Complex signal analysis is a challenging task in sensory processing for all animals, particularly for bats because they use echolocation for navigation in darkness. Recent studies proposed that the bat’s perceptional system might organize complex echo-acoustic information into auditory streams, allowing it to track specific auditory objects during flight. We show that in the auditory cortex of bats, neurons can selectively respond to echo streams from specific objects.
Keywords: bat, biosonar, auditory cortex, auditory scene analysis, cortical map
Abstract
Echolocating bats use echoes of their sonar emissions to determine the position and distance of objects or prey. Target distance is represented as a map of echo delay in the auditory cortex (AC) of bats. During a bat’s flight through a natural complex environment, echo streams are reflected from multiple objects along its flight path. Separating such complex streams of echoes or other sounds is a challenge for the auditory system of bats as well as other animals. We investigated the representation of multiple echo streams in the AC of anesthetized bats (Phyllostomus discolor) and tested the hypothesis that neurons can lock on echoes from specific objects in a complex echo-acoustic pattern while the representation of surrounding objects is suppressed. We combined naturalistic pulse/echo sequences simulating a bat’s flight through a virtual acoustic space with extracellular recordings. Neurons could selectively lock on echoes from one object in complex echo streams originating from two different objects along a virtual flight path. The objects were processed sequentially in the order in which they were approached. Object selection depended on sequential changes of echo delay and amplitude, but not on absolute values. Furthermore, the detailed representation of the object echo delays in the cortical target range map was not fixed but could be dynamically adapted depending on the temporal pattern of sonar emission during target approach within a simulated flight sequence.
NEW & NOTEWORTHY Complex signal analysis is a challenging task in sensory processing for all animals, particularly for bats because they use echolocation for navigation in darkness. Recent studies proposed that the bat’s perceptional system might organize complex echo-acoustic information into auditory streams, allowing it to track specific auditory objects during flight. We show that in the auditory cortex of bats, neurons can selectively respond to echo streams from specific objects.
segregating signals from specific objects in a complex environment is a ubiquitous challenge for sensory systems (Lamme 1995; Pawluk et al. 2010; Roelfsema et al. 1998; Rokni et al. 2014). In the auditory world, auditory scene analysis deals with the organization of complex sounds in perceptually meaningful elements (Bregman 1990). Perceptional organization of sounds into auditory streams has been studied in many different species, including humans (Fay 1998; Izumi 2002; MacDougall-Shackleton et al. 1998; Micheyl et al. 2005; van Noorden 1975; Schul and Sheridan 2006). Most neurophysiological studies investigated auditory stream segregation using fairly simplified acoustic stimuli such as alternating pure tones (Fishman et al. 2001; Kanwal et al. 2003; Micheyl et al. 2005); therefore, the neural basis of perceptional organization of more complex sounds in a natural environment is still not well understood.
For bats, evaluating and sorting complex sounds is most important, because they use echolocation for navigation in darkness. Echolocating bats use the time delay between emission of a pulse and reception of its reflected echoes to measure their distance from objects or prey. Neurons that selectively encode specific echo delays emerge in the ascending auditory pathway of bats from the midbrain on (Olsen and Suga 1991; O’Neill and Suga 1982; Portfors and Wenstrup 1999; Valentine and Moss 1997). In the auditory cortex (AC), these neurons have been shown to form chronotopically organized computational maps of target distance (Hagemann et al. 2010; Schuller et al. 1991; Suga and O’Neill 1979). Dear et al. (1993b) proposed that a concurrent representation of multiple echo delays in this map acts as the neuronal basis for a complex acoustic scene representation.
In a natural situation, during flight, a bat receives a sustained echo stream that provides continuously changing acoustic information (echo-acoustic flow) about the surrounding objects in a complex acoustic environment (Moss and Surlykke 2010). Echo-acoustic flow contains information not only about echo delay but also about the geometric relationship between objects and their motion relative to the bat (McKerrow 2008).
Recent studies simulating a flight using naturalistic pulse/echo sequences have shown that echo-acoustic flow fields significantly increase the selectivity of echo-delay-tuned neurons in the AC and modify the cortical target range representation (Bartenstein et al. 2014; Beetz et al. 2016b). However, receptive fields of adjacent echo-delay-tuned neurons largely overlap, and the cortical target distance map is more blurry than precise (Hechavarría et al. 2013b; O’Neill and Suga 1982; Schuller et al. 1991). Furthermore, in a dynamic and cluttered environment, bats have to deal with complex interfering patterns of incoming echo streams from multiple objects (Moss et al. 2006; Moss and Surlykke 2001). Moss and Surlykke (2010) proposed that the bat’s perceptional system might organize complex echo-acoustic information into auditory streams, allowing it to track specific auditory objects during flight. The temporal pattern of sonar emissions, such as grouping into so-called “strobe groups” (sequences of clustered pulses, containing two or more pulses at high repetition rate; Moss et al. 2006), might further facilitate echo stream segregation in cluttered environments (e.g., Kothari et al. 2014; Wheeler et al. 2016).
This study aims to investigate the cortical representation of echo streams from multiple objects during a simulated naturalistic pulse/echo sequence and the impact of dynamic stimulation on the cortical target range map. Our hypothesis was that neurons in the auditory cortex of anesthetized bats can lock onto echoes from specific objects in a complex echo-acoustic pattern while the representation of surrounding objects is suppressed.
We presented naturalistic pulse/echo sequences simulating a bat’s flight while passing two virtual objects positioned lateral to its flight path and recorded resulting neuronal responses evoked in the posterior dorsal auditory cortex of anesthetized bats. Specifically, we were interested in whether neurons in the AC were able to track specifically one of two objects that were presented in a complex sequence. We also varied the temporal pattern of sonar emissions during the simulated pulse/echo sequences to test their influence on the cortical target representation.
Our results show that neurons in the AC can selectively respond to streams of echoes from specific objects in a complex and dynamic echo-acoustic environment. Furthermore, variation of the temporal pattern of sonar emission revealed the highly adaptable nature of the cortical target range representation.
METHODS
Surgery.
All the experiments complied with the principles of laboratory animal care and were conducted under the regulations set out by the current version of the German Law on Animal Protection (approval 55.2-1-54-2532-147-13 Regierung von Oberbayern). The bats (Phyllostomus discolor; 3 adult females) originated from a breeding colony situated in the Department Biology II of the Ludwig-Maximilian University of Munich. For experiments, animals were kept separated from other bats under seminatural conditions (12:12-h day-night cycle, 65–70% relative humidity, 28°C) with free access to food and water.
The surgical procedures are described in detail by Hoffmann et al. (2008) and are mentioned only briefly. The bats were anesthetized using a combination of medetomidine (Dorbene; Zoetis), midazolam (Dormicum; Hoffmann-La Roche), and fentanyl (Fentadon; Albrecht) at a dosage of 0.4, 4.0, and 0.04 µg/g body wt, respectively. Anesthesia was maintained through additional injections containing two-thirds of the initial dose every 1.5 h. The skin overlying the skull was opened along the midline, and the skull surface was freed from tissue. A small metal tube was then fixed to the skull by using a microglass composite to fix the animal to a stereotaxic device. Details of the stereotaxic device and the procedure used to reconstruct the recording sites are described elsewhere (Schuller et al. 1986). In brief, the alignment of the animal’s skull and the underlying brain within the stereotaxic coordinate system was measured by scanning the characteristic profile lines of the skull in the parasagittal and frontal planes. These profiles were then fitted onto a standardized skull profile in a standardized coordinate system.
To alleviate postoperative pain, an analgesic (0.2 µg/g body wt; meloxicam, Metacam; Boehringer-Ingelheim) was administered after the surgery for 4 postoperative days. The anesthesia was antagonized with a mixture of atipamezole (Alzane; Novartis), flumazenil (flumazenil; HEXAL), and naloxone (Naloxon-ratiopharm; Ratiopharm) that was injected subcutaneously (2.5, 0.5, and 1.2 µg/g body wt, respectively). The bats were treated with antibiotics (0.5 µg/g body wt; enrofloxacin, Batril; Bayer) for 4 postoperative days.
Electrophysiological recordings.
After initial surgery, experiments were conducted in a sound-attenuated and heated (~35°C) chamber. Extracellular recordings were made with parylene-coated tungsten microelectrodes (5-MΩ impedance; Alpha Omega) in anesthetized bats (see Surgery). Note that the responses recorded from cortical units under this anesthesia regime reflect the behavioral performance of P. discolor well (Firzlaff et al. 2006). Recording sessions took place 3 days a week for up to 8 weeks (with at least 1 day off between consecutive experiments) and could last up to 5 h per day. Electrode penetrations in the AC were run obliquely to the brain surface with different mediolateral and rostrocaudal angles. The electrode signal was recorded using an analog-to-digital converter [RA16, RX5; Tucker-Davis Technologies (TDT); sampling rate 25 kHz, bandpass filter 400-3,000 Hz] and Brainware (TDT). The action potentials were threshold discriminated and saved for later offline analysis. We tried to isolate single neurons whenever possible. However, because it was not always possible to clearly discriminate the activity of a single neuron, the term “unit” is used in this article to describe the collective activity of one to three neurons recorded at a recoding site. A comparison of neuronal responses from single units and multiunits revealed no differences.
