The nucleus of the solitary tract contains significant neuronal structures responsible for control of 1) cough excitability, 2) motor drive during cough, 3) cough phase timing, and 4) cough rhythmicity. Significant elimination of neurons in the solitary tract nucleus results in cough apraxia (incomplete and/or disordered cough pattern). The mechanism of the cough impairment is different from that for the concomitant changes in breathing.
Keywords: kynurenate, nucleus of solitary tract, cough, abdominal muscle activity, cough phase timing
Abstract
The importance of neurons in the nucleus of the solitary tract (NTS) in the production of coughing was tested by microinjections of the nonspecific glutamate receptor antagonist kynurenic acid (kyn; 100 mM in artificial cerebrospinal fluid) in 15 adult spontaneously breathing anesthetized cats. Repetitive coughing was elicited by mechanical stimulation of the intrathoracic airway. Electromyograms (EMG) were recorded from inspiratory parasternal and expiratory transversus abdominis (ABD) muscles. Bilateral microinjections of kyn into the NTS rostral to obex [55 ± 4 nl total in 2 locations (n = 6) or 110 ± 4 nl total in 4 locations (n = 5)], primarily the ventrolateral subnucleus, reduced cough number and expiratory cough efforts (amplitudes of ABD EMG and maxima of esophageal pressure) compared with control. These microinjections also markedly prolonged the inspiratory phase, all cough-related EMG activation, and the total cough cycle duration as well as some other cough-related time intervals. In response to microinjections of kyn into the NTS rostral to the obex respiratory rate decreased, and there were increases in the durations of the inspiratory and postinspiratory phases and mean blood pressure. However, bilateral microinjections of kyn into the NTS caudal to obex as well as control vehicle microinjections in the NTS location rostral to obex had no effect on coughing or cardiorespiratory variables. These results are consistent with the existence of a critical component of the cough rhythmogenic circuit located in the rostral ventral and lateral NTS. Neuronal structures of the rostral NTS are significantly involved specifically in the regulation of cough magnitude and phase timing.
NEW & NOTEWORTHY The nucleus of the solitary tract contains significant neuronal structures responsible for control of 1) cough excitability, 2) motor drive during cough, 3) cough phase timing, and 4) cough rhythmicity. Significant elimination of neurons in the solitary tract nucleus results in cough apraxia (incomplete and/or disordered cough pattern). The mechanism of the cough impairment is different from that for the concomitant changes in breathing.
the nucleus of the solitary tract (NTS) includes complex neuronal circuits of high importance for the control of numerous body functions (Bauman and Wang 2004; Boscan et al. 2002; Li et al. 1999; Mifflin 1992; Paton et al. 1999; Zubcevic and Potts 2010). The NTS is a primary input and transmission site for afferent signals from baroreceptors, chemoreceptors, airways, and lung receptors (Hayakawa et al. 2001; Lipski et al. 1976, 1991; Mifflin 1992; Mifflin et al. 1988; Miura and Takayama 1986). Distinct airway sensors send their axons to the separate NTS subregions. In particular, slowly adapting receptors terminate rostral to obex, primarily in dorsal, lateral, and ventral subnucleus (Berger and Dick 1987; Davies et al. 1987); irritant rapidly adapting receptors terminate mostly in the commissural and medial subnucleus caudal to the obex (Davies and Kubin 1986; Kubin and Davies 1988; Lipski et al. 1991); and bronchopulmonary C fibers terminate in the medial and parvicellular subregions as well as caudally in the dorsal commissural NTS (Kubin et al. 1991). Most recently, novel sensory receptors located primarily in the large airways have been identified and termed “cough receptors” (Mazzone et al. 2005) and are proposed to project to medial, intermediate, central, dorsomedial, and ventrolateral NTS subnuclei (Canning and Mori 2010).
The NTS is not just a “transmission station” but is a significant site of central processing of several afferent signals (Boscan et al. 2002) and their integration. Neurons with cough-related activity patterns have been found in the rostral NTS (rNTS) (Haji et al. 2012). They are hypothesized to not only have relay functions but participate in more complex functions such as regulation of cough excitability and/or spatiotemporal control of the cough motor pattern. Furthermore, it is hypothesized that the primary mechanism of this integration is convergence of multiple sources of peripheral and central efferent information (e.g., efference copy) that modify the excitability of NTS neurons (Lipski et al. 1976; Mifflin et al. 1988; Paton 1998; Paton et al. 1999; Richter et al. 1986; Silva-Carvalho et al. 1998).
Airway protection’s proposed use of this extensive “wiring” among NTS neuronal populations located in the NTS includes behaviors such as cough (Canning 2007; Haji et al. 2012; Jakus et al. 2008; Mazzone et al. 2005), sneeze (Wallois et al. 1995, 1997), and swallow (Amirali et al. 2001). Injection of central antitussives in the NTS suppresses cough in the rabbit (Mutolo et al. 2008), and administration of neuroactive substances influences cough production (Cinelli et al. 2013, 2015; Mutolo et al. 2007, 2014).
To further test this hypothesis, we employed a method of reversible chemical depression of NTS neuronal activity with the broad-spectrum compound kynurenic acid (kyn). kyn is a nonselective blocker of ionotropic excitatory amino acid receptors and nicotinic α7 receptors and likely also has other activities (Alkondon et al. 2004; Yamanashi et al. 2002). As such, it represents a useful tool for investigation of the contribution of local circuits to the production of behaviors when little information is known regarding specific neurotransmitters and neuromodulators in that location. Nonspecific interventions have traditionally been first-line approaches to the investigation of circuits that are hypothesized to participate in rhythmogenesis. Moreover, kyn is endogenous and is elevated in the brains of patients with Alzheimer’s disease (Wu et al. 2013), a patient group who are known to suffer from significant deficits in airway protection.
We hypothesized that blocking of neuronal function in the region of NTS would result in a suppression of cough and in alterations of cough phase timing including perturbations of the cough rhythm during bouts of repetitive coughing.
MATERIALS AND METHODS
Experiments were performed on 15 cats of both sexes (4.46 ± 0.25 kg). Ten animals underwent a single microinjection protocol, and the remaining five underwent multiple randomized protocols (either control or at different NTS locations). At least 3 h elapsed between kyn microinjections and any other protocol (as suggested by, e.g., Mutolo et al. 2007). The animals were anesthetized with pentobarbital sodium (35 mg/kg iv initially, then 1–3 mg/kg iv supplementary as needed). Atropine (0.1 mg/kg iv) was given at the beginning of the experiment to reduce airway secretions. The trachea and femoral artery and vein were cannulated. The animals were allowed to spontaneously breathe oxygen-enriched air (∼40% oxygen, balance nitrogen). Arterial blood pressure, respiratory rate, end-tidal CO2 (ETCO2), and body temperature were continuously monitored (temperature was maintained at 38.0 ± 0.5°C by a heating pad). Periodically, samples of arterial blood were removed for blood gas and pH analysis. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the University of Florida Institutional Animal Care and Use Committee.
Electromyograms (EMG) were recorded with bipolar insulated fine-wire hook electrodes bilaterally from the expiratory transversus abdominis (ABD) muscle and inspiratory parasternal (PS) muscles. The PS electrodes were placed at T3 proximal to the sternum after the surface of the muscle was exposed. The ABD electrodes were placed 3–4 cm lateral to the linea alba. In addition, in some animals EMG recordings from the costal diaphragm were made. Diaphragm electrodes were placed percutaneously just ventral to the manubrium.
Animals were placed prone in a stereotaxic frame, and the dorsal surface of medulla was exposed by an occipital craniotomy. The surface of the brain stem was covered by warm paraffin oil. Microinjections of kyn (100 mM) in artificial cerebrospinal fluid (aCSF) were employed to suppress neuronal activity. Single-barrel glass micropipettes (tip diameter 18–40 μm) were used for pressure injection of the solutions. The tip of the micropipette was positioned under stereotaxic control into the region of caudal NTS (cNTS) or rNTS.
