Abstract
A neurochemical target at which cannabinoids interact to have global effects on behavior is brain noradrenergic circuitry. Acute and repeated administration of a cannabinoid receptor synthetic agonist is capable of increasing multiple indices of noradrenergic activity. This includes cannabinoid-induced 1) increases in norepinephrine (NE) release in the medial prefrontal cortex (mPFC); 2) desensitization of cortical α2-adrenoceptor-mediated effects; 3) activation of c-Fos in brainstem locus coeruleus (LC) noradrenergic neurons; and 4) increases in anxiety-like behaviors. In the present study, we sought to examine adaptations in adrenoceptor expression and function under conditions of cannabinoid receptor type 1 (CB1r) deletion using knockout (KO) mice and compare these to wild type (WT) controls. Electrophysiological analysis of α2-adrenoceptor-mediated responses in mPFC slices in WT mice showed a clonidine-induced α2-adrenoceptor-mediated increase in mPFC cell excitability coupled with an increase in input resistance. In contrast, CB1r KO mice showed an α2-adrenoceptor-mediated decrease in mPFC cell excitability. We then examined protein expression levels of α2- and β1-adrenoceptor subtypes in the mPFC as well as TH expression in the locus coeruleus (LC) of mice deficient in CB1r. Both α2- and β1-adrenoceptors exhibited a significant decrease in expression levels in CB1r KO mice when compared to WT in the mPFC, while a significant increase in TH was observed in the LC. To better define whether the same cortical neurons express α2A-adrenoceptor and CB1r in mPFC, we utilized highresolution immunoelectron microscopy. We localized α2A-adrenoceptors in a knock-in mouse that expressed a hemoagglutinin (HA) tag downstream of the α2A-adrenoceptor promoter. Although the α2A-adrenoceptor was often identified pre-synaptically, we observed colocalization of CB1r with α2-adrenoceptors post-synaptically in the same mPFC neurons. Finally, using receptor binding, we confirmed prior results showing that α2A-adrenoceptor is unchanged in mPFC following acute or chronic exposure to the synthetic cannabinoid receptor agonist, WIN 55,212-2, but is increased, following chronic treatment followed by a period of abstinence. Taken together, these data provide convergent lines of evidence indicating cannabinoid regulation of the cortical adrenergic system.
Keywords: cannabinoid receptor type 1, α2-adrenoceptors, cannabinoid receptor knock out mice, medial prefrontal cortex, locus coeruleus, tyrosine hydroxylase
Introduction
Norepinephrine (NE), a biogenic amine integral to the stress-response system and regulation of higher cognitive functions (Degenhardt et al., 2001, Arendt and Munk-Jorgensen, 2004, Pattij et al., 2008) is modulated by the endocannabinoid (eCB) system (Page et al., 2007; Page et al., 2008; Reyes et al., 2009; Carvalho et al., 2010b; Wyrofsky et al., 2015). Emerging lines of evidence indicate that the neurobehavioral consequences of exogenous cannabinoid exposure involve, in part, cannabinoid-induced cellular and molecular changes in adrenergic neurons. For example, pre-treatment of human subjects with the beta-adrenergic antagonist, propranolol, has been shown to prevent the acute effects of cannabis-induced impairtment of learning (Sulkowski and Vachon, 1977). Likewise, using an animal model, pre-test administration of the α1-adrenergic agonist, phenylephrine, reversed post-training cannabinoid-induced retrieval impairment (Moshfegh et al., 2011). The efficacy of nabilone, a cannabinoid receptor agonist, in the treatment of post-traumatic stress disorder (PTSD) symptoms is attributed to actions of cannabinoids on NE circuitry (Reyes et al., 2009; Villanueva et al., 2009; Carvalho et al., 2010a; Carvalho et al., 2010b). Finally, mice deficient in CB1 receptor exhibit anxiogenic-like responses in various behavioral paradigms including the elevated plus-maze, the open field test and the light-dark box (Haller et al., 2002; Maccarrone et al., 2002; Martin et al., 2002; Uriguen et al., 2004; Thiemann et al., 2009). These mice also exhibited depressive-like phenotypes (Viveros et al., 2005; Valverde and Torrens, 2012), cognitive impairments including memory and learning deficits (Martin et al., 2002; Varvel and Lichtman, 2002; Bilkei-Gorzo et al., 2005; Madronal et al., 2012) as well as impairment in the extinction of aversive memories (Marsicano et al., 2002) with no changes in locomotor function (Haller et al., 2002) suggesting an effect on biogenic amine circuitry.
Cannabinoid receptor agonists have been shown to both increase, and decrease, indices of brain noradrenergic activity. Activation of presynaptic CB1 receptor on terminals of sympathetic axons innervating blood vessels has been shown to reduce the release of NE (Ishac et al., 1996; Pfitzer et al., 2005). Incubation of synaptosomes with low concentrations of tetrahydrocannabinol (THC) results in reductions in NE release (Poddar and Dewey, 1980) while systemic administration of rimonabant (a CB1 receptor antagonist) increases NE in the anterior hypothalamus and medial prefrontal cortex (mPFC) not the nucleus accumbens (Tzavara et al., 2001; Tzavara et al., 2003). In addition to inhibition of NE release, several lines of evidence support cannabinoid-induced increases in NE release (Rodriguez de Fonseca et al., 1991; Molderings et al., 2002; Page et al., 2007). Acute systemic (Oropeza et al., 2005) or local (Page et al., 2008) administration of the synthetic cannabinoid receptor agonist, WIN 55,212-2, or administration of a FAAH inhibitor (Gobbi et al., 2005), increases NE efflux in the rat frontal cortex. Acute WIN 55,212-2 exposure stimulates c-Fos expression in noradrenergic neurons of the locus coeruleus (LC) (Oropeza et al., 2005; Page et al., 2008), enhances N-methyl-D- aspartate-induced firing of LC neurons (Mendiguren and Pineda, 2004) and increases NE synthesis (Moranta et al., 2009) and release in terminal regions (Oropeza et al., 2005). CB1 receptor have been localized to noradrenergic axon terminals in the mPFC (Oropeza et al., 2007) supporting the hypothesis that NE and eCBs can regulate each other’s function.
We have also described functional interactions between CB1 receptor and adrenoceptor systems in the mPFC. Whole cell patch clamp recordings of layer V/VI cortical pyramidal neurons in rats revealed that clonidine-induced α2-adrenoceptor-mediated elevations in cortical pyramidal cell excitability are significantly decreased following pre-treatment with the synthetic CB1 receptor agonist, WIN 55,212-2, suggesting cannabinoid stimulation of NE release and desensitization of α2-adrenoceptors (Reyes et al., 2012). The receptor interaction was both action potential and GABAA receptor-independent as the desensitization occurred similarly in the presence or absence of tetrodotoxin or the GABAA receptor antagonist bicuculline indicating that CB1-α2-AR interactions are likely direct rather than mediated by synaptic afferents. We also showed that α2A-adrenoceptors-immunoreactivity is distributed in axon terminals, somata and dendrites in the mPFC using immunoelectron microscopy (Cathel et al., 2014), consistent with other reports (Aoki et al., 1994; Venkatesan et al., 1996; Aoki et al., 1998; Aoki et al., 2000). Systemic administration of WIN 55,212-2, tetrahydrocannabinol (Δ9 THC) and CP 55940 increases the spontaneous firing rate of LC neurons in a dose dependent manner (Mendiguren and Pineda, 2006; Muntoni et al., 2006). These effects were prevented by pretreatment with the cannabinoid receptor (CB1 receptor) antagonist, SR 141716 (Oropeza et al., 2005; Mendiguren and Pineda, 2006) supporting the involvement of CB1 receptors.
Adaptations occur following chronic exposure to WIN 55212-2 and withdrawal. For example, repeated administration of WIN 55212-2 increases TH protein expression in the LC and this is accompanied by potentiated NE efflux in response to an acute injection of WIN 55212-2 without a change in baseline NE efflux (Page et al., 2007). Chronic WIN 55,212-2 administration produces an anxiogenic-like response that reverts to pre-drug levels following a period of abstinence (Page et al., 2007). Chronic WIN 55212-2 treatment completely abolishes the ability of clonidine to induce an increase in excitability of PFC neurons (Reyes et al., 2012) and reduces the binding site density of β1-adrenoceptors in neocortex (Hillard and Bloom, 1982; Reyes et al., 2009), and both α2- and β1-adrenoceptors in the accumbens (Carvalho et al., 2010b). Meanwhile withdrawal following chronic WIN 55212-2 exposure alters β1-adrenoceptors in the mPFC (Reyes et al., 2009). Taken together, these data indicate that sustained CB1 receptor activation results in a sustained increase in NE release which induces down-regulation of adrenergic receptors.
However, several gaps in knowledge remain that we addressed in the present study using a multidisciplinary approach. First, we measured electrophysiological properties of α2–adrenoceptor responses under conditions of CB1 receptor deletion using CB1 receptor-knockout (KO) mice and compared these to wild type (WT) controls. Next, we examined expression levels of α2- and β1-adrenoceptors in the mPFC in mice lacking the CB1 receptor as well as the catecholamine synthesizing enzyme, tyrosine hydroxylase (TH), in the LC, which provides the sole source of NE to the mPFC (Halliday, 2004, Aston-Jones and Cohen, 2005, Aston-Jones et al., 2007). To better define sites of cortical cannabinoid-adrenoceptor interactions, we examined the ultrastructural localization of CB1 receptors with respect to neurons expressing α2-adrenoceptors in a knock-in mouse that expressed a hemoagglutinin (HA)-tag downstream of the α2-adrenoceptor promoter (Lu et al., 2009). Given the results showing significant adaptations in the adrenergic system under conditions of CB1r deletion, we next investigated α2A-adrenoceptor binding in the mPFC following acute and chronic WIN 55,212-2 and following abstinence from chronic WIN 55,212-2 exposure in rats. These data are important considering the increasing prevalence of clinical studies examining exogenous cannabinoid administration for the treatment of a variety of pathophysiological conditions. Furthermore, because the noradrenergic and eCB systems are both dynamically regulated by stress, where stress decreases anandamide and CB1 receptor levels while increasing 2-arachidonoyl glycerol levels (Morena et al., 2016), understanding the mechanism underlying the cannabinoid-adrenoceptor interactions in the mPFC may help unravel the mechanism underlying cannabinoid-induced impairments in attention and cognition.
Methods
The procedures employed in the present study conformed to Drexel University Institutional Animal Care and Use Committee, National Institute of Health’s Guide for the Care and Use of Laboratory Animals (1996), the Health Research Extension Act (1985) and the PHS Policy on Humane Care and Use of Laboratory Animals (1986). All efforts were made to utilize only the minimum number of animals necessary to produce reliable scientific data, and experiments were designed to minimize any animal distress.
