Transgenic mice that express the blue light-sensitive protein channelrhodopsin2 (ChR2) in nociceptive nerve fibers that contain voltage-gated sodium channel Nav1.8 were used to determine functional properties of these afferent fibers. Electrophysiological recordings in vivo revealed that most nociceptive fibers that possess Nav1.8 are C-fiber nociceptors that respond to multiple stimulus modalities. Furthermore, responses evoked by blue light stimulation were comparable to those elicited by noxious mechanical, heat, and cold stimuli.
Keywords: electrophysiology, peripheral neuron, tibial nerve, channelrhodopsin2, C-fiber
Abstract
Optogenetic methods that utilize expression of the light-sensitive protein channelrhodopsin-2 (ChR2) in neurons have enabled selective activation of specific subtypes or groups of neurons to determine their functions. Using a transgenic mouse model in which neurons natively expressing Nav1.8 (a tetrodotoxin-resistant voltage-gated sodium channel) also express the light-gated channel ChR2, we have been able to determine the functional properties of Nav1.8-expressing cutaneous nociceptors of the glabrous skin in vivo. Most (44 of 53) of the C-fiber nociceptors isolated from Nav1.8-ChR2+ mice were found to be responsive to blue (470 nm) light. Response characteristics, including conduction velocity and responses to mechanical stimuli, were comparable between nociceptors isolated from Nav1.8-ChR2+ and control mice. Interestingly, while none of the non–light-responsive C-fibers were sensitive to heat or cold, nearly all (77%) light-sensitive fibers were excited by mechanical and thermal stimuli, suggesting that Nav1.8 is predominantly expressed by C-fiber nociceptors that are responsive to multiple stimulus modalities. The ability to activate peripheral nociceptors with light provides a method of stimulation that is noninvasive, does not require mechanical interruption of the skin, and accesses receptive fields that might be difficult or impossible to stimulate with standard stimuli while allowing repeated stimulation without injuring the skin.
NEW & NOTEWORTHY Transgenic mice that express the blue light-sensitive protein channelrhodopsin2 (ChR2) in nociceptive nerve fibers that contain voltage-gated sodium channel Nav1.8 were used to determine functional properties of these afferent fibers. Electrophysiological recordings in vivo revealed that most nociceptive fibers that possess Nav1.8 are C-fiber nociceptors that respond to multiple stimulus modalities. Furthermore, responses evoked by blue light stimulation were comparable to those elicited by noxious mechanical, heat, and cold stimuli.
the advent of neuronal stimulation through optogenetics has enabled selective activation of neurons that express the light-sensitive algal protein channelrhodopsin2 (ChR2) (Nagel et al. 2003, Zhang et al. 2006). Use of optogenetic methods to induce expression of ChR2 in the brain has enabled selective activation of genetically defined neurons to determine their specific functions. Although blue light stimulation has been used to characterize somatosensory neurons of hairy skin in an ex vivo preparation (Baumbauer et al. 2015), this approach has not been fully used to characterize primary somatosensory neurons in vivo. Expressing ChR2 in specific afferents based on different molecular targets could be useful in their reclassification, such as differentiating pruritic and painful afferents. Using a transgenic mouse model, we targeted sensory neurons natively expressing Nav1.8, a tetrodotoxin-resistant voltage-gated sodium channel, to express the opsin ChR2, enabling the selective activation of their fibers in the plantar skin of the hind paw with light stimulation in awake, freely moving animals (Stirling et al. 2005; Madisen et al. 2012; Daou et al. 2013). Nav1.8 is almost exclusively expressed in primary somatosensory neurons, most of which are nociceptive (Djourhri et al. 2003; Stirling et al. 2005; Shields et al. 2012), though the functional properties of these neurons have not been fully explored in vivo. Nav1.8-ChR2+ mice exhibit normal withdrawal responses to mechanical and thermal stimuli (Daou et al. 2013). Furthermore, stimulation of the plantar hind paw with a pulsing, 470-nm blue light elicited nocifensive behaviors (lifting, licking, jumping, and vocalization), and exposure to blue light produced conditioned place avoidance (Daou et al. 2013). Extended exposure of the hind paw to blue light (30 min under anesthesia) induced long-term behavioral sensitization to mechanical and thermal stimuli, and a 10-min exposure induced c-Fos expression in dorsal horn neurons ipsilateral to the stimulated hind paw (Daou et al. 2013). Thus, the ability to evoke nocifensive behaviors with light alone provides a novel method of stimulation that is noninvasive, does not require mechanical interruption of the skin, and can be repeated without producing tissue injury.