To search for acoustically driven neural activity, pairs of typical echolocation sounds of P. discolor (representing “pulse” and a virtual “echo”) were used (downward modulated, multiharmonic, main energy between 40 and 90 kHz, duration ~1.2 ms). The delay and amplitude ratio between the “pulse” and “echo” could be varied [digital-to-analog (DA) converter: RX6 (TDT); sampling rate 260 kHz; attenuator: PA5 (TDT)]. Search stimuli were presented binaurally with a repetition rate of 2 Hz. For units that responded to these presented pairs of search stimuli, basic delay response properties and responses to simulated naturalistic pulse/echo sequences were determined (see Characterization of basic delay-tuning properties). All acoustic stimuli were presented via custom-made transducer ear phones (Schuller 1997).
After the experiments were completed, a neuronal marker (BDA 3000; Sigma-Aldrich; 1 mg/20 µl phosphate buffer) was pressure-injected (Nanoliter 2000 injector; World Precision Instruments) into the brains to reconstruct the position of the recording sites in standardized stereotaxic coordinates of a brain atlas of P. discolor (Nixdorf A, Fenzl T, Schwellnus B, unpublished observations). The animals were then euthanized by an intraperitoneally applied lethal dose of pentobarbital (0.16 mg/g body wt) and subsequently perfused transcardially.
Characterization of basic delay-tuning properties.
To measure the basic echo delay-tuning properties, the following procedure was used: a prerecorded typical echolocation pulse of P. discolor was presented with a constant level of 70–85 dB SPL. An “echo” was simulated by presenting the same pulse while randomly changing the delays from 1 to 29 ms and the amplitudes from −40 to 0 dB relative to the level of the “pulse.” The resulting neuronal activity was measured within a time window of 20–50 ms, starting directly after the echo presentation. Units’ responses were classified as “facilitated” if their response to at least one of the presented pulse/echo pairs was at least 30% stronger than the sum of the responses for only pulses or only echoes. Only facilitated units were further analyzed with regard to the basic delay-tuning characteristics to ensure that responses were specific to pairs of pulses and echoes.
Delay response fields (DRFs) for facilitated units were visualized as filled contour plots, and threshold curves were calculated using a threshold of 50% of the maximum response for each unit. The DRF of each unit was further characterized by measuring the delay at the maximum response (best delay, BD) and the delay at the lowest tip of the threshold curve (characteristic delay, CD), corresponding to Hechavarría et al. (2013b). The BD and CD were used, together with the units’ positions in the AC projected onto the cortical surface, to calculate cortical delay-tuning maps. Furthermore, the distributions of the BD and CD on the cortical surface were statistically analyzed using Spearman’s rank correlation analysis (MATLAB Statistics Toolbox).
Stimulus generation for naturalistic flight sequences.
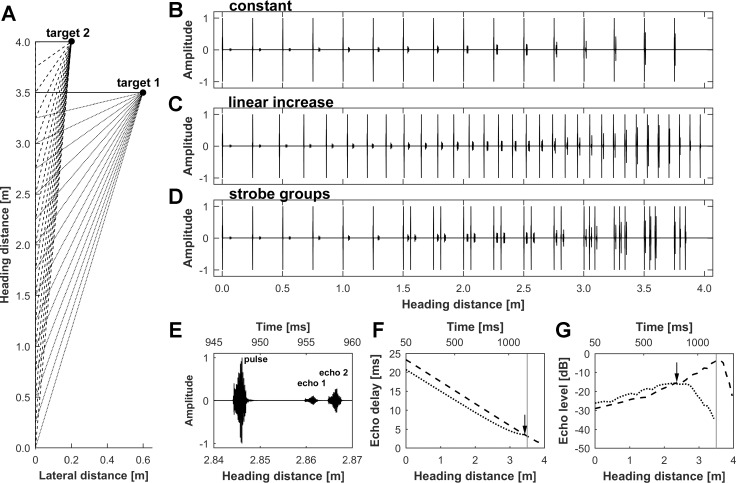
Naturalistic flight sequences were generated in the manner described by Bartenstein et al. (2014). In detail, flight trajectories were calculated for a bat flying laterally toward two objects (target 1 and target 2) over a flight distance of 4.0 m. The objects were positioned at distances of 3.5 and 4.0 m (in the direction of the heading, which we will call the heading distance) with lateral distances to the bat’s flight path of 0.6 and 0.2 m for target 1 and target 2, respectively (Fig. 1A). Both objects were positioned at the same height as the bat’s flight path. The obstacles were assumed to be perfect reflectors.
Fig. 1.
Generation of naturalistic pulse/echo sequences. A: spatial layout of the virtual flight sequence with 2 targets positioned at heading distances of 3.5 and 4.0 m and at lateral distances of 0.6 and 0.2 m, respectively. Dotted (target 1) and dashed lines (target 2) indicate the reflected echoes from the objects during the flight sequence. B–D: sequences of pulses and echoes from both targets are shown for a constant pulse rate (B), a linearly increasing pulse rate (C), and strobe groups (D). E: details of a pulse/echo sequence showing a single echolocation pulse and corresponding echoes from target 1 and target 2. F and G: progression of echo delay (F) and echo level (G) for target 1 (dotted line) and target 2 (dashed line) along the flight path with corresponding timescale. The acoustic stimulation is preceded by a 50-ms silent period to determine the level of spontaneous activity and is followed by another 200-ms silent period. Vertical gray lines mark the position where the bat passes target 1. F: the echo delay of target 2 exceeds the echo delay of target 1 until the 3.43-m heading distance (black arrow; 7 cm before passing target 1). G: the echo levels for target 1 and target 2 increase when the bat approaches the targets. After an ~2.4-m heading distance, the angle to target 1 increases considerably, and consequently, the target 1 echo level drops rapidly as target 1 leaves the beam of sonar emission.
To investigate the influence of naturalistic echolocation behavior, we used three different temporal echolocation pulse patterns: 1) a constant pulse rate of 12 Hz, 2) a linearly increasing pulse rate from 12 to 36 Hz, and 3) a simulation of typical strobe groups (Rother and Schmidt 1982) with a group frequency of 12 Hz and an increasing number of pulses in each group (1–3 calls per group; Fig. 1, B–D). The positions of the pulse emission and echo reception for both objects along the flight path were determined. The simulated flight speed was 3 m/s, which is in the range typically reported for phyllostomid bat species (Kugler et al. 2016; Morrison 1980).
To generate pulse/echo sequences, an echolocation pulse of P. discolor containing no directionality was used (see Bartenstein et al. 2014). The pulse was convolved with the left and right ear pair of the head-related transfer function (De Mey et al. 2008; Firzlaff and Schuller 2003) and the transfer function of pulse emission of P. discolor (Vanderelst et al. 2010), corresponding to the angular direction of the echo reflection for each position and object along the flight path. The resulting artificial echoes included all relevant spatial cues, such as interaural time differences (ITDs), frequency-dependent interaural intensity differences (IIDs), and spectral profiles as well as distance-dependent and frequency-dependent sound attenuation. We did not simulate the Doppler shifts caused by the bat’s movement because these effects are negligible in FM bats. To simulate the bat’s echolocation pulses, nondirectional pulses were attenuated by 26 dB to mimic the typical level of self-stimulation by vocalization (Pietsch and Schuller 1987). Pulses and echoes for both objects were then combined, including the respective echo delays representing the distance to each object at each position during the flight sequence. The final sequences of pulses and echoes were generated with a constant pulse rate, a linearly increasing pulse rate, and simulated strobe groups. The complete pulse/echo sequences were calculated using MATLAB functions custom built in our laboratory, DA converted (RX6; TDT), and presented via custom-made transducer ear phones (Schuller 1997). In the final sequences, all echo-acoustically important parameters such as echo delay, echo amplitude, echo reflection angle, and echo spectral content were changed dynamically as a function of flight time (i.e., the position of the bat along the flight path). The pulses were presented at 70–85 dB SPL, and the pulse level was constant within the sequence. We adapted the presented pulse sound pressure level to achieve stable neuronal responses to the presented sequences. Information for this was gained from the DRFs recorded for each cell. The echo delays decreased within the sequence from 20.7 to 3.5 ms and from 23.4 to 1.2 ms for target 1 and target 2, respectively. The echo amplitudes increased from −34.4 to −15.7 dB and from −29.1 to −3.4 dB relative to the pulse level for target 1 and target 2, respectively (Fig. 1, E–G). Pulses within the sequences never overlapped with preceding echoes (no pulse/echo ambiguity). We used exclusively the described spatial layout to keep the differences between the targets with respect to echo delay and echo level in a limited range. This was done to ensure that as many neurons as possible would respond to presentation of echoes from both targets when presented alone in a sequence, a prerequisite of our experiment.
