Abstract
Purpose of review
Urine is increasingly being investigated as a convenient clinical sample for the identification of mycobacterial products for the diagnosis of tuberculosis. The available literature on mycobacterial lipoarabinomannan (LAM) and urine mycobacterial DNA is reviewed.
Recent findings
The available data, despite being extracted from heterogeneous clinical populations and different clinical subgroups, indicate that urine LAM has little diagnostic utility in unselected tuberculosis suspects; however, test characteristics improve in HIV-infected patients, particularly those with advanced immunosuppression (CD4 cell count <200 cells/μl). Methodologies for urine PCR for detection of mycobacterial DNA vary across studies and focus is on standardizing assays with respect to specimen collection, assay design, and processing methodology.
Summary
Both the urine LAM and PCR for mycobacterial DNA are being evaluated in different geographical settings. Urine LAM currently offers little utility for the diagnosis of tuberculosis in unselected populations. However, urine LAM appears promising as a diagnostic tool in HIV-infected patients with CD4 cell counts less than 200 cells/μl in different clinical settings. Further developmental studies are required to enhance the performance of the assays, and their usefulness over sputum microscopy in HIV-infected patients with advanced immunosuppression requires definition in large cohort studies.
Keywords: diagnosis, lipoarabinomannan, mycobacterial DNA, PCR, tuberculosis, urine
Introduction
Tuberculosis (TB) remains a global health catastrophe. Annually, there are an estimated 9.3 million new TB cases and 2 million TB-related deaths [1]. In many parts of sub-Saharan Africa, up to 80% of TB patients are HIV-co-infected, and TB remains the leading cause of death among HIV-infected patients in this region [1,2]. HIV–TB-co-infected patients present with atypical clinical and radiological features [3]. Thus, HIV-infected patients frequently create diagnostic and management conundrums for practising clinicians with consequent misdiagnosis, diagnostic delay, and increased mortality and morbidity.
In high-burden settings, the cornerstone of TB diagnosis remains sputum smear microscopy. The sensitivity in routine clinical practice varies between 35 and 80% in HIV-negative patients, but drops to as low as 20% among HIV-infected patients [4]. Furthermore, in the latter group, sputum scarcity is common increasing the need for procedures such as sputum induction or bronchoscopy for sample acquisition. Conventional sputum microscopy is also of limited usefulness in children and those with extrapulmonary TB. Mycobacterial cultures take several weeks to provide results, are expensive, require specialized laboratory facilities, and remain unavailable in most high-burden settings [4]. Newer quantitative T-cell assays [interferon-gamma release assay (IGRAs)] have little current utility in high-burden settings and cannot distinguish latent from active TB [5], while serological tests have consistently demonstrated poor diagnostic accuracy [6]. Nucleic-acid amplification tests (NAATs) have limited sensitivity and are not widely available in high-burden countries [6]. There remains a need for inexpensive, rapid, easy to perform, point-of-care (POC) tests for TB diagnosis.
Recently, there have been attempts to use biological samples other than sputum such as urine, exhaled breath, and blood for the diagnosis of TB. Of these, urine is particularly appealing because of its availability, ease of access (easily obtained from children and adults), processing and storage, and the low infection risk to healthcare workers during sample collection.
In this review, we summarize the literature on the performance outcomes of urine diagnostics in patients with pulmonary and extrapulmonary TB (excluding urogenital TB) in different clinical settings and focus on the recent studies of urinary lipoarabinomannan (LAM) and Mycobacterium tuberculosis (MTB)-specific urine PCR tests for detection of transrenal DNA. The potential of the use of these tools in routine clinical practice, the gaps in the literature, and areas warranting further study are defined. Peer-reviewed publications were identified by searches of PubMed databases, up to and including December 2009, in all languages, using the search terms ‘tuberculosis’, ‘Mycobacterium tuberculosis DNA’, ‘urine’, ‘urinary’, ‘lipoarabinomannan’, and ‘transrenal PCR’. Other sources were experts in the field and the references of retrieved articles.
Conventional mycobacterial smear and culture using urine specimens
As early as the 1960s, it was postulated that MTB bacilli could be found in the urine of patients with active pulmonary TB, and for many years it was part of the microbiological work-up of patients with suspected TB. Currently, it is not recommended for the routine diagnosis of pulmonary TB as two large retrospective reviews indicated that the yield of urine smear and culture was less than 2% [7,8]. However, in HIV-infected patients with advanced immunosuppression, the yield of urine culture is improved (77% in one HIV-infected cohort [9]). However, even in this subgroup, the sensitivity of urine smear microscopy remains poor as does the incremental yield (~ 5%) of urine MTB culture over sputum or lymph node smear microscopy or culture [10]. Thus, conventional TB diagnostics applied to urine specimens has limited clinical usefulness.
