorescence in B21. B21 was either stimulated at 6 Hz (bottom trace in A1, B1, C1, and D1, top) or depolarized via current injection into the soma (D1, bottom). The resulting change in calcium fluorescence was monitored in the lateral process (top traces in A1, B1, C1, and D1, top). In all cases changes in calcium fluorescence are expressed as percentages of the magnitude of the signal before drug or vehicle application. Control values were obtained after preparations were incubated in the appropriate vehicle for the appropriate amount of time. Preparations were superfused with NiCl2 for 10 min. All other drugs and vehicles were applied for 30 min. Note that NiCl2 produced a significant decrease in the amplitude of the calcium signal (C2; n = 3), whereas CPA (A2; n = 5) and FCCP (B2; n = 5) did not. This suggests the involvement of a calcium current. This current, however, is not blocked by nifedipine (D1, top, and D2), despite the fact that nifedipine blocks the current induced by subthreshold depolarization (D1, bottom, and D2; n = 4). In D2, each signal was normalized to its own control. *P < 0.05; **P ≤ 0.01. ns, No significant difference.
When increases in [Ca2+]i result from subthreshold depolarization, they are very effectively reduced by prior exposure to nifedipine (Edmonds et al. 1990; Ludwar et al. 2009). This suggests a major contribution from a dihydropyridine (DHP)-sensitive calcium current. Consistent with this idea, a current of this nature has been characterized in B21 with the use of voltage-clamp techniques (Svensson et al. 2016). To determine whether this current makes a major contribution to the spike-induced increase in calcium fluorescence, we performed experiments in which we alternated between the injection of brief current pulses to trigger spiking in B21 and current injections that produced a subthreshold depolarization of ~20 mV. Changes in fluorescence were imaged before and after superfusion of 10 μM nifedipine reduced the magnitude of the calcium signal induced by subthreshold depolarization (i.e., after ~20 min). Nifedipine had no significant effect on the signal induced by spiking (i.e., the peak amplitude of the dF/F0 signal was 21.9 ± 3.5% before nifedipine and 19.3 ± 4.2% after) [Fig. 4D1; paired t-test, t(3) = 1.02, P = 0.38; n = 4]. On average, the spike-induced signal after nifedipine was 87.3 ± 11.5% of the signal before nifedipine (Fig. 4D2). In contrast, the subthreshold depolarization induced signal after nifedipine was only 17.3 ± 12.1% of the signal before drug addition (Fig. 4D2). Thus nifedipine more effectively reduced the size of the signal induced by subthreshold depolarization [paired t-test, t(3) = 4.9, P = 0.02; n = 4]. Taken together, these data suggest that increases in [Ca2+]i induced by spiking require calcium influx. Although nifedipine-sensitive channels may make a minor contribution to the magnitude of the induced signal, there is clearly an additional channel (or channels) involved. This contrasts with what is observed with subthreshold depolarization alone. With subthreshold depolarization, increases in [Ca2+]i primarily result from the induction of a DHP-sensitive current (Ludwar et al. 2009).
Increases in [Ca2+]i and changes in synaptic efficacy.
Previous experiments have established that synaptic transmission is potentiated by increases in [Ca2+]i that result from subthreshold depolarization. A question of interest was, Will transmission be modified by further increases in the [Ca2+]i induced by spiking? If so, we would expect to see greater potentiation of synaptic transmission when B21 is stimulated at a frequency that is sufficient to produce summation of spike-induced increases in fluorescence. To determine whether this is the case, we triggered a single spike in B21 when it was maximally depolarized. Postsynaptic potentials (PSPs) were, on average, 134.3 ± 11.6% of control values (Fig. 3, B1 and B2). (Control values were obtained by triggering a single spike with minimal central depolarization.) We then generated a burst of action potentials in B21 using parameters that we have shown generate a relatively large summated calcium signal. We measured the peak amplitude of the last PSP of the burst. This PSP is triggered at the point at which maximal summation of the calcium signal is expected (Fig. 3, A1 and A2). In all preparations the last PSP of the burst was significantly larger than the PSP evoked by a single spike; on average, it was 627.3 ± 68.7% of the control value [Fig. 3, B1 and B2; paired t-test; t(3) = −8.3, P = 0.004; n = 4].
