SUMMARY
SETTING
Antiretroviral therapy (ART) reduces pulmonary tuberculosis (PTB) in human immunodeficiency virus (HIV) infected children. Recent ART recommendations have increased the number of children on ART.
OBJECTIVE
To determine the prevalence and incidence of TB in HIV-infected children after the implementation of expanded ART guidelines.
DESIGN
A prospective cohort study including HIV-infected children aged 6 weeks to 14 years was conducted in Kenya. The primary outcome measure was clinically diagnosed TB. Study participants were screened for prevalent TB at enrollment using Kenya’s national guidelines and followed at monthly intervals to detect incident TB. Predictors of TB were assessed using logistic regression and Cox proportional hazards regression.
RESULTS
Of 689 participants (median age 6.4 years), 509 (73.9%) were on ART at baseline. There were 51 cases of prevalent TB (7.4%) and 10 incident cases, with over 720.3 child-years of observation (incidence 1.4 per 100 child-years). Months on ART (adjusted hazard ratio [aHR] 0.91, P = 0.003; aOR 0.91, P < 0.001) and months in care before ART (aHR 0.87, P = 0.001; aOR 0.92, P < 0.001) were protective against incident and prevalent TB.
CONCLUSIONS
ART was protective against TB in this cohort of HIV-infected children with high levels of ART use. Optimal TB prevention strategies should emphasize early ART in children.
Keywords: epidemiology, sub-Saharan Africa, pediatrics, HIV-1, tuberculosis
IN 2009, an estimated 2.5 million children aged <15 years were living with the human immunodeficiency virus (HIV), 80% of those in sub-Saharan Africa.1 In the absence of antiretroviral therapy (ART) or other preventive interventions, it is estimated that 20% or more of HIV-infected children living in high-burden areas will develop active tuberculosis (TB) disease, leading to a three-to six-fold higher mortality.2–6 Clinical diagnostic algorithms widely used in resource-limited settings overall perform poorly, particularly in children with HIV, leading to varying prevalence and incidence reports in this population.7–9
Retrospective studies among HIV-infected children from sub-Saharan Africa have demonstrated that the use of ART is associated with a 50–85% decrease in incident TB in HIV-infected children.10–12 A prospective randomized controlled trial in South Africa demonstrated that both isoniazid preventive therapy (IPT) and ART prevent TB separately and in combination in children with HIV.13
Recent updates in World Health Organization (WHO) guidelines for ART initiation in infants and children have led to even more HIV-infected children being treated.14 To inform policy aimed at reducing the impact of TB in HIV-infected children and to determine the potential utility of ART, we prospectively evaluated the incidence and prevalence of TB in HIV-infected children in Kenya, where national guidelines on early initiation of ART in children have been implemented.15
MATERIALS AND METHODS
Objectives
The objectives of this study were 1) to determine the baseline prevalence and incidence of TB in a cohort of HIV-infected children enrolled for HIV care, 2) to describe risk factors for TB diagnosis, and 3) to assess the impact of ART on TB in a pediatric population using expanded recommendations for ART initiation.
Study design
In a prospective cohort study, enrolled participants were screened for TB at baseline, then followed for development of incident TB until they were diagnosed with TB, transferred out of care, died or were lost to follow-up before the end date of the study.
Participants
The study included children aged 6 weeks to 14 years with confirmed HIV infection who were in care or who had initiated HIV care between February 2009 and December 2010. HIV infection was determined using DNA polymerase chain reaction testing for children aged <18 months and serologically for children aged ⩾18 months. Children were excluded if they had known active TB or a history of TB within 1 year before enrollment in the study. Study participants were recruited during routine clinic visits.
