Abstract
Background
Rheumatic heart disease (RHD) is the principal cause of valvular heart disease–related mortality and morbidity in low- and middle-income countries. The disease predominantly affects children and young adults. It is estimated that RHD may potentially be responsible for 1.4 million deaths annually worldwide and 7.5% of all strokes occurring in developing countries. Despite the staggering global burden, there are no contemporary data documenting the presentation, clinical course, complications, and treatment practices among patients with RHD.
Methods
The REMEDY study is a prospective, international, multicenter, hospital-based registry planned in 2 phases: the vanguard phase involving centers in Africa and India will enrol 3,000 participants with RHD over a 1-year period. We will document clinical and echocardiographic characteristics of patients at presentation. Over a 2-year follow-up, we will document disease progression and treatment practices with particular reference to adherence to secondary prophylaxis and oral anticoagulation regimens. With 3,000 patients, we will be able to reliably determine the incidence of all-cause mortality, worsening heart failure requiring hospitalization, systemic embolism (including stroke), and major bleeding individually among all participants. We will identify barriers to care in a subgroup of 500 patients.
Conclusion
The REMEDY study will provide comprehensive, contemporary data on patients with RHD and will help in the development of strategies to prevent and manage RHD and its complications. (Am Heart J 2012;163:535-540.e1.)
Rheumatic heart disease (RHD) is a consequence of the damage to heart valves as an immunologic sequel to antecedent group A streptococcal infections. Rheumatic heart disease predominantly affects children and young adults at the peak of their productivity because of their predilection to streptococcal infections. Although RHD has largely disappeared from developed countries, it continues to be a major cause of premature death and morbidity in low- and middle-income countries.1–3 Recent systematic reviews have found that the incidence of acute rheumatic fever (ARF) is >10/100,000 in some regions of Eastern Europe, the Middle East, Asia, and the Pacific regions.4,5 The prevalence of echocardiographically detected RHD in school-aged children has recently been estimated to be as high as 30.4 per 1,000 in school children in Mozambique.6 Current estimates suggest that 62 to 78 million individuals worldwide may have RHD, which could potentially result in 1.4 million deaths per year from the disease and its complications.7 Rheumatic valvular disease causes significant morbidity as a result of cardioembolic stroke, congestive failure, and infective endocarditis. Carapetis and colleagues1 estimated that up to 7.5% of all strokes occurring in less developed countries could be the direct result of RHD. Among women of reproductive age in Taiwan, nearly 30% of strokes occurred in patients with RHD.8 In a retrospective study from Ethiopia, 26.5% of all cardiovascular deaths were due to RHD, and in 70% of these patients, the death was attributable to heart failure.9 Rheumatic heart disease also adversely affects the outcomes of pregnancy. Data from South Africa suggest that of the 10.4% of women who died during pregnancy due to preexisting medical conditions, more than half had rheumatic valve disease.10 Rheumatic heart disease and its complications reduce quality of life, result in loss of income because of poor attendance at work, and, among children, poor scholastic performance.11 The need for chronic medical, surgical, and interventional treatment among those with RHD imposes an overwhelming burden on the already-limited health care resources available in poor countries.12 In addition, there is evidence that 2 proven secondary prevention therapies, namely, penicillin prophylaxis and oral anticoagulation therapy, may be underused in patients with RHD.13–15
Rationale
Despite the magnitude of the problem, there are few systematically collected contemporary data on disease characteristics and long-term outcomes in patients with RHD.16 The absence of contemporary, systematically collected data is a gap that needs to be urgently bridged, as an essential first step toward reducing the burden of RHD in developing countries.
Much of the morbidity due to RHD can be prevented by existing therapies. For example, there is good evidence to suggest that secondary prophylaxis with long-acting penicillin reduces the recurrence of episodes of ARF.17 Likewise, the role of oral anticoagulation in preventing stroke and systemic embolism in patients with rheumatic valve disease with atrial fibrillation (AF) is well established.18 However, several reports from developing countries have documented dismal adherence to secondary prophylaxis14 and the poor quality of oral anticoagulation.15 Reliable data on treatment practices and patient compliance with secondary prophylaxis and oral anticoagulation regimens will help policy makers in developing countries in formulating strategies to improve adherence to important treatments, thereby improving patient outcomes.
The REMEDY study is a collaborative study that aims to collect comprehensive data on disease characteristics, practice patterns, barriers to care, and outcomes of patients with RHD, in countries across the world where RHD remains endemic.