Other data consistent with the idea that bulk increases in the magnitude of the [Ca2+]i potentiate synaptic transmission were obtained in experiments that utilized A23187. A23187 is an ionophore that increases [Ca2+]i. We triggered bursts of action potentials in B21 and recorded PSPs in B8 under control conditions (no A23187), in the presence of 190 μM A23187, and after drug washout (Fig. 5A). B21 was stimulated using parameters that allow summation of the calcium signal to occur (6 Hz for 10 s). Nevertheless, addition of the ionophore further potentiated synaptic transmission [Fig. 5B; 1-way ANOVA, F(2,8) = 11.00, P < 0.01; n = 5]. PSPs in the presence of A23187 were, on average, 195.2 ± 19.6% of control values. Posthoc analyses conducted with a Bonferroni correction for repeated measures indicated that PSPs in the presence of A23187 were significantly larger than control PSPs [t(2) = 4.45, P < 0.01] or PSPs recorded after drug washout [t(2) = 3.50, P < 0.05].
Fig. 5.
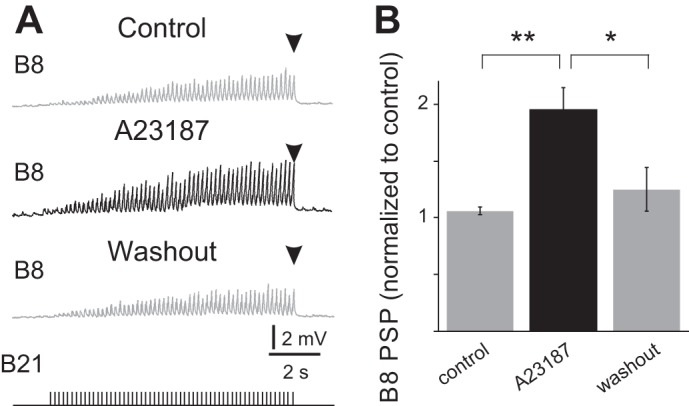
Effect of the calcium ionophore A23187 on B21-B8 synaptic transmission. A: B21 was centrally depolarized (to approximately −40 mV) and stimulated at 6 Hz for 10 s (bottom trace). PSPs were recorded in B8 at its normal resting potential (−65 mV) under control conditions (control), in the presence of 190 μM A23187, and after drug washout. B: group data for the experiment shown in A. Note that incubation in the ionophore produced a significant increase in PSP amplitude (n = 5). *P < 0.05; **P ≤ 0.01.
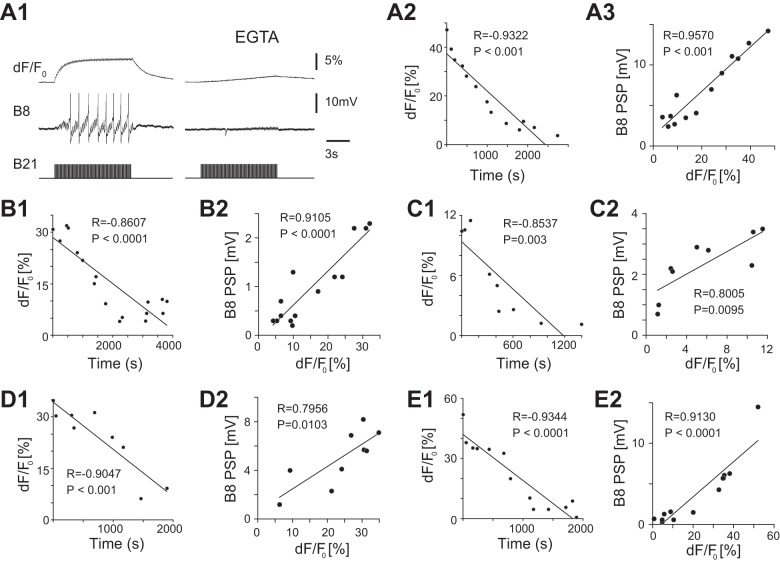
In another set of experiments we injected the calcium chelator EGTA into the soma of B21. EGTA effectively reduces increases in [Ca2+]i that result from subthreshold depolarization (Ludwar et al. 2009) and would be expected to similarly impact global (non-hot spot) levels of [Ca2+]i when B21 spikes. In these experiments we imaged progressive changes in fluorescence in B21’s “output region” as the EGTA moved from the soma to the lateral process. As expected, we observed progressive decreases in calcium fluorescence in the five preparations studied (Fig. 6A2 and Fig. 6, B1, C1, D1, E1; for the 5 preparations studied: R = −0.9322, P < 0.001; R = −0.8607, P < 0.0001; R = −0.8537, P = 0.003; R = −0.9047, P <0.001; and R = −0.9344, P < 0.0001). To determine whether there was a corresponding modification of synaptic transmission, we simultaneously monitored B21-induced PSPs in B8 (e.g., Fig. 6A1, middle trace). In all preparations there was a correlated decrease in PSP peak amplitude (Fig. 6A1, left vs. right, and Fig. 6, A3, B2, C2, D2, E2; for the 5 preparations studied: R = 0.9570, P < 0.001; R = 0.9105, P < 0.0001; R = 0.8005, P = 0.0095; R = 0.7956, P = 0.0103; and R = 0.9130, P < 0.0001). These data indicate that there is a direct relationship between the efficacy of synaptic transmission and the magnitude of [Ca2+]i, even when it is in the range observed when B21 spikes.