Setting
The study was conducted in Kenya at two urban Family AIDS Care and Education Services (FACES) supported HIV clinics—one in Nairobi and one in Nyanza Province—and the New Nyanza Provincial General Referral Hospital HIV Clinic, Nyanza. Both settings have a high burden of HIV and TB: in 2009, the adult case notification rate for all types of TB was 612 per 100 000 population in Nairobi and 400/100000 in Nyanza (national rates for children are not available).16
FACES is a collaboration between the University of California, San Francisco (UCSF), the Kenya Medical Research Institute (KEMRI), and the Kenya Ministries of Health (MoH), funded by the Presidential Emergency Plan for AIDS Relief. FACES supports comprehensive HIV prevention, care and treatment through the MoH of over 80000 HIV-infected patients. Children at all clinics receive free comprehensive HIV care and treatment following national HIV guidelines.15 Care includes routine screening and treatment for opportunistic infections, laboratory monitoring and provision of ART.
Since November 2008, Kenya has instituted national guidelines for early initiation of ART in children similar to current WHO guidelines, with the exception of the recent recommendation to start all HIV-infected children aged <2 years on ART (Table 1).14,15 As national guidelines in Kenya did not recommend the use of IPT in HIV-infected children at the time of this study, IPT was not offered to study participants.
Table 1.
Comparison of Kenya and WHO pediatric ART guidelines
| A Kenya National Guidelines for ART initiation in children, November 2008-November 201115
| ||||
|---|---|---|---|---|
| Age | <18 months | 18 months–5 years | 5–12 years | |
| CD4 % | All | <25% | <20% | |
| Total CD4 | All | <1000 | <350* | |
| WHO Stage | All | 3 and 4 | 3 and 4 | |
|
B WHO 2010 Guidelines for ART initiation in children14
| ||||
| Age | < 24 months | 24 months–5 years | 5–12 years | |
|
| ||||
| CD4 % | All | <25% | NA | |
| Total CD4 | All | <750 | <350 | |
| WHO Stage | All | 3 and 4 | 3 and 4 | |
In August 2010, the total CD4 threshold for children aged 5–12 years was changed to 500 cells/μl.
WHO = World Health Organization; ART = antiretroviral therapy; NA = not available.
Procedures
Children were enrolled consecutively from the study sites and underwent comprehensive TB screening at study entry. TB screening was performed by regular clinic staff in accordance with the Kenya Division of Leprosy, TB and Lung Disease 2008 TB diagnostic guidelines for children,17 and included clinical history, physical examination, chest radiograph (CXR), tuberculin skin test (TST), and modified Keith-Edwards Pediatric TB score chart.18 Clinic staff were trained on TB diagnosis in children, including CXR interpretation, TST placement and reading and use of the TB score chart. An anterior-posterior CXR was obtained for all children regardless of symptoms; findings were recorded on a standardized CXR reading form. TST was performed on all study participants using 0.1 ml of purified protein derivative (Statens Serum Institute, Copenhagen, Denmark) injected subdermally into the antero-lateral forearm. Reactions were read at 48–72 h. A positive result was defined as >5 mm, regardless of history of bacille Calmette-Guérin immunization, which is routinely administered at 2 weeks of age. Children without TB at enrollment were followed monthly or bi-monthly during routine clinic visits and screened for TB using clinical symptoms and history.
Outcomes
Our primary outcome was TB diagnosed clinically based on Kenya and WHO criteria for TB diagnosis in children.17,19 The following case definitions were used: 1) definite TB, for those sputum smear-positive for acid-fast bacilli, and 2) probable TB, for those with a TB score of at least 7 per the Kenya Pediatric TB score chart or a CXR abnormality consistent with possible TB infection and any of the following: positive TST, household contact, failure to thrive, or cough of >2 weeks not responding to standard antibiotic treatment. This algorithm, in particular the modified Keith Edwards Score chart, has many limitations, especially in areas with high HIV prevalence, where it has low specificity and likely leads to overdiagnosis of TB.7,20 However, it was the standard approach used nationally at the time this study was conducted. Standardized clinical case definitions for intrathoracic TB in children were released after implementation of the study, but were reviewed as supplementary categories in Table 2.21 TB cases diagnosed within 30 days of enrollment were defined as prevalent cases; subsequent diagnoses were defined as incident TB. All children with definitive or probable TB were treated with standard anti-tuberculosis treatment (2 months of rifampicin [RMP], isoniazid [INH] and pyrazinamide, followed by 4 months of RMP and INH).