Specificity of CB1 receptor antibody
Three male CB1r KO mice and three WT controls mice (9–12 weeks old) were deeply anesthetized with sodium pentobarbital (40 mg/kg) and perfused transcardially through the ascending aorta with heparinized saline followed with 25 ml of 4% formaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Immediately following perfusion/fixation, the brains were removed and postfixed for 30 min. Brains were sectioned in the coronal plane at a setting of 40 μm using a Vibratome (Technical Product International, St. Louis, MO, USA) through the forebrain and hippocampus, and collected into 0.1 M PB. Sections through the rostrocaudal extent of mPFC and hippocampus were processed for light microscopic detection of CB1 receptor in the mPFC. Tissue sections were incubated in rabbit anti-CB1 receptor at 1:1,000 in 0.1% bovine serum albumin (BSA), 0.25% Triton X-100 and 0.1M tris buffered saline (TBS; pH 7.6) for 15–18 h at room temperature. The following day, tissue sections were rinsed three times in 0.1 M TBS and incubated in biotinylated donkey anti-rabbit (1:400; Vector Laboratories, Burlingame, CA, USA) for 30 min followed by rinses in 0.1 M TBS. Subsequently, a 30-minute incubation of avidin-biotin complex (Vector Laboratories) was done. For all incubations and washes, sections were continuously agitated with a rotary shaker. CB1 receptor was visualized by a 4-minute reaction in 22 mg of 3,3′-diaminobenzidine (Sigma-Aldrich) and 10 μl of 30% hydrogen peroxide in 100 ml of 0.1 M TBS. Sections were collected, dehydrated and coverslipped with Permount (Sigma-Aldrich) for light microscopic analysis of CB1 receptor immunoreactivity.
In vitro electrophysiology
For electrophysiology experiments, male wild-type (WT) and CB1 receptor KO mice (9–12 weeks old) were housed three per cage in a controlled environment (12-hour light schedule, temperature at 20°C). CB1 receptor KO mice were originally generated on a C57Bl/6 background by Zimmer et al. (Zimmer et al., 1999) at the National Institutes of Health. Heterozygous breeding pairs were generously donated by Dr. Carl Lupica at the National Institutes of Health and were bred and genotyped at Temple University to obtain CB1 receptor KO mice and WT littermates. Food and water were provided ad libitum. The care and use of animals were approved by the Institutional Animal Care and Use Committee of Temple University and were conducted in accordance with the NIH Guide for the care and use of laboratory animals.
Drug preparation and administration
A stock solution of clonidine (Sigma-Aldrich, St. Louis, MO) was prepared in de-ionized water at a stock concentration of 10 mM and diluted on the day of the experiment to a final concentration in the perfusion bath of 10 μM.
Electrophysiology
Male CB1 receptor KO mice and WT controls (3–5 mo.) were euthanized, brains rapidly removed, 250μm slices were taken containing layer V/VI pyramidal neurons of the infralimbic region of mPFC, which is implicated in conditioning and extinction of fear (Giustifino and Maren, 2015), drug seeking behavior (Kalivas, 2009; Moorman et al., 2015) and resilience to social stress (Cooper et al., 2015). Whole-cell recordings under current clamp (I = 0pA) conditions were conducted as described previously (Reyes et al., 2012; Cathel et al., 2014). At baseline, membrane potential was recorded and input resistance calculated using the current-voltage relationship. Neuronal excitability was assessed in each cell by recording voltage responses to a series of current pulses (0–160 pA). Membrane potential, input resistance and neuronal excitability were also measured following bath application of the α2-adrenoceptor agonist, clonidine (10 μM). Recordings were analyzed and pyramidal cell morphology of recorded cells determined by immunohistochemistry as described previously (Reyes et al., 2012; Cathel et al., 2014).
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software. For electrophysiology data, membrane voltage, input resistance and excitability responses to individual current pulses were compared between genotypes by unpaired Student’s t-test and drug effects on these endpoints compared by paired Student’s t-test. Significance was set at P < 0.05.
Protein extraction and Western blot analysis
For Western blotting experiments, WT and CB1 receptor KO littermates (9–15 weeks old) were housed three per cage in a controlled environment (12-hour light schedule, temperature at 20°C). The CB1 receptor KO were generated in CD1 mice as previously reported (Ledent et al., 1999). Brain tissue was rapidly removed from each animal on ice. Using a trephine, the mPFC brain region was microdissected from each animal. mPFC was homogenized with a pestle and extracted in radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) on ice for 20 min. Lysates were cleared by centrifugation at 13,000 rpm for 12 min at 41°C. Supernatants or protein extracts were diluted with an equal volume of Novex 2® tris glycine sodium dodecyl sulfate sample buffer (Invitrogen, Carlsbad, CA, USA) containing dithiothreitol (Sigma-Aldrich Inc., St. Louis, MO, USA). Protein concentrations of the undiluted supernatants were quantified using the bicinchoninic acid protein assay reagent (Pierce, Rockford, IL, USA).
Cell lysates containing equal amounts of protein were separated on 4–12% tris-glycine polyacrylamide gels and then electrophoretically transferred to Immobilon-P polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). Membranes were incubated in rabbit antityrosine hydroxylase (1:4,000; Immunostar Inc., Hudson, WI), rabbit anti-α2-adrenoceptor (1:500; Sigma-Aldrich Inc.) or rabbit anti-β1-adrenoceptor (1:1,000) primary antibodies overnight at 4°C and then in alkaline phosphatase-conjugated secondary antibodies for 30 min to probe for the presence of proteins using a Western blotting detection system (Western Breeze Chemiluminescent Kit; Invitrogen). Membranes containing proteins obtained from LC were incubated with mouse anti-TH while membranes containing proteins obtained from the mPFC were incubated with rabbit anti-α2-adrenoceptor or rabbit anti-β1-adrenoceptor. Following incubation in a chemiluminescent substrate (Western Breeze Chemiluminescent Kit), blots were exposed to X-OMAT AR film (Kodak, Rochester, NY, USA) for different lengths of time to optimize exposures. CB1 receptor was readily detected by immunoblotting in rat mPFC extracts, and was visualized as a single band that migrates at approximately 60 kDA whereas α2-adrenoceptor, or β1-adrenoceptor, migrate at approximately 45 kDA and 64 kDA. Blots were incubated in stripping buffer (Restore Stripping Buffer, Pierce) to disrupt previous antibody-antigen interactions and then re-probed with β-actin (1:5,000, Sigma-Aldrich Inc.) with 1-hour incubation to ensure proper protein loading. The density of each band was quantified using Un-Scan-It blot analysis software (Silk Scientific Inc., Orem, UT, USA). CB1 receptor, α2-adrenoceptors, or β1-adrenoceptors was normalized to β-actin immunoreactivity on each respective blot. Student’s t-test was used to analyze the difference in CB1 receptor, α2-adrenoceptors, or β1-adrenoceptors protein expression between WT and CB1 receptor KO mice. Results are presented as mean ± SEM. Data on α2A-adrenoceptor binding and Western blot data were analyzed using Student’s t-test.
Immunoelectron microscopy
Five male hemagglutinin (HA) epitope-tagged wild type α2A-adrenoceptors knock-in mice, six months of age were kindly provided by Dr. Qin Wang of the University of Alabama in Birmingham, Alabama. The HA-α2A line is a gene targeting knock-in line not transgenic line. The HA tag was inserted right after the first ATG of the alpha2A coding sequence (this gene is intronless). Hence, α2A expression is at a level/localization/pattern identical to the endogenous one (same gene structure, under the same transcriptional control) (Lu et al., 2009). These mice were housed two or three to a cage (20°C, 12-h light, 12-h dark cycle lights on 0700) and were quarantined prior to perfusion. These mice were used for examining the subcellular localization of HA epitope-tagged α2A-adrenoceptor and CB1 receptor. Food and water were freely available. On the day of perfusion these male HA-α2A-adrenoceptor knock-in mice were deeply anesthetized with sodium pentobarbital (40 mg/kg) and transcardially through the ascending aorta with heparinized saline followed with 25 ml of 3.75% acrolein (Electron Microscopy Sciences) and formaldehyde in 0.1 M PB; pH 7.4, respectively. Brains were collected and sectioned as described earlier. Sections through the rostrocaudal extent of mPFC were processed for electron microscopic analysis of HA-α2A-adrenoceptor and CB1 receptor. Tissue sections were incubated in mouse anti-HA (Covance, Emeryville, CA, USA) at 1:1,000 and rabbit anti-CB1 receptor at 1:1,000 in 0.1% bovine serum albumin (BSA) and 0.1M TBS for 1518 h at room temperature. The following day, tissue sections were rinsed three times in 0.1 M TBS and incubated in biotinylated donkey anti-mouse (1:400; Vector Laboratories, Burlingame, CA, USA) for 30 min followed by rinses in 0.1 M TBS. Subsequently, a 30-minute incubation of avidin-biotin complex (Vector Laboratories) was done. For all incubations and washes, sections were continuously agitated with a rotary shaker. HA-α2A-adrenoceptor was visualized by a 4-minute reaction in 22 mg of 3,3′-diaminobenzidine (Sigma-Aldrich) and 10 μl of 30% hydrogen peroxide in 100 ml of 0.1 M TBS.
Following rinses in 0.1 M TBS, tissues sections were rinsed with 0.1 M PB and 0.01 M PBS, and were incubated in a 0.2% gelatin-PBS and 0.8% BSA buffer for 10 min. This was followed by incubation in goat anti-rabbit IgG conjugate in <1 nm gold particles (Amersham Bioscience Corp., Piscataway, NJ, USA) at room temperature for 2 hours. Sections were then rinsed in buffer containing the same concentration of gelatin and BSA as above and subsequently rinsed with 0.01 M PBS. Sections were then incubated in 2% glutaraldehyde (Electron Microscopy Sciences) in 0.01 M PBS for 10 min followed by washes in 0.01 M PBS and 0.2 M sodium citrate buffer (pH 7.4). A silver enhancement kit (Amersham Bioscience Corp.) was used for silver intensification of the gold particles. The optimal times for silver enhancement time were determined by empirical observation for each experiment and ranged 7–8 min. Following intensification, tissue sections were rinsed in 0.2 M citrate buffer and 0.1 M PB, and incubated in 2% osmium tetroxide (Electron Microscopy Sciences) in 0.1 M PB for 1 h, washed in 0.1 M PB, dehydrated in an ascending series of ethanol followed by propylene oxide and flat embedded in Epon 812. Thin sections of approximately 50–100 nm in thickness were cut with a diamond knife (Diatome-US, Fort Washington, PA, USA) using a Leica Ultracut (Leica Microsystems, Wetzlar, Germany). Sections were collected on copper mesh grids and counterstained stained with 5% uranyl acetate followed by Reynold’s lead citrate. Captured images of selected sections were compared with captured light microscopic images of the block face before sectioning. Sections were examined with a Morgagni transmission electron microscope (Fei Company, Hillsboro,OR, USA) and digital images were captured using the AMT advantage HR/HR-B CCD camera system (Advance Microscopy Techniques Corp., Danvers, MA, USA). Figures were assembled and adjusted for brightness and contrast in Adobe Photoshop.