Fig. 6.
Decreases in calcium fluorescence induced by injection of EGTA depress B21-B8 synaptic transmission. A1, left: B21 was centrally depolarized (held at approximately −40 mV) and brief current pulses were injected to trigger action potentials (bottom trace). B8 was held at its normal resting potential (−65 mV), and we simultaneously recorded induced PSPs (middle trace) and changes in calcium fluorescence in the lateral process of B21 (top trace). Right, EGTA was injected into the soma of B21. Approximately 15 min later, we observed a decrease in calcium fluorescence (top trace) and a corresponding decrease in the amplitude of PSPs (middle trace). A2 and A3 are plots of data obtained in the experiment shown in A1. A2 shows the change in calcium fluorescence in the lateral process of B21 vs. time. A3 shows the peak amplitude of the last PSP of the burst vs. the change in calcium fluorescence. B1, C1, D1, and E1 are plots of the change in calcium fluorescence in the lateral process of B21 vs. time for data obtained in 4 other preparations. B2, C2, D2, and E2 are plots of the peak amplitude of the last PSP of the burst vs. the change in calcium fluorescence from these same 4 preparations. In all preparations PSP amplitude decreased, as did calcium fluorescence.
EGTA has slow calcium-binding kinetics compared with a buffer such as BAPTA. Consequently, it generally acts as a high-pass temporal and spatial filter for calcium (Wang and Augustine 2015) and is commonly used to selectively manipulate global increases in [Ca2+]i. (e.g., Awatramani et al. 2005; Ivanov and Calabrese 2003). The effectiveness of EGTA in our system therefore suggests that the synaptic potentiation that we observe is mediated by a diffuse rather than highly localized calcium increase. Other data consistent with this idea were obtained in experiments that took advantage of the fact that global increases in [Ca2+]i induced by spiking in B21 have a slow time constant and persist after spiking has ceased (e.g., Fig. 2A2). More specifically, signals last for several seconds. It is unlikely that an increase in [Ca2+]i could remain highly localized for this length of time. We therefore sought to determine whether synaptic transmission was potentiated for several seconds after a burst. To accomplish this, we monitored the efficacy of B21-B8 synaptic transmission before and after B21 was stimulated at 12 Hz (a reasonable firing frequency for feeding neurons) for 3.5 s (a retraction duration observed when intact animals feed; Cropper et al. 1990) (inset in Fig. 7). These experiments were conducted with B8 voltage clamped at its normal resting membrane potential (−65 mV), since in current clamp it was difficult to control the B8 membrane potential after burst stimulation. EPSCs after the burst were potentiated (e.g., for the first few seconds, EPSCs were approximately twice the control amplitude) [Fig. 7; 1-way ANOVA run on all postburst data, F(8,27) = 5.206, P = 0.0005; n = 4]. Thereafter, there was a progressive decrease in EPSC amplitude. When intact animals feed, interburst intervals can be as short as 3.5 s (Cropper et al. 1990). Interestingly, EPSC amplitude was still significantly elevated at this point; i.e., the EPSC ratio was 1.97 ± 0.26, which was significantly above the control value (1.08 ± 0.04; gray line in Fig. 7; paired t-test, t = 4.00, P < 0.05; n = 4). This suggests that progressive summation of potentiating effects may occur when animals feed.