Table 2.
Clinical and diagnostic findings from incident and prevalent TB cases
| Incident TB (n = 10) mean ± SD or n (%) |
Prevalent TB (n = 51) mean ± SD or n (%) |
|
|---|---|---|
| Age, months* | 108.0 ± 49.9 | 66.8 ± 35.5 |
| Duration of ART before TB diagnosis, months | 13.2 ± 14.8 | 10.4 ± 15.2 |
| CD4 count | 716.6 ± 593.7 | 989.2 ± 737.6 |
| CD4 % | 14.5 ± 7.9 | 21.1 ± 10.7 |
| Persistent cough† | 10 (100) | 26 (51.0) |
| Weight loss/failure to thrive* | 2 (20) | 33 (64.7) |
| Persistent unexplained fever | 5 (50) | 21 (41.2) |
| Chest X-ray† | ||
| Normal | 0 | 7 (14.6) |
| Unlikely TB | 1 (10) | 9 (18.8) |
| Probable TB | 8 (80) | 24 (50.0) |
| Most likely TB | 0 | 8 (16.7) |
| Positive TST | 3 (30) | 22 (44.0) |
| TB score | 6.6 ± 2.1 | 6.7 ± 2.5 |
| History of TB contact | 4 (40) | 22 (43.1) |
| Clinical case definition‡ | ||
| Probable TB | 9 (90) | 29 (57) |
| Possible TB | 1 (10) | 20 (39) |
| Unlikely TB | 0 | 2 (4) |
P ≤ 0.01.
P ≤ 0.05.
Diagnostic categories taken from Graham et al.21
TB = tuberculosis; SD = standard deviation; ART = antiretroviral therapy; TST = tuberculin skin test.
Time to diagnosis of incident TB was measured in days from study enrollment to diagnosis or to date of censoring due to death, withdrawal, loss to follow-up or study end (31 December 2010). For analysis of both incident and prevalent TB diagnosis, predictor variables included sex, age in months at study enrollment, weight, body mass index (BMI), WHO stage, CD4 count, ART status and duration of HIV care before initiating ART. ART status was modeled in two forms: 1) duration of treatment in months, and 2) as an indicator variable for recent initiation of ART (<6 months) to separately test the potential protective effect of ART use and the unmasking of latent TB during immune reconstitution. Baseline weight and, for those aged >10 years, height, were used to generate Z-scores for weight for age or BMI according to the WHO Child Growth Standards.22 Underweight was defined as a Z-score of ≤−2.0 in either weight for age (<10 years) or BMI for age (⩾10 years). WHO stage was measured at baseline and at each follow-up visit; baseline scores were used for noncases and the latest scores prior to TB diagnosis for cases. Baseline CD4 count and CD4 % for children aged ≤5 years (when available) were used to categorize participants according to the age-adjusted WHO classification of HIV-associated immunodeficiency.23
Ethics
Ethical approval was obtained from both the KEMRI Ethical Review Committee and the UCSF Committee on Human Research. Consent was obtained for all study participants from at least one parent or primary care giver, and assent was obtained from children aged ⩾13 years. Study participants received a small transport stipend (equivalent to approximately US$2.50) for special study-related visits only.
Statistical methods
Clinic staff and the study nurse collected data using standardized clinic and study forms. Data were entered into Epi Info™ (Centers for Disease Control and Prevention, Atlanta, GA, USA) and analyzed using Stata 11.0 (Stata Corp, College Station, TX, USA). Descriptive characteristics were compared using χ2 tests for categorical variables and Student’s t-tests and one-way analysis of variance (ANOVA) tests for continuous variables. For the primary outcome, we modeled the association of baseline covariates with incident TB using Cox proportional hazards regression for time from study start to diagnosis. Records for individuals who initiated ART during the study were split at date of initiation; the resulting dependence among records was accounted for in analysis. All models were tested for proportional hazards; we addressed non-proportionality by including the interaction between the covariate and time in the model. The secondary outcome, prevalent TB, was modeled using logistic regression on baseline covariates. For both incident and prevalent case analysis, binary models were adjusted for site to account for differences in TB prevalence at each location. In multivariate analyses, we modeled the impact of time in care pre-ART and ART status on incident and prevalent TB, adjusting for hypothesized confounders (age, sex and site).