The purpose of the current study was to determine response characteristics of light-sensitive cutaneous nociceptors in vivo in Nav1.8-ChR2+ mice. By selectively activating neurons expressing ChR2 using blue light, we determined the functional properties of cutaneous nociceptors of the glabrous skin expressing Nav1.8. Recordings were made from single nociceptors innervating the glabrous skin of the plantar hind paw of mice and responses evoked by mechanical, heat, and cold stimuli in addition to light were determined. Additionally, we assessed nociceptor response characteristics in non–light-responsive littermates.
MATERIALS AND METHODS
Animals.
Thirty-one adult (>6 wk old) mice (n = 17 male, n = 14 female) were used in the current study. Of these, 15 were Nav1.8-ChR2+ and 16 were non–light-responsive controls. Nav1.8-ChR2+ mice were created as described previously (Daou et al. 2013). Conditional expression of ChR2 was targeted to Nav1.8+ sensory neurons in mice (namely, Nav1.8-ChR2) as previously reported (see Fig. 1 in Daou et al. 2013).
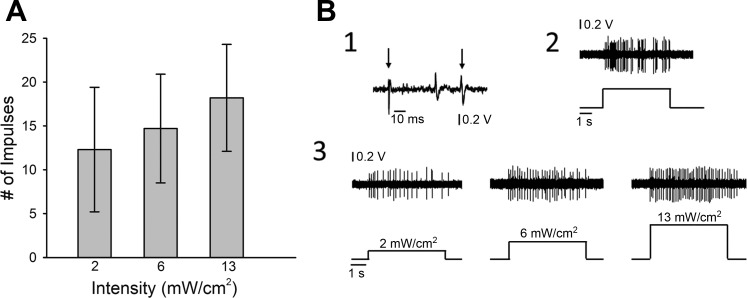
Fig. 1.
Light-responsive nociceptors respond with higher discharge rates as light intensity increases. A: mean responses of light-responsive C-fibers (n = 43) to increasing intensity-of-light stimuli. B: representative example of a light-responsive C-fiber nociceptor. 1: conduction latency: multiple traces were overlapped to show consistency of conduction latency (CV = 0.38 m/s). The left arrow indicates the onset of the stimulus and the right arrow indicates the action potential of the fiber of interest. 2: response evoked by 147 mN for 5 s. 3: responses evoked by 5 s stimulation with light stimuli of increasing intensity.
For our experiments, heterozygous Nav1.8-ChR2 mice were crossed with wild-type C57BL/6 mice purchased from The Jackson Laboratory. This cross yielded ~10% of offspring expressing ChR2 in Nav1.8+ primary afferent sensory neurons; these mice were identified by phenotyping for a nocifensive reaction to illumination of hind paws with 470-nm light from a light-emitting diode (LED, Plexon) with an attached fiber optic cable (1 mm diameter). The nonresponsive mice were used as control littermates. Mice were housed in cages of 3–4 on a 12:12-h light-dark cycle. All subjects had free access to food and water. All protocols and procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee and were conducted according to the guidelines established by the International Association for the Study of Pain (Zimmerman, 1983).
Surgical procedures and electrophysiological recording.
The surgical preparation to isolate and record from teased fibers of the tibial nerve has been described (Cain et al. 2001). Mice were anesthetized using inhaled isoflurane (2–3% induction, 1–2% maintenance). The level of anesthesia was evaluated by applying pressure to the right hind paw or tail and/or testing for corneal reflexes. Upon completion of the experiment, mice were killed by an intraperitoneal injection of euthasol (sodium pentobarbital, 390 mg/ml, and phenytoin sodium, 90 mg/ml).