Fig. 7.
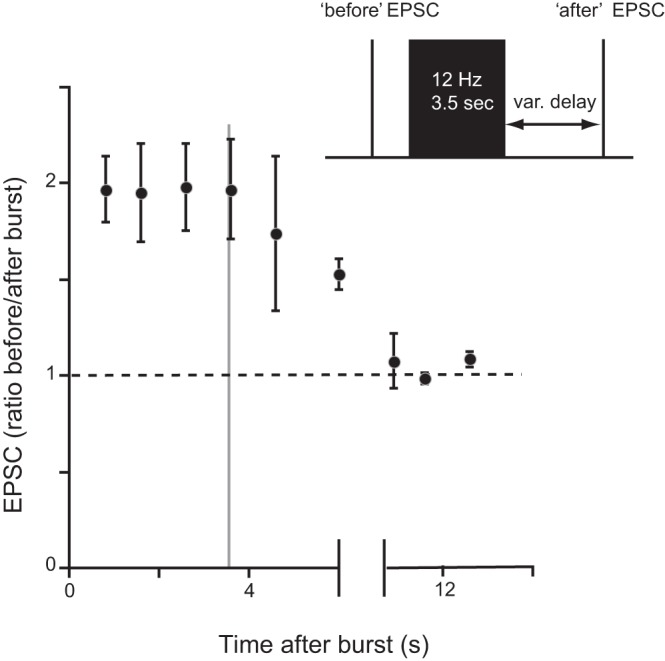
Persistent potentiation of B21-B8 synaptic transmission after a burst of action potentials in B21. Inset shows experimental protocol. B21 was centrally depolarized and a single spike elicited before and with a variable (var.) delay after a burst of action potentials. During the burst, B21 spikes were triggered at 12 Hz for 3.5 s. Induced synaptic potentials were recorded in B8 under voltage-clamp conditions. Plotted is the peak amplitude of the “after” EPSC as a fraction of the peak amplitude of the peak amplitude “before” burst. The dashed line indicates a ratio of 1 (the point at which the “after” EPSC is no longer larger than the “before” EPSC). The gray line at 3.5 s marks the approximate point at which a subsequent burst of activity might be generated when an intact animal feeds (see text for more details; n = 4).
DISCUSSION
A number of studies have demonstrated that subthreshold depolarization can produce global increases in “background” [Ca2+]i that potentiate subsequent spike-mediated synaptic transmission. This has been demonstrated in invertebrate systems (including at the B21-B8 synapse) (e.g., Ivanov and Calabrese 2003; Ludwar et al. 2009; Shapiro et al. 1980; Shimahara and Peretz 1978). Likewise, in mammals it has been described in the cortex (Shu et al. 2006), olfactory bulb (Fekete et al. 2014), auditory system (Awatramani et al. 2005), and cerebellum (Bouhours et al. 2011; Christie et al. 2011). In this study we show that similar increases in [Ca2+]i can be observed following spiking activity. Furthermore, we demonstrate that these changes in calcium concentration can alter the efficacy of synaptic transmission.
Source of widespread increases in intracellular calcium when B21 spikes.
The global increase in [Ca2+]i that we see with spiking is similar to the one induced by subthreshold depolarization in that influx of extracellular calcium makes a major contribution to the magnitude of the signal. Thus spike-induced increases in intracellular calcium are not observed when calcium channels are blocked (Fig. 4, C1 and C2) but are observed in the presence of drugs that deplete intracellular calcium stores (Fig. 4, A1–B2). Our data strongly suggest, however, that different currents are involved, at least to some extent. In B21, currents activated by subthreshold depolarization alone are DHP sensitive (Ludwar et al. 2009). This differs from what has been described in vertebrate preparations, but even in vertebrates there is variability in that different channels appear to be involved in different systems. For example, P/Q- and N-type channels appear to be primarily responsible for calcium increases induced by subthreshold induced depolarization in the cortex (Yu et al. 2010), P/Q-type channels in the cerebellar molecular layer interneurons (Bouhours et al. 2011) and in the calyx of Held (Awatramani et al. 2005), and T-type channels in the olfactory bulb (Fekete et al. 2014).