RESULTS
A total of 724 HIV-infected children aged 6 weeks to 14 years were assessed for study participation; 38 were excluded (27 due to TB in the last 1 year, 4 refusals and 7 for other reasons) and 686 apparently TB-negative children were enrolled (Figure), representing 61% of the 1121 HIV-infected children at the three clinics. During follow-up, 4 children died (<1%), 17 transferred care (2.5%) and 4 (<1%) were lost to follow-up. The median age of children who enrolled was 6.4 years (interquartile range 4.0–9.5). Of the 504 (73.5%) children on ART at study entry, approximately 37% were initiated due to updated ART guidelines.
Figure.
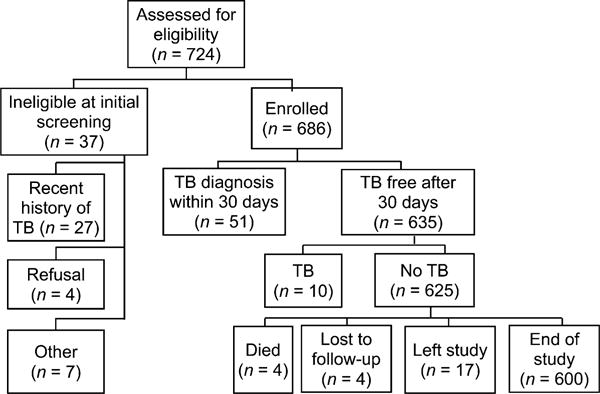
Study flow diagram. TB = tuberculosis.
Of the 686 child participants, TB was detected in 51 children through screening for TB at study enrollment, giving a prevalence of 7.4%. Table 3 shows the characteristics of the cohort at the time of enrollment. Significant differences between prevalent, incident and non-TB cases included site, age and clinical characteristics. The urban Nairobi site contributed 50% of the incident and 23% of the prevalent cases.
Table 3.
Characteristics of study participants without TB, with incident TB and with prevalent TB at study enrollment (n = 686)
| No TB n (%) or mean ± SD | Incident TB n (%) or mean ± SD | Prevalent TB n (%) or mean ± SD | |
|---|---|---|---|
| Demographic characteristic | |||
| District* | |||
| Kisumu | 551 (88) | 5 (50) | 37 (73) |
| Nairobi | 74 (12) | 5 (50) | 14 (27) |
| Site† | |||
| Lumumba Health Centre, Kisumu | 282 (45) | 4 (40) | 22 (43) |
| Provincial General Hospital, Kisumu | 269 (43) | 1 (10) | 15 (29) |
| FACES-Nairobi | 74 (12) | 5 (50) | 14 (27) |
| Sex | |||
| Male | 295 (47) | 3 (30) | 19 (37) |
| Female | 330 (53) | 7 (70) | 32 (63) |
| Age, months† | 81.2 ± 41.1 | 103.4 ± 49.3 | 66.8 ± 35.5 |
| Clinical characteristic | |||
| WHO Stage* | |||
| Stage I | 123 (21) | 1 (10) | 2 (4) |
| Stage II | 202 (35) | 3 (30) | 8 (16) |
| Stage III | 226 (39) | 4 (40) | 36 (71) |
| Stage IV | 34 (6) | 2 (20) | 5 (10) |
| Missing | 40 | 0 | 0 |
| CD4 | |||
| CD4 count, cells/mm3† (n = 448) | 1120.0 ± 675.3 | 716.6 ± 593.7 | 821.3 ± 620.7 |
| CD4 % (≤5 years, n = 197)* | 29.5 ± 11.5 | 21.0 ± 4.6 | 19.1 ± 11.5 |
| Immunodeficiency† | |||
| Not significant | 370 (64) | 2 (20) | 23 (46) |
| Mild | 68 (12) | 2 (20) | 6 (12) |
| Advanced | 62 (11) | 4 (40) | 5 (10) |
| Severe | 76 (13) | 2 (20) | 16 (32) |
| Missing | 49 | 0 | 1 |
| Weight | |||
| Z-score weight for age (n = 539)* | −0.8 ± 1.0 | −0.9 ± 1.2 | −1.5 ± 1.5 |
| Underweight (ZWFA or ZBMI ≤−2)* | 66 (11) | 1 (10) | 20 (41) |
| ART at baseline* | |||
| Yes | 472 (76) | 5 (50) | 27 (53) |
| Months on ART (n = 504) | 25.5 ± 15.9 | 19.4 ± 11.8 | 19.0 ± 16.0 |
| Outcome | |||
| Study duration* | |||
| Days in study | 418.7 ± 152.4 | 141.5 ± 70.3 | NA |
| Mortality* | |||
| Alive at end date | 621 (99) | 8 (80) | NA |
| Died | 4 (1) | 2 (20) | NA |
P ≤ 0.01.