Action potentials from individual fibers were amplified, audiomonitored, and visualized on an oscilloscope and personal computer using Spike 2.0 software (CED, Cambridge, UK). Nociceptors were initially identified by mild pinching and/or applying pressure to the glabrous skin of the hind paw. In Nav1.8-ChR2+ mice, 470-nm light from an LED (Plexon) with an attached fiber optic cable (1 mm diameter) was also used as a search stimulus and was applied to the skin for a few seconds at a time. Calibrated von Frey monofilaments were used to identify the precise location of the receptive field (RF), which was marked on the skin with a felt-tip pen.
Conduction velocity (CV) was determined for each fiber as described previously (Cain et al. 2001). Fibers with CV of 1.2 m/s or less were classified as C-fibers, whereas those with CV between 1.2 and 10 m/s were classified as Aδ-fibers and those with CV >10 were classified as Aβ-fibers. C- and Aδ-fiber nociceptors were preferentially studied.
Electrophysiological responses of nociceptors.
For each nociceptor, the rate of spontaneous activity was determined for a period of 2 min before any testing. Mechanical response threshold (mN) was obtained using von Frey monofilaments. The RF was stimulated multiple times with a single filament, and if no response was elicited, the next higher force (or lower force if there was a response) was applied. Response threshold was defined as the lowest force eliciting a response on 50% or more of the trials.
Responses evoked by suprathreshold mechanical stimuli were determined using a single suprathreshold von Frey monofilament that delivered a force of 147 mN. This monofilament was applied three times, each for 5 s with an interstimulus interval of 60 s, and the evoked response was defined as the mean number of impulses from the three trials.
Nociceptors were initially assessed for responses to light stimulation by positioning the fiber optic cable above the RF and applying the highest intensity (16 mW/cm2). If a fiber was responsive to this stimulus, the fiber optic cable was placed in a manipulator and positioned ~1 mm above the RF. The threshold was determined by slowly increasing the light intensity until an action potential was evoked. Threshold was defined as the minimal mean light intensity that evoked a response from three trials each separated by 60 s. Light stimuli were then delivered for 5 s each at three different intensities (2, 6, and 13 mW/cm2) with a 60-s interstimulus interval.
A Peltier device (contact area 25 mm2) was used to deliver cold and heat stimuli to the skin. The probe was maintained at a base temperature of 32°C, and cold stimuli from 28°C to 0°C were delivered in descending order in steps of 4°C. Each stimulus was applied for 10 s, with a 60-s interstimulus interval. Heat stimuli from 34°C to 50°C were delivered in ascending order of 2°C. Each stimulus was applied for 5 s every 60 s. Nociceptors that did not respond to thermal stimuli were classified as either C-mechanonociceptors (CM) or Aδ-mechanonociceptors (AδM). Nociceptors that responded to cold but not heat were classified as either C-mechanocold nociceptors (CMC) or Aδ-mechanocold nociceptors (AδMC), whereas those that responded to heat but not cold were classified as C-mechanoheat nociceptors (CMH) or Aδ-mechanoheat nociceptors (AδMH). Nociceptors that responded to both heat and cold were classified as either C-mechanoheatcold (CMHC) or Aδ-mechanoheatcold nociceptors (AδMHC). Nociceptors that did not respond to mechanical stimuli but were excited by heat were classified as mechanically insensitive heat (CH) nociceptors.
Data analysis.