Urinary lipoarabinomannan for diagnosis of tuberculosis
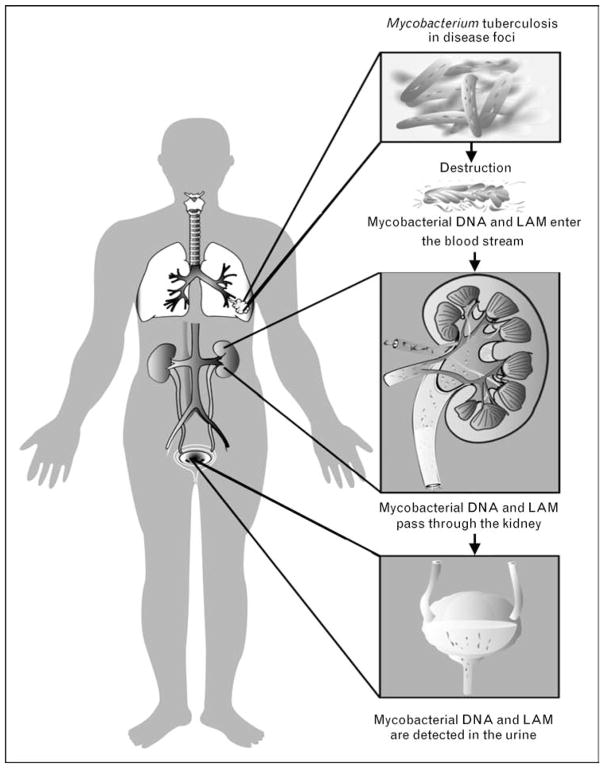
The detection of mycobacterial antigens in urine for diagnostic purposes has been suggested since the 1930s [11]. The 17.5 kD glycolipid LAM, found in the outer cell wall of mycobacterial species, has been investigated as a diagnostic antigen. LAM, an immunogenic virulence factor, is released from metabolically active or degrading bacterial cells [12] during TB infection [13]. LAM is heat stable, filtered by the kidney and detectable in the urine [14–17] (Fig. 1). Published data suggest that LAM is specific for mycobacterial species [18,19]. However, LAM-like glycolipids are found in several species of fungi and bacteria, including Corynebacterium diphtheria [20•]. We have recently found that anti-LAM polyclonal antibodies cross-react with various Actinobacteria, including different strains of Nocardia and Streptomyces, and Candida albicans, and Tsukamurella paurometabolum found in normal mouth flora [21].
Figure 1. Annotated diagram illustrating the passage of mycobacterial DNA and lipoarabinomannan antigen from infection site to urine.
LAM, lipoarabinomannan.
Hamasur et al. [14] developed an ELISA and dipstick test for the detection of LAM antigen in urine. These early developmental studies were followed by the creation of a first generation precommercial prototype [MTB LAM ELISA (Chemogen, Portland, USA)], which was clinically trialled in 2005 in Tanzania [16]. A second generation prototype using a different batch of polyclonal antibody was then developed. This has recently been superceded by a commercially available prototype, the Clearview TB ELISA (Inverness Medical Innovations, Waltham, Massachusetts, USA; see http://www.clear-view.com/tb_elisa.aspx), which uses the same polyclonal antibody used in the second generation Chemogen assay. Other investigators, including those at the Swedish Institute for Infectious Disease Control, are evaluating alternative urine assays using monoclonal antibodies (see http://www.cababstractsplus.org/abstracts/Abstract.aspx?AcNo=20013151047). Alternative urine LAM detection platforms are also being investigated (http://www.finddiagnostics.org/programs/tb/pipeline.html). Integrated commercial dipstick prototypes are currently undergoing validation studies as potential user-friendly and rapid POC diagnostic tests. One such prototype is being developed and validated by Inverness Medical Innovations.
Clinical utility of urine lipoarabinomannan in unselected patients with suspected tuberculosis from a primary care setting
Recent studies of urine LAM for TB diagnosis examined heterogeneous patient populations and performance has been disappointing. The best clinical utility seems to be in HIV-infected patients with advanced immunosuppression. Further studies evaluating this group are awaited.
Performance outcomes in clinical studies
In resource-poor TB countries, the primary care outpatient clinic shoulders the major burden of TB diagnosis and care. Tessema et al. [15] reported a sensitivity and specificity [95% confidence interval (CI)] of 74.0% (67–80) and 86.9% (84–89), respectively, in an Ethiopian cohort in which the HIV status was not determined.
Boehme et al. [16] using the first generation MTB LAM ELISA prototype (Chemogen) reported a sensitivity and specificity of 80.3 and 99%, respectively, in a Tanzanian cohort with an HIV seroprevalence of 69%. Unfortunately, the initial promise of these early studies has not been sustained. Four recently published studies show consistently poor overall test performance with sensitivities ranging between 13 and 51% [21,22•–24•] (Table 1). These disappointing results suggest that there is little clinical utility for the urine LAM assay in unselected outpatients with suspected TB. Nevertheless, across studies in high TB and HIV prevalent settings test sensitivity was higher (21–62%) in the HIV-infected patients with suspected TB [21,23•,24•]. However, which HIV-infected outpatients are most likely to benefit from the urine LAM assay is unclear because of the lack of stratification by CD4 cell counts in recent studies [22•–24•]. We recently found that urine LAM had a sensitivity of 25% in smear-negative culture-positive HIV-infected TB individuals with a CD4 cell count less than 200 cells/μl (Table 1).
Table 1.