Microinjections were made bilaterally at each rostrocaudal location. The depth and lateral stereotaxic coordinates on the injections fell into narrow ranges. For microinjections into the rNTS directed to ventrolateral subnucleus, the lateral and depth coordinates were 2.3–2.7 mm lateral to the midline and 1.6–1.9 mm below the dorsal medullary surface. For microinjections into the cNTS directed to commissural subnucleus the lateral and depth coordinates were 1.2–1.5 mm lateral to the midline and 1.4–1.7 mm below the dorsal medullary surface. Rostrocaudal coordinates for the rNTS sites included one or two locations, depending on whether one or two sets of bilateral microinjections were made. Microinjections in the rNTS were made only at 1.5–1.8 mm rostral to the obex in one group of animals (n = 6) or at 1.5–1.8 mm rostral to obex and at 0.8–0.9 mm rostral to obex in another group of animals (n = 5). Microinjections into the cNTS were made at two rostrocaudal locations, −0.8 to −1.0 and −1.6 to −1.9 mm caudal to obex (n = 5).
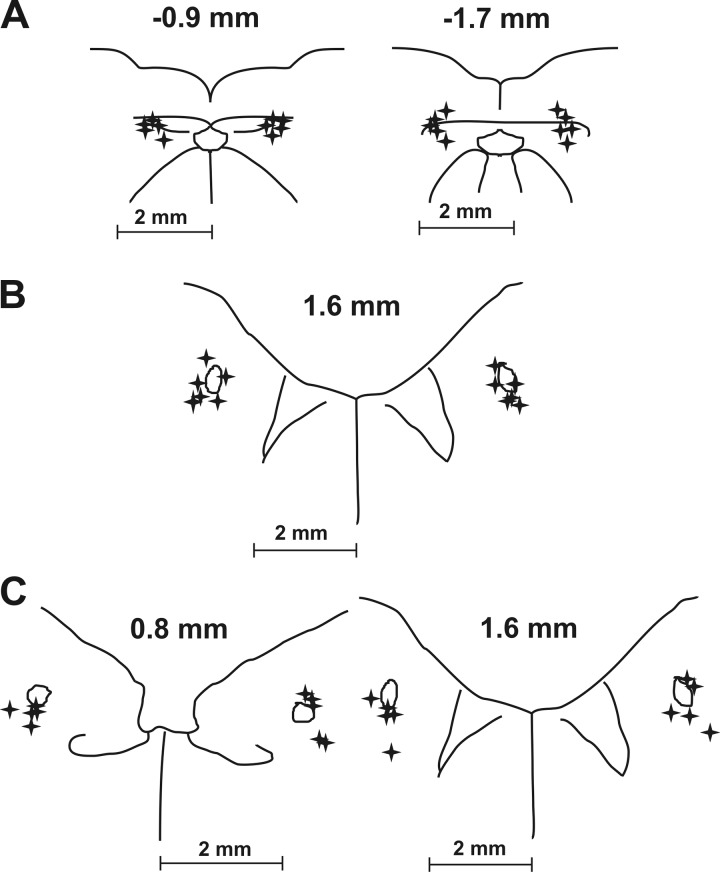
The injected volume was monitored by observation of movement of the meniscus in the micropipette with a microscope. Injection sites were labeled by fluorescent latex beads that were suspended in the injectate. After the experiment, the medulla was removed and the tissue was fixed in 4% paraformaldehyde followed by 30% sucrose solution. The frozen medulla was then cut into transverse slices (thickness 100 μm) by a freezing microtome. Sections were examined under light and UV microscopy for detection and localization of injection sites. Reconstruction of injected sites relative to the solitary tract and central canal is summarized in Fig. 1.
Fig. 1.
Reconstructions of kynurenic acid microinjection sites in the NTS. Drawings of transverse medullary sections are shown with microinjection locations labeled with stars. A: 5 caudal NTS series with 4 injections bilaterally (targeting commissural subnucleus) ~0.9 and ~1.7 mm caudal to the obex. B: 6 rostral NTS series (targeting ventrolateral subnucleus) with 2 injections bilaterally ~1.6 mm rostral to the obex. C: 5 rostral NTS series with 4 injections bilaterally ~0.8 and ~1.6 mm rostral to the obex.
Tracheobronchial cough was elicited by mechanical stimulation of the intrathoracic airways with a thin polyethylene catheter. This catheter was inserted into the trachea for 20 s (moved back and forth and rotated with frequency 1/s) to elicit repetitive coughing. Cough was defined by a large burst of inspiratory-related PS and/or diaphragm EMG activity immediately followed by a burst of expiratory ABD EMG activity. These criteria separated cough from other airway-defensive behaviors such as expiration reflex, augmented breath, and aspiration reflex (Poliacek et al. 2008).
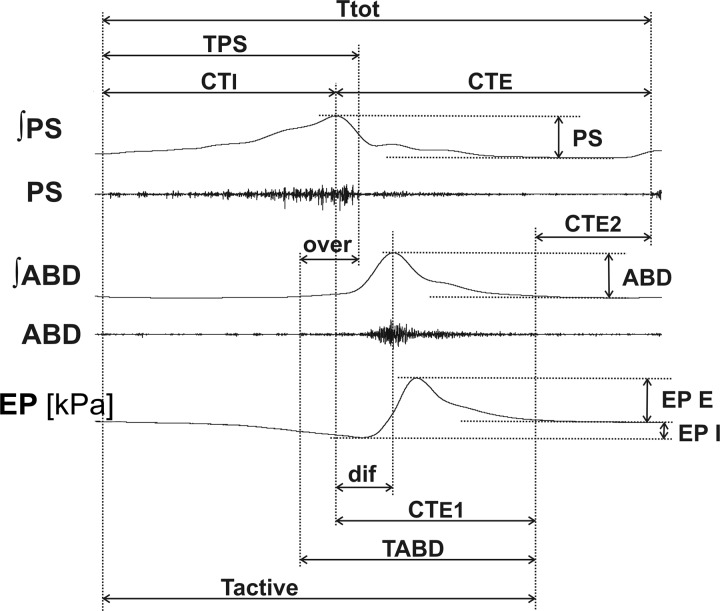
All EMGs were amplified, filtered (300–5,000 Hz), rectified, and integrated (moving average time constant 200 ms). The following cough data were analyzed: the number of coughs (CN) in response to mechanical stimulation of the trachea (cough number = average number of coughs per 20-s stimulation per trial), amplitudes of PS and ABD EMG moving averages and amplitudes of esophageal pressure during appropriate phases, the inspiratory (CTI, augmenting/elevating part of PS burst) and expiratory (CTE, from the peak of PS discharge to the end of cough cycle) phase durations, the active portion of cough expiration (CTE1, from the peak of PS activity to the end of cough-related ABD discharge), the expiratory “passive” period (CTE2, from the end of cough ABD discharge to the end of the cough cycle), total cough cycle time (Ttot), and duration of cough-related motor activity (Tactive).
In addition, other features of the cough motor pattern were analyzed: the decrementing (descending) parts of the PS bursts (desI), the overlap of cough-related PS and ABD discharge (over), the time between peaks of PS and ABD activity (dif), and the duration of cough-related burst of PS (TPS) and ABD (TABD). The variables for cough analysis are illustrated in Fig. 2. The data were compared in control preinjection and postinjection periods.
Fig. 2.
Illustration of cough measurements. ABD, abdominal muscle EMG; ʃABD, normalized moving average of the abdominal muscle EMG; CTE, duration of cough expiratory phase; CTE1, duration of active cough expiratory phase; CTE2, duration of quiescent period of cough expiration; CTI, duration of cough inspiratory phase; dif, time interval between peaks of parasternal and abdominal EMG activity; EP, esophageal pressure; EP E, EP I, expiratory and inspiratory esophageal pressure amplitudes, respectively; over, duration of parasternal and abdominal EMG coactivation; PS, parasternal muscle EMG; ʃPS, normalized moving average of parasternal muscle EMG; Tactive, duration of cough-related EMG activity; Ttot, total cough cycle duration; TABD, TPS, durations of abdominal and parasternal muscle discharge, respectively. Note that the decrementing part of the PS bursts (desI) corresponding to the interval TPS–CTI is not depicted.