Sequences were presented binaurally, with both objects positioned contralaterally to the recording site. Each stimulus sequence was preceded by a 50-ms silent period to determine the level of spontaneous activity and had a duration of ~1.4 s. Sequences were presented every 3 s.
To measure the responses for each individual target, pulse/echo sequences were generated 1) for both targets together, 2) for target 1 only, and 3) for target 2 only. To ensure that the units responded only to pairs of pulses and echoes specifically, additional sequences were presented for every unit and pulse emission pattern, containing 4) only pulses or 5) only echoes. Consecutive presentations of pulse/echo sequences were separated by at least 30 s to prevent interactions due to the order of presentation.
Data analysis for naturalistic flight sequences.
The spike responses evoked by the simulated flight sequences were arranged both as peristimulus time histograms (PSTHs; 2-ms bin width) and as raster plots. To determine the responses to each pulse/echo pair within a sequence, manually fixed response windows were set after each pulse/echo pair. Response windows started at the time of the first object echo and ended after the neuronal activity decreased below the level of the spontaneous activity, but not later than the time of the first echo of the subsequent pulse. To ensure that the response windows could not overlap between two successive pulse/echo pairs, the response window length never exceeded that of the minimal inter-pulse interval for each simulated pulse rate. The minimal interpulse interval was 83 ms for the constant pulse rate, 27 ms for the linearly increasing pulse rate, and 15 ms for the simulated strobe groups. The length of the response window was equal for all pulse/echo pairs within a sequence and for all records of a unit for each simulated pulse rate. For every presented sequence (both targets, only target 1, only target 2, only pulses, only echoes), spikes occurring in each response window were summed to get the response rate of a unit for every pulse/echo pair within the sequence.
Units’ responses were classified as “facilitated” if their response in a pulse/echo-sequence was at least 30% stronger than the sum of responses from only pulses and only echoes. To determine the specific response of a facilitated unit to each object, the pulse/echo pair evoking the maximum response rate within sequences presenting only target 1 or target 2 was identified (maximum response position). The echo delay of the corresponding pulse/echo pair was considered to be the “specific delay” of the unit for the simulated object and pulse rate. Only units that showed a distinct and facilitated response to both individual targets (“specific response”) were further analyzed regarding the cortical echo delay tuning and object representation. Cortical echo delay-tuning maps were calculated for each target and pulse rate based on the specific delays and stereotaxic coordinates of all of the facilitated and specific units. The distributions of the specific delays for both targets at all different pulse rates were statistically analyzed using a Kruskal-Wallis test, followed by a pairwise Wilcoxon-Mann-Whitney test (MATLAB Statistics Toolbox).
To investigate whether the neurons responded equally to the targets when they were presented alone in a sequence compared with when both targets being presented together in a complex sequence, we examined the response rate at the maximum response position for each object and compared the values between the individual presentations of the targets and the combined presentation (at the respective pulse/echo pairs within the sequence). It was essential for this comparison that the responses of both individual targets could be identified in the complex sequence. Therefore, units that responded to both targets on the same pulse/echo pair within the sequence (i.e., an overlap of the maximum responses) were excluded from further analysis (n = 3).
The response of a unit in the combined presentation to the respective object was classified as an “equal” response if the spike rate was at least 50% of the original response in the individual presentation. The unit’s response was classified as “decreased” if the spike rate was less than 50% of the original spike rate. By using such strict criteria, we ensured that the number of neurons focusing on specific targets was not overestimated. Units’ responses were then further categorized as “no focus” if they responded equally to both targets, as “focus on target 1” if only the response to target 2 decreased, and as “focus on target 2” if only the response to target 1 decreased. In some rare cases, the unit’s response decreased for both targets.
RESULTS
Delay response fields.
We used delay response fields (DRFs) to gather an initial insight into the basic echo delay-tuning properties of the AC neurons and to enable a direct comparison with the response characteristics of dynamic flight sequences as well as data obtained from other bats.
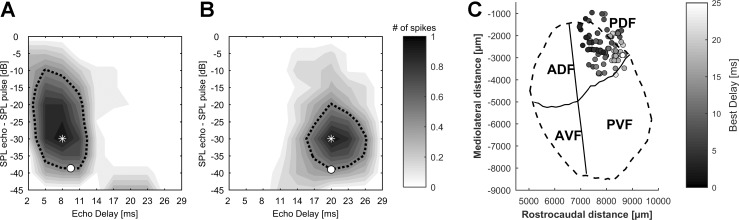
A total of 156 units were recorded in the posterior dorsal field (PDF) of the auditory cortex in 3 bats. Of these, 110 units (71%) exhibited a facilitated response to the presented pulse/echo pairs. Echo delays ranged from 1 to 29 ms. For all 110 delay-sensitive units, best delays (BD) and characteristic delays (CD) were determined. Figure 2, A and B, shows the DRFs of two exemplary delay-sensitive neurons. The unit in the frontal part of the PDF (Fig. 2A) was selective for shorter echo delays, with a BD of 8.0 ms at −30 dB echo level and a CD of 9.5 ms at −38 dB echo level. The unit in the caudal part of the PDF (Fig. 2B) responded to longer echo delays at a BD of 20.0 ms at −30 dB echo level and a CD of 20.0 ms at −39 dB echo level.
Fig. 2.
DRFs for 2 delay-sensitive units and cortical distribution of best delay. A and B: responses of 2 delay-sensitive units from the rostral (A) and caudal (B) part of the posterior dorsal field in the AC. The spike count in both DRFs is normalized and coded in grayscale; the black dotted contour line represents the response threshold at 50% of the unit’s maximal spike rate. The echo delay/echo level combination eliciting the maximal response is marked by an asterisk (best delay, BD). The echo delay with the lowest threshold (characteristic delay, CD) is marked by a circle. BDs were 8.0 (A) and 20.0 ms (B), and CDs were 9.5 (A) and 20.0 ms (B). C: BDs of all recorded delay-sensitive units projected on the flattened surface of the AC. The position of each unit is marked by circles; the BD is coded in grayscale. The outlines of the AC are indicated by dashed lines and the borders of the 4 subfields by solid lines. AC outlines and subfields are according to Hoffmann et al. (2008). ADF, anterior dorsal field; AVF, anterior ventral field; PVF, posterior ventral field; PDF, posterior dorsal field.
The BDs of all 110 delay-sensitive units ranged from 3.0 to 26.0 ms (median: 9.2 ms), and the CDs from 2.0 to 29.0 ms (median: 9.5 ms). For both the BD and CD, short delays where overrepresented. Units in the frontal part of the PDF were mainly tuned to short echo delays, and both BD and CD increased along the rostrocaudal axis.
We calculated a cortical echo delay-tuning map for the PDF of the AC using the BDs and the stereotaxic coordinates of all delay-sensitive units (Fig. 2C). The echo delay-tuning map clearly shows an increase in the BD along the rostrocaudal axis as well as slightly increasing delays along the mediolateral axis. A correlation analysis (Spearman’s rank correlation) revealed a highly significant correlation between the BDs and the units’ rostrocaudal positions (correlation coefficient ρ = 0.66, P = 8.14 × 10−14) as well as a significant but weak correlation between the BDs and the units’ mediolateral positions (ρ = 0.34, P = 7.60 × 10−4).
The units’ CDs showed a similar distribution to that of the BDs along the rostrocaudal axis (ρ = 0.65, P = 2.77 × 10−14) with only slightly increasing delays along the mediolateral axis (ρ = 0.33, P = 1.00 × 10−3).
Object focusing in naturalistic flight sequences.
In the simulations of naturalistic flight sequences, data were recorded from a total of 107 units. For every presented sequence, the sum of spikes following each presented pulse/echo pair within the sequence was analyzed. Spike responses were determined for sequences presenting both targets individually and in combination. To determine the specific response of a unit to each target, the pulse/echo pair evoking the maximum response within sequences presenting only target 1 or target 2, respectively, was identified. Eighty-six of 107 units (80%) showed a facilitated (see methods) and specific response to both individually presented targets for at least one of the simulated echolocation pulse rates. In detail, 83/107 (78%) units showed specific responses for both individually presented objects in sequences with a constant pulse rate, 58/107 (54%) responded to the sequences with a linearly increasing pulse rate, and 58/107 (54%) to the sequences with simulated strobe groups, respectively.