Identification of gold-silver labeling in profiles
Selective gold-silver labeled profiles were identified by the presence, in single thin sections, of at least two gold particles within a cellular compartment. As were previously reported (Reyes et al., 2006), spurious silver grains can contribute to false positive labeling and can be detected on blood vessels, myelin or nuclei. Considering that a minimal spurious labeling was identified, we have set the criteria that a process is immunolabeled if two immunogold-silver particles were present. Thus, a profile containing only one gold particle in adjacent thin sections was designated as lacking detectable immunoreactivity. Whenever possible, the more lightly labeled axonal labeling for HA-α2A-adrenoceptors was confirmed by detection in at least two serial sections. As observed in low magnification electron micrographs, background labeling in the neuropil, deemed spurious, was not commonly encountered.
Data analysis for electron microscopy
Tissue sections were taken from four male HA epitope-tagged wild type α2A-adrenoceptor knock-in mice with the best immunocytochemical labeling and preservation of ultrastructural morphology. These tissue sections were used in the analysis and the information is presented in Table 1. At least three Vibratome sections were examined per mouse. Vibratome sections were selected from non-adjacent 40-μm-thick tissue sections. At least 8 grids containing containing four to eight ultrathin sections were collected from the surface of individual Vibratome sections. For quantification of labeled profiles in 40 μm-thick sections immunolabeled before embedding for electron microscopy, we have observed that the collection of sections only from the surface of the section minimizes artifacts that may be associated with incomplete penetration of antisera. The analysis of tissue sections collected at the plastic-tissue interface ensured that both markers were detectable in all sections used for analysis (Chan et al., 1990).
Table 1.
Experiments conducted for the semi-quantitative analysis.
| Mouse number | Number of Vibratome tissue sections analyzed | Number of grids analyzed per Vibratome tissue section |
|---|---|---|
| Mouse 1 | Three | 8 |
| Mouse 2 | Three | 8 |
| Mouse 6 | Three | 8 |
| Mouse 7 | Three | 8 |
The classification of identified cellular elements was based on the method of Peters et al. (Peters et al., 1991). Neuronal perikarya were distinguished from proximal dendrites by the presence of a nucleus. Dendrites usually contained endoplasmic reticulum and were postsynaptic to axon terminals. They were defined as proximal if their size was larger than 0.7 μm in diameter. Axon terminals contained synaptic vesicles and were at least 0.3 μm in diameter. A varicosity was considered as synaptic when it showed a junctional complex, a restricted zone of apposed parallel membranes with slight enlargement of the intercellular space, and/or associated postsynaptic thickening. Asymmetric synapses were identified by thick postsynaptic densities (Gray’ type I), whereas symmetric synapses had thin densities (Gray’ type II). The terms ‘contact’ or ‘apposition’ were also used to denote close parallel membrane associations of axon terminals with perikarya and/or dendrites, which lacked recognizable specializations, but were otherwise not separated by glial processes.
Drug preparation and administration
WIN 55,212-2, a synthetic cannabinoid agonist, (Sigma-Aldrich Inc., St. Louis, MO) was dissolved in 5% dimethyl sulfoxide (DMSO) in 0.9% sodium chloride solution at a concentration of 3 mg/ml. Experimental and control rats were injected intraperitoneally (i.p.) with 0.1 ml/100 g body weight of either WIN 55,212-2 or DMSO, respectively.
Rats were randomly divided into three groups at the beginning of the study. For the acute group, animals were administered WIN 55,212-2 at 3.0 mg/kg once. For the chronic group, animals were administered WIN 55,212-2 at 3.0 mg/kg once daily for seven days. Another group consisted of animals that were administered WIN 55,212-2 for seven days and were subsequently abstinent from WIN 55,212-2 for seven days. For each group examined, the control animals received a solution of DMSO solution i.p. at 0.1 ml/100 g body weight. Thirty minutes following the last WIN 55,212-2 injection, rats were briefly exposed to isoflurane (Abbott Laboratories, North Chicago, IL; 0.5–1.0%, in air) and euthanized by decapitation. Brains were removed for subsequent α2A-AR mRNA expression and binding analysis.
α2-adrenoceptor binding
3H-RX821002 (55.0 Ci/mmol, Perkin Elmer, Waltham, MA) was used to quantitate α2-adrenoceptor binding sites in mPFC. 3H-RX821002 binding was performed as previously described (Szot et al., 2006, Szot et al., 2010). Briefly, slides were thawed, and then 600 μl/slide of incubation buffer (~2 nM 3H-RX 821002 in 50 mM NaPO4 buffer, pH 7.4) was placed over the tissue. Non-specific binding was defined in the presence of 10 μM rauwolscine. Slides were incubated for 45 min at room temperature and then washed for 2 min in ice-cold 50 mM NaPO4 buffer, pH 7.4, dipped in ice-cold distilled water and dried rapidly under a stream of cool air. Slides were apposed to Biomax MR film (Eastman Kodak Co., Horsham, PA) for 2 months. Films were developed by standard procedures (Szot et al., 1997, Szot et al., 2007). 3H-RX821002 binding sites were quantitated as optical density (OD) using MCID system (InterFocus Imaging Ltd., Cambridge, England).
Results
Specificity of antibodies and control experiments
The characterization of all the primary antibodies used in the present study is presented in Table 2. Immunoperoxidase detection of CB1 receptor antibody was conducted in tissue sections obtained from mice with CB1 receptor deletion in parallel with the tissue sections obtained from the wild-type mice. CB1 receptor immunoperoxidase labeling was detected in multiple brain regions in WT mice including the cerebral cortex and hippocampus (not shown) consistent with previous studies (Katona et al., 2006; Scavone et al., 2010; Fitzgerald et al., 2012; Cathel et al., 2014; Reyes et al., 2015). Furthermore, the specificity of CB1 receptor was also demonstrated using immunoblotting where a protein of the expected molecular size was detected (Suarez et al., 2008). CB1 receptor specificity was also verified using preabsorption of the primary antisera with the blocking peptide corresponding to the last 15 amino acids of the CB1 receptor C-terminus. Tissue sections obtained from the rat forebrain incubated in this solution did not exhibit CB1 receptor immunoreactivity compared with tissue sections in which standard immunohistochemistry was performed. In addition, preabsorption experiment was also performed using Western blot analysis in which CB1 receptor immunoreactivity was also abolished (Scavone et al., 2010; Reyes et al., 2015). Tissue sections obtained from the rat forebrain incubated in this solution did not exhibit CB1 receptor immunoreactivity compared with tissue sections in which standard immunohistochemistry was performed. In addition, preabsorption experiment was also performed using Western blot analysis in which CB1 receptor immunoreactivity was also abolished (Scavone et al., 2010; Reyes et al., 2015). The monoclonal antibody against HA-α2A-adrenoceptor was generated in mouse against the 12-amino-acid peptide CYPYDVPDYASL The specificity of the TH antibody has been examined by preabsorption of the antibody with a high concentration of TH (Van Bockstaele and Pickel, 1993). The specificity of the α2-adrenoceptor and β1-adrenoceptor was investigated using immunoperoxidase, immunofluorescence and Western blotting. We showed specific immunoreactivity of β1-adrenoceptor in the amygdala using light, immunofluorescence and electron microscopy as well as Western blotting (Rudoy et al., 2009). We also showed specific immunoreactivity of α2-adrenoceptor in the mPFC using light and immunofluorescence (Reyes et al., 2009; Carvalho et al., 2010b). Preabsorption of α2-adrenoceptor and β1-adrenoceptor with the antigenic peptide at 10 μM blocked the α2-adrenoceptor and β1-adrenoceptor immunoreactivities in the forebrain. Blots incubated with 10 μg/ml of affinity-purified α2-adrenoceptor and β1-adrenoceptor antisera preincubated with antigenic peptide blocked the α2-adrenoceptor and β1-adrenoceptor expression in rat FC extracts (Reyes et al., 2009). Furthermore, some sections were processed in parallel with the rest of the immunohistochemical procedures identical except that one of the primary antisera was omitted. Sections processed with the omission of primary antibody (CB1 receptor, α2-adrenoceptor, β1-adrenoceptor) abolished any detectable immunoreactivity (CB1 receptor, α2-adrenoceptor- or β1-adrenoceptor-immunoreactivity). Furthermore, control of specificity for CB1 receptor, HA- α2A-adrenoceptor, TH, α2-adrenoceptor and β1-adrenoceptor was also carried out, including control of the secondary antibody where control sections were also processed without primary antibody/ies but with secondary antibody and control for primary antibody/ies where some sections were processed with primary antibody/ies but without secondary antibody/ies. In those control tissue sections, run in parallel, peroxidase immunoreactivity or immunogold-silver particles were not demonstrated in tissue sections from which primary or secondary antibody/ies was/were omitted. To evaluate the possible cross-immunoreactivity of secondary antibodies with the primary antibodies in the dual-labeling experiment, some sections were processed for dual immunolabeling with omission of one of the primary antibodies.
Table 2.