Analyzed cardiorespiratory data included respiratory rate (RR), amplitudes of the PS EMG moving average and esophageal pressure during breathing, inspiratory, postinspiratory and expiratory phase durations (TI, TE1, TE2), mean arterial blood pressure, heart rate, and ETCO2. Monitored cardiorespiratory parameters were measured in the control period before the first microinjection and in the postinjection period ~5 min after the last microinjection was performed. To compare breathing and cough alterations, observations were also taken at approximately 10 and 20 min after microinjection and in the recovery period. RR and respiratory phase durations were calculated from three consecutive breathing cycles.
First, 15–25 consecutive cough stimulation trials, separated by ~1 min, were conducted to establish a stable cough baseline. Then, two control cough trials (20 s) were completed, separated by 10 min. After the microinjection, two trials were completed at 1) 5–15 min post, 2) 15–25 min post, 3) 90 min post, and 4) 180 min post.
Magnitudes of the moving averages during coughing (and breathing) were normalized relative to the mean intensities of control preinjection coughs. All cough parameters were averaged over all coughs induced during two trials during each segment of the protocol. Each of three protocols, cNTS kyn, rNTS kyn, and rNTS vehicle (aCSF) microinjections, was analyzed independently.
Results are expressed as means ± SE. For statistical analysis an ANOVA was applied with Student-Neuman-Keuls posttests, Kruskal-Wallis test with Dunn posttest, paired test, or Wilcoxon matched paired test as appropriate. The differences of variables were considered significant if P < 0.05.
RESULTS
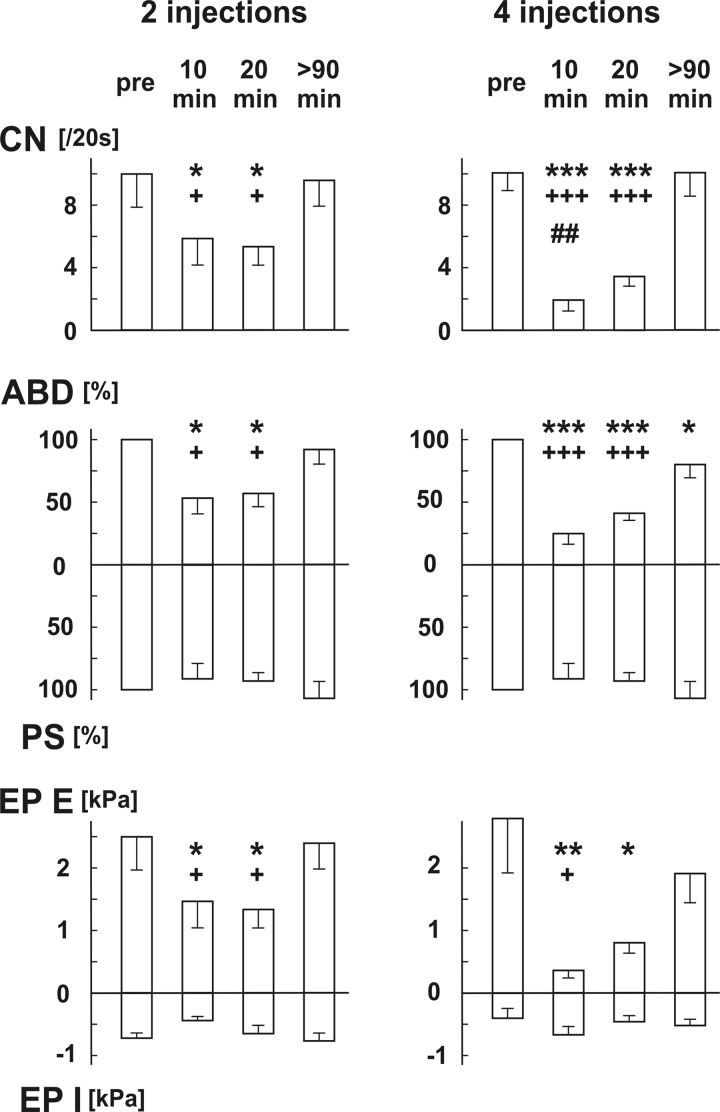
Microinjections of kyn in the rNTS (single injection 20–40 nl, total 7.99 ± 0.90 nmol, n = 11) noticeably reduced CN, cough expiratory efforts (Table 1; Fig. 3, Fig. 4), and prolonged cough temporal parameters (Table 1; Fig. 4), with the exception of CTE2. Cough changes were more pronounced in the 4-injection (23–34 nl, total 10.96 ± 0.36 nmol, n = 5) group compared with the 2-injection (20–40 nl, total 5.5 ± 0.4 nmol, n = 6; Fig. 3) group, statistically confirmed for CN within the 5–15 min interval (Fig. 3).
Table 1.
Cough parameters after kynurenic acid microinjections in rostral NTS
| Control | 5–15 min | 15–25 min | Recovery | P Value | |
|---|---|---|---|---|---|
| Cough number, motor drive, and mechanics | |||||
| CN (per 20 s) | 10.0 ± 1.2 | 4.1 ± 1.2***+++ | 4.5 ± 0.7***+++ | 9.8 ± 1.1 | <0.001 |
| ABD, % | 100 | 40 ± 9***+++ | 50 ± 7***+++ | 87 ± 8 | <0.001 |
| PS, % | 100 | 89 ± 11 | 90 ± 9 | 110 ± 11 | >0.05 |
| EP E, kPa | 24.1 ± 5.2 | 5.8 ± 1.7***++ | 8.1 ± 1.8***++ | 20.0 ± 4.3 | <0.001 |
| EP I, kPa | −5.6 ± 1.0 | −5.4 ± 0.8 | −5.5 ± 0.9 | −6.5 ± 0.9 | >0.05 |
| Cough phase durations | |||||
| CTI, s | 1.08 ± 0.11 | 3.31 ± 0.68***++ | 3.39 ± 0.52***++ | 1.21 ± 0.10 | <0.001 |
| CTE, s | 1.40 ± 0.14 | 2.76 ± 0.83 | 2.28 ± 0.43 | 1.32 ± 0.19 | >0.05 |
| CTE1, s | 0.63 ± 0.07 | 2.24 ± 0.87 | 1.77 ± 0.42 | 0.75 ± 0.06 | <0.05 |
| CTE2, s | 0.76 ± 0.12 | 0.52 ± 0.21 | 0.52 ± 0.26 | 0.57 ± 0.17 | >0.05 |
| Ttot, s | 2.47 ± 0.24 | 6.07 ± 1.36**++ | 5.47 ± 0.84*++ | 2.53 ± 0.26 | 0.001 |
| Tactive, s | 1.71 ± 0.15 | 5.55 ± 1.43**++ | 4.96 ± 0.88*+ | 1.96 ± 0.13 | <0.01 |
| Other temporal metrics of cough | |||||
| desI, s | 0.14 ± 0.01 | 1.64 ± 0.73*+ | 1.30 ± 0.40 | 0.21 ± 0.02 | <0.05 |
| dif, s | 0.18 ± 0.01 | 1.65 ± 0.70*+ | 1.19 ± 0.41 | 0.25 ± 0.02 | <0.05 |
| over, s | 0.55 ± 0.08 | 3.14 ± 1.16*+ | 2.96 ± 0.88*+ | 0.66 ± 0.12 | <0.01 |
| TPS, s | 1.21 ± 0.12 | 4.95 ± 1.31**++ | 4.44 ± 0.86*++ | 1.42 ± 0.11 | <0.001 |
| TABD, s | 1.04 ± 0.12 | 3.73 ± 1.31*+ | 3.48 ± 0.91*+ | 1.21 ± 0.15 | <0.05 |
| Cardiorespiratory data | |||||
| TI, s | 0.77 ± 0.07 | 2.57 ± 0.57***++ | 2.15 ± 0.32**++ | 0.88 ± 0.11 | <0.001 |
| TE1, s | 0.63 ± 0.09 | 2.64 ± 0.84*+ | 2.30 ± 0.86 | 0.77 ± 0.19 | <0.01 |
| TE2, s | 1.40 ± 0.18 | 0.96 ± 0.14* | 1.13 ± 0.19 | 0.92 ± 0.11* | <0.05 |
| RR, min−1 | 22.6 ± 1.5 | 13.3 ± 2.3***+++ | 14.0 ± 1.8***+++ | 26.6 ± 3.0 | <0.001 |
| PS ampl, % | 13.1 ± 2.2 | 24.3 ± 7.5 | 11.9 ± 2.4 | 18.4 ± 8.3 | >0.05 |
| EP ampl, kPa | 0.33 ± 0.08 | 0.44 ± 0.17 | 0.43 ± 0.16 | 0.26 ± 0.06 | >0.05 |
| ETCO2, mmHg | 31.7 ± 1.8 | 33.4 ± 2.4 | 35.0 ± 2.0 | 32.2 ± 2.1 | >0.05 |
| BP, mmHg | 130 ± 5 | 175 ± 9***+++ | 165 ± 7***+++ | 126 ± 5 | <0.001 |
| HR, min−1 | 188 ± 10 | 213 ± 11*** | 203 ± 11* | 203 ± 11* | <0.01 |