Performance of standardized urine lipoarabinomannan assays in different clinical subgroups of patients with suspected tuberculosis stratified by HIV status and, where available, CD4 T cell count
| Study | Clinical subgroup and country | Evaluable patients tested/total number recruited | HIV (%positive) | Overall sensitivity (CI), HIV-positive patients, HIV-positive patients with CD4 <200 cells/μl | Overall specificity (CI), HIV-positive patients, HIV-positive patients with CD4 <200 cells/μl | Overall PPV (CI), HIV-positive patients, HIV-positive patients with CD4 <200 cells/μl | Overall NPV (CI), HIV-positive patients, HIV-positive patients with CD4 <200 cells/μl | Sensitivity (CI) in smear-negative (S), culture-positive (C) TB cases [n] (n = S–ve, C+ve) |
|---|---|---|---|---|---|---|---|---|
| Boehme et al.a [16] | Outpatient clinic and healthy USA/local controls (Tanzania) | 235/333 | 69.0% | Overall, 80 | 99 (N/R) | 100 (N/R) | 80 (N/R) | [n = 50] 76 (N/R) |
| HIV+, 81 | N/R | N/R | N/R | N/R | ||||
| CD4<200, N/R | N/R | N/R | N/R | N/R | ||||
| Daley et al.b [22•] | Outpatient clinic (India) | 200/200 | 8.5% | Overall, 18 (9–33) | 88 (81–92) | 30 (15–50) | 79 (72–84) | [n = 12] 25 (4–64) |
| HIV+, 20 (1–77) | 83 (51–97) | 33 (2–88) | 71 (42–90) | N/R | ||||
| CD4 <200, N/R | N/R | N/R | N/R | N/R | ||||
| Reither et al.b [24•] | Outpatient clinic (Tanzania) | 151/291 | 59.1% | Overall, 51 (38–63) | 88 (79–94) | 78 (63–89) | 68 (58–77) | [n = 21] 38 (N/R) |
| HIV+, 62 (47–75) | 84 (68–94) | 84 (68–94) | 62 (47–75) | 42 (N/R) | ||||
| CD4<200, N/R | N/R | N/R | N/R | N/R | ||||
| Mutetwa et al.b [23•] | Outpatient clinic (Zimbabwe) | 261/397 | 77% | Overall, 44(36–54) | 89(81–94) | 84(74–91) | 54(46–61) | [n = 40] 28 (13–43) |
| HIV+, 52 (43–62) | 86 (73–93) | 84 (73–92) | 57 (48–66) | N/R | ||||
| CD4<200, N/R | N/R | N/R | N/R | N/R | ||||
| Dheda et al.c [21] | Outpatient clinic (South Africa) | 427/500 | 31% | Overall, 13 (8–19) | 99 (97–100) | 94 (74–99) | 59 (53–64) | [n = 70] 9 (3–20) |
| HIV+, 21 (11–35) | 100 (91–100) | 100 (70–100) | 55 (43–65) | 18 (7–39) | ||||
| CD4<200, 37 (19–59) | 100 (84–100) | 78 (45–94) | 76 (63–86) | 29 (8–64) | ||||
| Lawn et al.b [26••] | ARV clinic, asymptomatic HIV patients (South Africa) | 235/235 | 100% | Overall, 38 (27–51) | 100 (N/R) | 100 (82–100) | 83 (77–88) | [n = 50] 36d (N/R) |
| HIV+, 38 (27–51) | 100 (N/R) | 100 (82–100) | 83 (77–88) | 36d (N/R) | ||||
| CD4 50–100, 41 (22–64) | N/R | N/R | N/R | 35d (N/R | ||||
| CD4< 50, 67 (44–84) | N/R | N/R | N/R | 56d (N/R) | ||||
| Shah et al.c [25•] | Inpatients (South Africa) | 315/499 | 85% | Overall, 59 (52–66) | 96 (91–99) | 73 (65–80) | 34 (29–39) | [n = 111] 56 (N/R) |
| HIV+, 67 (59–74) | 94 (87–98) | 75 (67–82) | 30 (25–36) | N/R | ||||
| CD4 50–100, 71 (51–87) | N/R | N/R | N/R | N/R | ||||
| CD4 <50, 85 (73–93) | N/R | N/R | N/R | N/R |
ARV, antiretroviral; CI, confidence interval; NPV, negative predictive value; N/R, not reported in study manuscript; PPV, positive predictive value.
Used the first version of the MTB LAM ELISA prototype (Chemogen, Portland, USA).
Used a second version (different polyclonal antibody) of the MTB LAM ELISA prototype (Chemogen, Portland, USA).
Used the commercially available Clearview TB ELISA assay (Inverness Medical Innovations, Waltham, Massachusetts, USA).
These values were calculated using patient numbers reported in the manuscript by Lawn et al.
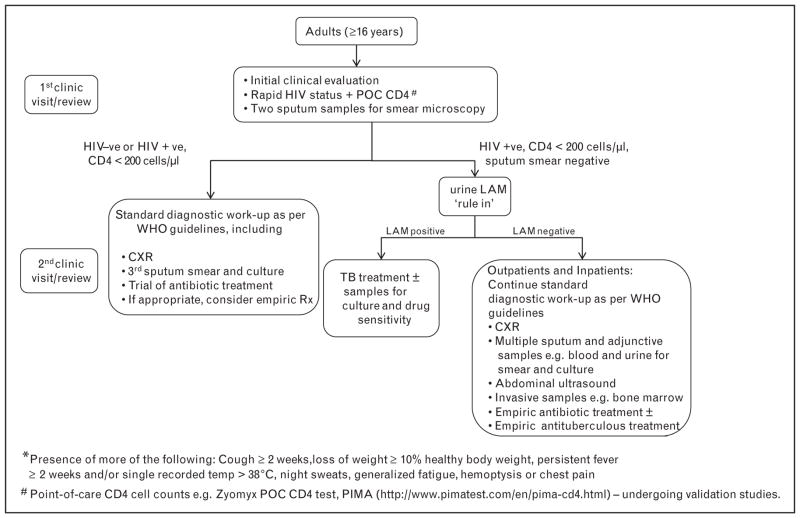
Although the evidence is currently insufficient to make a strong recommendation, urinary LAM may potentially be a useful ‘rule-in’ test in HIV-infected patients with advanced immunosuppression and suspected active TB (see Fig. 2 for how urine LAM may in the future be integrated into patient management). To clarify matters, future outpatient studies should now specifically target HIV-infected patients with advanced immunosuppression who are suspected of having active TB.