Characterization of the primary antibodies
| Antigen | Immunogen | Manufacturer | Host, monoclonal/polyclonal | References |
|---|---|---|---|---|
| CB1r | Directed against the last 15 amino acids of the C-terminus of the rat CB1r | Generated in the laboratory of Dr. Ken Mackie. | Rabbit polyclonal |
Scavone et al., 2010 Katona et al., 2006; Fitzgerald et al., 2012; Reyes et al., 2015; Cathel et al., 2014 |
| HA | Directed against the 12-amino-acid peptide CYPYDVPDYASL | Covance | Mouse monoclonal |
Lu et al., 2009 Wang et al., 1997 |
| TH | Raised against denatured TH from rat pheochromocytoma | Immunostar | Mouse monoclonal | Van Bockstaele et al., 1993 |
| α2A-AR | Synthetic peptide, amino acids 218–235 of human, rat and pig | Sigma-Aldrich | Rabbit polyclonal |
Reyes et al., 2009 Carvalho et al., 2010 |
| β1-AR | Synthetic peptide, amino acid 392–408 | Sigma-Aldrich | Rabbit polyclonal |
Reyes et al., 2009 Carvalho et al., 2010 |
Indices of adrenergic activity in CB1 receptor KO mice
To define the adrenergic activity in CB1 receptor KO mice, we compared α2-adrenoceptor-mediated responses of pyramidal neurons in mPFC slices from WT and CB1 receptor KO mice (Fig. 1). Here, we used clonidine, an α2-adrenoceptor agonist that increased the excitability and input resistance of rat mPFC pyramidal neurons in vitro (Cathel et al., 2014). In the presence of WIN 55,212-2 the effects of clonidine on neuronal excitability and input resistance were blocked (Cathel et al., 2014). Figure 1 depicts data that represent N=14 cells from seven WT mice and N=7 cells from three CB1 receptor KO mice. Baseline membrane voltage was not different between CB1 receptor KO and WT mice (WT: −58.7 ± 2.8 mV; CB1r KO: −56.0 ± 3.0 mV) and neither group showed a significant clonidine-induced change in membrane voltage. Similar to results that we and others reported for rats (Carr et al., 2007; Reyes et al., 2012; Cathel et al., 2014), WT mice exhibited a clonidine-induced α2-adrenoceptor-mediated increase in mPFC cell excitability [leftward shift of the stimulus-response curve; significantly increased excitability responses to 40, 60, 80 (P < 0.05), 100 and 120 pA (P< 0.01) current pulses] coupled with a significantly increased input resistance (252 ± 27 to 303 ± 37 MΩ, P < 0.01). In contrast, CB1 receptor KO mice showed reduced basal cortical excitability compared to WTs and an α2-adrenoceptor-mediated decrease in mPFC cell excitability (rightward shift of the stimulus-response curve; significantly decreased excitability response to 160 pA current pulse (P < 0.05) coupled with a slight decrease in input resistance (243 ± 27 to 206 ± 12 MΩ, n.s.). Clonidine effects on excitability and input resistance both reflect α2-adrenoceptor-mediated modulation of a hyperpolarization/cyclic nucleotide-gated (HCN) current in mPFC pyramidal dendrites that influences synaptic integration in these neurons (Carr et al., 2007). These data provide evidence that genetic deletion of CB1 receptor reduces basal mPFC pyramidal neuron excitability and desensitizes cortical α2-adrenoceptors, decreasing the ability of cortical neurons to respond to excitatory synaptic inputs.
Figure 1.
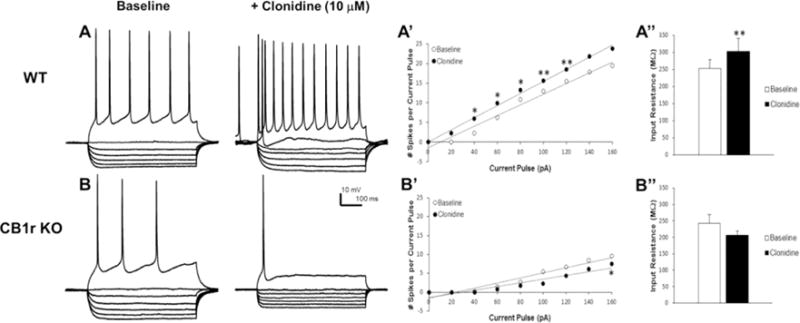
α2-adrenoceptor responses in mPFC pyramidal neurons in WT and CB1 receptor KO mice. Panels A and B indicate voltage responses to 700 ms current steps (pA; −100, −80, −60, − 40, −20, 0, +80) from a representative WT (A) and CB1 receptor KO mouse (B) both before and after bath application of clonidine (10 μM). Panels A’ and B’ summarize mean excitability data as stimulus-response curves to a range of current steps (0–160 pA) in mice from each genotype. Panels A” and B” show clonidine effects on membrane input resistance in mice from each genotype. Acute stimulation of the α2-adrenoceptor with clonidine increases excitability of mPFC neurons (A and rightward shift in A’) and increases input resistance (A”) in WT mice. In contrast, clonidine decreases excitability of mPFC neurons (B and leftward shift B’) and slightly decreases input resistance (B”) in CB1 receptor KO mice. Data represent mean ± SEM. Asterisks indicate a significant difference between baseline and clonidine by paired Student’s t-test (*P < 0.05; **P < 0.01).
In addition, to define adaptations in adrenergic systems under conditions of CB1 receptor deletion, we examined expression levels of the catecholamine synthesizing enzyme, TH (Figure 2A) in the LC, as well as two adrenergic receptor subtypes, β1-adrenoceptor (Figure 2B) and α2-adrenoceptor (Figure 2C) in the mPFC of mice lacking the CB1 receptor. This data is important considering that the release of NE is related to arousal states that have profound effects on cognitive and behavioral processes involving mPFC functions (Arnsten, 2007; Lapiz and Morilak, 2006). These modulatory systems engage multiple receptor subtypes, including α1-adrenoceptor, α2-adrenoceptor and β1-adrenoceptor (Ramos et al., 2005; Lapiz and Morilak, 2006; Arnsten, 2007). In mice deficient in CB1 receptor (Fig. 2), a significant increase in TH was observed in the LC (P < 0.05) while both α2-AR (P < 0.05) and β1-AR (P < 0.01) receptors in the mPFC exhibited a significant decrease in expression levels when compared to WT.
Figure 2.
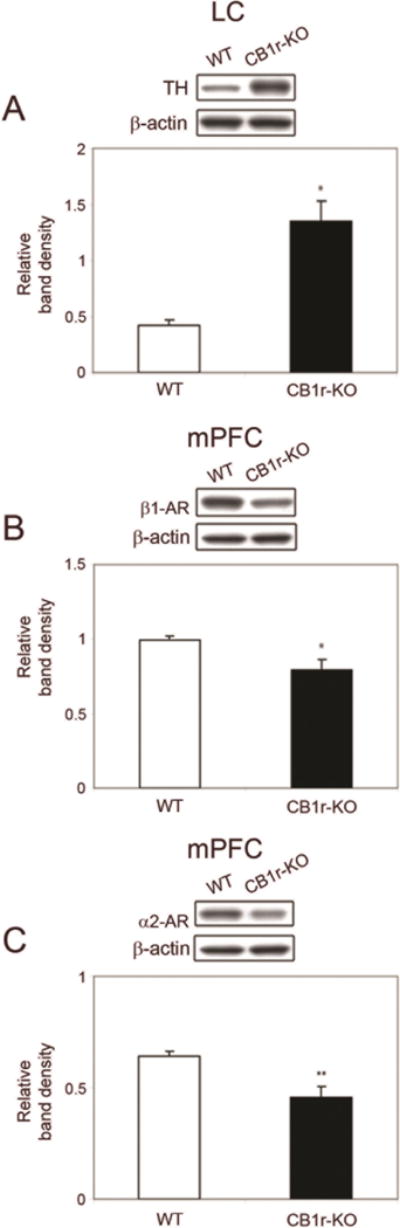
Western blot analysis of tyrosine hydroxylase (TH) in the locus coeruleus (LC), β1-adrenoceptor (β1-AR) and α2-adrenoceptor expression in the mPFC in WT and CB1 receptor KO mice. Bands shown are representative of one sample obtained from one animal per group. A. Western blot for TH in protein extracts from the LC in WT and CB1 receptor KO mice. TH expression is significantly higher in CB1 receptor KO mice compared with WT mice CB1 receptor KO mice. B. Western blot analysis of β1-adrenoceptor expression in the mPFC of WT and CB1 receptor KO mice. β1-adrenoceptor expression is significantly lower in CB1 receptor KO mice compared with WT mice. C. Western Blot analysis of α2-adrenoceptor expression in the mPFC in WT and CB1r-KO mice. α2-adrenoceptor is significantly lower in CB1 receptor KO mice compared with WT. Data are presented as mean ± SEM of change in band intensity. β-actin immunoblotting was used as a control to verify equal protein loading. Data were analyzed using paired Student’s t-test. *P 0.05 vs WT mice, **P 0.01 vs WT mice.
Ultrastructural localization of CB1 and HA-α2A-AR in frontal cortex
The region analyzed for ultrastructural analysis included the infralimbic portion of the mPFC as illustrated in the rat brain atlas (Paxinos and Watson, 1997) at the antero-posterior level of bregma 2.20–3.20mm. The infralimbic region of the mPFC is bounded anteriorly by the medial orbital cortex, dorsally by the prelimbic cortex, laterally by the forceps minor of corpus callosum, as well as ventrally and caudally by the dorsal peduncular cortex and lateral septal nucleus. Control tissue sections for immunoelectron microscopy were processed to determine the specificity of the primary antibodies used in the present study. Control tissue sections from mice with CB1 receptor deletion incubated with CB1 receptor antibody did not show any immunoreactivity at ultrastructural level (Figure 3A). In addition, tissue section obtained from HA-α2A-adrenoceptor-tagged mouse showed absence of HA-α2A-adrenoceptor immunoreactivity when tissue sections were incubated without HA-α2A-adrenoceptor primary antibody (Figure 3B). HA-α2A-adrenoceptor was also not present when tissue sections from non- HA-α2A-adrenoceptor-tagged mouse were incubated with HA-α2A-adrenoceptor primary antibody (Figure 3C).
Figure 3.
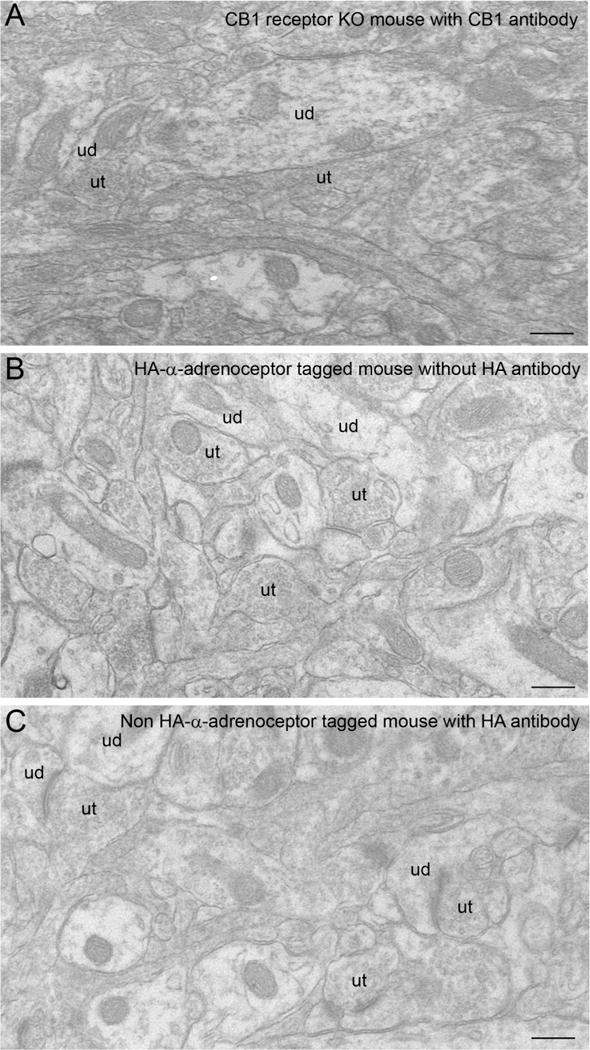
A. Electron photomicrograph showing the absence of CB1 receptor immunolabeling in mPFC in a representative tissue section obtained from a CB1 receptor KO mouse incubated with CB1 receptor primary antibody and processed for silver enhancement to visualize immunogold silver labeling. B. Electron photomicrograph showing the absence of HA-α2A-adrenoceptor immunogold-silver labeling in mPFC of a representative tissue section obtained from an HA epitope-tagged HA-α2A-adrenoceptor knock-in mouse incubated without HA-α2A-adrenoceptor primary antibody and processed for immunoperoxidase detection. C. Electron photomicrograph showing the absence of HA-α2A-adrenoceptor immunogold-silver labeling in mPFC of a representative tissue section obtained from a non-HA epitope-tagged mice incubated with HA-α2A-adrenoceptor primary antibody and processed for immunoperoxidase detection. ud: unlabeled ut: unlabeled terminal. Scale bars, 0.5 μm.