Values are means ± SE (n = 11). Data were pooled for both rNTS microinjection groups. Control, preinjection control; 5–15 min, 15–25 min, data within the time intervals after completion of microinjections; Recovery, data >90 min after microinjections; CN, number of coughs per 20-s stimulus duration (per 1 trial); ABD, amplitudes of abdominal muscle EMG; PS, amplitudes of parasternal muscle EMG; EP E, EP I, expiratory and inspiratory esophageal pressure amplitudes, respectively; CTI, duration of cough inspiratory phase; CTE, duration of cough expiratory phase; CTE1, duration of active cough expiratory phase; CTE2, duration of quiescent period of cough expiration; Ttot, total cough cycle duration; Tactive, duration of cough-related EMG activity; desI, descendent portion of cough parasternal bursting; dif, time interval between the peaks of parasternal and abdominal EMG activity; over, duration of parasternal and abdominal activity overlap; TPS, TABD, durations of cough-related parasternal and abdominal discharge, respectively; TI, inspiratory phase duration; TE1, postinspiratory phase duration; TE2, expiratory phase 2 duration; RR, respiratory rate; PS ampl, amplitudes of parasternal muscle EMG; EP ampl, amplitudes of esophageal pressure; ETCO2, end-tidal CO2 content; BP, mean arterial blood pressure; HR, heart rate. P value is for ANOVA.
P < 0.05,
P < 0.01,
P < 0.001 compared with preinjection control;
P < 0.05,
P < 0.01,
P < 0.001 compared with recovery >90 min after injection.
Fig. 3.
Changes in coughing induced by 2 (n = 6) and 4 (n = 5) microinjections of kynurenic acid in the rostral NTS. Values are means ± SE. pre, Preinjection control; 10 min, data 5–15 min after completion of microinjections; 20 min, data 15–25 min after completion of microinjections; >90 min, data in late recovery period; CN, number of coughs per 20-s stimulus duration (per 1 trial); ABD, normalized amplitudes of abdominal muscle EMG; PS, normalized amplitudes of parasternal muscle EMG; EP E, EP I, expiratory and inspiratory esophageal pressure amplitudes, respectively. *P < 0.05, **P < 0.01, ***P < 0.001 compared with preinjection; +P < 0.05, +++P < 0.001 compared with recovery >90 min after injection, respectively; ##P < 0.01 compared with 2 microinjections.
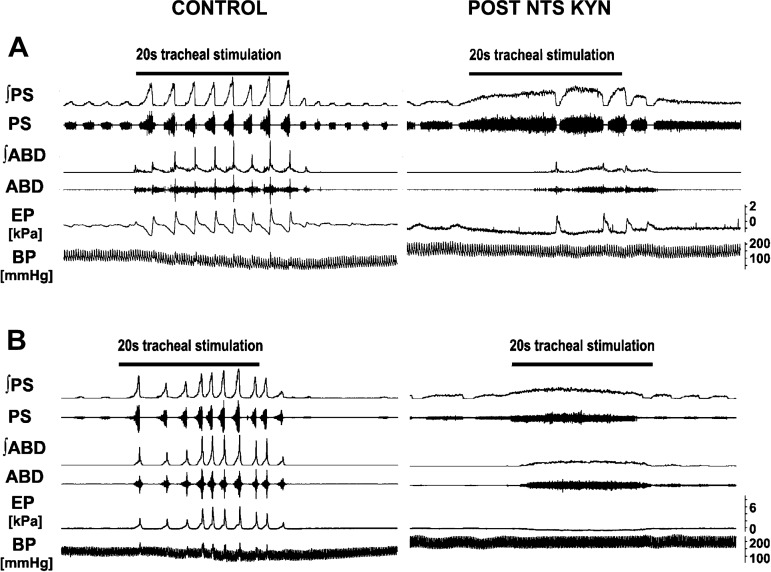
Fig. 4.
Changes in cough response induced by microinjections of kynurenic acid into the rostral NTS. Control: repetitive coughing was induced by mechanical stimulation of the intrathoracic trachea. Post NTS KYN: A: coughing ~12 min after 4 microinjections of kynurenic acid (on right). Note extremely long preparatory cough inspirations with “tonic” preexpulsive ABD activation during inspiratory cough phase starting from the 2nd cough and temporary reduction of this activity before the transition into cough expiration in the 3rd cough in the trial. B: within 10 min after microinjections of kynurenic acid, mechanical stimulation of the intrathoracic trachea frequently did not elicit coughing but produced apneusis and long-lasting coactivation of inspiratory and expiratory muscles (on right). ʃ, EMG moving average; PS, parasternal muscle EMG; ABD, abdominal muscle EMG; EP, esophageal pressure; BP, arterial blood pressure.
A profound CTI prolongation was evident in both groups, with ABD coactivation beginning in early CTI (see long-lasting coactivation of PS and ABD in Table 1; Fig. 4). Of note, a similar pattern was observed when the responses could not be classified as “cough” (Fig. 4B; typically early after microinjections). The cough inspiratory-expiratory phase transition was sometimes accompanied by depression of ABD activity before expulsion began.
Additionally, nonrhythmic “apneustic or apraxic” responses (disordered and/or incomplete “cough” motor pattern) to mechanical stimulation of the tracheobronchial tree and/or apneustic bursts of cough inspiratory activity (Fig. 4) were observed within 15 min in all 4-microinjection animals and 50% of 2-microinjection animals. These apneustic/apraxic cough episodes occurred even when the breathing rhythm had mostly recovered (Fig. 4B). Two animals had a complete elimination of the cough motor pattern within 5–15 min after microinjection (1 from each group).
At 90 min after rNTS microinjections cough parameters recovered (Table 1; Fig. 3), with the exception of three animals (2 with 4 microinjections and 1 with 2 microinjections) that recovered after 180 min.
Cardiorespiratory changes within 5 min included apneustic breathing (100% of animals with 4 injections and 50% of animals with 2 injections) lasting 5–10 min (in 2 cases up to 25 min); RR reduction from 22.6 ± 1.5 to 13.2 ± 1.5 min−1 (P < 0.001); prolongation of TI from 0.76 ± 0.07 to 2.52 ± 0.81 s (P < 0.05) and TE1 from 0.63 ± 0.09 to 2.51 ± 0.81 s (P < 0.05); and mean arterial blood pressure increase from 129 ± 4 to 152 ± 4 mmHg (P < 0.01). In the 4-microinjection group, heart rate increased from 170 ± 7 to 181 ± 6 min−1 (P < 0.01) and esophageal pressure amplitudes during breathing rose from 0.26 ± 0.09 to 0.82 ± 0.19 kPa (P < 0.05). A slight increase in ETCO2 (from 29 ± 2 to 33 ± 2 mmHg; P < 0.05) was seen in the 2-microinjection group. Data obtained during the stimulation periods at approximately 10 min and 20 min after microinjection and the recovery period are listed in Table 1.