Figure 2. Putative clinical algorithm for the diagnostic work-up of outpatients and inpatients with suspected tuberculosis* in either a primary care clinic or referral hospital in a resource-poor high-tuberculosis burden and high-HIV prevalence setting.
This suggested algorithm will require prospective validation. LAM, lipoarabinomannan; TB, tuberculosis.
Relationship between test performance and level of immunosuppression
Interestingly, a crude inverse relationship can be demonstrated between improved urine LAM sensitivity and declining CD4 cell count. Thus, when several settings are considered, the test performs best in patients with advanced immunosuppression, that is, CD4 cell count less than 200 cells/μl [21,25•,26••]. Why is there an increase in LAM positivity in those with advanced immunosuppression? The reasons are not entirely clear. HIV-infected patients with advanced immunosuppression have more severe disease and a likely higher antigen burden [27]. Boehme et al. [16] found a correlation between degrees of smear positivity (1+, 2+, etc.) and LAM positivity, suggesting that burden of disease is related to the degree of urine LAM positivity. We hypothesize that changes in glomerular filtration secondary to HIV-related podocyte dysfunction [28], more common in advanced HIV, may account for the observed increase in urinary LAM. Population-specific genetic heterogeneity may also play a role [21]. Whether LAM is only filtered in those with advanced immunosuppression or whether suboptimal test sensitivity is the main reason limiting detection in other patients remains unclear. The latter seems more likely and thus improved and better detection platforms are likely required in the future.
Concerns about urine lipoarabinomannan specificity
A major concern when considering the application of urine LAM to routine clinical practice is the poor test specificity (83–89%) found in three recent clinical studies with varying HIV prevalence [22•–24•]. This contrasted with the excellent test specificity (99–100%) found in three other recent trials [21,25•,26••]. The poorer specificity in some studies may possibly be explained by undiagnosed occult TB disease, detection of antigen from latently infected patients (unlikely given the suboptimal test sensitivity), contamination of the sample by environmental mycobacteria or other bacteria (particularly if the sample was collected at home and brought to the clinic), or contamination by nonbacterial species, for example, Candida, which is prevalent in HIV-infected populations. These possibilities are tenable, given our findings that several organisms, including Candida species, cross-react with the polyclonal LAM antibodies within the assay [21]. Sample collection methodology and its possible impact on test performance will be important to consider in future studies.
Prototype variations of commercially available urine lipoarabinomannan assay and their potential impact
There remains concern about the comparability of different versions of the urine LAM assay. As previously mentioned, different batches of polyclonal antibodies and versions using the same polyclonal antibody have been studied [21,22•–25•,26••]. Although the polyclonal antibody used in the Clearview LAM ELISA and second generation precommercial Chemogen prototype is the same, there are differences in the manufacturing protocols used (e.g. antibody coating technologies, etc.). Whether this may impact test performance seems unlikely but remains a possibility, given that several technical factors may impact on ELISA test performance [29].
In summary, in unselected HIV-infected or uninfected outpatients suspected of having active TB, urinary LAM currently has limited clinical utility in a high-burden primary care setting. There is, however, the potential to integrate urine LAM into patient management in out-patients who are smear-negative, HIV-positive, and have a CD4 cell count less than 200 cells/μl (Fig. 2). The same considerations apply to the POC versions when fully developed. Given the widespread availability of rapid HIV testing, and in the near future, POC CD4 cell tests, for example, Zyomyx POC CD4 cell test (http://www.zyomyx.com/) and PIMA (http://www.pimatest.com/en/pima-cd4.html), we believe that urine LAM could easily be incorporated into an integrated diagnostic management algorithm for TB. The overall high specificity of the assay, its modest cost, the lack of other alternative rapid diagnostic options, and the diagnostic conundrum that these patients pose for practising primary care physicians, lends support to this possibility. However, further data are required before any firm recommendations can be made. Future studies should evaluate the impact of the test within an integrated algorithm rather than LAM as an isolated test. Additionally, the cost-effectiveness of the assay in this context will require evaluation.
Tuberculosis screening in HAART-naive ambulatory HIV-positive patients
In sub-Saharan Africa, TB is the leading cause of death among HIV-infected patients [2]. Highly active antiretroviral therapy (HAART) has substantially reduced the risk of HIV-infected individuals acquiring TB [30,31] and mortality in patients with active TB [31,32]. However, HIV-infected patients commencing HAART, because of several considerations, including unmasking immune reconstitution inflammatory syndrome (IRIS), must first be screened for active TB [33,34]. In Africa, approximately 25% of patients without a known TB diagnosis and referred for HAART were found to have culture-positive TB when investigated further; the majority of patients had smear-negative TB [26••,35,36]. Lawn et al. in a South African study, evaluating 235 HAART-naive patients with a median CD4 cell count of 125 cells/μl, demonstrated a urine LAM sensitivity (95% CI) of 33% (22–46%) using unconcentrated urine [26••]. In this study, standard diagnostic tools such as TB symptom screening and chest radiograph showed a sensitivity of 64%, but a specificity of just 39%, whereas smear microscopy showed a sensitivity of only 14% [26••]. Combined sensitivity of urine LAM and sputum smear microscopy was 45%, but this increased to 67% if confined to patients with a CD4 cell count less than 50 cells/μl. Given the limited data, it is not possible to make clinical recommendations, but these preliminary results suggest that urine LAM may be a useful adjunctive diagnostic tool for TB screening in antiretroviral (ARV) roll-out clinics in high-TB burden countries where patients often present with advanced immunosuppression. Further studies are now needed to clarify these findings.