Methods
Study design
Study population
This is a prospective, international, multicenter, hospital-based registry planned in 2 phases: the vanguard phase involving centers in Africa, India, and the Middle East will enrol 3,000 patients with RHD over a 1-year period. This phase will assess the feasibility and the logistics of conducting such a registry in resource-poor settings and also provide preliminary data that will inform the design of the full-scale study. The full-scale registry will involve additional centers in South and Central Asia, Latin America, the Middle East, Eastern Europe, and the Asia Pacific region and will enrol a total of 10,000 patients with RHD. We will consider enrolling patients from a broader population, including those from the community, in the full-scale study.
Objectives
The specific objectives of the registry are as follows:
To describe the demographic characteristics of the affected patients and their presenting features, with particular reference to the pattern and severity of valvular involvement, the prevalence of AF, and other comorbid conditions specific to the populations being studied (eg, HIV infection and sickle cell disease)
To describe the pharmacologic treatments used, particularly secondary antibiotic prophylaxis, oral anticoagulation therapy, and antiarrhythmic therapy for AF
To document the frequency of the following outcomes over a 2-year follow-up: death (due to any cause), recurrence of rheumatic fever/carditis, worsening heart failure requiring hospitalization, systemic embolism (including stroke), and major bleeding. In addition, we will determine the occurrence of other complications such as infective endocarditis and prosthetic valve thrombosis.
To identify risk factors for adverse outcomes
To document the natural history of specific valvular lesions over the period of follow-up
To describe barriers to care, with particular reference to the use of secondary prophylaxis and oral anticoagulation, using qualitative and quantitative instruments, in a subset of 500 patients
To compare regional differences in the use of percutaneous and surgical interventional procedures in the treatment of valvular heart disease.
Study eligibility
Patients with a primary diagnosis of RHD (clinical or echocardiographic) seen in outpatient clinics, emergency departments, and inpatient facilities of participating hospitals will be eligible to participate. The registry proposes to be broad in its scope, and therefore, there are no other criteria for enrollment.
Inclusion criteria
Patients with a clinical diagnosis of RHD seen at outpatient facilities, emergency departments, or inpatient facilities of the participating hospitals are included in the study. Inpatients will be eligible if RHD is the reason for their admission to hospital. This will include conditions directly attributable to RHD, such as, heart failure, cardiac arrhythmia, and stroke.
Exclusion criteria
Patients are excluded from the study if they have the following:
A primary diagnosis of valvular disease other than RHD (eg, degenerative disease)
No informed consent/assent.
Data collection
Patient demographic data, clinical findings, and details of electrocardiographic (ECG) and echocardiographic findings will be recorded on structured case record forms. Considerable emphasis will be placed on obtaining details of medications prescribed, with particular reference to penicillin prophylaxis, antithrombotic and antiplatelet drugs, and antiarrhythmic medications, at study entry, at 1-year follow-up, and at the end of 2 years. The adequacy of antithrombotic therapy will be assessed by retrieving and recording the international normalized ratio (INR) values at recruitment and at follow-up. Adherence to penicillin prophylaxis will be assessed by obtaining injection records and/or direct questioning.
Follow-up
The schedule of visits and the minimum recommended study procedures during follow-up are shown in Table I. Patients will undergo 2 follow-up visits, one at 12 months and one at 24 months. An echocardiogram will be performed at both follow-up visits. Assessment for outcomes will be performed at follow-up visits and at any other visits that the patient makes to the hospital as part of usual care. Outcomes will be based on standard definitions. The study outcomes and their definitions are listed in Table II. Deaths will be recorded, and the cause will be ascertained by review of the relevant source documents. Morbidity will be ascertained by review of hospitalizations, new diagnoses and treatments, investigations, and surgical procedures. Any additional information needed will be obtained by contacting one of the patient’s physicians or next of kin. A random sample of 10% of the locally adjudicated events will be audited by an independent committee for consistency with the study definitions.
Table I.
Schedule of visits and minimum recommended study procedures
| Data collected | Visit 0 | Visit 1 (12 mo) |
Visit 2 (24 mo) |
|---|---|---|---|
| Informed consent | X | – | – |
| Medical history and physical examination | X | X | X |
| ECG | X | X* | X* |
| Echocardiography | X | X | X |
| Assessment for outcome events | – | X | X |
| Blood tests | X† | – | – |
Performance of ECGs at each follow-up visit is not mandated by the protocol, but data will be collected if done as part of routine practice.
Include hemogram, basic biochemistry, and INR; performance of blood investigations are not mandated by the protocol, but data will be collected if tests are done as part of routine practice.