In principle, spike-induced increases in [Ca2+]i in B21 could be similar to subthreshold-induced increases in [Ca2+]i in that they result from influx via a DHP-sensitive current. In many systems these currents are activated at relatively depolarized potentials (e.g., Benham et al. 1987). Our data indicate, however, that this is not the case. Although nifedipine very effectively blocks increases in [Ca2+]i induced by subthreshold depolarization, there is no significant effect of nifedipine on the amplitude of spike-induced increases in calcium fluorescence in B21 (Fig. 4, D1 and D2). This strongly suggests the involvement of two different currents.
Going forward, it will be of interest to determine the physiological significance of this arrangement. One possibility is that the two types of currents are differentially modulated. The feeding circuitry is subjected to a great deal of modulation because of the release of neuropeptides and monoamines (Due et al. 2004; Friedman and Weiss 2010; Friedman et al. 2015; Jing et al. 2007; Jing et al. 2010; Koh et al. 2003; Koh and Weiss 2007; 2005; Morgan et al. 2000; Morgan et al. 2002; Siniscalchi et al. 2016). For example, neuropeptides play an important role in determining whether motor programs are ingestive or egestive. A previous study demonstrated that the nifedipine-sensitive current in B21 is modulated by peptides (Svensson et al. 2016). It is increased by a peptide released during the priming of ingestive activity and decreased by a peptide released during the priming of egestive activity. It will therefore be of interest to determine whether the current activated during spiking is similarly modulated or whether differential modulation occurs. Differential modulation could lead to an interesting arrangement in which the relative contribution of the two sources of calcium (subthreshold depolarization vs. spiking) could vary in a behavior-dependent manner.
Physiological role of widespread increases in intracellular calcium induced via subthreshold depolarization vs. spiking.
The present study was conducted in ganglia that were removed from the animal and were relatively inactive before experimental manipulations. In this situation there is presumably relatively little modulatory input to the feeding circuitry. Under these conditions, increases in the [Ca2+]i induced by a single spike are similar in magnitude to those induced by subthreshold depolarization (i.e., are not large). Interestingly, however, we show that spike-induced increases in calcium fluorescence decay with a very slow time constant. Consequently, they can very effectively summate when a burst of spikes is generated, even when B21 fires at a relatively low frequency (e.g., Fig. 2C). B21’s firing pattern can vary greatly because it is determined by the nature of the peripheral stimulus that activates it (e.g., Evans et al. 2011b). Bursts of action potentials such as those that produce summating calcium signals are, however, within the range of what has been described; e.g., when it is peripherally activated, B21 can fire at frequencies as high as ~35 Hz (Evans et al. 2011b). It is therefore highly likely that there are physiologically relevant conditions under which spike-induced increases in [Ca2+]i will be considerably larger than increases in [Ca2+]i induced by subthreshold depolarization alone.
We present several lines of data that suggest that increases in [Ca2+]i in this higher range can have an impact on synaptic transmission. For example, we show that PSPs are larger when B21 is stimulated so that summation of the calcium signal occurs (Fig. 3, B1 and B2). This suggests that the global increase in [Ca2+]i that we image at least makes a contribution to the homosynaptic frequency facilitation that we observe at the B21-B8 synapse (Evans et al. 2011a). Furthermore, we show that progressive decreases in the magnitude of the calcium signal produce progressive decreases in PSP size (Fig. 6, A1, A3, B2, C2, D2, E2). In other words, there is a direct relationship between the magnitude of the summated signal and the extent to which potentiation is observed.
An interesting consequence of a change in the magnitude of the calcium signal is that it impacts its persistence. Time constants of the relatively small increases in [Ca2+]i induced by a single spike or subthreshold depolarization were highly variable, and therefore, it was difficult to compare them to the time constant of the summated signal. Nevertheless, even without a time constant difference, the striking difference in the magnitude of the signal leads to an increase in the time it takes for the signal to decay (Fig. 2C). This leads to persistent potentiation of synaptic transmission (Fig. 7). We show that synaptic transmission was significantly potentiated 3.5 s after B21 was stimulated for 3.5 s. This particular burst duration and after stimulation time point are of particular interest. During feeding, B21 is presumably activated during the radula retraction phase of the motor program. When animals ingest strips of food, radula retraction is, on average, 3.5 s and is repeated every 3.5 s (Cropper et al. 1990). Our data therefore suggest that cumulative effects of repeated bursting activity are likely to occur under behaviorally relevant conditions.