Hospitalized tuberculosis suspects with advanced immunosuppression
In countries devastated by the synergistic co-epidemics of HIV and TB, a large burden of hospital admissions are related to ‘ruling in’ or ‘ruling out’ TB in HIV-infected patients. Co-infected patients suffer from increased rates of extrapulmonary and disseminated forms of TB with atypical presentations [37] and pose dilemmas for clinicians with consequent increased mortality and transmission, and prolonged hospital stay. Standard tests perform poorly and there is an urgent need for rapid convenient diagnostic tools. Shah et al. [25•] in a nested cohort study of 499 South African inpatients with suspected TB (85% HIV-infected) found the overall sensitivity (95% CI) of the Clearview LAM ELISA among confirmed TB cases to be 59% (52–66). This sensitivity improved to 71% (51–87%) in patients with a CD4 cell count 50–100 cells/μl and 85% (73–93) in patients with CD4 cell count less than 50 cells/μl. It is significant that the urine LAM sensitivity in smear-negative TB patient group was 56%. Factors associated with LAM positivity included HIV positivity, positive sputum smear [adjusted odds ratio (AOR) 2.42], and a positive mycobacterial blood culture (AOR 3.2), suggesting that a high antigenic burden and disseminated disease were related to improved test sensitivity. Anti-TB treatment was found to have a negative impact on test outcomes with sensitivity decreasing to 33% (43–77) in this group (n = 23).
The findings of this study, together with the advantages of urine LAM mentioned previously, lead us to suggest the possible inclusion of urine LAM as a ‘rule-in’ diagnostic test when evaluating HIV-infected patients with suspected TB (Fig. 2). Before firm recommendations can be made, larger studies in different geographic regions are required to confirm these preliminary findings.
Point-of-care dipstick format of urine lipoarabinomannan assay
The possibility of a POC urine dipstick test is an exciting recent development. We recently found that a point-of-care dipstick urine LAM prototype (Determine TB, Inverness Medical Innovations) had a sensitivity of 49.2% compared to 34.9% with the Clearview TB ELISA in a cohort of HIV–TB-infected patients (South African TB conference 2010; submitted). Furthermore, in patients with CD4 cell count less than 100 cells/μl, the Determine TB had a sensitivity of 91%, whereas the comparative sensitivity for Clearview TB ELISA was only 73%. The specificity of Determine TB and Clearview TB ELISA in TB-negative urines was 98 and 100%, respectively. These preliminary results are promising and larger studies will be required to clarify these findings when a finalized prototype is available.
Clinical utility of lipoarabinomannan ELISA using biological samples other than urine
Although the majority of studies have evaluated the diagnostic utility of the LAM ELISA using urine in different clinic settings, the detection of LAM antigen has now been explored in several studies using various biological samples, including serum [38,39], sputum [40], cerebrospinal fluid (CSF) [41], and pleural fluid [42••]. In serum, detection of LAM is complicated by immune complex formation [38], whereas utility in sputum and induced sputum detection is complicated by cross-reactivity with mouth flora, for example, Candida spp. [21]. In pleural fluid, it has been shown to be diagnostically unhelpful [42••], but a recent proof-of-concept study showed that, when used on CSF, it maybe a promising tool for the diagnosis of TB meningitis [41]. In a completed but unpublished prospective study evaluating patients with suspected TB meningitis, LAM antigen detection in the CSF appeared to be promising (V. Patel and K. Dheda; personal communication).
Urine PCR for diagnosis of tuberculosis
Alongside the cell wall components of MTB, nucleic acid fragments have also provided a target for detection in the diagnosis of pulmonary and extrapulmonary TB. Initial reports of detecting MTB DNA in the urine of patients without detecting whole MTB bacilli first reached the literature in 1997 [43] and since then a number of studies have reported MTB DNA detection in patients without genitourinary TB disease (reviewed in [44••]). PCR remains a modestly sensitive and highly specific tool that can accurately quantify MTB load and make a speedy diagnosis. These factors have led to the development of commercial platforms such as the self-contained Gene-Xpert (Cepheid, Sunnyvale, CA, USA) for near bedside diagnosis of TB and drug resistance from sputum. Yet urine PCR remains unutilized. The one unifying characteristic of this field is the variability of reported sensitivity, specificity, and methodology. Unlike LAM, no commercial assay has been developed specifically for the detection of urinary transrenal DNA and all methods remain in-house. Direct comparisons of published methods are missing, and when such a study was conducted by our group, the results were not comparable to the previous reports [45].
Considering the extent of confusion, are there any hard and fast rules for applying PCR technology to urine in a diagnostic setting? Certainly, a greater understanding of the phenomenon leading to the presence of DNA in urine [46] (Fig. 1) has provided consensus that, for PCR diagnosis of nongenitourinary disease, urine should not be centrifuged prior to nucleic acid extraction [47•]. The sensitivity of PCR detection is also improved by reducing the size of the target [48], though careful assay design is essential to preserve specificity. HIV infection does seem to correlate with an increase in detection of MTB DNA in urine, though the reason for this has not been determined or accurately correlated with severity of either disease.
At present, urine PCR for the routine diagnosis of pulmonary TB is not recommended, but this approach should not be completely discarded. Where current diagnostic gold standards are substandard in many patient groups, urine could be an adjunct sample. A recent study of pulmonary TB in India [49] demonstrated that of 11 bacteriologically negative but urine PCR-positive patients, nine (81.8%) responded to anti-TB therapy (ATT). This study circumvented any temporal changes in MTB DNA in urine by pooling specimens collected over a 3-day period, but did extract from the pellet of centrifuged samples and amplify a large target (786 bp) possibly reducing detection sensitivity. We are currently working to determine the impact of temporally focusing collection and ATT on MTB DNA in urine.