Table II.
Event definitions
| Outcome | Definition |
|---|---|
| Death | Death due to any cause |
| Congestive heart failure | Any 2 of the 3 following criteria: (i) signs (rales, increased iugular venous pressure or ankle edema) or symptoms (dyspnea on exertion or at rest, orthopnea, nocturnal paroxysmal dyspnea, or ankle edema) of congestive heart failure, (ii) radiologic signs of pulmonary congestion, and (iii) treatment with diuretics |
| Stroke | Diagnosis of stroke by a physician based on sudden onset of neurologic deficit consistent with ischemia/infarction of a vascular territory, lasting ≥24 h, with or without confirmation by neuroimaging |
| Transient ischemic attack | Deficits diagnosed by a physician lasting <24 h |
| Non-CNS systemic embolism | Diagnosed clinically in patients with loss of arterial pulse and/or evidence of end-organ ischemia (eg, ischemic limb pain, gangrene, etc) with or without confirmation by Doppler studies or arteriography |
| Major bleeding | Bleeding that (i) is fatal, (ii) involves a critical site (intracranial, retroperitoneal, intraspinal, intra ocular, pericardial, or intra-articular), or (iii) leads to a reduction in hemoglobin level ≥2 g/dL, or requires transfusion of ≥2 units of whole blood or packed red cells |
| New-onset AF or flutter | Physician diagnosis with or without ECG evidence of AF or flutter |
| ARF Infective endocarditis Valve surgery | Diagnosed by the current WHO criteria25 |
| Diagnosed by the modified Duke criteria26 | |
| Performance of any valve repair, or replacement of valve with a tissue, or mechanical prosthesis | |
| Percutaneous valvular interventions | Percutaneous balloon dilatation of stenosed mitral, aortic tricuspid, or pulmonary valves |
| Prosthetic valve thrombosis | Recent onset (≤2 wk) symptoms of valve dysfunction (dyspnea, angina, or congestive heart failure) accompanied by new onset of restricted valve leaflet motion on cinefluoroscopy with or without increased valve gradients on Doppler echocardiography27 |
CNS indicates Central nervous system; WHO, World Health Organization.
Analysis plan (vanguard phase)
Over a period of 1 year, 3,000 patients will be recruited from centers in Africa, India, and the Middle East. These numbers are based primarily on feasibility. The rates of the following outcomes will be determined over the period of follow-up: all-cause mortality, recurrence of rheumatic fever, worsening heart failure requiring hospitalization, systemic embolism (including stroke), and major bleeding. We estimate that about 6.6% of patients will die over a follow-up period of 24 months. This is a conservative estimate derived from a community-based prospective study done in India.19 We anticipate similar or higher rates of systemic embolism (including stroke) and major bleeding, based on extrapolations from studies of patients with valvular heart disease with and without AF, those with prosthetic heart valves, and those with nonvalvular AF.20–23 With a sample size of 3,000 patients followed up for 2 years, we will be able to determine the rates of all outcomes individually (except recurrence of rheumatic fever), with 95% confidence and a precision greater than ±1%. Although we will attempt to minimize loss to follow-up, this calculation allows for incomplete follow-up information in up to 20% of patients.
Descriptive data will be summarized using means (standard deviations) for continuous variables and percentages for categorical data. The incidence of all the individual outcome events will be presented as rates using Kaplan-Meier estimates. Adherence to secondary prophylaxis will be computed as the proportion of penicillin injections received or oral doses taken expressed as a percentage of the total prescribed. The association of the adherence to penicillin prophylaxis with the occurrence of a composite of death, rheumatic fever recurrence, or worsening heart failure requiring hospitalization will be tested using a Cox proportional hazard regression analysis. We will determine the percentage of INR measurements in the therapeutic range as a measure of adherence to oral anticoagulation. The association of adherence to oral anticoagulation with a composite of the occurrence of death, systemic embolism (including stroke), or major bleeding will be tested similarly using a Cox regression model. Patients who have undergone percutaneous or surgical intervention for valve disease will be analyzed as prespecified subgroups. We will characterize the progression of individual valve lesions at 2-year follow-up using clinical and echocardiographic parameters. Comparisons of baseline and outcome variables between regions will be performed using Student t tests or χ2 tests as appropriate.