Data analyses were conducted using SPSS statistical software. Data were evaluated for normality and the significance level was set at P < 0.05. Mean CVs were compared using independent t-tests, median mechanical response thresholds were compared using Mann-Whitney U-tests, and the mean number of impulses evoked by the 147-mN von Frey stimulus were compared using independent t-tests. Comparisons were performed for C-fiber nociceptors to evaluate whether there were any differences between control and Nav1.8-ChR2+ mice and to assess whether there were any differences between fibers that responded to blue light and those that did not. For fibers that responded to blue light stimulation, the median response threshold was expressed in mW/cm2, and the response to blue light stimulation was expressed as the number of impulses evoked during the 5-s stimulus after subtracting any spontaneous impulses that occurred during the 5-s period before the onset of the stimulus. Responses were determined for stimulation at 2, 6, and 13 mW/cm2, expressed as the number of impulses (mean ± SE), and were compared using one-way ANOVA. For C-fiber nociceptors that responded to thermal stimuli, the mean response thresholds for cold and heat were compared using independent t-tests. The cumulative number of impulses evoked by all heat and cold stimuli was compared between control and Nav1.8-ChR2+ mice using independent t-tests. Responses to individual temperatures were compared using repeated-measures ANOVA. Post hoc tests (Fisher’s least significant difference) were used to determine differences between groups at specific temperatures or among the range of temperatures. All evoked responses were determined by subtracting spontaneous activity that occurred 5–10 s before the onset of the stimulus.
RESULTS
A total of 107 cutaneous nociceptors innervating the glabrous skin of the hind paw were studied, 62 from Nav1.8-ChR2+ mice and 45 from control mice. We characterized nine Aδ-fiber nociceptors from control mice and nine from Nav1.8-ChR2+ mice, of which we characterized only four that were responsive to blue light. These numbers were not adequate for statistical analysis, so we focused our analysis of response properties on C-fiber nociceptors only. Conduction velocities for C-fiber nociceptors did not differ between the groups [t87 = 1.4, not significant (ns)]. The median mechanical response threshold for C-fiber nociceptors did not differ between groups (Mann-Whitney U-test = 573.0, n1 = 25, n2 = 49, ns), nor did the responses evoked by the 147-mN von Frey monofilament (t61 = 0.26, ns) (see Table 1). Among C-fibers studied in control mice, only 2 out of 36 (6%) had spontaneous activity (mean rate, 0.04 ± 0.00 Hz), whereas 5 out of 53 (9%) C-fibers from Nav1.8-ChR2+ mice were spontaneously active (mean rate, 0.09 ± 0.01 Hz). We also determined that there were no differences in nociceptor responses between male and female mice in control and Nav1.8-ChR2+ mice (data not shown), and so their data were combined for analyses. Nociceptors recorded from Nav1.8-ChR2+ mice were classified as either light responsive (OP+) or light nonresponsive (OP−), and were further classified by their responses to mechanical and thermal stimuli (see Table 2). There were no differences between OP+ and OP− nociceptors in CV (t49 = 1.3, ns), median mechanical threshold (Mann-Whitney U-test = 174.5, n1 = 9, n2 = 40, ns), or responses to 147-mN von Frey stimulation (t38 = 1.2, ns; see Table 2).
Table 1.
Conduction velocity and responses to mechanical stimuli of C-fiber nociceptors in control and Nav1.8-ChR2+ mice
| Group | Fiber Type | Number | CV, m/s | Threshold, mN | Response to 147 mN |
|---|---|---|---|---|---|
| Control | C | 36 | 0.58 ± 0.03 | 19.6 [13.7,39.2] | 45.5 ± 5.7 |
| Nav1.8-ChR2+ | C (all) | 53 | 0.52 ± 0.03 | 19.6 [13.7,39.2] | 45.5 ± 5.9 |
| C (OP+) | 9 | 0.49 ± 0.03 | 19.6 [13.7,39.2] | 41.4 ± 4.2 | |
| C (OP−) | 44 | 0.59 ± 0.02 | 13.7 [13.7,39.2] | 53.7 ± 11.2 |
CV, conduction velocity; OP+, light responsive; OP−, light nonresponsive; mN, mechanical response threshold.
Table 2.
Functional classification of C-fiber nociceptors from control and Nav1.8-ChR2+ mice (OP+ and OP− nociceptors)
| Group | Fiber Type* | Number | Percent |
|---|---|---|---|
| Control | CM | 14 | 39 |
| CMC | 10 | 28 | |
| CMH | 3 | 8 | |
| CMHC | 9 | 25 | |
| Nav1.8-ChR2+ (OP+) | CM | 10 | 23 |
| CMC | 9 | 20 | |
| CMH | 13 | 30 | |
| CMHC | 11 | 25 | |
| CH | 1 | 2 | |
| Nav1.8-ChR2+ (OP−) | CM | 9 | 100 |
C-fiber nociceptors are classified by temperature sensitivity:
CM, C-mechanonociceptors; CMC, C-mechanocold nociceptors; CMH, C-mechanoheat nociceptors; CMHC, C-mechanoheatcold nociceptors.