P ≤ 0.05.
TB = tuberculosis; SD = standard deviation; FACES = Family AIDS Care and Education Services; WHO = World Health Organization; ZWFA = Z-score weight for age; ZBMI = Z-score body mass index; ART = antiretroviral therapy; NA = not available.
The 635 children without prevalent TB were followed for a mean of 13.6 months (range 0.4–22.6, standard deviation 5.1). Among these children, 485 (76.4%) were on ART at study enrollment and an additional 63 (9.9%) initiated ART during follow-up. Ten incident cases of TB, including one case of extra-pulmonary TB, were identified during a total of 720.3 child-years of follow-up, giving an overall incidence rate of 1.39 per 100 child-years (95% confidence interval [CI] 0.75–2.58).
The diagnostic characteristics of the TB cases are shown in Table 2. Mean age at time of diagnosis was older in incident cases (108.0 vs. 66.8 months, P < 0.01). Time on ART and mean CD4 counts were not significantly different between the groups. All incident cases (100%) and the majority of prevalent cases had chronic cough (51%), and approximately 40% of cases had documented TB contact.
In bivariate analysis adjusted for site, the rate of incident TB was elevated in children with advanced or severe levels of immunosuppression (respectively hazard ratio [HR] 17.2, P = 0.001, 95%CI 3.2–92.9; and HR 9.2, P = 0.028, 95%CI 1.3–65.7) and in those recently initiating ART (<6 months on ART) (HR 28.2, P < 0.001, 95%CI 4.8–167.8; Table 4). The odds of prevalent TB were increased with underweight (odds ratio [OR] 5.6, P < 0.001, 95%CI 3.0–10.6), WHO Stage 3 (OR 10.3, P = 0.002, 95%CI 2.4–44.0) and WHO Stage 4 (OR 10.6, P = 0.007, 95%CI 1.9–57.7), severe immunosuppression (OR 4.2, P < 0.001, 95%CI 2.1–8.6) and recent ART initiation (OR 7.8, P < 0.001, 95%CI 3.2–18.8). Months on ART was protective for both incident (HR 0.92, P = 0.058, 95%CI 0.85–1.00) and prevalent cases (OR 0.94, P < 0.001, 95%CI 0.92–0.96), but was significant only for prevalent cases.
Table 4.