Using dual immunolabeling, HA-α2A-adrenoceptor was detected with immunoperoxidase and CB1 receptor was detected with immunogold-silver particles (Fig. 4A). Our present study confirms previous reports demonstrating that CB1 receptor is abundant in axon terminals in the infralimbic portion of the mPFC (Kawamura et al., 2006; Monory et al., 2006; Oropeza et al., 2007; Kano et al., 2009). Likewise, our results show that HA-α2A-adrenoceptor is prominently localized in axon terminals confirming results from our previous analysis (Cathel et al., 2014). There were several interactions observed between HA-α2A-adrenoceptor and CB1r (Figures 4–5). First, it was observed that HA-α2A-adrenoceptor-immunoreactive axon terminals also express CB1 receptor (Figure 4B–C). Semi-quantitative analysis conducted showed that out of 276 HA-α2A-adrenoceptor-labeled axon terminals, 26% (72/276) also exhibited CB1 receptor immunoreactivity. The dual-labeled axon terminals were directly contacting unlabeled dendrites. When synaptic specializations were observed, 64% (46/72) were of the symmetric type (Figure 4C) and 15% (11/72) formed asymmetric synapses with unlabeled dendrites (Figure 4B). The remaining 21% (15/72) of synaptic contacts did not form detectable synaptic specializations. This type of interaction showing both HA-α2A-adrenoceptor and CB1 receptor in axon terminals indicates that CB1 receptor could modulate NE release in the mPFC. It was also observed that appositions between HA-α2A-adrenoceptor-labeled axon terminals and CB1 receptor-labeled dendrite were present (10%; 27/276). When synaptic specializations were distinguishable, 56% (15/27) were of the symmetric type (Figure 4F) and 18% (5/27) formed asymmetric type synapses (Figure 4F). The remaining 26% (7/27) did not form detectable synaptic specializations. In some instances, HA-α2A-adrenoceptor-labeled dendrites also exhibited CB1 receptor immunoreactivity (15%; 40/276). Of 40 HA-α2A-adrenoceptor and CB1 receptor co-labeled dendrites, 60% (24/40) received symmetric type synapses (Figure 5B–D), 23% (9/40) received asymmetric type synapses (Figure 5B) and 17% (7/40) did not form detectable synaptic specializations. In very few cases, 3% (7/276) of HA-α2A-adrenoceptor-labeled dendrites received a synaptic contact from a CB1 receptor-labeled axon terminal (Figure 5D–E). Singly labeled HA-α2A-adrenoceptor and CB1 receptor axon terminals converging onto unlabeled dendrites was infrequently observed (1%; 2/276). These data add to our growing knowledge of cannabinoid modulation of noradrenergic circuitry and indicate that CB1 receptor-containing afferents are positioned to impact cortical activity via multiple synaptic configurations.
Figure 4.
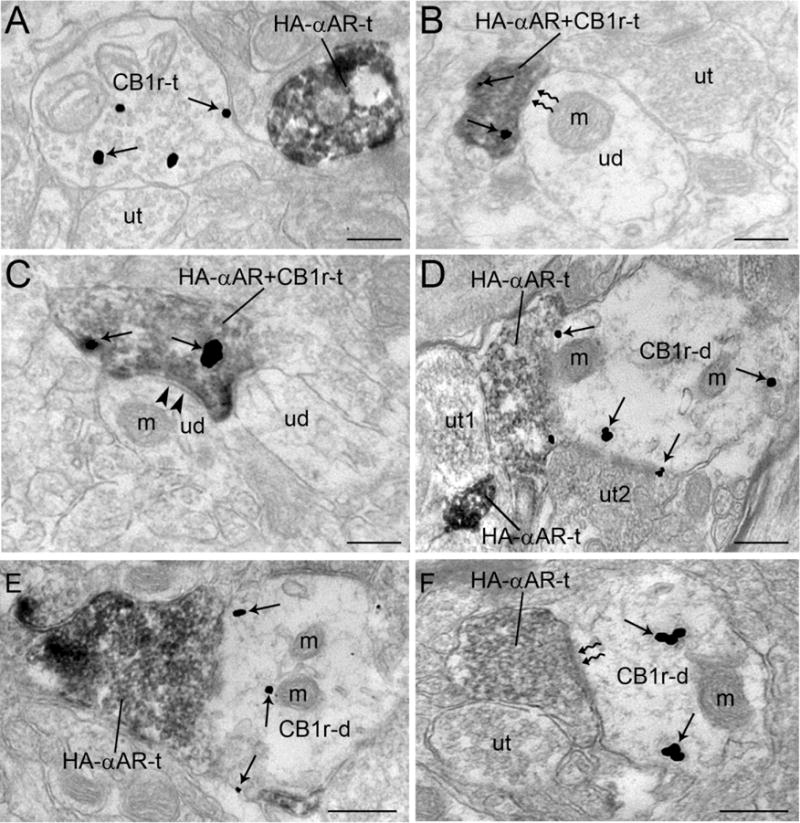
A. Electron photomicrograph showing immunoperoxidase labeling of HA-α2A-adrenoceptor (HA-αAR-t) and immunogold-silver labeling (arrows) of CB1r in separate axon terminals in the mPFC of an HA epitope-tagged HA-α2A-adrenoceptor knock-in mouse. B. An immunoperoxidase-labeled axon terminal contains dense immunoreactivity for HA-α2A-adrenoceptor and immunogold-silver labeling for CB1 receptor (HA-αAR+ CB1r-t). The dually labeled terminal forms an asymmetric type synapse (zigzag arrows) with an unlabeled dendrite (ud) that contains a mitochondria (m) and is apposed by an unlabeled terminal (ut). C. A dually labeled HA -α-adrenoceptor +CB1r-t axon terminal forms a symmetric type synapse (arrowheads) with an unlabeled dendrite (ud) containing a mitochondria (m). D. A densely peroxidase labeled HA-αAR-t is shown contacting an unlabeled axon terminal (ut1) that contacts another HA-αAR-t that in turn contacts a CB1 receptor-labeled dendrite (CB1r-d) containing several mitochondria (m). Arrows point to immunogold-silver labeled particles. The CB1r-t also receives a synapse from another unlabeled terminal (ut2). E. HA-αAR-t is shown contacting a CB1r-d with several mitochondria (m). F. A HA-αAR-t forms an asymmetric synapse (zigzag arrows) with a CB1r-d and is directly apposed by an unlabeled terminal (ut). m: mitochondria; ut: unlabeled terminal. Scale bars, 0.5 μm.
Figure 5.
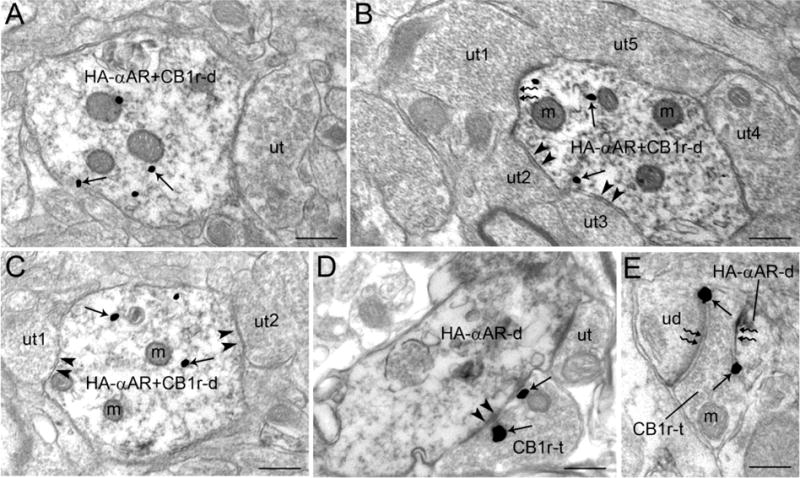
Ultrastructural evidence for co-localization of HA-α-adrenoceptor and CB1 receptor in dendrites. A. An immunoperoxidase-labeled dendrite contains HA-α2A-adrenoceptor immunoreactivity as well as immunogold-silver labeling for CB1 receptor (HA-αAR+CB1r-d). This dendrite is directly contacted by an unlabeled terminal (ut). B. A dually labeled HA-αAR+CB1r dendrite receives an asymmetric type synapse (zigzag arrows) from an unlabeled terminal (ut1) and a symmetric type synapse (arrowheads) from ut2 and ut3. A fourth unlabeled terminal (ut4) directly contacts the HA-αAR+CB1r dendrite. C. A dually labeled HA-αAR+CB1r-d receives two symmetric type synapses (arrowheads) from ut1 and ut2. D. A CB1 receptor labeled axon terminal (CB1r-t) forms a symmetric type synapse (arrowheads) with HA-α-adrenoceptor labeled dendrite (HA-αAR-d). The same HA-αAR-d is contacted by an unlabeled terminal (ut). E. A CB1r-t forms an asymmetric type synapse (zigzag arrows) with an unlabeled dendrite (uD) and a HA-αAR-d. m: mitochondria; ut: unlabeled terminal. Scale bars, 0.5 μm.