Responses to light stimulation.
Eighty-three percent of C-fiber nociceptors responded to blue light stimulation. The mean number of impulses for C-fiber nociceptors evoked by light is shown in Fig. 1. Light-evoked responses tended to increase with light intensity, but this was not significant. For OP+ C-fiber nociceptors, the median threshold was 0.79[0.67,1.22] mW/cm2 with a range of 0.15 to 12.86 mW. The majority of C-fibers tested (37 of 40) had thresholds less than 2 mW/cm2. The number of impulses (means ± SE) evoked by the highest light intensity was lower than that evoked by the 147-mN stimulus (38.6 ± 3.96 impulses). A representative example of responses of a C-fiber to mechanical and light stimulation is shown in Fig. 1.
Responses to thermal stimuli.
The majority (34 of 53) of C-fiber nociceptors isolated in Nav1.8-ChR2+ mice were responsive to heat and/or cold. Furthermore, 77% (34 of 44) of light-sensitive C-fiber nociceptors were responsive to heat and/or cold, and all of the OP− C-fibers in Nav1.8-ChR2+ mice were classed as CM. This indicates that in glabrous skin Nav1.8 is expressed predominantly on C-fiber nociceptors that respond to more than one stimulus modality. Figure 2 shows responses of an OP+ nociceptor (CMHC) to responsive heat, cold, and light. We were also able to isolate a mechanically insensitive C-fiber that responded to heat but not cold (CH). This nociceptor was identified when using the light source as the search stimulus (see Fig. 2).
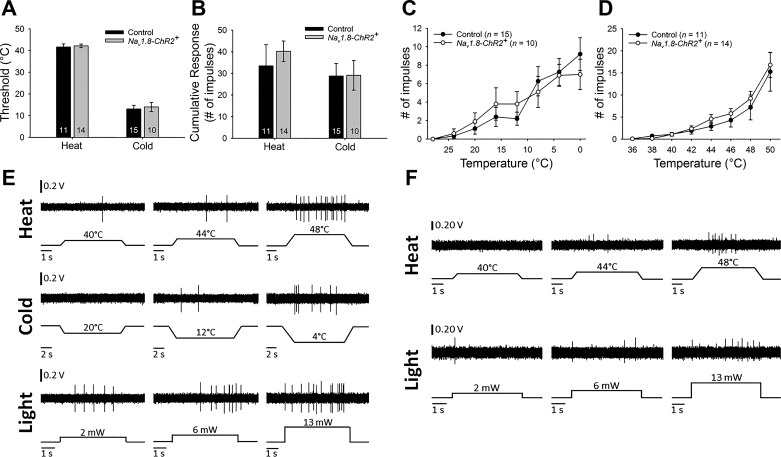
Fig. 2.
Responses to thermal stimuli did not differ between control and Nav1.8-ChR2+ mice. A: means ± SE response thresholds of C-fibers responsive to heat and/or cold did not differ between control and Nav1.8-ChR2+ mice. B: means ± SE cumulative responses of C-fibers responsive to heat and/or cold did not differ between control and Nav1.8-ChR2+ mice. C: responses to a range of temperatures did not differ for cold-responsive C-fibers in control (n = 15) and Nav1.8-ChR2+ (n = 10) mice. D: responses to a range of temperatures did not differ for heat-responsive C-fibers in control (n = 11) and Nav1.8-ChR2+ (n = 14) mice. E: representative examples of evoked responses of a light-responsive C-mechanoheatcold nociceptor fiber. Top: responses to 5-s heat stimuli of increasing temperature; middle: responses to 10-s cold stimuli of decreasing temperature; bottom: responses to 5-s light stimuli of increasing intensity. F: evoked responses from a single light-responsive CH fiber. Top: responses to 5-s heat stimuli of increasing temperature; bottom: responses to 5-s light stimuli of increasing intensity.