Study management
Research personnel at individual centers will be responsible for collecting patient data and entry into paper case record forms. Data will be transmitted to the regional coordinating center and, subsequently, to the central coordinating center at the Population Health Research Institute (PHRI), through the i-Datafax system (Clinical Datafax Systems Inc., Hamilton, ON). The PHRI will oversee the coordination of all the participating sites. Each country will have a national coordinator for the purpose of the registry. In the vanguard phase, the All India Institute of Medical Sciences at New Delhi, India, and the University of Cape Town at Cape Town, South Africa, will serve as the regional coordinating centers. The PHRI will be responsible for developing and maintaining the study database, receiving data from sites, and ensuring data quality and performing data analysis. The regional coordinating centers at Cape Town and New Delhi will have access to data from sites coordinated by these centers, and the project office at PHRI Hamilton will have access to all the study data. Individual sites will have access to their own centers’ data and will also be provided site-specific data summaries periodically by the global coordinating center at Hamilton. Publication policy will be decided by a steering committee consisting of the principal investigators from all participating sites.
Ethical issues
The protocol will be reviewed and approved by the local ethics committee at each of the recruiting centers. Using standardized patient information forms tailored to local requirements, patients will be informed about the registry, and their consent to participate will be recorded. Patients will be identified throughout the study duration by the study number allotted to them at the time of enrollment. All data will be handled in the strictest confidence.
Status of the study and study participants
The vanguard phase commenced on April 1, 2011. We have enrolled 773 patients till the time of writing. We expect that the enrollment of the first 3,000 participants will be complete by March 31, 2012, and 2-year (average) follow-up data will be available by the middle of 2013. The participating countries as on August 1, 2011, are shown in Figure 1, and the investigators by site and country are listed in the online Appendix.
Figure 1.
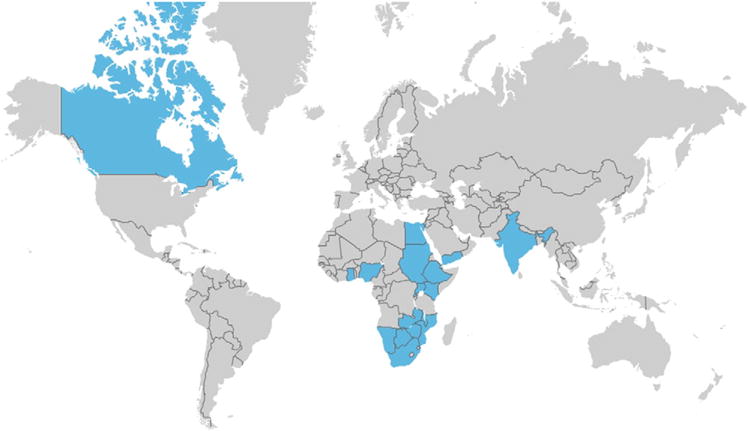
Countries participating in the vanguard phase of REMEDY individual sites, and their investigators are listed in the online Appendix.
Discussion
The REMEDY study is the first attempt at comprehensive characterization of RHD in countries where it is most prevalent. We will collect detailed data on clinical and echocardiographic features both at presentation and follow-up and meticulously document clinical and echocardiographic progression of disease and the occurrence of disease-related adverse events during the course of the study. We will, thus, have contemporary data on disease progression and the incidence of major adverse events such as stroke, congestive heart failure, major bleeding, and death. Much of the data related to disease progression and incidence of adverse events is from the preechocardiographic era. The REMEDY study will also document current practices in treating rheumatic valve disease, with particular reference to prescription of and adherence to secondary prophylaxis and oral anticoagulation therapy. Finally, REMEDY, because of its international nature, presents the scope for examining regional similarities and differences in the clinical features and outcomes of RHD.
Strengths and limitations
The REMEDY study is a hospital-based registry and will not address the issue of disease burden in the community. Participating hospitals have been selected based on the availability of expertise in recognizing and managing RHD and may, thus, result in overrepresentation of advanced disease and difficult to treat patients, thus overestimating disease-related adverse events. On the other hand, because of the same reasons, we may overestimate the use of surgery, percutaneous interventions, and other guideline-recommended treatments. Limitations notwithstanding, the vanguard phase of REMEDY will provide us with valuable feasibility data for the subsequent larger registry study.