Association of covariates and TB compared to those without TB
| Incident TB*
|
Prevalent TB†
|
|||
|---|---|---|---|---|
| HR (95%CI)‡ | P value | OR (95%CI)‡ | P value | |
| Sex | ||||
| Male | 1.00 | 1.00 | ||
| Female | 2.02 (0.53–7.80) | 0.306 | 1.50 (0.83–2.71) | 0.182 |
| Age, months | 1.01 (0.99–1.03) | 0.306 | 0.99 (0.98–1.00) | 0.003 |
| Z-score weight | ||||
| ⩾−2 | 1.00 | 1.00 | ||
| <−2 | 0.94 (0.12–7.30) | 0.952 | 5.63 (3.00–10.59) | <0.001 |
| WHO Stage | ||||
| Stage I | 1.00 | 1.00 | ||
| Stage II | 0.81 (0.10–6.78) | 0.844 | 1.47 (0.30–7.30) | 0.635 |
| Stage III | 2.76 (0.30–25.71) | 0.372 | 10.32 (2.42–44.03) | 0.002 |
| Stage IV | 14.52 (0.89–237.68) | 0.061 | 10.55 (1.93–57.65) | 0.007 |
| Immunological classification | ||||
| Not significant | 1.00 | 1.00 | ||
| Mild | 6.09 (0.88–42.25) | 0.068 | 1.57 (0.61–4.06) | 0.350 |
| Advanced | 17.20 (3.18–92.85) | 0.001 | 1.45 (0.52–4.00) | 0.475 |
| Severe | 9.16 (1.28–65.74) | 0.028 | 4.22 (2.06–8.62) | <0.001 |
| ART | ||||
| Recent ART initiation (<6 months) | 28.23 (4.75–167.76) | <0.001 | 7.76 (3.20–18.80) | <0.001 |
| Duration of ART, months§ | 0.92 (0.85–1.00) | 0.058 | 0.94 (0.92–0.96) | <0.001 |
| Duration of pre-ART care, months | 0.96 (0.91–1.01) | 0.092 | 0.98 (0.95–1.00) | 0.060 |
Incident cases (n = 10) compared to enrolled TB-free children throughout the study (n = 625); see Figure.
TB diagnosed within 30 days of study enrollment (n = 51) compared to TB-free at enrollment (n = 635).
Adjusted for site.
Adjusted for interaction between covariate and time.
TB = tuberculosis; HR = hazard ratio; CI = confidence interval; OR = odds ratio; WHO = World Health Organization; ART = antiretroviral therapy.
In multivariable analysis, recent ART initiation remained a significant predictor for incident TB (adjusted HR [aHR] 13.8, P = 0.003, 95%CI 2.4–79.8), although it was not significantly associated with prevalent TB. Duration of care pre-ART and months on ART were significantly protective for both incident TB (aHR 0.87, P = 0.001, 95%CI 0.80–0.94; aHR 0.91, P < 0.001, 95%CI 0.86–0.95) and prevalent TB (adjusted OR [aOR] 0.92, P < 0.001, 95%CI 0.89–0.95; aOR 0.91, P < 0.001, 95%CI 0.89–0.94; Table 5).
Table 5.
Adjusted association of ART duration and time in care in incident and prevalent TB
| Incident TB
|
Prevalent TB
|
|||
|---|---|---|---|---|
| aHR (95%CI)* | P value | aOR (95%CI)* | P value | |
| Recent ART initiation (<6 months) | 13.82 (2.39–79.83) | 0.003 | 2.34 (0.84–6.54) | 0.103 |
| Duration of ART, months | 0.91 (0.86–0.95) | <0.001 | 0.91 (0.89–0.94) | <0.001 |
| Duration of pre-ART care, months | 0.87 (0.80–0.94) | 0.001 | 0.92 (0.89–0.95) | <0.001 |
Adjusted for site, age, and sex.
ART = antiretroviral therapy; TB = tuberculosis; aHR = adjusted hazard ratio; CI = confidence interval; aOR = adjusted odds ratio.
DISCUSSION
This prospective, multisite cohort study documented an incidence of 1.4/100 child-years, with a TB prevalence of 7.4%. While ART initiation within 6 months was associated with an elevated incidence of TB, overall duration of ART and duration of pre-ART care appeared to protect against TB. ART is thought to reduce the risk of TB by improving immune response and containing infection.24 Our results support several other retrospective studies showing that ART reduces TB incidence in children infected with HIV.10–12 The benefits of ART may be greater than they appear due to the risk of immune reconstitution inflammatory syndrome (IRIS), as shown by the increased risk of TB within the first 6 months of ART in this study.