Among C-fibers that responded to cold and/or heat, some fibers were stimulated for classification purposes but did not receive the entire range of stimuli and were not included in analyses. Among C-fibers that responded to cold stimuli, their mean cold response threshold did not differ between control and Nav1.8-ChR2+ mice (t23 = 0.34, ns). The cumulative number of impulses evoked by all cold stimuli also did not differ between groups (t23 = 0.03, ns). Furthermore, responses evoked by stimuli within the range of cold stimuli delivered did not differ between control and Nav1.8-ChR2+ mice (F1,23 = 0.001, ns). Cold-evoked responses in both groups increased as the stimulus temperature decreased (F1,23 = 19.53, P < 0.001; see Fig. 2). Similarly, mean thresholds did not differ between fibers isolated in control and Nav1.8-ChR2+ mice (t23 = 0.32, ns), nor did the cumulative number of impulses evoked by all heat stimuli (t23 = 0.66, ns). Responses to heat stimuli within the range of heat stimuli did not differ between control and Nav1.8-ChR2+ mice (F1,23 = 0.41, ns), and responses increased with increasing stimulus temperature in both groups (F1,23 = 28.20, P < 0.001; see Fig. 2).
DISCUSSION
In the present study, the use of a Nav1.8-ChR2 mouse line enabled selective activation of Nav1.8-expressing cutaneous nociceptors in vivo. A large proportion of C-fiber nociceptors innervating the glabrous skin of the plantar hind paw responded to light stimulation. This allowed us to evaluate the response to light and to compare the response characteristics of OP+ nociceptors with those isolated from control mice that did not express ChR2. C-fibers did not differ with respect to CV, response thresholds, or responses to suprathreshold mechanical and thermal stimuli, demonstrating that the genetic manipulation that enabled activation of Nav1.8-expressing nociceptors by light did not have an effect on normal nociceptor characteristics or function. Furthermore, response characteristics of C-fiber nociceptors evoked by light stimulation were similar to those evoked by physical stimuli and include defined response thresholds, increasing discharge rates with increases in stimulus intensity, and light-evoked response frequencies that were similar to those elicited by high (48–50°C) or low (0–4°C) temperatures in thermally responsive nociceptors. This finding supports the use of blue light stimulation in Nav1.8-ChR2+ mice as an equivalent nociceptive stimulus to noxious heat, cold, or mechanical stimuli. Our results are consistent with studies conducted ex vivo in the hairy skin of mice expressing ChR2 in somatosensory neurons, where the majority of C-fibers were responsive to more than one stimulus modality, and responses to blue light stimulation were comparable to those elicited by noxious mechanical and heat stimulation (Baumbauer et al. 2015).
We also found that OP+ nociceptors were predominantly C-fibers that were responsive to more than one stimulus modality. By contrast, the 17% of C-fiber nociceptors that failed to respond to light responded only to mechanical stimuli, reinforcing the interpretation that Nav1.8 is mainly expressed in nociceptors that respond to more than one stimulus modality. The proportion of C-fibers found in Nav1.8-ChR2+ mice that responded to more than one stimulus modality (64%) was comparable to that observed in control mice (61%). These proportions parallel our previous results from tibial nerve recordings in mice (Cain et al. 2001; Potenzieri et al. 2009). A large proportion of C-fibers respond to more than one stimulus modality, regardless of the presence of ChR2, which is consistent with studies showing that a large population of C-fiber nociceptors express Nav1.8 (Djouhri et al. 2003). This finding does, however, contrast with a recent report suggesting that the majority of C-fiber nociceptors are modality-specific, and that fibers responding to more than one modality represent a small portion of nociceptors (Emery et al. 2016). Several methodological considerations could explain the discrepancy between our results and those of Emery et al., such as our use of teased fiber vs. dorsal root ganglia (DRG) recording techniques, the use of different mechanical stimuli to identify nociceptors, differences in protocols and approaches using thermal stimuli, and our direct recording of electrophysiological responses vs. calcium imaging. Importantly, Nav1.8 knockout mice showed altered behavioral profiles in response to both mechanical and thermal stimuli, indicating the involvement of this channel in multiple aspects of nociception (Emery et al. 2016). These mice had normal responses to noxious mechanical stimulation, but required higher amounts of pressure to evoke withdrawal in the tail. Withdrawal latencies in the hot plate test were normal, but withdrawal latencies in the paw flick and tail flick tests were higher in knockout mice (see Fig. 6 in Akopian et al. 1999). The polymodal nature of nociceptors, which has been shown in many earlier investigations (see Green, 2004 and Belmonte and Viana, 2008 for reviews) together with the present results, suggest that most Nav1.8-expressing nociceptors are responsive to more than one stimulus modality. It should be noted, however, that our studies focused exclusively on nociceptors innervating glabrous skin, and functional properties of fibers in hairy skin as well as those in deeper tissues may possess different functional characteristics. Studies in the glabrous skin of the hind paw allow us to draw parallels with our behavior studies, which are typically conducted with mechanical, heat, and cold stimuli applied to the plantar hind paws.