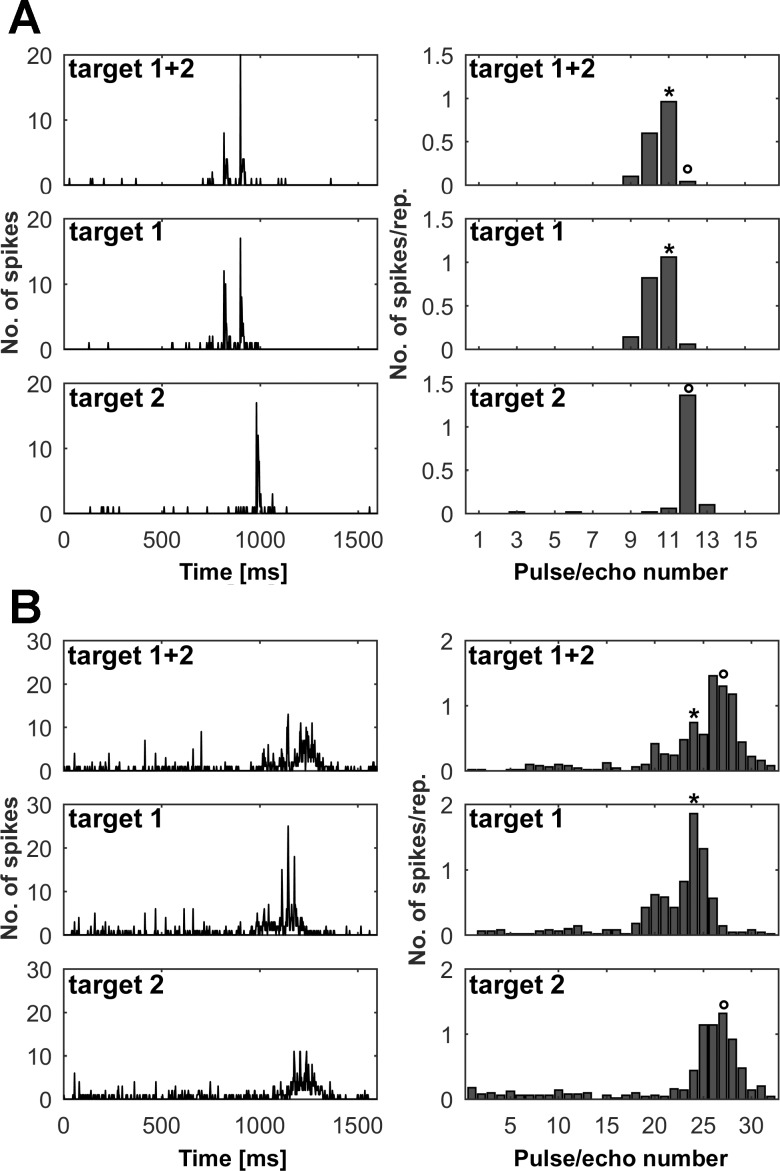
Figure 3 shows two examples of units responding in the presented pulse/echo sequences. The PSTHs in Fig. 3A, left, show clear responses of a unit to the individual presentations of target 1 and target 2, respectively (middle and bottom panels). The PSTH of the combined presentation of both targets (top panel), however, clearly resembles the individual presentation of target 1, whereas the response to target 2 is missing. The analysis of the sum of spikes for each pulse/echo pair within the sequence (Fig. 3A, right) in the combined presentation of both targets shows that the unit responded equally to target 1 (>50% of the response in the individual presentation) but that the response to target 2 was strongly reduced (<50% of the response in the individual presentation). This means that the unit focused to target 1 in the complex flight sequence. Figure 3B illustrates an example of a unit focusing on the second target while the response to target 1 is significantly reduced (<50% of the response in the individual presentation).
Fig. 3.
Examples of units focusing on different targets. A and B: neuronal responses of 2 different units (A and B, respectively) in naturalistic flight sequences. For each, left panels show the PSTH (bin width = 2 ms) and right panels show the mean number of spikes per repetition (50 repetitions) for every pulse/echo pair within the sequence. Top row shows the response to the sequence where both targets were presented together. Middle and bottom rows show the responses of the sequences where only target 1 or target 2 were presented, respectively. Asterisks mark the pulse/echo pair evoking the highest spike rate for target 1, and circles indicate the response to target 2. The acoustic stimulation in each sequence is preceded by a 50-ms silent period to determine the level of spontaneous activity and is followed by another 200-ms silent period. The unit in A shows a clear response to both targets in the individual presentations while focusing on target 1 in the complex flight sequence. The sequence was simulated with a constant pulse rate. The unit in B is focusing on target 2 in a sequence with a linearly increasing pulse rate.
In total, in the simulations of flight sequences with a constant pulse rate, 32/83 units (39%) responded equally to both targets in the complex sequence, 29/83 (35%) focused their response on target 1, and 9/83 (11%) focused on target 2. In the records of 10/83 (12%) units, the response decreased for both targets. In 3/83 records (4%), the responses for both targets overlapped.
Because we were interested in determining whether increasing pulse rates simulating a target approach phase had any impact on the cortical object representation, we also tested sequences with linearly increasing pulse rates and strobe groups for the same units whenever possible. Since not all neurons exhibited a facilitated and specific response for all simulated pulse rates with respect to our strict criteria (see methods), fewer neurons could be analyzed in sequences with increasing pulse rates.
In flight sequences with linearly increasing pulse rates, 26/58 (45%) units responded equally to both targets, 18/58 (31%) focused on target 1, and 11/58 (19%) focused on target 2. Only 3 units (5%) showed a decrease in their spike rates for both presented objects. An example of a unit responding to a sequence with a linearly increasing pulse rate is shown in Fig. 3B. Note that as the interval between the pulses decreases from 83 to 27 ms within the sequence, the differences between successive pulse/echo pairs with respect to echo delay and echo level decline. Thus units usually responded in the last part of those sequences to more successive pulse/echo pairs compared with sequences with a constant pulse rate.
In the sequences simulating strobe groups, units responded similarly as in the sequences with linearly increasing pulse rates. Twenty-nine of 58 (50%) responded equally to both targets, 16/58 (28%) focused on target 1, and 9/58 (16%) focused on target 2. In the records of 4 units (7%), the responses decreased for both targets.
The presentation of the sequences at different pulse rates revealed no substantial differences in object selection. The overall tendency of neurons locking onto either target 1 or target 2 is similar for all pulse rates. In total, ~46% of the recorded neurons focused on one of the two targets in a complex flight sequence, and from these about two-thirds responded only to target 1 and about one-third focused on target 2.
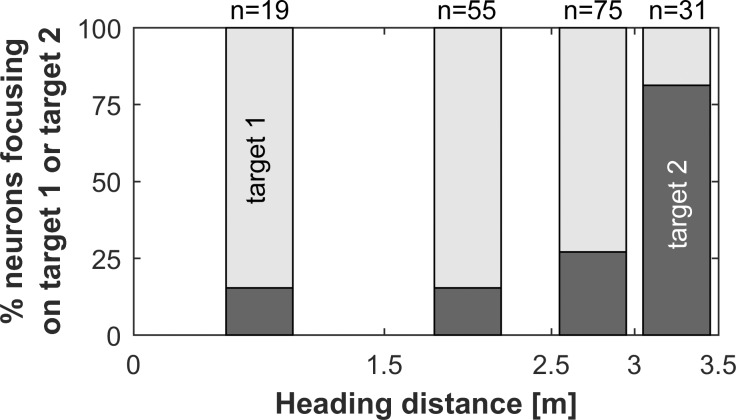
In general, neurons tended to focus mainly on target 1 if they responded during the first and middle part of the flight sequence (i.e., neurons tuned to long or medium echo delays). If they responded in the last section of the sequence (i.e., heading distance >3.0 m, short echo delays), where the angle to target 1 increased rapidly, they mainly focused on target 2 (Fig. 4). This indicates that the targets are processed sequentially in the auditory cortex of P. discolor.
Fig. 4.
Units’ focus depending on heading distance. Data are percentages of records where the units’ focus is on either target 1 (open bars) or target 2 (filled bars). Neurons in the first part of the flight sequence tend to focus on target 1. In the final part of the sequence (heading distance >3.0 m), neurons exhibit a preference for focusing on target 2. At a heading distance of 3.5 m, the bat passes target 1.
To investigate whether the selective response to specific objects within the sequence depended on single parameters, such as echo delay or echo level, alone or is a result of a complex interaction of multiple dynamically changing parameters, we further analyzed the echo levels and delays at the maximum response positions for all the units that focused on either target 1 or target 2. Because we had found no differences between different pulse rates in respect to object focusing, we combined the results from all of the simulated temporal pulse patterns for this analysis.