Microinjection of kyn into the cNTS (4 microinjections of 23–95 nl, total 12.22 ± 1.56 nmol, n = 5, all P > 0.28, paired test) induced no significant changes in cardiorespiratory (RR from 20.8 ± 0.9 to 21.3 ± 1.8 min−1, TI 0.83 ± 0.07 to 0.84 ± 0.08 s, TE1 0.62 ± 0.10 to 0.74 ± 0.12 s, TE2 1.47 ± 0.17 to 1.33 ± 0.15 s, mean arterial blood pressure 131 ± 5 to 138 ± 6 mmHg, heart rate 177 ± 12 to 169 ± 11 min−1, respiratory esophageal pressure amplitudes 0.40 ± 0.12 to 0.40 ± 0.18 kPa, and ETCO2 35 ± 1 to 33 ± 2 mmHg; all P ≥ 0.15) and cough parameters relative to aCSF (vehicle) microinjected into the rNTS (4 microinjections of 27–33 nl, total 121.2 ± 1.9 nl, n = 6, all P > 0.17, paired test).
DISCUSSION
The unique finding of this study is that microinjections of kyn into the rNTS induced cough suppression, sequencing disruptions, and extreme prolongation of phase durations. The changes recovered within 2–3 h. Additionally, these cough alterations were more profound and longer-lasting than changes in the cardiorespiratory parameters. Of note, kyn had no effect on cough when microinjected into the cNTS.
The significant role of the NTS in the production of cough has been documented by studies in which neuroactive substances (including antitussives) were microinjected in rabbits and guinea pigs (Canning and Mori 2010; Cinelli et al. 2013, 2015; Haji and Ohi 2010; Mutolo et al. 2007, 2008, 2014). These findings are not surprising, given that pulmonary vagal afferents terminate on second-order neurons in NTS (Davies et al. 1987; Kalia and Mesulam 1980; Lipski et al. 1991). The identification of specific neurotransmitters/neuromodulators that contribute to regulation of the excitability of coughing by NTS circuits represents an important outcome of these prior experiments. For example, both NMDA and non-NMDA glutaminergic neurotransmission are important in cough regulation by the NTS (Canning and Mori 2010; Haji et al. 2012; Mutolo et al. 2007; Ohi et al. 2014; Saha et al. 1995). Although kyn is a well-known antagonist at ionotropic excitatory amino acid receptors (Motekaitis et al. 1996), its antagonist activity also extends to nicotinic α7 receptors, and the drug has other activities as well (Alkondon et al. 2004; Yamanashi et al. 2002). Our results generally support prior reports indicating the importance of excitatory amino acids in the production of cough by NTS pathways. However, the main purpose of using this particular drug was its broad-spectrum effect (i.e., nonspecificity). Our intent was to transiently disrupt neuronal activity, without cell death. Although functions of some cough-related neurons have been proposed (Haji et al. 2012), the actual roles, their connectivity, and the neuromodulators that control this network are poorly understood. The use of kyn represents a first step in a long-term approach to understanding the role of NTS circuits in the production of cough.
The micropipette tip was directed in the rNTS to the region of the ventrolateral subnucleus. Assuming a diffusion pattern of up to 500 μm from the pipette tip, the ventrolateral NTS and surrounding areas, mostly the solitary tract, dorsolateral (including interstitial) and ventral subnuclei were significantly covered by injectate (Fig. 1) in all cases. Depending on the actual positions of the micropipette tip (Fig. 1), in few cases the lateral, dorsal and medial (including intermediate) subnuclei of the NTS were also affected (Claps and Torrealba 1988; Davies and Kalia 1981). Our methodology does not allow more precise localization of the NTS subnuclei that were affected by the microinjections. However, cough-related neurons in the NTS are not restricted to a single subnucleus (Haji et al. 2012). Moreover, microinjections in the 2- and 4-microinjection groups targeted the same NTS subnuclei. We attribute the more pronounced effects of four microinjections on coughing to larger numbers of neurons being affected within the aforementioned subnuclei.
The activity patterns of several classes of neurons in the NTS are related to the cough motor pattern (Haji et al. 2012, 2013). Their significance in the production of cough is supported by plasticity of their responses to peripheral cough-provoking stimuli (Bonham et al. 2006). Central sensitization of coughing can be induced in anesthetized guinea pigs (executed via cough receptors; Canning and Mori 2011) involving administration of TRPV1 and neurokinin receptor agonists within NTS (Mazzone et al. 2005). Haji et al. (2012) proposed the presence of neurons that could contribute to the regulation of coughing in cats located 1.5 mm caudal to 2 mm rostral to obex in an area corresponding to sites that produced cough during microstimulation. Our data revealed no significant contribution of neurons affected by kyn caudal to obex on cough in the cat, consistent with results obtained in the guinea pig (Canning and Mori 2010).
In support of both Canning and Mori (2010) and Haji et al. (2012), our results indicate that the rNTS is an important area controlling the excitability and execution of coughing. We have proposed that there is a functional system that controls cough excitability in the brain stem, which has been termed a cough gate (Bolser 1996; Bolser et al. 1999; Bolser and Davenport 2002). This system is proposed to mediate the antitussive effect of centrally acting cough-suppressant drugs. However, in the cat the mode of action of virtually all of those drugs, whether administered systemically, centrally via the vertebral artery, or locally by microinjection into the brain stem, is to decrease CN and expiratory motor drive but not inspiratory activity during coughing (Bolser 1996; Bolser et al. 1999; Bolser and Davenport 2002). The present results do not rule out the participation of the rNTS in the proposed cough gating mechanism (Canning and Mori 2010), but the prolongation of cough phase durations and disruption of cough rhythmicity (Fig. 3) indicate a more expansive, possibly commanding role of this area in the cough generation circuit.
The most widely accepted model of the respiratory/cough neuronal network incorporates cough-related input from putative second-order cough neurons in NTS to many components of the cough network (Bolser et al. 2006; Shannon et al. 1998). Alterations in this widespread input result in modified cough phase timing (O’Connor et al. 2012). The primary substrate for generating the breathing and cough motor rhythm is proposed to be located in the rostral ventral respiratory column. However, microinjection of kyn into the rNTS resulted in significant prolongation of cough temporal features, in particular inspiratory, PS, all cough EMG, as well as the total cough cycle durations and the duration of phase transition from PS to ABD activity maxima during cough (Table 1). Moreover, we observed a high variability in the durations of individual coughs within a trial (see SE values in Table 1), reflecting significant disruption in cough rhythmicity. Note that all of the prolonged cough timing characteristics that we observed in response to microinjection of kyn into the rNTS were related to the very long cough inspiratory PS activation (Table 1) and to a transient cessation of cough rhythmicity when NTS function was disrupted by kyn (Fig. 4).
Interestingly, the duration of CTE2 was not significantly affected (Table 1). When cough receptors were activated within 10 min of the microinjections, very long cough inspiratory cycles with significant coactivation of expiratory muscles occurred, consistent with cough apraxia (inability to execute a normal cough motor pattern). Intervals between cough trials were marked by rhythmic breathing. However, activation of the cough receptor population resulted in transient cessation of all rhythmogenicity (neither rhythmic breathing nor rhythmic coughing occurred; Fig. 4B). This finding shows that in the absence of a cough rhythm there was not a “default” to a breathing rhythm. Thus the disruption of rNTS function resulted in an inability to produce rhythmic coughing and prompts us to suggest a revised role of NTS and the rostral ventral respiratory column neuronal circuits in the control of cough.