Our primary interest in the current study was to define functional characteristics of OP+ fibers, but it is notable that none of the OP+ fibers were Aβ-fibers. We also did not find any nonnociceptive mechanoreceptors that were excited by light, despite the fact that previous studies of Nav1.8 expression in the DRG have shown that Nav1.8 is expressed by low-threshold mechanoreceptors, including those with Aβ fibers (Shields et al. 2012). Although we could not detect any response from low-threshold mechanoreceptors to light stimulation, further studies are needed to determine the proportions and response characteristics of OP+ Aβ or C-LTMR fibers. We were also unable to characterize an adequate number of OP+ Aδ-fiber nociceptors to perform statistical analyses, and further studies are needed to determine whether this is due to a low proportion of Nav1.8 expression in these fibers or differences in response properties to blue light stimulation in myelinated fibers. Epidermal nerve fibers, known to be predominantly unmyelinated C-fibers (Simone et al. 1998; Bianchi et al. 2004; Chen et al. 2005), may be easier to reliably stimulate due to their depth. A higher light intensity may be needed to stimulate fibers at a lower depth, and the branching patterns of the nerve could contribute to variability in the responses to blue light stimulation.
One aspect of light-evoked responses that was not addressed in the present study is how the use of extended exposure to high-intensity light might affect nociceptor functions. A 30-min exposure to pulsed blue light in anesthetized Nav1.8-ChR2+ mice triggered hyperalgesia to mechanical and thermal stimuli that lasted up to 24 h beyond the period of light stimulation, whereas a 10-min exposure induced c-Fos expression in ipsilateral dorsal horn neurons (Daou et al. 2013), suggesting that prolonged light stimulation caused sensitization of Nav1.8-expressing nociceptors and/or central sensitization. Optogenetic silencing of Nav1.8-expressing nociceptors has been used to inhibit activity through the use of inhibitory archaerhodopsin-3 (Arch) proton pumps, which hyperpolarize neurons when they are exposed to yellow (589 nm) light (Daou et al. 2016). Mechanical and thermal hyperalgesia produced by inflammation and nerve injury were reduced in hind paws illuminated with yellow light without altering withdrawal responses under control conditions (Daou et al. 2016). Combining Arch with ChR2 in Nav1.8-expressing nociceptors resulted in inhibition of light-induced activation of ChR2 following extended exposure to inhibitory yellow light and prevented sensitization following prolonged exposure to blue light (Daou et al. 2016). These results support our findings that the majority of Nav1.8-expressing nociceptors are responsive to multiple stimulus modalities. Electrophysiological responses of primary afferent nociceptors and dorsal horn neurons following stimulation with blue or yellow light could provide information on the exact nature of the changes evoked by selective activation or inhibition of Nav1.8-expressing nociceptors.