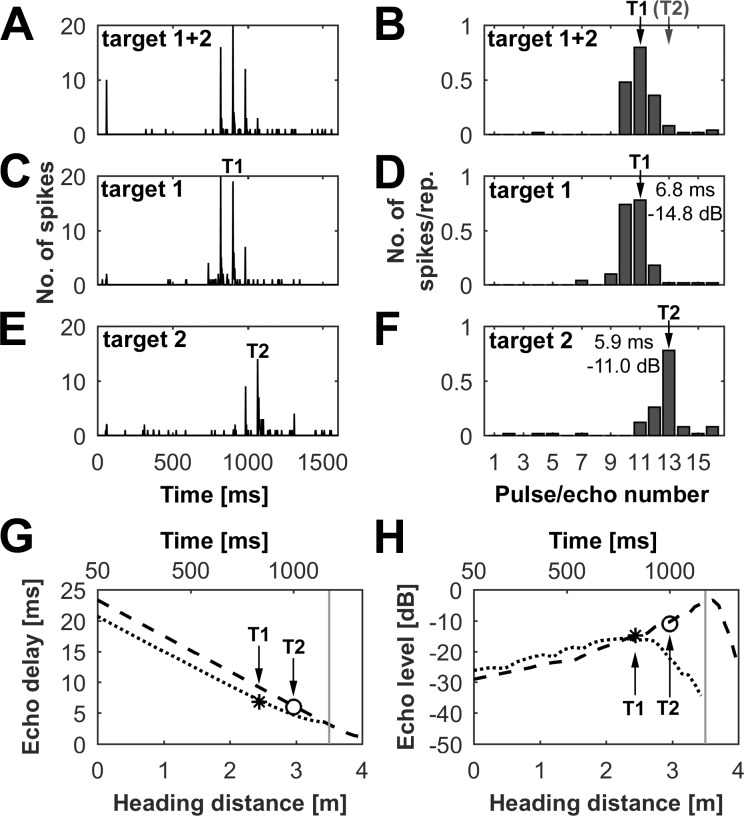
At first glance it seems most likely that in a complex pattern of incoming echoes, units might specifically respond to the loudest or earliest object echoes. However, our data point in the opposite direction: Fig. 5 shows an example of a unit specifically responding to target 1. The unit showed its maximum response to target 1 at an echo delay of 6.8 ms at an echo level of −14.8 dB. The unit’s maximum response to target 2 was at an echo delay of 5.9 ms at an echo level of −11.0 dB. This means that by locking onto target 1, the neuron selectively responded to the object that had the longer echo delay but the fainter echo level. It did not select the target with the loudest echoes within the sequence.
Fig. 5.
Echo delay and level of unit responding in naturalistic flight sequence. A–F: neuronal responses of cortical units in naturalistic flight sequences focusing on target 1. Shown are simulations with a sequence presenting both targets (A and B), a sequence presenting only target 1 (C and D), and a sequence with only target 2 (E and F). For each sequence, the PSTH (bin width = 2 ms; A, C, E) and the mean number of spikes per repetition (B, D, F) are shown for every pulse/echo pair within the sequence. For each target (T1, target 1; T2, target 2), the maximum response within the sequence is indicated by an arrow. The echo delay and echo level at each maximum response position are indicated in the bar plots in D and F. The acoustic stimulation in each sequence is preceded by a 50-ms silent period to determine the level of spontaneous activity and is followed by another 200-ms silent period. G and H: progression of echo delay (G) and echo level (H) for target 1 (dotted line) and target 2 (dashed line). The delay and level at the maximum response of T1 (asterisk) and T2 (circle) are indicated.
To analyze the temporal relationship of pulse and echoes of the two objects in detail, one can look at two independent measures: 1) relative timing of echoes after each pulse and 2) absolute echo delay (representing the physical distance to the target) at maximum response.
-
1)
In all presented virtual pulse/echo sequences, the bat receives after each pulse first the echo from target 1 and after that the echo from target 2 (Fig. 1E) until it begins to pass target 1 at a 3.50-m heading distance. When the bat reaches a heading distance of 3.43 m, the temporal order of the echoes is reversed. After a 3.43-m heading distance, the bat receives the echo from target 2 before the echo from target 1 (see Fig. 1F). However, note that only in sequences with linearly increasing pulse rate, a single pulse/echo pair was simulated between the 3.43- and 3.50-m heading distances. Our data clearly show that about one-third of the recorded neurons have already focused on target 2 before the bat passes target 1 at the 3.5-m heading distance (see Fig. 4). This means most of these neurons selectively responded not to the first but to the second echo after the pulse. Because of this, we can exclude forward suppression or synaptic depression as underlying mechanisms for target preference, at least for units that lock onto target 2.
-
2)
In the analysis of all recorded units in respect to echo delay at each maximum response position (see Fig. 5, G and H), about one-half of the neurons (48%) selectively responded to the object with the shorter echo delay (i.e., shorter target distance), and the other half (52%) to the object with the longer echo delay (i.e., longer target distance). These results further indicate that the focusing on specific objects within the sequences cannot be a direct consequence of the echo delay.
The analysis of the echo levels revealed that in most of the records (92%), the target 2 echo level exceeded that of target 1 at the maximum response positions. Since more neurons locked onto target 1 than on target 2, ~61% of the units selected the object with the lower echo level. Consequently, the response to specific objects cannot result from echo level alone.
These results show that neurons did not focus on the earliest, loudest, or most dominant object echo, but rather that target focusing is caused by a complex interaction consisting of multiple dynamically changing parameters (i.e., echo-acoustic flow).
Cortical delay tuning in naturalistic flight sequences.
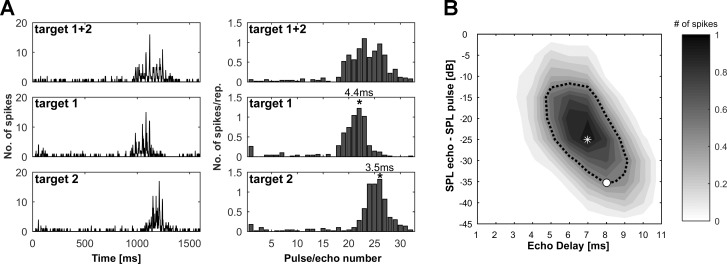
In addition to the cortical object representation, we analyzed the specific delays of all units that showed facilitated and specific responses to both individually presented targets for each pulse rate. Figure 6A shows an example of a unit’s response in sequences with a linearly increasing pulse rate. In the sequence where only target 1 was presented, the highest spike rate was evoked at pulse/echo pair 22, which corresponded to an echo delay of 4.4 ms. In the simulation presenting only the target 2, the highest response is evoked later in the sequence at pulse/echo pair 26, which corresponded to an echo delay of 3.5 ms. Note that the specific delay for target 2, which is the object closer to the bat’s flight path (see Fig. 1A) is shorter than the specific delay for target 1. Figure 6B shows the DRF from the same unit. In the static delay response field, the unit is selective for significantly longer delays than in the dynamic flight sequence (BD = 7.0 ms, CD = 8.0 ms).
Fig. 6.
Specific delay in naturalistic flight sequence compared with the static delay response field. A: neuronal response of a facilitated unit to naturalistic flight sequences with linearly increasing pulse rates. Left panels show the PSTH (bin width = 2 ms), and right panels show the mean number of spikes per repetition for each pulse/echo pair. Top row shows the response to the sequence where both targets were presented together; middle and bottom rows show the responses of sequences where only target 1 or target 2 was presented, respectively. The acoustic stimulation in each sequence is preceded by a 50-ms silent period to determine the level of spontaneous activity and is followed by another 200-ms silent period. An asterisk marks the pulse/echo pair evoking the highest spike rate for the sequences with only target 1 or target 2. The specific delays are indicated at each maximum response position. B: the DRF for the same unit as shown in A. The asterisk marks BD, and the circle marks CD. Note that the BD of 7.0 ms is significantly longer than the specific delays in the naturalistic flight sequence.
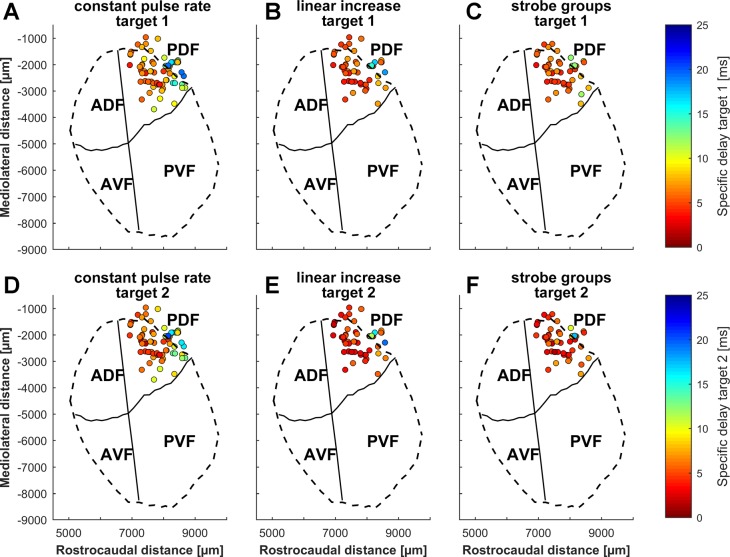
Cortical echo delay-tuning maps were calculated using the specific delays of all units tested for the different targets and pulse rates (Fig. 7). All maps for both targets and all simulated pulse rates show units with shorter specific delays in the frontal part of the PDF and increasing specific delays along the rostrocaudal axis. A correlation analysis revealed that there was a significant relationship between the rostrocaudal position and the specific delay for both objects and all simulated pulse rates (P < 0.001). The units’ mediolateral positions and specific delays showed no significant correlation. All calculated echo delay maps in Fig. 7 are tuned to noticeably shorter echo delays than the maps constructed from the delay response fields (Fig. 2C), and they show a striking overrepresentation of short delays.
Fig. 7.