Our observations support one of two hypotheses: 1) the cough rhythm is produced solely by an NTS circuit, and the rhythmogenic circuit for breathing is effectively “conscripted” by it during coughing or 2) conditionally active elements of NTS circuit constitute a segment of the cough rhythmogenic circuit that cooperates with the rhythmogenic circuit for breathing (presumably including the pre-Bötzinger complex) to generate the cough rhythm, forming an anatomically distributed network that, like that for breathing, is driven by core elements in the rostral ventral respiratory column.
Both hypotheses are consistent with previous findings that inspiratory and expiratory neurons throughout the ventral respiratory column are modulated during coughing, with only one major neuron type (a subset of expiratory augmenting neurons in the Bötzinger complex: expiratory augmenting, E-AUG late) that does not undergo a significant increase in discharge rate during this behavior (Shannon et al. 1998). Both hypotheses also hold that this NTS circuit would have a command function in the production of rhythmic coughing, which is similar to that described for other behaviors in lower vertebrate and invertebrate circuits (Daghfous et al. 2016; Eaton et al. 2001). We believe the purpose for this circuit arrangement in the NTS is to place the rhythmogenic mechanism for coughing close to where the relevant sensory afferent input is processed in the brain stem. Consistent with this hypothesis, the rhythmogenic area for sneezing is proposed to be located in the ventromedial part of the spinal trigeminal nucleus and nearby lateral reticular formation (Nonaka et al. 1990), which are near to where sneeze-related afferent input enters the brain stem via the trigeminal nerve.
Microinjections of kyn into the rNTS reduced RR, prolonged TI and TE1, and shortened TE2 (Table 1 and results), consistent with other experiments that perturbed the function of neurons in the rNTS (Brodie and Borison 1957; Budzińska et al. 1985; Jakus et al. 1990; Wasserman et al. 2000). Cardiorespiratory changes induced by microinjection of kyn into the rNTS at the 10 min time point correspond well to those seen at 5 min after microinjection (Table 1 and results). The differences declined at 20 min and resolved in the recovery period (Table 1). Comparison of respiratory and cough alterations does not allow us to evaluate contribution of respiratory (certainly participating in cough control) vs. cough-specific neuronal components in the NTS to the cough changes observed. The sites of kyn microinjections in our experiments are near to the locations of pump cells in the cat (Davies et al. 1987). We propose that perturbation of pump cell activity by our microinjections resulted in the observed changes in breathing. However, it is unlikely that disruption of pump cell activity affected cough in the same manner. Pulmonary stretch receptor activity, essential in regulation of breathing pattern in vagal intact animals, has a different role in coughing. Modulation of the cough pattern by volume-related feedback has been observed only when cough volumes/airflows were very low or timed outside of the inspiratory phase (Poliacek et al. 2016). Under normal conditions there is no effect of volume-related feedback on phase timing during coughing in the anesthetized cat (Bolser and Davenport 2000). It has been proposed that slowly adapting stretch receptor feedback has a permissive effect on cough in the rabbit (Hanácek et al. 1984). However, this conclusion has been questioned recently (Canning and Mori 2011) because of the nonspecific effects of sulfur dioxide that was employed in those experiments.
Although there were significant changes in the breathing pattern induced by microinjections of kyn in the rNTS (Table 1), alterations in ETCO2 (Table 1) were not statistically significant. The lower RR was likely sufficiently compensated by deeper breathing (Table 1). In this regard, significantly higher respiratory amplitudes of EP in the 4-microinjection group correspond to more prominent respiratory changes induced by kyn in the rNTS. Given that ETCO2 did not change, it is unlikely that our microinjections significantly altered blood gases. Furthermore, alterations in coughing occur only during large changes in CO2 or O2 (Tatár et al. 1986; Nishino et al. 1989).
Microinjections of kyn into the cNTS had no effect on parameters of cardiorespiratory function or coughing in our anesthetized cats. The micropipette tips were directed to the commissural subnucleus of the cNTS (Claps and Torrealba 1988), and this area was significantly covered by our microinjections in all cats (Fig. 1). The cNTS is an important site for pulmonary C-fiber (Kubin et al. 1991) and rapidly adapting receptor (Davies and Kubin 1986; Kubin and Davies 1988; Lipski et al. 1991) interneurons. The nonspecific activity of kyn supports the concept that second-order interneurons mediating the sensory effects of these receptor types are not important in the neurogenesis of mechanically induced tracheobronchial cough in the anesthetized cat. These findings also support the recent work of Canning and coworkers, showing that a separate cough sensory receptor exists that has axonal projections to the rNTS, not the cNTS (Canning and Mori 2010). Mutolo and coworkers (Cinelli et al. 2013, 2015; Mutolo et al. 2007, 2008, 2014) have shown that microinjection of various receptor agonists and antagonists into the cNTS disrupts coughing in rabbits, partially through a mechanism involving changes in phase timing. Our results suggest that the neural substrate for cough that is affected by microinjections of kyn in the cat is localized primarily in the rNTS. The rabbit may have a more distributed NTS neural network related to cough, or it may be located more caudally than in the cat (Cinelli et al. 2013, 2015; Mutolo et al. 2007, 2008, 2014). Significant uncertainty exists regarding the full extent and neuronal identity of the NTS neural network for coughing in any species that has been employed to study cough.
The dosage and concentration of kyn employed in our experiments were selected on the basis of experiences with microinjection and the use of kyn by our group and others (Lipski et al. 1988; Mutolo et al. 2007; Poliacek et al. 2010) to alter activity of many neurons but within a restricted area of the NTS. The results of this study indicate that the dose of kyn that was chosen was sufficient to perturb the cough reflex. Our microinjections were unlikely to have modified the activity of neurons >0.5 mm away from the micropipette tip. For example, when kyn microinjections were placed more ventral in the lateral tegmental field (1 case; data excluded from the present analysis) there were no temporal alterations of cough motor pattern (although the cough number and expiratory cough efforts were reduced, consistent with Jakus et al. 2000). For more detailed discussion of mechanisms and limitation of the microinjection technique see, e.g., Lipski et al. (1988) and Poliacek et al. (2010).
In conclusion, our results are consistent with the existence of significant cough control components located in the rNTS distinct from processing of the primary cough afferent signals. These cough control elements are involved in the regulation of the cough excitability, cough efforts, and cough phase timing and are essential for the production of rhythmic coughing.
GRANTS
This project was supported by National Heart, Lung, and Blood Institute Grant HL-103415, VEGA Project 1/0072/16, and VEGA Project 1/0253/15.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
I.P., T.P., P.W.D., and D.C.B. conceived and designed research; I.P., T.P., M.J.R., M.S., M.V., Z.K., and D.C.B. performed experiments; I.P., M.S., M.V., and Z.K. analyzed data; I.P. and D.C.B. interpreted results of experiments; I.P., M.J.R., and D.C.B. prepared figures; I.P. and D.C.B. drafted manuscript; I.P., T.P., M.J.R., P.W.D., M.S., M.V., Z.K., and D.C.B. approved final version of manuscript; T.P. edited and revised manuscript.