Optimizing PCR sensitivity and specificity by addressing specimen collection, storage [47•], and extraction are the first steps to producing a urine PCR that delivers for pulmonary TB diagnosis. The next steps are to determine the relevance of MTB DNA in urine to TB disease. It is clear that latency and persistent mycobacteria [50] are challenges for the current diagnostic repertoire. Techniques do exist, such as quantitative PCR, which provide extremely accurate measurements of MTB DNA, but the correlation between the amount of fragmented MTB DNA in urine and TB disease needs to be determined. The diagnostic capability of any new test should also not be considered in isolation of the point of application. The highest burden of TB still remains in the most resource poor countries, where the application of advanced molecular techniques requires specific considerations [51•]. A POC test for transrenal MTB DNA would be a huge step forward for molecular-based diagnosis from urine.
Additional experimental approaches for urine-based diagnostics
Given the advantages of using urine as a diagnostic sample, together with the fact that urine likely contains a number of host and organism-related proteins and metabolites, the identification of signature molecules for TB diagnosis in the urine holds much promise and warrants ongoing interest. We, and others, are currently using proteomic and metabolomic technologies to screen for urinary peptides and metabolites in an attempt to uncover candidate molecules and/or characteristic TB-specific protein signatures. Napolitano et al. [52], using these techniques, identified four TB-specific antigens, including ornithine carboamyltransferase, which may be useful for the diagnosis and monitoring of pulmonary TB. Additionally, investigators are evaluating monoclonal antibodies against known TB antigens, including LAM and the region of difference (RD)-1 antigens, for the diagnosis of TB using urine samples. Other urine-based approaches, including use of an electronic nose (E-nose) [53] and gas chromatography-mass spectrometry to characterize volatile organic compounds specific to TB are also being investigated. Urine PCR, if found to be useful, will likely be improved by simpler and user-friendlier NAAT platforms such as loop-mediated isothermal amplification (LAMP) [54].
Conclusion
The advantages of using urine as a clinical specimen and the current evaluation of POC dipstick formats make urine diagnostics an exciting frontier of TB research. Unfortunately, limited data make it difficult to provide firm recommendations for clinical practice. It is, however, clear from current studies that urine LAM, and likely urine PCR, will only be useful in certain specific subgroups of patients with suspected TB. Urine LAM has little role in the diagnosis of active disease in unselected patients. However, it could be useful, in various clinical settings, as rule-in tests in HIV-infected patients with advanced immunosuppression. We have suggested how these tools could possibly be integrated into patient management in the future. Adequately powered prospective studies are now required in specific subgroups, including HIV-infected patients with advanced immunosuppression, to determine how these assays should be optimally used in clinical practice. It is likely that alternative antigens, and better detection platforms, including field-friendly formats will make urine diagnostics a valuable adjunct to TB diagnosis. Indeed, as a famous nephrologist once stated, ‘There is little that examination of the urine cannot reveal’.
Acknowledgments
J.P. is supported by a Discovery Foundation Fellowship and by the Fogarty International Clinical Research Scholars/Fellows Support Centre National Institutes of Health grant R24TW007988. K.D. is supported by a South African MRC Career Development Award and the South African Research Chair Initiative (SARChi), EU (FW7) and the EDCTP. A.Z., M.H., P.M., and C.G. received support from EuropeAID, EUFW7, EDCTP, and A.Z. from the NIHR CBRC and UK MRC. The authors have no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 293–294).
- 1.WHO. Global tuberculosis control: surveillance, planning, financing: WHO report 2009. World Health Organisation; Geneva: 2009. [Google Scholar]
- 2.Harries AD, Hargreaves NJ, Kemp J, et al. Deaths from tuberculosis in sub-Saharan African countries with a high prevalence of HIV-1. Lancet. 2001;357:1519–1523. doi: 10.1016/S0140-6736(00)04639-0. [DOI] [PubMed] [Google Scholar]
- 3.Garcia GF, Moura AS, Ferreira CS, Rocha MO. Clinical and radiographic features of HIV-related pulmonary tuberculosis according to the level of immunosuppression. Rev Soc Bras Med Trop. 2007;40:622–626. doi: 10.1590/s0037-86822007000600004. [DOI] [PubMed] [Google Scholar]
- 4.Davies PD, Pai M. The diagnosis and misdiagnosis of tuberculosis. Int J Tuberc Lung Dis. 2008;12:1226–1234. [PubMed] [Google Scholar]
- 5.Dheda K, Smit RZ, Badri M, Pai M. T-cell interferon-gamma release assays for the rapid immunodiagnosis of tuberculosis: clinical utility in high-burden vs. low-burden settings. Curr Opin Pulm Med. 2009;15:188–200. doi: 10.1097/MCP.0b013e32832a0adc. [DOI] [PubMed] [Google Scholar]
- 6.Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: Part II. Active tuberculosis and drug resistance. Expert Rev Mol Diagn. 2006;6:423–432. doi: 10.1586/14737159.6.3.423. [DOI] [PubMed] [Google Scholar]