Investigators working in other systems have also noted the slow decay of calcium signals and postulated that persistent calcium-dependent facilitation of synaptic transmission could serve as a basis for working memory (Mongillo et al. 2008). It has been argued that this would be a more energy efficient arrangement than one in which working memory is mediated via persistent reverberation (Mongillo et al. 2008). Persistent reverberation obviously requires persistent spike generation, which requires energy. Our results demonstrate that summation of spike-induced increases in intracellular calcium can occur under physiological conditions, and can be of sufficient magnitude to potentiate synaptic transmission. Furthermore, we show that there are at least two factors that are likely to determine the extent to which this occurs: the intraburst firing frequency of a neuron and the interburst-interval. In other systems these factors may impact memory retention.
GRANTS
This work was supported by National Institutes of Health Grants NS066587, NS070583, and MH051393.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.C.L. and E.C.C. conceived and designed research; B.C.L., C.G.E., and M.C. performed experiments; B.C.L., C.G.E., and E.C.C. analyzed data; B.C.L., C.G.E., and E.C.C. interpreted results of experiments; B.C.L., M.C., and E.C.C. prepared figures; B.C.L. and E.C.C. drafted manuscript; B.C.L., C.G.E., and E.C.C. edited and revised manuscript; B.C.L., C.G.E., M.C., and E.C.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Klaudiusz R. Weiss for discussions and valuable comments on this manuscript.
REFERENCES
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science 311: 1290–1293, 2006. doi: 10.1126/science.1119055. [DOI] [PubMed] [Google Scholar]
- Alle H, Geiger JR. Analog signalling in mammalian cortical axons. Curr Opin Neurobiol 18: 314–320, 2008. doi: 10.1016/j.conb.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Andjelic S, Torre V. Calcium dynamics and compartmentalization in leech neurons. J Neurophysiol 94: 4430–4440, 2005. doi: 10.1152/jn.00695.2005. [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron 48: 109–121, 2005. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Benham CD, Hess P, Tsien RW. Two types of calcium channels in single smooth muscle cells from rabbit ear artery studied with whole-cell and single-channel recordings. Circ Res 61: I10–I16, 1987. [PubMed] [Google Scholar]
- Borovikov D, Evans CG, Jing J, Rosen SC, Cropper EC. A proprioceptive role for an exteroceptive mechanoafferent neuron in Aplysia. J Neurosci 20: 1990–2002, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhours B, Trigo FF, Marty A. Somatic depolarization enhances GABA release in cerebellar interneurons via a calcium/protein kinase C pathway. J Neurosci 31: 5804–5815, 2011. doi: 10.1523/JNEUROSCI.5127-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L, Chase PB, Stimers JR. Permeation and interaction of divalent cations in calcium channels of snail neurons. J Gen Physiol 85: 491–518, 1985. doi: 10.1085/jgp.85.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G, Eilers J, Konnerth A. Axonal calcium entry during fast ‘sodium’ action potentials in rat cerebellar Purkinje neurones. J Physiol 495: 641–647, 1996. doi: 10.1113/jphysiol.1996.sp021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Chiu DN, Jahr CE. Ca2+-dependent enhancement of release by subthreshold somatic depolarization. Nat Neurosci 14: 62–68, 2011. doi: 10.1038/nn.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B, Häusser M. Neural coding: hybrid analog and digital signalling in axons. Curr Biol 16: R585–R588, 2006. doi: 10.1016/j.cub.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Cropper EC, Evans CG, Rosen SC. Multiple mechanisms for peripheral activation of the peptide-containing radula mechanoafferent neurons B21 and B22 of Aplysia. J Neurophysiol 76: 1344–1351, 1996. [DOI] [PubMed] [Google Scholar]
- Cropper EC, Kupfermann I, Weiss KR. Differential firing patterns of the peptide-containing cholinergic motor neurons B15 and B16 during feeding behavior in Aplysia. Brain Res 522: 176–179, 1990. doi: 10.1016/0006-8993(90)91598-B. [DOI] [PubMed] [Google Scholar]