Cortical distribution of specific delays from naturalistic flight sequences. Specific delays of all analyzed units are projected onto the flattened surface of the auditory cortex. A–C: specific delays of units for sequences where only target 1 was presented. D–F: specific delays of units for sequences where target 2 was presented. The sequences were presented for simulations with a constant pulse rate (A and D), a linearly increasing pulse rate (B and E), and strobe groups (C and F). The outlines of the auditory cortex are indicated by dashed lines and the borders of the 4 subfields by solid lines. ADF, anterior dorsal field; AVF, anterior ventral field; PVF, posterior ventral field; PDF, posterior dorsal field.
Both calculated delay-tuning maps for simulations with a constant pulse rate (Fig. 7, A and D) revealed a tuning to considerably longer specific delays than in sequences with a linearly increasing pulse rate (Fig. 7, B and E) or strobe groups (Fig. 7, C and F). Furthermore, units, as shown in Fig. 6A, tended to respond at shorter echo delays for target 2 than for target 1.
To study these effects in detail, the distributions of the specific delays for both targets and the different pulse rates were statistically analyzed. The analysis included all units that responded in sequences for at least one of the simulated pulse rates. The tests revealed that there were significantly longer specific delays in simulations with a constant pulse rate than in those with a linearly increasing pulse rate (target 1: P < 0.001; target 2: P < 0.001) or for the strobe groups (target 1: P < 0.001; target 2: P < 0.001) for targets 1 and 2 (Fig. 8B). The specific delays in the sequences with a linearly increasing pulse rate were not significantly different from those of the strobe groups. Furthermore, units tended to respond at shorter delays to target 2 (the object nearer to the bats flight path) than to target 1 for all simulated pulse rates. The specific delays for a linearly increasing pulse rate (P < 0.001) and for the strobe groups (P < 0.001) differed significantly between both targets.
Fig. 8.
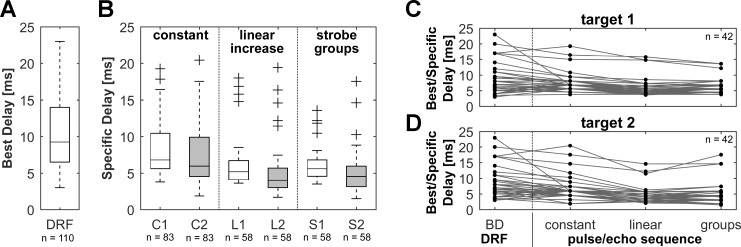
Distribution of specific delays for different targets and pulse rates. A: box plot with BDs from all units for which static delay response fields were recorded. The box represents the median (center line) and interquartile ranges (IQR) of the BDs. Whiskers indicate values within 1.5 × IQR. B: box plots with specific delays from all units that responded in naturalistic flight sequences with at least one of the simulated pulse rates. The boxes show the specific delays for each target and pulse rate. Whiskers indicate values within 1.5 × IQR; crosses represent outliers. Open boxes show specific delays for target 1 and gray boxes for target 2. Units in flight sequences with a constant pulse rate (C1, C2) responded at significantly longer delays than in flight sequences with linearly increasing pulse rates (L1, L2) or strobe groups (S1, S2). Note the different delay range of specific delays from the dynamic naturalistic flight sequences compared with the BDs from the static DRFs. C and D: BDs and specific delays for units that responded to static DRFs as well as to the presented flight sequences at all different pulse rates for target 1 (C) and target 2 (D). Note the consistent trend of decreasing echo delays in natural dynamic pulse/echo sequences at increasing pulse rates.
In total, the median specific delays for targets 1 and 2, respectively, were 6.8 and 6.0 ms for a constant pulse rate, decreased to 5.2 and 4.0 ms for a linearly increasing pulse rate, and were 5.6 and 4.5 ms for the simulated strobe groups. All of the units showed considerable shorter delays in the naturalistic flight sequences than in the static delay response fields (Fig. 8, A and B). The distribution of the BDs from the delay response fields differed significantly from those of the specific delays in all flight sequences (P < 0.001), except in the simulation of target 1 at a constant pulse rate.
The trend of decreasing best/specific delays could also be seen on a neuron-by-neurons basis, when we analyzed only neurons that responded in DRFs as well as in dynamic pulse/echo sequences for all simulated pulse rates (Fig. 8, C and D). The BDs from the static DRFs differed significantly from the specific delays in sequences with linearly increasing pulse rates (target 1: P < 0.01; target 2: P < 0.001) and at least for target 2 in sequences with strobe groups (target 1: not significant; target 2: P < 0.001). Furthermore, the specific delays in sequences presented at constant pulse rate differed significantly from those in sequences with linearly increasing pulse rates (target 1: P < 0.001; target 2: P < 0.001) and strobe groups (target 1: P < 0.01; target 2: P < 0.001). Because of the limited number of units in this analysis (n = 42), no significant differences could be found between BDs from the DRFs and specific delays in sequences with constant pulse rate.
DISCUSSION
In the present study, we investigated the cortical representation of echo streams originating from two simultaneously presented objects in simulated naturalistic flight sequences. Our data show that cortical neurons in bats can selectively respond to streams of echoes from specific objects in complex dynamic acoustic scenes. Furthermore, our results show that the dynamic stimulus presentation and changing temporal patterns of sonar emissions can have a substantial influence on the target range representation in the cortical map.
Basic delay-tuning properties.
We used a standard approach (see Hagemann et al. 2010; Hechavarría et al. 2013a; O’Neill and Suga 1979) of statically presented pairs of echolocation pulses and echoes with changing echo delays and echo levels to investigate the basic delay-tuning characteristics of cortical neurons. Neurons selective to such pairs of pulses and echoes were mainly found in the posterior dorsal field of the AC in P. discolor. Recent studies strongly indicate a chronotopic arrangement of delay-tuned neurons in this region (Bartenstein et al. 2014; Hoffmann et al. 2013). However, a standard approach to determine delay tuning was still missing.
Our results clearly show chronotopically arranged delay-tuned neurons in the PDF of the AC in P. discolor. BDs ranged from 3 ms in the rostral part and up to 26 ms in the caudal part of the PDF (median: 9.2 ms). This corresponds well to earlier findings on the spatiotemporal response characteristics in P. discolor (delay: 2–26 ms, median: 9.0 ms; Hoffmann et al. 2013) and other bats (Dear et al. 1993a; Hagemann et al. 2010; Hechavarría et al. 2013a; O’Neill and Suga 1982; Schuller et al. 1991; Wong et al. 1992).
Cortical object representation in dynamic flight sequences.
It has been proposed that bats perceptually organize acoustic information into echo streams to track specific objects during flight in complex environments. Many different acoustic parameters such as echo direction, intensity, timing, duration, and frequency are discussed to contribute to echo stream segregation in bats (Kanwal et al. 2003; Moss and Surlykke 2010). Although attention-based mechanisms might also play a role, neurophysiological studies on auditory stream segregation often suggest rather basic underlying neural processes such as frequency selectivity and short-term adaptation (e.g., Fishman et al. 2001; Micheyl et al. 2005). Recent studies further showed that forward suppression induced by naturalistic echo streams leads to a sharper tuning of cortical delay-tuned neurons (Beetz et al. 2016b).
In our study we used the simulation of naturalistic flight sequences to investigate the cortical representation of objects in complex, dynamic acoustic scenes and to study a possible neural basis of echo stream segregation in bats using naturalistic stimuli. Therefore, in our dynamic simulation many parameters covaried during acoustic stimulation (e.g., echo level, echo delay, echo reflection angles, and pulse rate). In the following, we discuss the possible influence of some of these parameters on echo stream segregation and cortical object representation.
Although all of the analyzed neurons could respond to both targets when presented individually, about half of them selectively responded to only one of these in a complex situation. Neurons tended to lock on the first target in the first and middle part of the pulse/echo sequence, whereas in the final part of the pulse/echo sequence, shortly before target 1 was passed, neurons showed a preference in responding only to target 2. This suggests that different acoustic targets are processed sequentially in a fly-by situation and that target preference is not directly influenced by any single parameters such as echo delay or level, but rather by a complex interaction of multiple dynamically changing parameters. To test this hypothesis, we analyzed echo amplitudes and timings for the neurons selectively to only one of the targets. At first glance, it seems likely that due to forward suppression, neurons might always respond only to the first echo in a series of arriving echoes from multiple objects (Luan et al. 2003; Wehr and Zador 2005). In our virtual flight sequences, after each pulse, the bats first receive the echo from target 1 and then the echo from target 2, until they pass target 1 at a 3.5-m heading distance. However, our data revealed that even before the bat in the simulated flight sequence actually passed target 1 (at 3.5-m heading distance), increasing numbers of neurons started to lock on target 2 (Fig. 4). This excludes a simple forward suppression mechanism due to the timing of the echoes.