- 7.Mortier E, Pouchot J, Girard L, et al. Assessment of urine analysis for the diagnosis of tuberculosis. BMJ. 1996;312:27–28. doi: 10.1136/bmj.312.7022.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nour EM, Cherian BP, Quantrill SJ. Diagnostic yield of early morning urine samples in the diagnosis of tuberculosis. QJM. 2007;100:464–465. doi: 10.1093/qjmed/hcm047. [DOI] [PubMed] [Google Scholar]
- 9.Shafer RW, Kim DS, Weiss JP, Quale JM. Extrapulmonary tuberculosis in patients with human immunodeficiency virus infection. Medicine (Baltimore) 1991;70:384–397. doi: 10.1097/00005792-199111000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Monkongdee P, McCarthy KD, Cain KP, et al. Yield of acid-fast smear and mycobacterial culture for tuberculosis diagnosis in people with human immunodeficiency virus. Am J Respir Crit Care Med. 2009;180:903–908. doi: 10.1164/rccm.200905-0692OC. [DOI] [PubMed] [Google Scholar]
- 11.Parker M. Compliment fixation with urine in tuberculosis. Am Rev Tuberc. 1931;23:733–738. [Google Scholar]
- 12.Chan J, Fan XD, Hunter SW, et al. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamasur B, Bruchfeld J, Haile M, et al. Rapid diagnosis of tuberculosis by detection of mycobacterial lipoarabinomannan in urine. J Microbiol Methods. 2001;45:41–52. doi: 10.1016/s0167-7012(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 15.Tessema TA, Hamasur B, Bjun G, et al. Diagnostic evaluation of urinary lipoarabinomannan at an Ethiopian tuberculosis centre. Scand J Infect Dis. 2001;33:279–284. doi: 10.1080/003655401300077306. [DOI] [PubMed] [Google Scholar]
- 16.Boehme C, Molokova E, Minja F, et al. Detection of mycobacterial lipoarabinomannan with an antigen-capture ELISA in unprocessed urine of Tanzanian patients with suspected tuberculosis. Trans R Soc Trop Med Hyg. 2005;99:893–900. doi: 10.1016/j.trstmh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Hunter SW, Gaylord H, Brennan PJ. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986;261:12345–12351. [PubMed] [Google Scholar]
- 18.Daffe M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microb Physiol. 1998;39:131–203. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- 19.Lee RE, Brennan PJ, Besra GS. Mycobacterium tuberculosis cell envelope. Curr Top Microbiol Immunol. 1996;215:1–27. doi: 10.1007/978-3-642-80166-2_1. [DOI] [PubMed] [Google Scholar]
- 20•.Moreira LO, Mattos-Guaraldi AL, Andrade AF. Novel lipoarabinomannan-like lipoglycan (CdiLAM) contributes to the adherence of Corynebacterium diphtheriae to epithelial cells. Arch Microbiol. 2008;190:521–530. doi: 10.1007/s00203-008-0398-y. Important study pointing out other bacterial sources of LAM. [DOI] [PubMed] [Google Scholar]
- 21.Dheda K, Davids V, Lenders L, et al. Clinical Utility of a Commercial LAM-ELISA Assay for TB Diagnosis in HIV-Infected Patients Using Urine and Sputum Samples. PLoS ONE. 2010;5:e9848. doi: 10.1371/journal.pone.0009848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Daley P, Michael JS, Hmar P, et al. Blinded evaluation of commercial urinary lipoarabinomannan for active tuberculosis: a pilot study. Int J Tuberc Lung Dis. 2009;13:989–995. This study evaluates a commercial LAM assay for urine LAM. [PMC free article] [PubMed] [Google Scholar]
- 23•.Mutetwa R, Boehme C, Dimairo M, et al. Diagnostic accuracy of commercial urinary lipoarabinomannan detection in African tuberculosis suspects and patients. Int J Tuberc Lung Dis. 2009;13:1253–1259. This study evaluates a commercial LAM assay for urine LAM. [PMC free article] [PubMed] [Google Scholar]
- 24•.Reither K, Saathoff E, Jung J, et al. Low sensitivity of a urine LAM-ELISA in the diagnosis of pulmonary tuberculosis. BMC Infect Dis. 2009;9:141. doi: 10.1186/1471-2334-9-141. Recent study from Africa showing low sensitivity of LAM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Shah M, Variava E, Holmes CB, et al. Diagnostic accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalized patients in a high HIV prevalence setting. J Acquir Immune Defic Syndr. 2009 doi: 10.1097/QAI.0b013e3181b98430. A study of LAM in HIV-infected hospitalized patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Lawn SD, Edwards DJ, Kranzer K, et al. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS. 2009:23. doi: 10.1097/qad.0b013e32832e05c8. First demonstration that the LAM assay may be more sensitive than sputum microscopy in HIV-infected patients with high mycobacterial load. [DOI] [PubMed] [Google Scholar]
- 27.Lawn SD, Butera ST, Shinnick TM. Tuberculosis unleashed: the impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect. 2002;4:635–646. doi: 10.1016/s1286-4579(02)01582-4. [DOI] [PubMed] [Google Scholar]
- 28.Doublier S, Zennaro C, Spatola T, et al. HIV-1 Tat reduces nephrin in human podocytes: a potential mechanism for enhanced glomerular permeability in HIV-associated nephropathy. AIDS. 2007;21:423–432. doi: 10.1097/QAD.0b013e328012c522. [DOI] [PubMed] [Google Scholar]
- 29.Sittampalam GS, Smith WC, Miyakawa TW, et al. Application of experimental design techniques to optimize a competitive ELISA. J Immunol Methods. 1996;190:151–161. doi: 10.1016/0022-1759(95)00262-6. [DOI] [PubMed] [Google Scholar]
- 30.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 31.Lawn SD, Kranzer K, Wood R. Antiretroviral therapy for control of the HIV-associated tuberculosis epidemic in resource-limited settings. Clin Chest Med. 2009;30:685–699. viii. doi: 10.1016/j.ccm.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dheda K, Booth H, Huggett JF, et al. Lung remodeling in pulmonary tuberculosis. J Infect Dis. 2005;192:1201–1209. doi: 10.1086/444545. [DOI] [PubMed] [Google Scholar]