- Debanne D, Bialowas A, Rama S. What are the mechanisms for analogue and digital signalling in the brain? Nat Rev Neurosci 14: 63–69, 2013. doi: 10.1038/nrn3361. [DOI] [PubMed] [Google Scholar]
- Delaney KR, Tank DW. A quantitative measurement of the dependence of short-term synaptic enhancement on presynaptic residual calcium. J Neurosci 14: 5885–5902, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due MR, Jing J, Weiss KR. Dopaminergic contributions to modulatory functions of a dual-transmitter interneuron in Aplysia. Neurosci Lett 358: 53–57, 2004. doi: 10.1016/j.neulet.2003.12.058. [DOI] [PubMed] [Google Scholar]
- Edmonds B, Klein M, Dale N, Kandel ER. Contributions of two types of calcium channels to synaptic transmission and plasticity. Science 250: 1142–1147, 1990. doi: 10.1126/science.2174573. [DOI] [PubMed] [Google Scholar]
- Evans CG, Jing J, Rosen SC, Cropper EC. Regulation of spike initiation and propagation in an Aplysia sensory neuron: gating-in via central depolarization. J Neurosci 23: 2920–2931, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CG, Ludwar BC, Askanas J, Cropper EC. Effect of holding potential on the dynamics of homosynaptic facilitation. J Neurosci 31: 11039–11043, 2011a. doi: 10.1523/JNEUROSCI.2361-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CG, Ludwar BC, Kang T, Cropper EC. Effect of presynaptic membrane potential on electrical vs. chemical synaptic transmission. J Neurophysiol 106: 680–689, 2011b. doi: 10.1152/jn.00340.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete A, Johnston J, Delaney KR. Presynaptic T-type Ca2+ channels modulate dendrodendritic mitral-mitral and mitral-periglomerular connections in mouse olfactory bulb. J Neurosci 34: 14032–14045, 2014. doi: 10.1523/JNEUROSCI.0905-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson AL, Zucker RS. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys J 48: 1003–1017, 1985. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Weiss KR. Repetition priming of motoneuronal activity in a small motor network: intercellular and intracellular signaling. J Neurosci 30: 8906–8919, 2010. doi: 10.1523/JNEUROSCI.1287-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AK, Weiss KR, Cropper EC. Specificity of repetition priming: the role of chemical coding. J Neurosci 35: 6326–6334, 2015. doi: 10.1523/JNEUROSCI.4562-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JE, Magoski NS. Ca2+-induced Ca2+ release in Aplysia bag cell neurons requires interaction between mitochondrial and endoplasmic reticulum stores. J Neurophysiol 100: 24–37, 2008. doi: 10.1152/jn.90356.2008. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Calabrese RL. Modulation of spike-mediated synaptic transmission by presynaptic background Ca2+ in leech heart interneurons. J Neurosci 23: 1206–1218, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Sweedler JV, Cropper EC, Alexeeva V, Park JH, Romanova EV, Xie F, Dembrow NC, Ludwar BC, Weiss KR, Vilim FS. Feedforward compensation mediated by the central and peripheral actions of a single neuropeptide discovered using representational difference analysis. J Neurosci 30: 16545–16558, 2010. doi: 10.1523/JNEUROSCI.4264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing J, Vilim FS, Horn CC, Alexeeva V, Hatcher NG, Sasaki K, Yashina I, Zhurov Y, Kupfermann I, Sweedler JV, Weiss KR. From hunger to satiety: reconfiguration of a feeding network by Aplysia neuropeptide Y. J Neurosci 27: 3490–3502, 2007. doi: 10.1523/JNEUROSCI.0334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol 195: 481–492, 1968. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh HY, Vilim FS, Jing J, Weiss KR. Two neuropeptides colocalized in a command-like neuron use distinct mechanisms to enhance its fast synaptic connection. J Neurophysiol 90: 2074–2079, 2003. doi: 10.1152/jn.00358.2003. [DOI] [PubMed] [Google Scholar]
- Koh HY, Weiss KR. Peptidergic contribution to posttetanic potentiation at a central synapse of Aplysia. J Neurophysiol 94: 1281–1286, 2005. doi: 10.1152/jn.00073.2005. [DOI] [PubMed] [Google Scholar]