Time in care pre-ART may be protective for several reasons. A substantial duration of pre-ART care may indicate slow progression of disease and thus better overall health. If the parent is concurrently in care, they themselves may have TB prevented, treated early or otherwise improved health cascading to their children. The high TB prevalence found in this study despite being in care may be related to challenges in diagnosing TB prior to the study as well as lower rates of ART initiation prior to the expanded ART guidelines.
Although these results should be interpreted with some caution, they corroborate the protective effect of ART in HIV-infected children for preventing TB reported in other published studies.4,6,10–12 As described by Frigati et al., IPT may confer an additional benefit even in those children on ART.13 Notably, 40% of TB cases had a TB contact, revealing missed opportunities for TB prevention. Recent WHO guidelines recommend universal preventive TB therapy for HIV-infected children aged >1 year. However, the low TB incidence in children on ART, the high rates of TB IRIS in the first 6 months of ART, and the mixed results for pre-exposure prophylaxis in children should all be considered given the difficulties in implementing IPT in low-resource settings.25–27
The strengths of this study are its prospective nature, the low rate of loss to follow-up and the context of an operational setting. The primary limitation is the lack of a gold standard (microscopy or culture) for confirmatory diagnosis of TB. However, despite its limitations, this type of diagnostic algorithm is used extensively in sub-Saharan Africa for the diagnosis of TB in children, making our results more generalizable in similar settings. Secondary limitations include the lack of a historical baseline of TB incidence and the fact that the older mean age of this cohort is not reflective of HIV-infected children overall. Exclusion of those with recent history of TB may have led to a lower incidence rate, as these individuals are at higher risk of recurrent TB. Finally, the low number of incident cases resulted in poor power to detect associations.
CONCLUSION
The importance of preventing TB in HIV-infected children remains a priority given the increased morbidity, mortality and complexity of treating dual infections. Implementation of current recommendations for initiation of ART, along with rigorous TB screening in HIV care, may significantly reduce the burden of TB in this population.
Acknowledgments
The authors acknowledge the invaluable contributions of the study participants, their care givers, as well as the dedicated staff at all study clinics, and the Director of KEMRI for permission to publish the results. Support for this study was provided by Fogarty International Center National Institutes of Health 5-R24-TW007988-03 and Center for AIDS Prevention Studies through the National Institute of Mental Health 5-P30-MH062246.
Footnotes
Conflict of interest: none declared.
References
- 1.United Nations International Children’s Emergency Fund. Children and AIDS. Geneva, Switzerland: UNICEF; 2010. (5th Stocktaking Report 2010). [Google Scholar]
- 2.Elenga N, Kouakoussui KA, Bonard D, et al. Diagnosed tuberculosis during the follow-up of a cohort of human immunodeficiency virus-infected children in Abidjan, Cote d’Ivoire: ANRS 1278 study. Pediatr Infect Dis J. 2005;24:1077–1082. doi: 10.1097/01.inf.0000190008.91534.b7. [DOI] [PubMed] [Google Scholar]
- 3.Berggren Palme I, Gudetta B, Degefu H, Muhe L, Bruchfeld J, Giesecke J. A controlled estimate of the risk of HIV infection in Ethiopian children with tuberculosis. Epidemiol Infect. 2001;127:517–525. doi: 10.1017/s0950268801006215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walters E, Cotton MF, Rabie H, Schaaf HS, Walters LO, Marais BJ. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on antiretroviral therapy. BMC Pediatr. 2008;8:1. doi: 10.1186/1471-2431-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. Lancet Infect Dis. 2008;8:498–510. doi: 10.1016/S1473-3099(08)70182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braitstein P, Nyandiko W, Vreeman R, et al. The clinical burden of tuberculosis among human immunodeficiency virus-infected children in Western Kenya and the impact of combination antiretroviral treatment. Pediatr Infect Dis J. 2009;28:626–632. doi: 10.1097/INF.0b013e31819665c5. [DOI] [PubMed] [Google Scholar]