REFERENCES
- Alkondon M, Pereira EF, Yu P, Arruda EZ, Almeida LE, Guidetti P, Fawcett WP, Sapko MT, Randall WR, Schwarcz R, Tagle DA, Albuquerque EX. Targeted deletion of the kynurenine aminotransferase ii gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via alpha7 nicotinic receptors in the hippocampus. J Neurosci 24: 4635–4648, 2004. doi: 10.1523/JNEUROSCI.5631-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirali A, Tsai G, Schrader N, Weisz D, Sanders I. Mapping of brain stem neuronal circuitry active during swallowing. Ann Otol Rhinol Laryngol 110: 502–513, 2001. doi: 10.1177/000348940111000603. [DOI] [PubMed] [Google Scholar]
- Bauman NM, Wang D. Laryngeal electromyographic, cardiovascular, and respiratory effects of neuropeptide injections into the nucleus tractus solitarius of rats. Ann Otol Rhinol Laryngol 113: 809–820, 2004. doi: 10.1177/000348940411301007. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Dick TE. Connectivity of slowly adapting pulmonary stretch receptors with dorsal medullary respiratory neurons. J Neurophysiol 58: 1259–1274, 1987. [DOI] [PubMed] [Google Scholar]
- Bolser DC. Mechanisms of action of central and peripheral antitussive drugs. Pulm Pharmacol 9: 357–364, 1996. doi: 10.1006/pulp.1996.0047. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Davenport PW. Volume-timing relationships during cough and resistive loading in the cat. J Appl Physiol (1985) 89: 785–790, 2000. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Davenport PW. Functional organization of the central cough generation mechanism. Pulm Pharmacol Ther 15: 221–225, 2002. doi: 10.1006/pupt.2002.0361. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Hey JA, Chapman RW. Influence of central antitussive drugs on the cough motor pattern. J Appl Physiol (1985) 86: 1017–1024, 1999. [DOI] [PubMed] [Google Scholar]
- Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: a holarchical system? Respir Physiol Neurobiol 152: 255–265, 2006. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham AC, Sekizawa S, Chen CY, Joad JP. Plasticity of brainstem mechanisms of cough. Respir Physiol Neurobiol 152: 312–319, 2006. doi: 10.1016/j.resp.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Boscan P, Pickering AE, Paton JF. The nucleus of the solitary tract: an integrating station for nociceptive and cardiorespiratory afferents. Exp Physiol 87: 259–266, 2002. doi: 10.1113/eph8702353. [DOI] [PubMed] [Google Scholar]
- Brodie DA, Borison HL. Evidence for a medullary inspiratory pacemaker; functional concept of central regulation of respiration. Am J Physiol 188: 347–354, 1957. [DOI] [PubMed] [Google Scholar]
- Budzińska K, von Euler C, Kao FF, Pantaleo T, Yamamoto Y. Effects of graded focal cold block in the solitary and para-ambigual regions of the medulla in the cat. Acta Physiol Scand 124: 317–328, 1985. doi: 10.1111/j.1748-1716.1985.tb07667.x. [DOI] [PubMed] [Google Scholar]
- Canning BJ. Encoding of the cough reflex. Pulm Pharmacol Ther 20: 396–401, 2007. doi: 10.1016/j.pupt.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Mori N. An essential component to brainstem cough gating identified in anesthetized guinea pigs. FASEB J 24: 3916–3926, 2010. doi: 10.1096/fj.09-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Mori N. Encoding of the cough reflex in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 300: R369–R377, 2011. doi: 10.1152/ajpregu.00044.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinelli E, Bongianni F, Pantaleo T, Mutolo D. Suppression of the cough reflex by α2-adrenergic receptor agonists in the rabbit. Physiol Rep 1: e00122, 2013. doi: 10.1002/phy2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinelli E, Bongianni F, Pantaleo T, Mutolo D. The cough reflex is upregulated by lisinopril microinjected into the caudal nucleus tractus solitarii of the rabbit. Respir Physiol Neurobiol 219: 9–17, 2015. doi: 10.1016/j.resp.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Claps A, Torrealba F. The carotid body connections: a WGA-HRP study in the cat. Brain Res 455: 123–133, 1988. doi: 10.1016/0006-8993(88)90121-7. [DOI] [PubMed] [Google Scholar]
- Daghfous G, Green WW, Alford ST, Zielinski BS, Dubuc R. Sensory activation of command cells for locomotion and modulatory mechanisms: lessons from lampreys. Front Neural Circuits 10: 18, 2016. doi: 10.3389/fncir.2016.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RO, Kalia M. Carotid sinus nerve projections to the brain stem in the cat. Brain Res Bull 6: 531–541, 1981. doi: 10.1016/S0361-9230(81)80028-7. [DOI] [PubMed] [Google Scholar]
- Davies RO, Kubin L. Projection of pulmonary rapidly adapting receptors to the medulla of the cat: an antidromic mapping study. J Physiol 373: 63–86, 1986. doi: 10.1113/jphysiol.1986.sp016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RO, Kubin L, Pack AI. Pulmonary stretch receptor relay neurones of the cat: location and contralateral medullary projections. J Physiol 383: 571–585, 1987. doi: 10.1113/jphysiol.1987.sp016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton RC, Lee RK, Foreman MB. The Mauthner cell and other identified neurons of the brainstem escape network of fish. Prog Neurobiol 63: 467–485, 2001. doi: 10.1016/S0301-0082(00)00047-2. [DOI] [PubMed] [Google Scholar]
- Haji A, Kimura S, Ohi Y. A model of the central regulatory system for cough reflex. Biol Pharm Bull 36: 501–508, 2013. doi: 10.1248/bpb.b13-00052. [DOI] [PubMed] [Google Scholar]
- Haji A, Ohi Y. Inhibition of spontaneous excitatory transmission induced by codeine is independent on presynaptic K+ channels and novel voltage-dependent Ca2+ channels in the guinea-pig nucleus tractus solitarius. Neuroscience 169: 1168–1177, 2010. doi: 10.1016/j.neuroscience.2010.05.077. [DOI] [PubMed] [Google Scholar]
- Haji A, Ohi Y, Kimura S. Cough-related neurons in the nucleus tractus solitarius of decerebrate cats. Neuroscience 218: 100–109, 2012. doi: 10.1016/j.neuroscience.2012.05.053. [DOI] [PubMed] [Google Scholar]
- Hanácek J, Davies A, Widdicombe JG. Influence of lung stretch receptors on the cough reflex in rabbits. Respiration 45: 161–168, 1984. doi: 10.1159/000194614. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Takanaga A, Maeda S, Seki M, Yajima Y. Subnuclear distribution of afferents from the oral, pharyngeal and laryngeal regions in the nucleus tractus solitarii of the rat: a study using transganglionic transport of cholera toxin. Neurosci Res 39: 221–232, 2001. doi: 10.1016/S0168-0102(00)00218-2. [DOI] [PubMed] [Google Scholar]
- Jakus J, Poliacek I, Halasova E, Murin P, Knocikova J, Tomori Z, Bolser DC. Brainstem circuitry of tracheal-bronchial cough: c-fos study in anesthetized cats. Respir Physiol Neurobiol 160: 289–300, 2008. doi: 10.1016/j.resp.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus J, Stránsky A, Nagyová B, Oravec A, Bosel’ová L, Baráni H. Effects of focal cooling of medulla oblongata structures on quiet breathing in cats. Physiol Bohemoslov 39: 171–184, 1990. [PubMed] [Google Scholar]
- Jakus J, Stránsky A, Poliacek I, Baráni H, Bosel’ová L. Kainic acid lesions to the lateral tegmental field of medulla: effects on cough, expiration and aspiration reflexes in anesthetized cats. Physiol Res 49: 387–398, 2000. [PubMed] [Google Scholar]
- Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol 193: 467–508, 1980. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Kubin L, Davies RO. Sites of termination and relay of pulmonary rapidly adapting receptors as studied by spike-triggered averaging. Brain Res 443: 215–221, 1988. doi: 10.1016/0006-8993(88)91615-0. [DOI] [PubMed] [Google Scholar]