WIN 55,212-2 effects on α2A-adrenoceptor binding in the mPFC
Here, we examined the influence of WIN 55,212-2 on cortical adrenoceptor binding of α2A-adrenoceptor in the mPFC. We previously published protein analysis data (Reyes et al., 2009) showing that acute or chronic treatment with WIN 55,212-2 did not produce any change in α2A-adrenoceptor expression in the mPFC. However, this prior study did show a trend for an increase in α2A-adrenoceptor expression following abstinence from chronic exposure to WIN 55,212-2. Using receptor binding, we confirm that acute or chronic treatment with WIN 55,212-2 does not affect α2A-adrenoceptor binding in the mPFC; however, α2A-adrenoceptor binding was increased following abstinence from chronic WIN 55,212-2 exposure. Figure 6 shows a representative autoradiogram of α2A-adrenoceptor binding in the mPFC following acute or chronic WIN 55,212-2 exposure and following abstinence from repeated WIN 55,212-2 treatment. The effect of abstinence on the α2-adrenoceptor binding in the mPFC was measured 7 days following withdrawal from 7-day WIN 55,212-2 administration. Acute or chronic WIN 55,212-2 exposure did not affect α2A-adrenoceptor binding in the mPFC; however, α2-adrenoceptor binding in the mPFC was significantly increased (P < 0.05) following abstinence (Figure 6).
Figure 6.
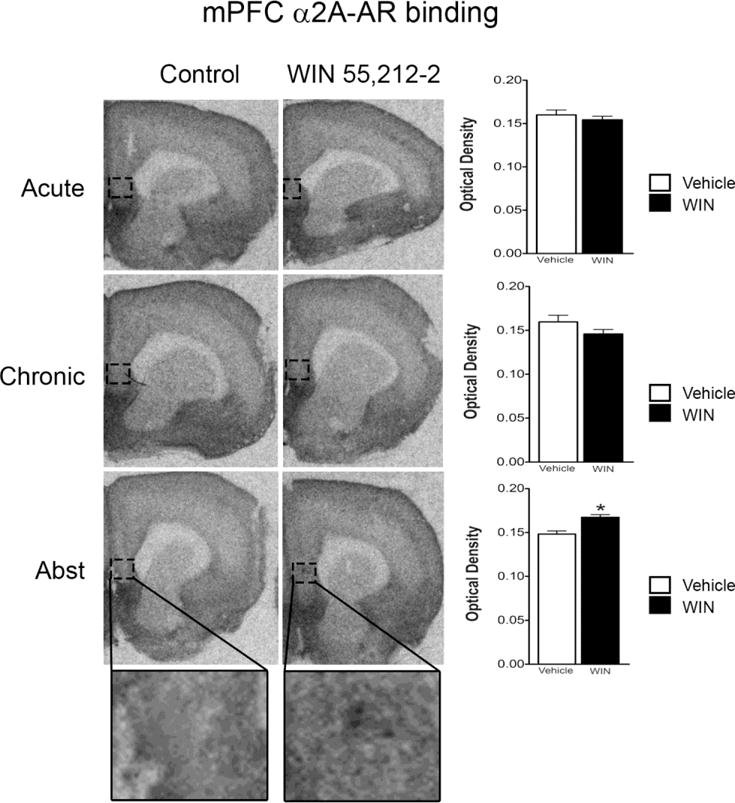
α2-adrenoceptor binding in mPFC of rats exposed to acute or chronic treatment with WIN 55,212-2. There was no change in the α2-adrenoceptor mRNA expression in the mPFC following acute or repeated administration of WIN 55,212-2 compared to control. However, following a period of abstinence from chronic WIN 55,212-2 exposure, there was a significant increase in α2-adrenoceptor mRNA binding in the mPFC. *P 0.05 vs WIN 55,212-2. Bottom panels show boxed region at a higher magnification.
Discussion
Electrophysiological studies in a slice preparation reveal that acute stimulation of α2-adrenoceptor with clonidine generated a differential response in CB1 receptor-KO mice compared to WT mice. Acute stimulation of the α2-adrenoceptor with clonidine significantly increased excitability of mPFC neurons in the WT mice while decreasing excitability of mPFC neurons in the CB1 receptor-KO mice. Our results also indicate significant decreases in two adrenoceptor subtypes, α2- and β1-adrenoceptors, in the mPFC of CB1 receptor-KO mice when compared to WT mice. Interestingly, the catecholamine synthesizing enzyme, TH, is significantly increased in the LC of CB1 receptor-KO mice compared with WT mice. Ultrastructural analysis revealed co-localization of the α2-adrenoceptor with CB1 receptor in mPFC neurons in addition to other synaptic configurations. Finally, a significant increase in α2-adrenoceptor mRNA expression was only observed in the mPFC following abstinence from exposure to a chronic cannabinoid receptor agonist in rats suggesting a potential alterations of NE at the synapse. These data add to the accumulating evidence supporting a significant impact of cannabinoids on brain adrenergic function.
Methodological considerations
Experiments were carried out in both rats and mice. In order to examine adaptations in the noradrenergic system under conditions of CB1 receptor deletion, a mouse model is necessary. Moreover, due to the lack of availability of specific antibodies directed against adrenoceptor subtypes for anatomical analysis in perfused brain tissue (Jensen et al., 2009; Bohmer et al., 2014), an HA-epitope tagged wild-type α2-adrenoceptor knock-in mice was used to determine the anatomical relationship between the α2a-adrenoceptor and CB1 receptor in the mPFC.
Approaches that combine immunohistochemical detection of an antibody with electron microscopy offers certain advantages. Specifically, the pre-embedding immunohistochemistry maintains morphological preservation of the neuropil while allowing detection of proteins that are present in low levels. A potential limitation associated with the pre-embedding immunohistochemical technique is the limited and/or differential penetration of immunoreagents in thick Vibratome sections (Leranth and Pickel, 1989; Chan et al., 1990). In order to circumvent this caveat, analysis of ultrathin section was carried out exclusively near the tissue-plastic interface where penetration of immunoreagents is maximal. Another caveat associated with the procedure is the specificity of immunogold-silver labeling. This was controlled by quantifying profiles that contained a minimum of two immunogold-silver particles (Carvalho et al., 2010; Cathel et al., 2014; Oropeza et al., 2007; Reyes et al., 2006; Reyes et al., 2015; Scavone et al., 2010; Van Bockstaele and Pickel, 1993).
Western blot analysis presents some experimental caveats that must be considered when interpreting data. These caveats include the accuracy of sampling of the region of interest and the comparison of equal protein quantities across treatment groups. In order to circumvent variability in tissue excision, a single investigator obtained the brain samples for each experiment. Moreover, to ensure equivalent loading of protein, blots were re-probed with β-actin and results were normalized to this internal standard. β-actin expression was comparable across treatment groups examined.
Desensitization of cortical α2-adrenoceptor under conditions of CB1 receptor deletion
Several studies have reported that CB1 receptor KO mice exhibit anxiogenic-like and depressive-like phenotypes (Haller et al., 2002; Viveros et al., 2005; Thiemann et al., 2009; Valverde and Torrens, 2012) with no changes in locomotor function (Haller et al., 2002). Administration of the CB1 receptor antagonist SR141716A in CB1 receptor-KO mice reduced anxiety-like behavior (Haller et al., 2002) while the CB1 receptor inverse agonist AM251 did not induce any significant alterations in anxiety-like behavior in CB1 receptor-KO mice (Thiemann et al., 2009). Our present findings that show an increase TH and decrease in α2-AR and β1-AR expression levels in CB1 receptor-KO mice may suggest involvement of a non-CB1 receptor and adrenergic receptors including vanilloid type 1 receptors (TRPV1) and GPR55 (Hong et al., 2009; Laricchiuta et al., 2013; Biernacki and Skrzydlewska, 2016;Marichal-Cancino et al., 2016; Zhou et al., 2016). It has been shown that eCBS can act to TRPV1 and GPR55 (Chavez et al., 2010; Hong et al., 2009; Laricchiuta et al., 2013; Biernacki and Skrzydlewska, 2016;Marichal-Cancino et al., 2016; Zhou et al., 2016). Our electrophysiology data in WT mice confirmed findings from earlier studies in rats indicating that stimulation of α2-adrenoceptors enhances the excitability of mPFC pyramidal neurons coupled with an increase in cellular input resistance (Andrews and Lavin, 2006; Carr et al., 2007; Reyes et al., 2012; Cathel et al., 2014). These effects are consistent with an inhibition of a HCN channel-mediated inward current in mPFC pyramidal neurons (Carr et al., 2007). Although we acknowledge that inter-species difference is critical (Ferreira et al., 2012; Liu et al., 2009), using rat (Cathel et al., 2014) and mice (present study) tissue with similar conditions demonstrate that we have obtained consistently similar data regardless of using mouse or rat tissue. α2-adrenoreceptors on mPFC pyramidal dendrites play a key role in the synaptic integration function of these neurons. α2-adrenoreceptor activation suppresses an HCN current that has two effects in rats: slight hyperpolarization of the pyramidal cell but increased temporal summation of distally evoked excitatory synaptic inputs that enhances the cell’s excitability (Carr et al, 2007). The net effect of this α2-HCN interaction could explain the ability of norephinephrine to promote attentional mechanisms through enhancement of the signal-to-noise function of mPFC neurons (Sawaguchi et al., 1990; Li et al., 1999; Aston-Jones and Cohen, 2005; Wang et al., 2007; Carr et al., 2007).
CB1 receptor KO mice showed a reduction of basal excitability of mPFC neurons, consistent with our anatomical data indicating reduced cortical α2-adrenoceptor expression. This reduced excitability likely reflects tonic adrenergic activity at α2-adrenoceptors, which normally maintains cortical cell excitability, an effect that is diminished in CB1 receptor KO mice. This reduction of cortical α2-adrenoceptors in CB1r KO mice further supports the finding that they do not show the normal α2-adrenoceptor response exhibited by WT controls. Indeed, the effects of clonidine in CB1 receptor KO mice is opposite of that observed in controls: an α2-adrenoceptor-mediated reduction of mPFC excitability coupled with a slight reduction of input resistance. Together, these results provide evidence for functional desensitization of cortical α2-adrenoceptors in CB1 receptor KO mice and more broadly, evidence of cannabinoid regulation of cortical α2-adrenoceptor-HCN interactions using a transgenic mouse model.
Interestingly, it has been previously reported that CB1 receptor activation using chronic WIN 55,212-2 treatment decreases cortical β1-adrenoceptor levels (Page et al., 2007) and binding site density (Hillard and Bloom, 1982; Reyes et al., 2009) as well as completely abolishing the clonidine-induced excitation of mPFC neurons (Reyes et al., 2012). Our present results indicate that CB1 receptor deficient mice show a decrease in α2- and β1-adrenoceptor expression levels and α2-adrenoceptor desensitization to clonidine exposure. Considering that these are two opposite manipulations (CB1 receptor activation and deletion) which seem to induce equivalent consequences with regard to expression and function of adrenoceptors, it is possible that chronic WIN 55,212-2 treatment downregulates CB1 receptors and that this effect may somehow contribute to adrenoceptor downregulation which CB1 receptor deficient mice may mimic producing similar effect on the NE system. It is also likely that the constitutive genetic deletion of CB1 receptors may produce some kind of developmental adaptation that have their own effects on NE neurotransmission, downregulating adrenoceptors. Chronic CB1 receptor agonism might be producing the same effect but via a different mechanism.