- 7.Pearce EC, Woodward JF, Nyandiko WM, Vreeman RC, Ayaya SO. A systematic review of clinical diagnostic systems used in the diagnosis of tuberculosis in children. AIDS Res Treat. 2012;2012:401896. doi: 10.1155/2012/401896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coovadia HM, Jeena P, Wilkinson D. Childhood human immunodeficiency virus and tuberculosis co-infections: reconciling conflicting data. Int J Tuberc Lung Dis. 1998;2:844–851. [PubMed] [Google Scholar]
- 9.Verhagen LM, Warris A, van Soolingen D, de Groot R, Hermans PW. Human immunodeficiency virus and tuberculosis coinfection in children: challenges in diagnosis and treatment. Pediatr Infect Dis J. 2010;29:e63–70. doi: 10.1097/INF.0b013e3181ee23ae. [DOI] [PubMed] [Google Scholar]
- 10.Edmonds A, Lusiama J, Napravnik S, Kitetele F, Van Rie A, Behets F. Anti-retroviral therapy reduces incident tuberculosis in HIV-infected children. Int J Epidemiol. 2009;38:1612–1621. doi: 10.1093/ije/dyp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakeera-Kitaka S, Conesa-Botella A, Dhabangi A, et al. Tuberculosis in human immunodeficiency virus infected Ugandan children starting on antiretroviral therapy. Int J Tuberc Lung Dis. 2011;15:1082–1086. doi: 10.5588/ijtld.10.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinson NA, Moultrie H, van Niekerk R, et al. HAART and risk of tuberculosis in HIV-infected South African children: a multi-site retrospective cohort. Int J Tuberc Lung Dis. 2009;13:862–867. [PMC free article] [PubMed] [Google Scholar]
- 13.Frigati LJ, Kranzer K, Cotton MF, Schaaf HS, Lombard CJ, Zar HJ. The impact of isoniazid preventive therapy and antiretroviral therapy on tuberculosis in children infected with HIV in a high tuberculosis incidence setting. Thorax. 2011;66:496–501. doi: 10.1136/thx.2010.156752. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Antiretroviral therapy for HIV infection in infants and children: towards universal access. Geneva, Switzerland: WHO; 2010. 2010 Revision. [PubMed] [Google Scholar]
- 15.Ministry of Medical Services Kenya. Change in paediatric ART recommendations—early initiation of ART in infants. Nairobi, Kenya: Ministry of Health; 2008. [Google Scholar]
- 16.Ministry of Public Health and Sanitation Kenya. Annual report 2010. Nairobi, Kenya: Division of Leprosy TB and Lung Disease, Ministry of Health; 2010. [Google Scholar]
- 17.National Leprosy and Tuberculosis Programme Kenya. NLTP guidelines on management of leprosy and tuberculosis. Nairobi, Kenya: Ministry of Health; 2008. [Google Scholar]
- 18.Edwards K. The diagnosis of childhood tuberculosis. PNG Med J. 1987;30:169–178. [PubMed] [Google Scholar]
- 19.World Health Organization. TB-HIV A clinical manual. Geneva, Switzerland: WHO; 2004. (WHO/HTM/TB/2004.329). [Google Scholar]
- 20.Hesseling AC, Schaaf HS, Gie RP, Starke JR, Beyers N. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. Int J Tuberc Lung Dis. 2002;6:1038–1045. [PubMed] [Google Scholar]
- 21.Graham SM, Ahmed T, Amanullah F, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis. 2012;205(Suppl 2):S199–S208. doi: 10.1093/infdis/jis008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. WHO child growth standards. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 23.World Health Organization. WHO case definitions for HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 24.Hung CC, Chen MY, Hsiao CF, et al. Improved outcomes of HIV-1-infected adults with tuberculosis in the era of highly active antiretroviral therapy. AIDS. 2003;17:2615–2622. doi: 10.1097/00002030-200312050-00008. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 26.Zar HJ, Cotton MF, Strauss S, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ. 2007;334:136. doi: 10.1136/bmj.39000.486400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madhi SA, Nachman S, Violari A, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. N Engl J Med. 2011;365:21–31. doi: 10.1056/NEJMoa1011214. [DOI] [PMC free article] [PubMed] [Google Scholar]