GRANTS
This work was supported by National Institute on Drug Abuse Grant R01 DA-015438-10 to G. L. Wilcox and training grant T32 DA-007097-32 to D. J. Bruce, and by Canadian Institutes of Health Research Grant MOP-130239 to P. Séguéla. Mouse colony costs were also supported by the RW Goltz Research Professorship in Dermatology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.L.W. and D.A.S. conceived and designed research; M.L.U., D.J.B., and D.A.S. performed experiments; M.L.U. analyzed data; M.L.U., G.L.W., and D.A.S. interpreted results of experiments; M.L.U. prepared figures; M.L.U. and D.A.S. drafted manuscript; M.L.U., D.J.B., P.S., G.L.W., and D.A.S. edited and revised manuscript; M.L.U., D.J.B., P.S., G.L.W., and D.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Catherine Harding-Rose for technical assistance, Dr. Anders Asp for providing the LED apparatus, and Dr. Hélène Beaudry for assistance with the description of the mouse genotype.
REFERENCES
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci 2: 541–548, 1999. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, DeBerry JJ, Adelman PC, Miller RH, Hachisuka J, Lee KH, Ross SE, Koerber HR, Davis BM, Albers KM. Keratinocytes can modulate and directly initiate nociceptive responses. eLife 4: e09674, 2015. doi: 10.7554/eLife.09674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Viana F. Molecular and cellular limits to somatosensory specificity. Mol Pain 4: 14, 2008. doi: 10.1186/1744-8069-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi R, Buyukakilli B, Brines M, Savino C, Cavaletti G, Oggioni N, Lauria G, Borgna M, Lombardi R, Cimen B, Comelekoglu U, Kanik A, Tataroglu C, Cerami A, Ghezzi P. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci USA 101: 823–828, 2004. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol 85: 1561–1574, 2001. [DOI] [PubMed] [Google Scholar]
- Chen YS, Chung SS, Chung SK. Noninvasive monitoring of diabetes-induced cutaneous nerve fiber loss and hypoalgesia in thy1-YFP transgenic mice. Diabetes 54: 3112–3118, 2005. doi: 10.2337/diabetes.54.11.3112. [DOI] [PubMed] [Google Scholar]
- Daou I, Beaudry H, Ase AR, Wieskopf JS, Ribeiro-da-Silva A, Mogil JS, Séguéla P. Optogenetic silencing of Nav1. 8-positive afferents alleviates inflammatory and neuropathic pain. eNeuro 3: ENEURO-0140, 2016. doi: 10.1523/ENEURO.0140-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou I, Tuttle AH, Longo G, Wieskopf JS, Bonin RP, Ase AR, Wood JN, De Koninck Y, Ribeiro-da-Silva A, Mogil JS, Séguéla P. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J Neurosci 33: 18631–18640, 2013. doi: 10.1523/JNEUROSCI.2424-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol 550: 739–752, 2003. doi: 10.1113/jphysiol.2003.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery EC, Luiz AP, Sikandar S, Magnúsdóttir R, Dong X, Wood JN. In vivo characterization of distinct modality-specific subsets of somatosensory neurons using GCaMP. Sci Adv 2: e1600990, 2016. doi: 10.1126/sciadv.1600990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG. Temperature perception and nociception. J Neurobiol 61: 13–29, 2004. doi: 10.1002/neu.20081. [DOI] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ III, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci 15: 793–802, 2012. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA 100: 13940–13945, 2003. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenzieri C, Brink TS, Simone DA. Excitation of cutaneous C nociceptors by intraplantar administration of anandamide. Brain Res 1268: 38–47, 2009. doi: 10.1016/j.brainres.2009.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Ahn HS, Yang Y, Han C, Seal RP, Wood JN, Waxman SG, Dib-Hajj SD. Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain 153: 2017–2030, 2012. doi: 10.1016/j.pain.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci 18: 8947–8959, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling LC, Forlani G, Baker MD, Wood JN, Matthews EA, Dickenson AH, Nassar MA. Nociceptor-specific gene deletion using heterozygous NaV1.8-Cre recombinase mice. Pain 113: 27–36, 2005. doi: 10.1016/j.pain.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods 3: 785–792, 2006. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16: 109–110, 1983. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]