Anatomical relationship of cortical α2-adrenoceptor and CB1 receptor in mPFC
Endogenous and exogenous cannabinoids acting through the CB1 receptor have been implicated in a plethora of physiological functions (Busquets-Garcia et al., 2015; Wyrofsky et al., 2015). In humans and rodents, convergent studies suggest that exogenous cannabinoids and the eCB system is an essential regulator of executive functions, mood and emotions (Fagundo et al., 2013; McLaughlin et al., 2014). As an integral part of the reward system, the mPFC regulates executive and cognitive functions. Dysfunction of the mPFC leads to dysregulated neurotransmission in limbic regions including the hippocampus and amygdala. This demonstrates the vital role of the mPFC in the development of neuropsychiatric disorders. Likewise, the role of cannabinoids in neuropsychiatric disorders including anxiety or depression has been described (Cortes-Briones et al., 2015; Habibisaravi et al., 2015; Panlilio et al., 2015). Our data are consistent with others (Zarate et al., 2008; Richter et al., 2012) showing presynaptic localization of α2-adrenoceptor in the mPFC (Pudovkina et al., 2001; Flugge et al., 2004). Using electron microscopy, we had previously described the pre-synaptic distribution of α2-adrenoceptor and CB1 receptor in the mPFC, in independent studies (Oropeza et al., 2007; Cathel et al., 2014). We also used confocal triple immunofluorescence miscroscopy to reveal that HA-α2-adrenoceptor immunoreactive fibers co-localized dopamine-β-hydroxylase, a marker of NE axon terminals, and CB1 receptor in the mPFC (Cathel et al., 2014). To our knowledge, this is the first subcellular demonstration of co-existence between α2-adrenoceptor and CB1 receptor in common cortical axonal profiles. In addition, our immunoelectron microscopy results demonstrate different types of CB1r and α2-adrenoceptor interactions in the mPFC. CB1 receptor-containing axon terminals can be found pre-synaptic to α2-adrenoceptor-containing dendrites and CB1 receptor and α2-adrenoceptor-can be found post-synaptically in common dendrites. Despite a low level of synaptic configurations (e.g. 15% of α2-adrenoceptor and CB1 receptor co-expression is symmetric), this interaction may still exert some significant behavioral consequences since small synaptic modifications applied to synapses in a given neuron disrupt the inhibitory and excitatory input and produce large effect for modifications induced by a single stimulus, and that few synaptic contacts may still exert a direct synaptic influence (Del Cid-Pellitero and Garzon, 2014, Yger et al., 2015).
The interaction of α2-adrenoceptor and CB1 receptor may have several functional consequences. Our previous electrophysiology findings of cortical pyramidal neurons showed that cannabinoid administration desensitized α2-adrenoceptor (Reyes et al., 2012; Cathel et al., 2014). Desensitization of presynaptic α2-adrenoceptor autoreceptors may result in the stimulation of NE efflux as removal of the agonist rapidly restores receptor function (Hein and Kobilka, 1995). Desensitization does not seem to depend on protein degradation and as such no differences in the total receptor protein would be expected during desensitization and this is consistent with the previous results showing no difference in α2-adrenoceptor protein levels following chronic WIN 55,212-2 exposure (Reyes et al., 2009). α2-adrenoceptor desensitization may occur through sequestration, downregulation and decreases in Gi that have been demonstrated following agonist exposure to α2-adrenoceptor (Jones et al., 1990; Jewell-Motz et al., 1998). Another possibility is that α2-adrenoceptor is phosphorylated by G-protein-coupled receptor kinases and/or second messenger-dependent kinases including protein kinases A and C (Milligan et al., 1995) because persistent activation of phospholipase C and protein kinase C signaling pathways induces firing (Carr et al., 2007). Alternatively, desensitization of α2-adrenoceptor may take place when it is sequestered to the intercellular compartment (Milligan et al., 1995). While our current and previous physiological evidence (Reyes et al., 2012; Cathel et al., 2014) suggests functional desensitization of α2-adrenoceptor in the mPFC through CB1 receptor agonism, future investigation is required to unravel the mechanism. Interestingly, while alterations of α2A-adrenoceptor binding in the mPFC occurred following abstinence from chronic WIN 55,212-2 exposure, the α2A-adrenoceptor mRNA expression was unchanged in the LC (data not shown) indicating that despite significant high densities of α2A-adrenoceptor in these two important nuclei (Probst et al., 1984), it is not dysregulated following acute or chronic WIN 55,212-2 exposure.
Adaptations in adrenoceptors following CB1 receptor agonist administration
Using Western blot analysis, we previously reported that WIN 55,212-2 administration affected the expression of cortical adrenoceptors (Reyes et al., 2009). We showed that chronic WIN 55,212-2 exposure, with or without abstinence, influences β1-adrenoceptor and that abstinence also affects α2-adrenoceptor protein levels in the mPFC (Reyes et al., 2009). In the present study, α2-adrenoceptor binding was significantly increased following abstinence from WIN 55,212-2 exposure. The increase in α2-adrenoceptor binding in the present study supports a mechanism whereby WIN 55,212-2 administration activates LC neurons with a concomitant increase of NE efflux in the mPFC (Oropeza et al., 2005; Page et al., 2007). Increases in NE efflux may contribute to an increase α2-adrenoceptor binding as more NE binds to α2-adrenoceptor. Although α2-adrenoceptor functions as an autoreceptor to inhibit NE release (Richter et al., 2012), we and others have provided evidence for postsynaptic localization of α2-adrenoceptor (Cathel et al., 2014); thus it is possible that increases in α2-adrenoceptor binding could represent α2-adrenoceptor that are located postsynaptically. However, further investigation is needed to elucidate this. In addition, an increase in α2-adrenoceptor binding may occur as a response to altered NE level at the synapse (Page et al., 2007). After a period of abstinence increased α2-adrenoceptor binding may result as a consequence of a rebound in receptor expression similar to that reported after a sudden cessation of using a beta blocker (Pratt and Bowery, 1993). Following period of abstinence increases in α2-adrenoceptor expression might be a consequence of normalization of NE levels as it has been shown that anxiety-like behavior and NE levels return to normal levels after chronic WIN 55,212-2 treatment followed by a cessation of drug use (Page et al., 2007).
Functional implications
In summary, the results of our present study highlight the diversity of cellular interactions of the endocannabinoid and noradrenergic systems in the mPFC. Moreover, these anatomical results provide a cellular basis for the functional desensitization of cortical α2-adrenoceptors in CB1 receptor KO mice providing evidence of cortical α2-adrenoceptor regulation by the endocannabinoid system. Considering that nabilone’s efficacy in the treatment of PTSD is attributed to the actions of cannabinoids on NE circuitry (Reyes et al., 2009; Villanueva et al., 2009; Carvalho et al., 2010a; Carvalho et al., 2010b) and that cortical NE neurotransmission is involved in cognitive and behavioral states including anxiety (Arnsten, 2007; Ramos and Arnsten, 2007; Arnsten and Pliszka, 2011), cannabinoid and adrenergic receptor interactions may underlie the regulation of cortical function by cannabinoids. Interestingly, recent studies have shown that CB1 and CB2 receptors also play a role in splenic contraction (Simkins et al., 2016), that both peripheral and central CB1 receptors control stress-induced impairment of memory consolidation (Busquets-Garcia et al., 2016) and that a memory acquisition deficit induced by CB1 receptor agonist, arachidonylcyclopropylamide, involved α2-adrenoceptors in the mPFC (Beiranvand et al., 2016). Thus, these findings may have implications for advancing our understanding of the circuitry underlying α2-adrenoceptor and CB1 receptor interactions that may be targeted in the development of pharmacological treatment for neuropsychiatric disorders.
Highlights.