Summary
Acute rheumatic fever and RHD continue to be widely prevalent in the developing world. This staggering burden of disease attests to the need for effective RHD control programs in developing countries. The REMEDY study represents, to the best of our knowledge, the first attempt to collect contemporary data on RHD from all the high-prevalence regions of the world. The REMEDY study will help quantify the burden of disease, document the prevalent approaches to patient management, and identify gaps and barriers in the care of these patients. It will provide information that will be critical for the development of locally sensitive guidelines, research programs, and policies, which will have the potential to improve the management of individuals with RHD.24
Acknowledgments
Funding Support
The REMEDY study is, in part, funded by the Canadian Network and Center for Trials Internationally, which is funded by the Canadian Institutes of Health Research (CIHR); the South African Medical Research Council; the Lily and Ernst Hausmann Research Trust; and Medtronic Foundation. G.K. was the recipient of the CIHR Canada-HOPE Scholarship, during which the planning and design of the study were initiated. L.Z. is a Fogarty International Clinical Research Fellow and is supported by the National Institutes of Health Office of the Director; Fogarty International Center; Office of AIDS Research; National Cancer Center; National Eye Institute; National Heart, Blood, and Lung Institute; National Institute of Dental and Craniofacial Research; National Institute on Drug Abuse; National Institute of Mental Health; National Institute of Allergy and Infectious Diseases Health; and National Institutes of Health Office of Women’s Health and Research through the International Clinical Research Scholars and Fellows Program at Vanderbilt University (R24 TW007988) and the American Relief and Recovery Act. The authors are solely responsible for the design and conduct of this study, the drafting and editing of the paper, and its final contents.
Appendix. List of participating sites and investigators by country
| Country | Investigator | Institution |
|---|---|---|
| Botswana | Dr Loeto Mzhani | University of Botswana, Gaborone |
| Egypt | Prof Azza Abul-Fadl | Benha University, Cairo |
| Prof Sahar Shaker Sheta | Cairo University Children’s Hospital, Cairo | |
| Ethiopia | Prof Abraham Haileamlak | Jimma University Hospital, Jimma |
| Ghana | Prof Albert Amoah | University of Ghana, Accra |
| Mozambique | Dr Ana Olga Mocumbi | Instituto Do Coracao, Maputo |
| Nigeria | Dr Fidelia Bode-Thomas | Jos University Teaching Hospital, Jos |
| Dr Okechukwu Ogah | University College Hospital, Ibadan | |
| Dr Dike Ojji | University of Abuja Teaching Hospital, Abuja | |
| Dr Mahmoud Sani | Aminu Kano Teaching Hospital, Kano | |
| Rwanda | Dr Joseph Mucumbitsi | King Faisal Hospital, Kigali |
| Sudan | Prof Ahmed El-Sayed | AlShaab Hospital, Khartoum |
| Uganda | Dr Charles Mondo | Mulago Hospital, Kampala |
| Kenya | Prof Stephen Ogendo | Kenyatta Hospital, Nairobi |
| Namibia | Dr Chris Hugo-Hamman | Windhoek Central Hospital, Namibia |
| Yemen | Prof Mohammed Al-Kebsi | Sana’a University, Sana’a |
| South Africa | Prof Bongani Mayosi | Groote Schuur Hospital, Cape Town |
| Dr Chris Sutton | Mankweng Hospital, Polokwane | |
| Dr Liesl Zuhlke | Red Cross War Memorial Children’s Hospital, Cape Town | |
| India | Dr Ganesan Karthikeyan | All India Institute of Medical Sciences, New Delhi |
| Dr Santhosh Satheesh | Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry | |
| Zambia | Dr John Musuku | University Teaching Hospital, Lusaka |
| Zimbabwe | Prof James Hakim and Prof Jonathan Matenga | Parirenyatwa Hospital, Harare |
Footnotes
Disclosure
None of the authors have any disclosure to make.