In addition, echo level might have an influence on object focusing. In a complex stream of incoming echoes, a response of the neurons to the loudest or most dominant echoes seems likely. Our results, however, show that more than half of the focusing neurons specifically responded to the fainter echoes in the sequence. Only in the final part of the simulated flight sequence, shortly before target 1 was passed, might the increasing number of neurons focusing on target 2 be substantially influenced by the echo level. When the bats started to pass target 1, the echo angle rapidly increased, which led to drastically decreasing echo levels for target 1. However, because all of these neurons responded significantly to target 1 in the individual presentation, the echo levels were still above response threshold. Our data suggest that the focusing onto a specific object in a complex flight sequence depends not merely on a single parameter such as echo delay or level alone, but on complex interactions of many different dynamically changing parameters that, in their sum, make up echo-acoustic flow information.
Classical studies in auditory physiology using pairs or sequences of pure tones or click stimuli have investigated the influence of mechanisms such as forward suppression, synaptic depression, and facilitation or lateral inhibition on auditory processing (Oswald et al. 2006; Scholes et al. 2011; Wehr and Zador 2005). As mentioned above, we can exclude forward suppression as an underlying mechanism at least for all cells that specifically responded to target 2 while the response to target 1 was reduced. However, intracellular recordings would be required to provide more insights about mechanistic details.
It would be interesting to see if responses during the pulse/echo sequence could be predicted from the static DRFs. However, delay-tuning characteristics of neurons in the AC also critically depend on the pulse repetition rate (Wong et al. 1992). Therefore, such a prediction would most probably fail.
As shown above, increasing numbers of neurons started to lock on target 2 even before the bat in the simulated flight sequences actually passed target 1. In a recent study, Fujioka et al. (2016) showed in flight room experiments that bats also attended future target information to optimize their flight paths. Our findings may be interpreted in a similar way: when the bat has almost reached the position of the more closely located target 1, target 2 becomes more important for the bat and the neural representation of target 2 consequently starts to increase. Therefore, our findings may describe some kind of non-attention-driven neural mechanism that is important in bats for planning flight paths in complex environments.
Dear et al. (1993b) suggested a concurrent cortical representation of multiple objects at different distances. Our data, however, indicate a sequential processing of targets at the level of single neurons. It needs to be shown in future experiments whether the population of delay-tuned neurons can process multiple objects at different positions in the auditory cortex simultaneously. Therefore, simultaneous recordings from multiple positions in the cortical target distance map by using multielectrode arrays might help to clarify this question. Beetz et al. (2016a) showed that neurons respond best to echoes from the nearest target in sequences containing echoes from multiple objects positioned behind each other and that the representation of the other objects is suppressed in larger parts of cortical target range map.
Impact of pulse emission pattern on object representation and delay tuning.
We simulated different pulse rates to investigate the influence of different temporal pulse patterning on object representation. Other studies reported a greater prevalence of sonar sound groups for bats hunting close to a cluttered background than in open space. It was suggested that these sonar sound groups have immediate consequences for the bat’s perception of space and enhance spatiotemporal accuracy in tracking and figure ground segregation (Kothari et al. 2014; Moss et al. 2006; Moss and Surlykke 2001). In our study, the different simulated pulse rates had no direct influence on target focusing, but they did have a significant impact on the target range distribution, thereby restricting the cortical object representation at high pulse rates to close range objects.
The distribution of specific delays and thereby the target range map is shifted in a shorter range at increasing pulse rates compared with a constant pulse rate and especially to the statically presented pulse/echo pairs (see Fig. 8). These findings corroborate earlier studies on the influence of pulse repetition rate on delay-tuned neurons (O’Neill and Suga 1982; Tanaka and Wong 1993; Wong et al. 1992) and further emphasize the functional significance of this delay shift: the bat focuses its target range map to the most relevant objects and suppresses irrelevant background clutter while increasing the pulse rate during target approach.
Auditory scene analysis in complex natural environment.
Over the past 20 years, auditory scene analysis and stream segregation has been intensively studied in behavioral and neurophysiological studies in humans and different vertebrates, in most cases using a classical pattern of alternating pure tones (Bregman 1990; Fay 1998; MacDougall-Shackleton et al. 1998; van Noorden 1975). Neurophysiological studies suggest that in addition to an influence of attention on the organization of sounds, basic neural processes such as short-term adaptation and frequency selectivity play a major role in stream segregation (Scholes et al. 2015). In the present study, we show that selective focusing on one object in two streams of biosonar information cannot simply be related to short-term adaptation during processing of echo delay or echo amplitude alone: neither the object reflection with the shortest delay nor the object reflecting the echo with the largest amplitude was the one the neurons were focusing to in their response. Focusing rather depended on the complex dynamic changes of echo parameters occurring during flight, i.e., echo-acoustic flow information.
Conclusion.
This study provides insights into the neural processing underlying auditory scene analysis in bats. Neurons in the auditory cortex of anesthetized bats can separate different echo streams and selectively lock onto echoes from specific objects in a complex dynamic environment while the representation of other objects is suppressed. Furthermore, our data show a crucial influence of dynamic naturalistic stimulations on the cortical target range representation. Our results demonstrate that the selective representation of streams of acoustic signals in mammals depends on the integration of multiple dynamically changing acoustic parameters.
GRANTS
This work was supported by German Research Foundation (DFG) Grant FI 1546/4-1 (to U. Firzlaff).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.G. and U.F. conceived and designed research; W.G. performed experiments; W.G. analyzed data; W.G. and U.F. interpreted results of experiments; W.G. prepared figures; W.G. and U.F. drafted manuscript; W.G. and U.F. edited and revised manuscript; W.G. and U.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Prof. Dr. L. Wiegrebe for providing the experimental animals.
REFERENCES
- Bartenstein SK, Gerstenberg N, Vanderelst D, Peremans H, Firzlaff U. Echo-acoustic flow dynamically modifies the cortical map of target range in bats. Nat Commun 5: 4668, 2014. doi: 10.1038/ncomms5668. [DOI] [PubMed] [Google Scholar]
- Beetz MJ, Hechavarría JC, Kössl M. Cortical neurons of bats respond best to echoes from nearest targets when listening to natural biosonar multi-echo streams. Sci Rep 6: 35991, 2016a. doi: 10.1038/srep35991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beetz MJ, Hechavarría JC, Kössl M. Temporal tuning in the bat auditory cortex is sharper when studied with natural echolocation sequences. Sci Rep 6: 29102, 2016b. doi: 10.1038/srep29102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman AS. Auditory Scene Analysis. The Perceptual Organization Of Sound. Cambridge, MA: MIT Press, 1990. [Google Scholar]
- De Mey F, Reijniers J, Peremans H, Otani M, Firzlaff U. Simulated head related transfer function of the phyllostomid bat Phyllostomus discolor. J Acoust Soc Am 124: 2123–2132, 2008. doi: 10.1121/1.2968703. [DOI] [PubMed] [Google Scholar]
- Dear SP, Fritz J, Haresign T, Ferragamo M, Simmons JA. Tonotopic and functional organization in the auditory cortex of the big brown bat, Eptesicus fuscus. J Neurophysiol 70: 1988–2009, 1993a. http://jn.physiology.org/content/70/5/1988. [DOI] [PubMed] [Google Scholar]
- Dear SP, Simmons JA, Fritz J. A possible neuronal basis for representation of acoustic scenes in auditory cortex of the big brown bat. Nature 364: 620–623, 1993b. doi: 10.1038/364620a0. [DOI] [PubMed] [Google Scholar]
- Fay RR. Auditory stream segregation in goldfish (Carassius auratus). Hear Res 120: 69–76, 1998. doi: 10.1016/S0378-5955(98)00058-6. [DOI] [PubMed] [Google Scholar]
- Firzlaff U, Schörnich S, Hoffmann S, Schuller G, Wiegrebe L. A neural correlate of stochastic echo imaging. J Neurosci 26: 785–791, 2006. doi: 10.1523/JNEUROSCI.3478-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firzlaff U, Schuller G. Spectral directionality of the external ear of the lesser spear-nosed bat, Phyllostomus discolor. Hear Res 181: 27–39, 2003. doi: 10.1016/S0378-5955(03)00164-3. [DOI] [PubMed] [Google Scholar]