- Kubin L, Kimura H, Davies RO. The medullary projections of afferent bronchopulmonary C fibres in the cat as shown by antidromic mapping. J Physiol 435: 207–228, 1991. doi: 10.1113/jphysiol.1991.sp018506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Morris KF, Baekey DM, Shannon R, Lindsey BG. Multimodal medullary neurons and correlational linkages of the respiratory network. J Neurophysiol 82: 188–201, 1999. [DOI] [PubMed] [Google Scholar]
- Lipski J, Bellingham MC, West MJ, Pilowsky P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Methods 26: 169–179, 1988. doi: 10.1016/0165-0270(88)90166-5. [DOI] [PubMed] [Google Scholar]
- Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J Physiol 443: 55–77, 1991. doi: 10.1113/jphysiol.1991.sp018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, McAllen RM, Trzebski A. Carotid baroreceptor and chemoreceptor inputs onto single medullary neurones. Brain Res 107: 132–136, 1976. doi: 10.1016/0006-8993(76)90101-3. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol 569: 559–573, 2005. doi: 10.1113/jphysiol.2005.093153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin SW. Arterial chemoreceptor input to nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol 263: R368–R375, 1992. [DOI] [PubMed] [Google Scholar]
- Mifflin SW, Spyer KM, Withington-Wray DJ. Baroreceptor inputs to the nucleus tractus solitarius in the cat: postsynaptic actions and the influence of respiration. J Physiol 399: 349–367, 1988. doi: 10.1113/jphysiol.1988.sp017085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Takayama K. The functional subdivisions of the nucleus tractus solitarii of the cat in relation to the carotid sinus nerve reflex. J Auton Nerv Syst 15: 79–92, 1986. doi: 10.1016/0165-1838(86)90081-0. [DOI] [PubMed] [Google Scholar]
- Motekaitis AM, Solomon IC, Kaufman MP. Blockade of glutamate receptors in CVLM and NTS attenuates airway dilation evoked from parabrachial region. J Appl Physiol (1985) 81: 400–407, 1996. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Cinelli E, Fontana GA, Pantaleo T. Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am J Physiol Regul Integr Comp Physiol 295: R243–R251, 2008. doi: 10.1152/ajpregu.00184.2008. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Bongianni F, Fontana GA, Pantaleo T. The role of excitatory amino acids and substance P in the mediation of the cough reflex within the nucleus tractus solitarii of the rabbit. Brain Res Bull 74: 284–293, 2007. doi: 10.1016/j.brainresbull.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Mutolo D, Cinelli E, Bongianni F, Pantaleo T. Inhibitory control of the cough reflex by galanin receptors in the caudal nucleus tractus solitarii of the rabbit. Am J Physiol Regul Integr Comp Physiol 307: R1358–R1367, 2014. doi: 10.1152/ajpregu.00237.2014. [DOI] [PubMed] [Google Scholar]
- Nishino T, Hiraga K, Honda Y. Inhibitory effects of CO2 on airway defensive reflexes in enflurane-anesthetized humans. J Appl Physiol (1985) 66: 2642–2646, 1989. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Unno T, Ohta Y, Mori S. Sneeze-evoking region within the brainstem. Brain Res 511: 265–270, 1990. doi: 10.1016/0006-8993(90)90171-7. [DOI] [PubMed] [Google Scholar]
- O’Connor R, Segers LS, Morris KF, Nuding SC, Pitts T, Bolser DC, Davenport PW, Lindsey BG. A joint computational respiratory neural network-biomechanical model for breathing and airway defensive behaviors. Front Physiol 3: 264, 2012. doi: 10.3389/fphys.2012.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi Y, Kimura S, Haji A. Modulation of glutamatergic transmission by metabotropic glutamate receptor activation in second-order neurons of the guinea pig nucleus tractus solitarius. Brain Res 1581: 12–22, 2014. doi: 10.1016/j.brainres.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Paton JF. Pattern of cardiorespiratory afferent convergence to solitary tract neurons driven by pulmonary vagal C-fiber stimulation in the mouse. J Neurophysiol 79: 2365–2373, 1998. [DOI] [PubMed] [Google Scholar]
- Paton JF, Li YW, Kasparov S. Reflex response and convergence of pharyngoesophageal and peripheral chemoreceptors in the nucleus of the solitary tract. Neuroscience 93: 143–154, 1999. doi: 10.1016/S0306-4522(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Poliacek I, Rose MJ, Corrie LW, Wang C, Jakus J, Barani H, Stransky A, Polacek H, Halasova E, Bolser DC. Short reflex expirations (expiration reflexes) induced by mechanical stimulation of the trachea in anesthetized cats. Cough 4: 1, 2008. doi: 10.1186/1745-9974-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliacek I, Simera M, Veternik M, Kotmanova Z, Pitts T, Hanacek J, Plevkova J, Machac P, Visnovcova N, Misek J, Jakus J. The course of lung inflation alters the central pattern of tracheobronchial cough in cat—the evidence for volume feedback during cough. Respir Physiol Neurobiol 229: 43–50, 2016. doi: 10.1016/j.resp.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliacek I, Wang C, Corrie LW, Rose MJ, Bolser DC. Microinjection of codeine into the region of the caudal ventral respiratory column suppresses cough in anesthetized cats. J Appl Physiol (1985) 108: 858–865, 2010. doi: 10.1152/japplphysiol.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Jordan D, Ballantyne D, Meesmann M, Spyer KM. Presynaptic depolarization in myelinated vagal afferent fibres terminating in the nucleus of the tractus solitarius in the cat. Pflugers Arch 406: 12–19, 1986. doi: 10.1007/BF00582946. [DOI] [PubMed] [Google Scholar]
- Saha S, Batten TF, Mcwilliam PN. Glutamate, gamma-aminobutyric acid and tachykinin-immunoreactive synapses in the cat nucleus tractus solitarii. J Neurocytol 24: 55–74, 1995. doi: 10.1007/BF01370160. [DOI] [PubMed] [Google Scholar]
- Shannon R, Baekey DM, Morris KF, Lindsey BG. Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J Appl Physiol (1985) 84: 2020–2035, 1998. [DOI] [PubMed] [Google Scholar]
- Silva-Carvalho L, Paton JF, Rocha I, Goldsmith GE, Spyer KM. Convergence properties of solitary tract neurons responsive to cardiac receptor stimulation in the anesthetized cat. J Neurophysiol 79: 2374–2382, 1998. [DOI] [PubMed] [Google Scholar]
- Tatár M, Korpás J, Polácek H, Záhradný V. Changes induced by severe hypoxia in respiratory defence reflexes in anaesthetized cats. Respiration 49: 114–121, 1986. doi: 10.1159/000194868. [DOI] [PubMed] [Google Scholar]
- Wallois F, Bodineau L, Macron JM, Marlot D, Duron B. Role of respiratory and non-respiratory neurones in the region of the NTS in the elaboration of the sneeze reflex in cat. Brain Res 768: 71–85, 1997. doi: 10.1016/S0006-8993(97)00602-1. [DOI] [PubMed] [Google Scholar]
- Wallois F, Gros F, Masmoudi K, Larnicol N. C-Fos-like immunoreactivity in the cat brainstem evoked by sneeze-inducing air puff stimulation of the nasal mucosa. Brain Res 687: 143–154, 1995. doi: 10.1016/0006-8993(95)00487-B. [DOI] [PubMed] [Google Scholar]
- Wasserman AM, Sahibzada N, Hernandez YM, Gillis RA. Specific subnuclei of the nucleus tractus solitarius play a role in determining the duration of inspiration in the rat. Brain Res 880: 118–130, 2000. doi: 10.1016/S0006-8993(00)02782-7. [DOI] [PubMed] [Google Scholar]
- Wu W, Nicolazzo JA, Wen L, Chung R, Stankovic R, Bao SS, Lim CK, Brew BJ, Cullen KM, Guillemin GJ. Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS One 8: e59749, 2013. doi: 10.1371/journal.pone.0059749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi K, Miyamae T, Sasaki Y, Maeda M, Hirano H, Misu Y, Goshima Y. Involvement of nitric oxide production via kynurenic acid-sensitive glutamate receptors in DOPA-induced depressor responses in the nucleus tractus solitarii of anesthetized rats. Neurosci Res 43: 231–238, 2002. doi: 10.1016/S0168-0102(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Zubcevic J, Potts JT. Role of GABAergic neurones in the nucleus tractus solitarii in modulation of cardiovascular activity. Exp Physiol 95: 909–918, 2010. doi: 10.1113/expphysiol.2010.054007. [DOI] [PubMed] [Google Scholar]