- 33.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and ‘unmasking’ of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177:680–685. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhasmana DJ, Dheda K, Ravn P, et al. Immune reconstitution inflammatory syndrome in HIV-infected patients receiving antiretroviral therapy: pathogenesis, clinical manifestations and management. Drugs. 2008;68:191–208. doi: 10.2165/00003495-200868020-00004. [DOI] [PubMed] [Google Scholar]
- 35.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 36.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–719. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 37.Frieden TR, Sterling TR, Munsiff SS, et al. Tuberculosis. Lancet. 2003;362:887–899. doi: 10.1016/S0140-6736(03)14333-4. [DOI] [PubMed] [Google Scholar]
- 38.Sada E, Brennan PJ, Herrera T, Torres M. Evaluation of lipoarabinomannan for the serological diagnosis of tuberculosis. J Clin Microbiol. 1990;28:2587–2590. doi: 10.1128/jcm.28.12.2587-2590.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sada E, Aguilar D, Torres M, Herrera T. Detection of lipoarabinomannan as a diagnostic test for tuberculosis. J Clin Microbiol. 1992;30:2415–2418. doi: 10.1128/jcm.30.9.2415-2418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereira Arias-Bouda LM, Nguyen LN, Ho LM, et al. Development of antigen detection assay for diagnosis of tuberculosis using sputum samples. J Clin Microbiol. 2000;38:2278–2283. doi: 10.1128/jcm.38.6.2278-2283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel VB, Bhigjee AI, Paruk HF, et al. Utility of a novel lipoarabinomannan assay for the diagnosis of tuberculous meningitis in a resource-poor high-HIV prevalence setting. Cerebrospinal Fluid Res. 2009;6:13. doi: 10.1186/1743-8454-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Dheda K, Van-Zyl Smit RN, Sechi LA, et al. Clinical diagnostic utility of IP-10 and LAM antigen levels for the diagnosis of tuberculous pleural effusions in a high burden setting. PLoS One. 2009;4:e4689. doi: 10.1371/journal.pone.0004689. This study illustrates the potential value for the detection of LAM in pleural effusions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sechi LA, Pinna MP, Sanna A, et al. Detection of Mycobacterium tuberculosis by PCR analysis of urine and other clinical samples from AIDS and non-HIV-infected patients. Mol Cell Probes. 1997;11:281–285. doi: 10.1006/mcpr.1997.0119. [DOI] [PubMed] [Google Scholar]
- 44••.Green C, Huggett JF, Talbot E, et al. Rapid diagnosis of tuberculosis through the detection of mycobacterial DNA in urine by nucleic acid amplification methods. Lancet Infect Dis. 2009;9:505–511. doi: 10.1016/S1473-3099(09)70149-5. Excellent review of transrenal detection of mycobacterial DNA and the variables affecting its use. [DOI] [PubMed] [Google Scholar]
- 45.Kafwabulula M, Ahmed K, Nagatake T, et al. Evaluation of PCR-based methods for the diagnosis of tuberculosis by identification of mycobacterial DNA in urine samples. Int J Tuberc Lung Dis. 2002;6:732–737. [PubMed] [Google Scholar]
- 46.Melkonyan H, Feaver JW, Meyer E, et al. Transrenal nucleic acids: from proof of principle to clinical tests. Problems and solutions. Ann N Y Acad Sci. 2008;1137:73–81. doi: 10.1196/annals.1448.015. [DOI] [PubMed] [Google Scholar]
- 47•.Cannas A, Goletti D, Giradi E, et al. Mycobacterium tuberculosis DNA detection in soluble fraction of urine from pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2008;12:146–151. First report of the use of transrenal detection of mycobacterial DNA using PCR. [PubMed] [Google Scholar]
- 48.Shekhtman EM, Kalpana A, Melkonyan H, et al. Optimization of transrenal DNA analysis: detection of fetal DNA in maternal urine. Clin Chem. 2009;55:723–729. doi: 10.1373/clinchem.2008.113050. [DOI] [PubMed] [Google Scholar]
- 49.Gopinath K, Singh S. Urine as an adjunct specimen for the diagnosis of active pulmonary tuberculosis. Int J Infect Dis. 2009;107:425–435. doi: 10.1016/j.ijid.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 50.Garton NJ, Waddell SJ, Sherratt AL, et al. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 2008;5:e75. doi: 10.1371/journal.pmed.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51•.Huggett JF, Green C, Zumla A. Nucleic acid detection and quantification in the developing world. Biochem Soc Symp. 2009;37:419–423. doi: 10.1042/BST0370419. This study discusses the problems associated with PCR work in the tropics. [DOI] [PubMed] [Google Scholar]
- 52.Napolitano DR, Pollock N, Kashino SS, et al. Identification of Mycobacterium tuberculosis ornithine carboamyltransferase in urine as a possible molecular marker of active pulmonary tuberculosis. Clin Vaccine Immunol. 2008;15:638–643. doi: 10.1128/CVI.00010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fend R, Kolk AH, Bessant C, et al. Prospects for clinical application of electronic-nose technology to early detection of Mycobacterium tuberculosis in culture and sputum. J Clin Microbiol. 2006;44:2039–2045. doi: 10.1128/JCM.01591-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boehme CC, Nabeta P, Henostroza G, et al. Operational feasibility of using loop-mediated isothermal amplification for diagnosis of pulmonary tuberculosis in microscopy centers of developing countries. J Clin Microbiol. 2007;45:1936–1940. doi: 10.1128/JCM.02352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]