- Fishman YI, Reser DH, Arezzo JC, Steinschneider M. Neural correlates of auditory stream segregation in primary auditory cortex of the awake monkey. Hear Res 151: 167–187, 2001. doi: 10.1016/S0378-5955(00)00224-0. [DOI] [PubMed] [Google Scholar]
- Fujioka E, Aihara I, Sumiya M, Aihara K, Hiryu S. Echolocating bats use future-target information for optimal foraging. Proc Natl Acad Sci USA 113: 4848–4852, 2016. doi: 10.1073/pnas.1515091113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann C, Esser K-H, Kössl M. Chronotopically organized target-distance map in the auditory cortex of the short-tailed fruit bat. J Neurophysiol 103: 322–333, 2010. doi: 10.1152/jn.00595.2009. [DOI] [PubMed] [Google Scholar]
- Hechavarría JC, Macías S, Vater M, Mora EC, Kössl M. Evolution of neuronal mechanisms for echolocation: specializations for target-range computation in bats of the genus Pteronotus. J Acoust Soc Am 133: 570–578, 2013a. doi: 10.1121/1.4768794. [DOI] [PubMed] [Google Scholar]
- Hechavarría JC, Macías S, Vater M, Voss C, Mora EC, Kössl M. Blurry topography for precise target-distance computations in the auditory cortex of echolocating bats. Nat Commun 4: 2587, 2013b. doi: 10.1038/ncomms3587. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Firzlaff U, Radtke-Schuller S, Schwellnus B, Schuller G. The auditory cortex of the bat Phyllostomus discolor: localization and organization of basic response properties. BMC Neurosci 9: 65, 2008. doi: 10.1186/1471-2202-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S, Warmbold A, Wiegrebe L, Firzlaff U. Spatiotemporal contrast enhancement and feature extraction in the bat auditory midbrain and cortex. J Neurophysiol 110: 1257–1268, 2013. doi: 10.1152/jn.00226.2013. [DOI] [PubMed] [Google Scholar]
- Izumi A. Auditory stream segregation in Japanese monkeys. Cognition 82: B113–B122, 2002. doi: 10.1016/S0010-0277(01)00161-5. [DOI] [PubMed] [Google Scholar]
- Kanwal JS, Medvedev AV, Micheyl C. Neurodynamics for auditory stream segregation: tracking sounds in the mustached bat’s natural environment. Network 14: 413–435, 2003. doi: 10.1088/0954-898X_14_3_303. [DOI] [PubMed] [Google Scholar]
- Kothari NB, Wohlgemuth MJ, Hulgard K, Surlykke A, Moss CF. Timing matters: sonar call groups facilitate target localization in bats. Front Physiol 5: 168, 2014. doi: 10.3389/fphys.2014.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler K, Greiter W, Luksch H, Firzlaff U, Wiegrebe L. Echo-acoustic flow affects flight in bats. J Exp Biol 219: 1793–1797, 2016. doi: 10.1242/jeb.139345. [DOI] [PubMed] [Google Scholar]
- Lamme VA. The neurophysiology of figure-ground segregation in primary visual cortex. J Neurosci 15: 1605–1615, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan R, Wu F, Jen PHS, Sun X. Effects of forward masking on the responses of the inferior collicular neurons in the big brown bats, Eptesicus fuscus. Chin Sci Bull 48: 1748–1752, 2003. doi: 10.1360/02wc0570. [DOI] [Google Scholar]
- MacDougall-Shackleton SA, Hulse SH, Gentner TQ, White W. Auditory scene analysis by European starlings (Sturnus vulgaris): perceptual segregation of tone sequences. J Acoust Soc Am 103: 3581–3587, 1998. doi: 10.1121/1.423063. [DOI] [PubMed] [Google Scholar]
- McKerrow PJ. Acoustic flow. 2008 IEEE/RSJ International Conference on Intelligent Robots and Systems, Nice, 2008, p. 1365–1370. doi: 10.1109/IROS.2008.4650800. [DOI] [Google Scholar]
- Micheyl C, Tian B, Carlyon RP, Rauschecker JP. Perceptual organization of tone sequences in the auditory cortex of awake macaques. Neuron 48: 139–148, 2005. doi: 10.1016/j.neuron.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Morrison DW. Flight speeds of some tropical forest bats. Am Midl Nat 104: 189–192, 1980. doi: 10.2307/2424971. [DOI] [Google Scholar]
- Moss CF, Bohn K, Gilkenson H, Surlykke A. Active listening for spatial orientation in a complex auditory scene. PLoS Biol 4: e79, 2006. doi: 10.1371/journal.pbio.0040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss CF, Surlykke A. Auditory scene analysis by echolocation in bats. J Acoust Soc Am 110: 2207–2226, 2001. doi: 10.1121/1.1398051. [DOI] [PubMed] [Google Scholar]
- Moss CF, Surlykke A. Probing the natural scene by echolocation in bats. Front Behav Neurosci 4: 33, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JF, Suga N. Combination-sensitive neurons in the medial geniculate body of the mustached bat: encoding of target range information. J Neurophysiol 65: 1275–1296, 1991. [DOI] [PubMed] [Google Scholar]
- O’Neill WE, Suga N. Target range-sensitive neurons in the auditory cortex of the mustache bat. Science 203: 69–73, 1979. doi: 10.1126/science.758681. [DOI] [PubMed] [Google Scholar]
- O’Neill WE, Suga N. Encoding of target range and its representation in the auditory cortex of the mustached bat. J Neurosci 2: 17–31, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald A-MM, Schiff ML, Reyes AD. Synaptic mechanisms underlying auditory processing. Curr Opin Neurobiol 16: 371–376, 2006. doi: 10.1016/j.conb.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Pawluk D, Kitada R, Abramowicz A, Hamilton C, Lederman SJ. Haptic figure-ground differentation via a haptic glance. 2010 IEEE Haptics Symposium, Waltham, MA, 2010, p. 63–66. doi: 10.1109/HAPTIC.2010.5444676. [DOI] [Google Scholar]
- Pietsch G, Schuller G. Auditory self-stimulation by vocalization in the CF-FM bat, Rhinolophus rouxi. J Comp Physiol 160: 635–644, 1987. doi: 10.1007/BF00611936. [DOI] [Google Scholar]
- Portfors CV, Wenstrup JJ. Delay-tuned neurons in the inferior colliculus of the mustached bat: implications for analyses of target distance. J Neurophysiol 82: 1326–1338, 1999. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Lamme VA, Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature 395: 376–381, 1998. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- Rokni D, Hemmelder V, Kapoor V, Murthy VN. An olfactory cocktail party: figure-ground segregation of odorants in rodents. Nat Neurosci 17: 1225–1232, 2014. doi: 10.1038/nn.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rother G, Schmidt U [The influence of visual information on echolocation in Phyllostomus-discolor (Chiroptera).] Z Saugetierkd 47: 324–334, 1982. [Google Scholar]
- Scholes C, Palmer AR, Sumner CJ. Forward suppression in the auditory cortex is frequency-specific. Eur J Neurosci 33: 1240–1251, 2011. doi: 10.1111/j.1460-9568.2010.07568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes C, Palmer AR, Sumner CJ. Stream segregation in the anesthetized auditory cortex. Hear Res 328: 48–58, 2015. doi: 10.1016/j.heares.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schul J, Sheridan RA. Auditory stream segregation in an insect. Neuroscience 138: 1–4, 2006. doi: 10.1016/j.neuroscience.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Schuller G. A cheap earphone for small animals with good frequency response in the ultrasonic frequency range. J Neurosci Methods 71: 187–190, 1997. doi: 10.1016/S0165-0270(96)00142-2. [DOI] [PubMed] [Google Scholar]
- Schuller G, O’Neill WE, Radtke-Schuller S. Facilitation and delay sensitivity of auditory cortex neurons in CF-FM bats, Rhinolophus rouxi and Pteronotus p.parnellii. Eur J Neurosci 3: 1165–1181, 1991. doi: 10.1111/j.1460-9568.1991.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Schuller G, Radtke-Schuller S, Betz M. A stereotaxic method for small animals using experimentally determined reference profiles. J Neurosci Methods 18: 339–350, 1986. doi: 10.1016/0165-0270(86)90022-1. [DOI] [PubMed] [Google Scholar]
- Suga N, O’Neill WE. Neural axis representing target range in the auditory cortex of the mustache bat. Science 206: 351–353, 1979. doi: 10.1126/science.482944. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Wong D. The influence of temporal pattern of stimulation on delay tuning of neurons in the auditory cortex of the FM bat, Myotis lucifugus. Hear Res 66: 58–66, 1993. doi: 10.1016/0378-5955(93)90260-8. [DOI] [PubMed] [Google Scholar]
- Valentine DE, Moss CF. Spatially selective auditory responses in the superior colliculus of the echolocating bat. J Neurosci 17: 1720–1733, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderelst D, De Mey F, Peremans H, Geipel I, Kalko E, Firzlaff U. What noseleaves do for FM bats depends on their degree of sensorial specialization. PLoS One 5: e11893, 2010. doi: 10.1371/journal.pone.0011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noorden L. Temporal Coherence in the Perception of Tone Sequences (Dissertation). Eindhoven, The Netherlands: Technische Hogeschool Eindhoven, 1975. doi: 10.6100/IR152538. [DOI] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron 47: 437–445, 2005. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Wheeler AR, Fulton KA, Gaudette JE, Simmons RA, Matsuo I, Simmons JA. Echolocating big brown bats, Eptesicus fuscus, modulate pulse intervals to overcome range ambiguity in cluttered surroundings. Front Behav Neurosci 10: 125, 2016. doi: 10.3389/fnbeh.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D, Maekawa M, Tanaka H. The effect of pulse repetition rate on the delay sensitivity of neurons in the auditory cortex of the FM bat, Myotis lucifugus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 170: 393–402, 1992. doi: 10.1007/BF00191456. [DOI] [PubMed] [Google Scholar]