- Koh HY, Weiss KR. Activity-dependent peptidergic modulation of the plateau-generating neuron B64 in the feeding network of Aplysia. J Neurophysiol 97: 1862–1867, 2007. doi: 10.1152/jn.01230.2006. [DOI] [PubMed] [Google Scholar]
- Ludwar BC, Evans CG, Cropper EC. Monitoring changes in the intracellular calcium concentration and synaptic efficacy in the mollusc Aplysia. J Vis Exp: e3907, 2012. doi: 10.3791/3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwar BC, Evans CG, Jing J, Cropper EC. Two distinct mechanisms mediate potentiating effects of depolarization on synaptic transmission. J Neurophysiol 102: 1976–1983, 2009. doi: 10.1152/jn.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Neurobiology: extending influence. Nature 441: 702–703, 2006. doi: 10.1038/441702a. [DOI] [PubMed] [Google Scholar]
- McFarlane MB, Gilly WF. State-dependent nickel block of a high-voltage-activated neuronal calcium channel. J Neurophysiol 80: 1678–1685, 1998. [DOI] [PubMed] [Google Scholar]
- Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science 319: 1543–1546, 2008. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Jing J, Vilim FS, Weiss KR. Interneuronal and peptidergic control of motor pattern switching in Aplysia. J Neurophysiol 87: 49–61, 2002. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Perrins R, Lloyd PE, Weiss KR. Intrinsic and extrinsic modulation of a single central pattern generating circuit. J Neurophysiol 84: 1186–1193, 2000. [DOI] [PubMed] [Google Scholar]
- Nicholls J, Wallace BG. Modulation of transmission at an inhibitory synapse in the central nervous system of the leech. J Physiol 281: 157–170, 1978. doi: 10.1113/jphysiol.1978.sp012414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Delaney KR, Tank DW. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci 14: 523–537, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E, Castellucci VF, Kandel ER. Presynaptic membrane potential affects transmitter release in an identified neuron in Aplysia by modulating the Ca2+ and K+ currents. Proc Natl Acad Sci USA 77: 629–633, 1980. doi: 10.1073/pnas.77.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimahara T, Peretz B. Soma potential of an interneurone controls transmitter release in a monosynaptic pathway in Aplysia. Nature 273: 158–160, 1978. doi: 10.1038/273158a0. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature 441: 761–765, 2006. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- Simon SM, Llinás RR. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J 48: 485–498, 1985. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniscalchi MJ, Cropper EC, Jing J, Weiss KR. Repetition priming of motor activity mediated by a central pattern generator: the importance of extrinsic vs. intrinsic program initiators. J Neurophysiol 116: 1821–1830, 2016. doi: 10.1152/jn.00365.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E, Evans CG, Cropper EC. Repetition priming-induced changes in sensorimotor transmission. J Neurophysiol 115: 1637–1643, 2016. doi: 10.1152/jn.01082.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swandulla D, Hans M, Zipser K, Augustine GJ. Role of residual calcium in synaptic depression and posttetanic potentiation: fast and slow calcium signaling in nerve terminals. Neuron 7: 915–926, 1991. doi: 10.1016/0896-6273(91)90337-Y. [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Meyer zum Alten Borgloh S, Astori S, Yang Y, Bausen M, Kügler S, Palmer AE, Tsien RY, Sprengel R, Kerr JN, Denk W, Hasan MT. Single-spike detection in vitro and in vivo with a genetic Ca2+ sensor. Nat Methods 5: 797–804, 2008. doi: 10.1038/nmeth.1242. [DOI] [PubMed] [Google Scholar]
- Wang LY, Augustine GJ. Presynaptic nanodomains: a tale of two synapses. Front Cell Neurosci 8: 455, 2015. doi: 10.3389/fncel.2014.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Maureira C, Liu X, McCormick D. P/Q and N channels control baseline and spike-triggered calcium levels in neocortical axons and synaptic boutons. J Neurosci 30: 11858–11869, 2010. doi: 10.1523/JNEUROSCI.2651-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]