References
- 1.Carapetis JR, Steer AC, Mulholland EK, et al. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 2.Padmavati S. Rheumatic heart disease: prevalence and preventive measures in the Indian subcontinent. Keywords: rheumatic heart disease; rheumatic fever Heart. 2001;86:127. doi: 10.1136/heart.86.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Essop MR, Nkomo VT. Rheumatic and nonrheumatic valvular heart disease: epidemiology, management, and prevention in Africa. Circulation. 2005;112:3584–91. doi: 10.1161/CIRCULATIONAHA.105.539775. [DOI] [PubMed] [Google Scholar]
- 4.Tibazarwa KB, Volmink JA, Mayosi BM. Incidence of acute rheumatic fever in the world: a systematic review of population-based studies. Heart. 2008;94:1534–40. doi: 10.1136/hrt.2007.141309. [DOI] [PubMed] [Google Scholar]
- 5.Seckeler MD, Hoke TR. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol. 2011;3:67–84. doi: 10.2147/CLEP.S12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marijon E, Ou P, Celermajer DS, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470–6. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 7.Paar JA, Berrios NM, Rose JD, et al. Prevalence of rheumatic heart disease in children and young adults in Nicaragua. Am J Cardiol. 2010;105:1809–14. doi: 10.1016/j.amjcard.2010.01.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeng JS, Tang SC, Yip PK. Stroke in women of reproductive age: comparison between stroke related and unrelated to pregnancy. J Neurol Sci. 2004;221:25–9. doi: 10.1016/j.jns.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Nkomo VT. Epidemiology and prevention of valvular heart diseases and infective endocarditis in Africa. Heart. 2007;93:1510–9. doi: 10.1136/hrt.2007.118810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moodley J, Pattinson RC, Bennun M, et al. A review of maternal deaths in South Africa during 1998. S Afr Med J. 2000;90:367–73. [PubMed] [Google Scholar]
- 11.Terreri MT, Ferraz MB, Goldenberg J, et al. Resource utilization and cost of rheumatic fever. J Rheumatol. 2001;28:1394–7. [PubMed] [Google Scholar]
- 12.Krishnaswami S, Joseph G, Richard J. Demands on tertiary care for cardiovascular diseases in India: analysis of data for 1960–89. Bull World Health Organ. 1991;69:325–30. [PMC free article] [PubMed] [Google Scholar]
- 13.Eissa S, Lee R, Binns P, et al. Assessment of a register-based rheumatic heart disease secondary prevention program in an Australian Aboriginal community. Aust N Z J Public Health. 2005;29:521–5. doi: 10.1111/j.1467-842x.2005.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 14.Ravisha MS, Tullu MS, Kamat JR. Rheumatic fever and rheumatic heart disease: clinical profile of 550 cases in India. Arch Med Res. 2003;34:382–7. doi: 10.1016/S0188-4409(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 15.Kakkar N, Kaur R, John M. Outpatient oral anticoagulant management–an audit of 82 patients. J Assoc Physicians India. 2005;53:847–52. [PubMed] [Google Scholar]
- 16.Carapetis JR. Rheumatic heart disease in Asia. Circulation. 2008;118:2748–53. doi: 10.1161/CIRCULATIONAHA.108.774307. [DOI] [PubMed] [Google Scholar]
- 17.Manyemba J, Mayosi BM. Penicillin for secondary prevention of rheumatic fever. Cochrane Database Syst Rev. 2002:CD002227. doi: 10.1002/14651858.CD002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salem DN, Stein PD, Al-Ahmad A, et al. Antithrombotic therapy in valvular heart disease—native and prosthetic. Chest. 2004;126:457S–82S. doi: 10.1378/chest.126.3_suppl.457S. [DOI] [PubMed] [Google Scholar]
- 19.Kumar R, Raizada A, Aggarwal AK, et al. A community-based rheumatic fever/rheumatic heart disease cohort: twelve-year experience. Indian Heart J. 2002;54:54–8. [PubMed] [Google Scholar]
- 20.Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–41. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- 21.Chan TY, Miu KY. Hemorrhagic complications of anticoagulant therapy in Chinese patients. J Chin Med Assoc. 2004;67:55–62. [PubMed] [Google Scholar]
- 22.Chiang CW, Lo SK, Ko YS, et al. Predictors of systemic embolism in patients with mitral stenosis. A prospective study Ann Intern Med. 1998;128:885–9. doi: 10.7326/0003-4819-128-11-199806010-00001. [DOI] [PubMed] [Google Scholar]
- 23.Jones M, McEwan P, Morgan CL, et al. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non-valvar atrial fibrillation: a record linkage study in a large British population. Heart. 2005;91:472–7. doi: 10.1136/hrt.2004.042465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayosi BM. A proposal for the eradication of rheumatic fever in our lifetime. S Afr Med J. 2006;96:229–30. [Google Scholar]
- 25.Expert Consultation on Rheumatic Fever and Rheumatic Heart Disease, Report of a WHO Expert Consultation on Rheumatic Fever and Rheumatic Heart Disease 29 October–1 November 2001. World Health Organization; Geneva: 2001. [Google Scholar]
- 26.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 27.Karthikeyan G, Math RS, Mathew N, et al. Accelerated infusion of streptokinase for the treatment of left-sided prosthetic valve thrombosis: a randomized controlled trial. Circulation. 2009;120:1108–14. doi: 10.1161/CIRCULATIONAHA.109.876706. [DOI] [PubMed] [Google Scholar]