CB1r KO mice showed an α2-adrenoceptor-mediated decrease in mPFC cell excitability
α2- and β1-adrenoceptor levels decreased in mPFC while TH increased in LC in CB1r KO mice
CB1r and α2-adrenoceptors are co-localized post-synaptically in the same mPFC neurons
α2A-adrenoceptor binding is unchanged in mPFC following acute or chronic WIN 55,212–2 but increased following withdrawal
These data provide convergent lines of evidence indicating cannabinoid regulation of the cortical adrenergic system
Acknowledgments
This project was supported by the National Institutes of Health grants DA020129 to E.J. Van Bockstaele and P30 DA013429 to L.G. Kirby.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews GD, Lavin A. Methylphenidate increases cortical excitability via activation of alpha-2 noradrenergic receptors. Neuropsychopharmacology. 2006;31:594–601. doi: 10.1038/sj.npp.1300818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Go CG, Venkatesan C, Kurose H. Perikaryal and synaptic localization of alpha 2A-adrenergic receptor-like immunoreactivity. Brain Res. 1994;650:181–204. doi: 10.1016/0006-8993(94)91782-5. [DOI] [PubMed] [Google Scholar]
- Aoki C, Rodrigues S, Kurose H. Use of electron microscopy in the detection of adrenergic receptors. Methods Mol Biol. 2000;126:535–563. doi: 10.1385/1-59259-684-3:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go CG, Forman R, Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb Cortex. 1998;8:269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Ann Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones GS, Iba M, Clayton E, Rajkowski J, Cohen JD. Locus coeruleus and regulation of behavioral flexibility and attention: clinical implications. In: Ordway GA, Schwartz MA, editors. Brain norepinephrine - neurobiology and therapeutics. Cambridge: University Press; 2007. pp. 196–235. [Google Scholar]
- Arendt M, Munk-Jorgensen P. Heavy cannabis users seeking treatment- prevalence of psychiatric disorders. Soc Psychiatry Psychiatr Epidemiol. 2004;39:97–105. doi: 10.1007/s00127-004-0719-7. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex. 2007;17(Suppl 1):i6–15. doi: 10.1093/cercor/bhm033. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99:211–216. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiranvand A, Nasehi M, Zarrindast MR, Moghaddasi M. Involvement of medial prefrontal cortex alpha-2 adrenoceptors on memory acquisition deficit induced by arachidonylcyclopropylamide, a cannabinoid CB1 receptor agonist, in rats; possible involvement of Ca2+ channels. J Psychopharmacology. 2016;30(9):945–954. doi: 10.1177/0269881116652585. [DOI] [PubMed] [Google Scholar]
- Biernacki M, Skrzydlewska E. Metabolism of endocannabinoids. Postepy Hig Med Dosw (Online) 2016;70:830–843. doi: 10.5604/17322693.1213898. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Valverde O, Otto M, Michel K, Sastre M, Zimmer A. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc Natl Acad Sci USA. 2005;102:15670–15675. doi: 10.1073/pnas.0504640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmer T, Pfeiffer N, Gericke A. Three commercial antibodies against alpha1-adrenergic receptor subtypes lack specificity in paraffin-embedded sections of murine tissues. Naunyn-Schmiedebergs Arch Pharmacol. 2014;387:703–706. doi: 10.1007/s00210-014-0992-2. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Desprez T, Metna-Laurent M, Bellocchio L, Marsicano G, Soria-Gomez E. Dissecting the cannabinergic control of behavior: The where matters. BioEssays. 2015;37:1215–1225. doi: 10.1002/bies.201500046. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, Gomis-Gonzalez M, Srivastava RK, Cutando L, Ortega-Alvaro A, Ruehle S, Ozaita A. Peripheral and central CB1 cannabinoid receptors control stress-induced impairment of memory consolidation. Proc Natl Acad Sci USA. 2016;113(35):9904–9909. doi: 10.1073/pnas.1525066113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Andrews GD, Glen WB, Lavin A. Alpha2-Noradrenergic receptors activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN currents. J Physiol. 2007;584:437–450. doi: 10.1113/jphysiol.2007.141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Mackie K, Van Bockstaele EJ. Cannabinoid modulation of limbic forebrain noradrenergic circuitry. Eur J Neurosci. 2010a;31:286–301. doi: 10.1111/j.1460-9568.2009.07054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Reyes AR, Sterling RC, Unterwald E, Van Bockstaele EJ. Contribution of limbic norepinephrine to cannabinoid-induced aversion. Psychopharmacology (Berl) 2010b;211:479–491. doi: 10.1007/s00213-010-1923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathel AM, Reyes BAS, Wang Q, Palma J, Mackie K, Van Bockstaele EJ, Kirby LG. Cannabinoid modulation of alpha2 adrenergic receptor function in rodent medial prefrontal cortex. Eur J Neurosci. 2014;40:3202–3214. doi: 10.1111/ejn.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nature Neurosci. 2010;13:1511–1518. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Clinard CT, Morrison KE. Neurobiological mechanisms supporting experience-dependent resistance to social stress. Neuroscience. 2015;291:1–14. doi: 10.1016/j.neuroscience.2015.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Briones JA, Cahill JD, Skosnik PD, Mathalon DH, Williams A, Sewell RA, Roach BJ, Ford JM, Ranganathan M, D’Souza DC. The Psychosis-like Effects of Delta-Tetrahydrocannabinol Are Associated with Increased Cortical Noise in Healthy Humans. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. The relationship between cannabis use, depression and anxiety among Australian adults: findings from the National Survey of Mental Health and Well-Being. Soc Psychiatry Psychiatr Epidemiol. 2001;36:219–227. doi: 10.1007/s001270170052. [DOI] [PubMed] [Google Scholar]
- Del Cid-Pellitero E, Garzon M. Hypocretin1/orexinA-immunoreactive axons form few synaptic contacts on rat ventral tegmental area neurons that project to the medial prefrontal cortex. BMC Neuroscience. 2014;15:105. doi: 10.1186/1471-2202-15-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806:127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- Fagundo AB, de la Torre R, Jimenez-Murcia S, Aguera Z, Pastor A, Casanueva FF, Granero R, Banos R, Botella C, Del Pino-Gutierrez A, Fernandez-Real JM, Fernandez-Garcia JC, Fruhbeck G, Gomez-Ambrosi J, Menchon JM, Moragrega I, Rodriguez R, Tarrega S, Tinahones FJ, Fernandez-Aranda F. Modulation of the Endocannabinoids N-Arachidonoylethanolamine (AEA) and 2-Arachidonoylglycerol (2-AG) on Executive Functions in Humans. PloS one. 2013;8:e66387. doi: 10.1371/journal.pone.0066387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ML, Shobin E, Pickel VM. Cannabinoid modulation of the dopaminergic circuitry: implications for limbic and striatal output. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:21–29. doi: 10.1016/j.pnpbp.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugge G, Van Kampen M, Mijnster MJ. Perturbations in brain monoamine systems during stress. Cell Tissue Res. 2004;315:1–14. doi: 10.1007/s00441-003-0807-0. [DOI] [PubMed] [Google Scholar]
- Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Front Behav Neurosci. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibisaravi R, Navaeinia S, Farnia S, Zarghami M. Alcohol, Cannabinoids, and Opioids Abuse and Dependence Among Psychiatric Inpatients. Iranian J Psychiatry Behav Sci. 2015;9:e229. doi: 10.17795/ijpbs229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Halliday G. Substantia nigra and locus coeruleus. In: Paxinos G, Mai JK, editors. The human nervous system. San Diego (CA): Elsevier Academic Press; 2004. pp. 449–463. [Google Scholar]
- Hein L, Kobilka BK. Adrenergic receptor signal transduction and regulation. Neuropharmacology. 1995;34:357–366. doi: 10.1016/0028-3908(95)00018-2. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Bloom AS. delta 9-Tetrahydrocannabinol-induced changes in beta-adrenergic receptor binding in mouse cerebral cortex. Brain Res. 1982;235:370–377. doi: 10.1016/0006-8993(82)91016-2. [DOI] [PubMed] [Google Scholar]
- Hong S, Fan J, Kemmerer ES, Evans S, Li Y, Wiley JW. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut. 2009;58:202–210. doi: 10.1136/gut.2008.157594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson BD, Hebert TE, Kelly ME. Physical and functional interaction between CB1 cannabinoid receptors and beta2-adrenoceptors. Br J Pharmacol. 2010;160:627–642. doi: 10.1111/j.1476-5381.2010.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BC, Swigart PM, Simpson PC. Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn-Schmiedebergs Arch Pharmacol. 2009;379:40–412. doi: 10.1007/s00210-008-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell-Motz EA, Donnelly ET, Eason MG, Liggett SB. Agonist-mediated downregulation of G alpha i via the alpha 2-adrenergic receptor is targeted by receptor-Gi interaction and is independent of receptor signaling and regulation. Biochemistry. 1998;37:15720–15725. doi: 10.1021/bi980999r. [DOI] [PubMed] [Google Scholar]
- Jones SB, Leone SL, Bylund DB. Desensitization of the alpha-2 adrenergic receptor in HT29 and opossum kidney cell lines. J Pharmacol Exp Ther. 1990;254:294–300. [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature Rev Neuroscience. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laricchiuta D, Centonze D, Petrosini L. Effects of endocannabinoid and endovanilloid systems on aversive memory extinction. Behav Brain Res. 2013;256:101–107. doi: 10.1016/j.bbr.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci. 2006;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Leranth C, Pickel VM. Electron microscopic preembedding double-labeling methods. In: Heimer L, Zaborszky L, editors. Neuroanatomical tracing methods. 1st. Vol. 2. New York: Plenum Press; 1989. pp. 129–172. [Google Scholar]
- Li BM, Mao ZM, Wang M, Mei ZT. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity realted to spatial working memory in monkeys. Neuropsychopharmacol. 1999;21:601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- Lu R, Li Y, Zhang Y, Chen Y, Shields AD, Winder DG, Angelotti T, Jiao K, Limbird LE, Zhou Y, Wang Q. Epitope-tagged receptor knock-in mice reveal that differential desensitization of alpha2-adrenergic responses is because of ligand-selective internalization. J Biol Chem. 2009;284:13233–13243. doi: 10.1074/jbc.M807535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Valverde O, Barbaccia ML, Castane A, Maldonado R, Ledent C, Parmentier M, Finazzi-Agro A. Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. Eur J Neurosci. 2002;15:1178–1186. doi: 10.1046/j.1460-9568.2002.01957.x. [DOI] [PubMed] [Google Scholar]
- Madronal N, Gruart A, Valverde O, Espadas I, Moratalla R, Delgado-Garcia JM. Involvement of cannabinoid CB1 receptor in associative learning and in hippocampal CA3-CA1 synaptic plasticity. Cereb Cortex. 2012;22:550–566. doi: 10.1093/cercor/bhr103. [DOI] [PubMed] [Google Scholar]
- Marichal-Cancino A, Fajardo-Valdez A, Ruiz-Contreras AE, Mendez-Diaz M, Prospero-Garcia O. Advances in the Physiology of GPR55 in the Central Nervous System. Current Neuropharmacology. 2016 doi: 10.2174/1570159X14666160729155441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Gorzalka BB. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev. 2014;42:116–131. doi: 10.1016/j.neubiorev.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. Cannabinoids enhance N-methyl-D-aspartate-induced excitation of locus coeruleus neurons by CB1 receptors in rat brain slices. Neurosci Lett. 2004;363:1–5. doi: 10.1016/j.neulet.2004.02.073. [DOI] [PubMed] [Google Scholar]
- Mendigurenm A, Pineda J. Systemic effect of cannabinoids on the spontaneous firing rate of locus coeruleus neurons in rats. Eur J Pharmacol. 2006;534:83–88. doi: 10.1016/j.ejphar.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Milligan G, Wise A, MacEwan DJ, Grassie MA, Kennedy FR, Lee TW, Adie EJ, Kim GD, McCallum JF, Burt A, et al. Mechanisms of agonist-induced G-protein elimination. Biochem Soc Trans. 1995;23:166–170. doi: 10.1042/bst0230166. [DOI] [PubMed] [Google Scholar]
- Molderings GJ, Bonisch H, Hammermann R, Gothert M, Bruss M. Noradrenaline release-inhibiting receptors on PC12 cells devoid of alpha(2(−)) and CB(1) receptors: similarities to presynaptic imidazoline and edg receptors. Neurochemistry Int. 2002;40:157–167. doi: 10.1016/s0197-0186(01)00076-6. [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Wolfel B, Dodt HU, Zieglgansberger W, Wotjak CT, Mackie K, Elphick MR, Marsicano G, Lutz B. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, Aston-Jones G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2015;1628:130–146. doi: 10.1016/j.brainres.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moranta D, Esteban S, Garcia-Sevilla JA. Chronic treatment and withdrawal of the cannabinoid agonist WIN 55,212-2 modulate the sensitivity of presynaptic receptors involved in the regulation of monoamine syntheses in rat brain. Naunyn-Schmiedebergs Arch Pharmacol. 2009;379:61–72. doi: 10.1007/s00210-008-0337-0. [DOI] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, Hill MN. Neurobiological Interactions Between Stress and the Endocannabinoid System. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] Neuropsychopharmacology. 2016;41(1):80–102. doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshfegh A, Babaei P, Oryan S, Soltani B, Zarrindast MR. Involvement of dorsal hippocampal alpha1-adrenergic receptors in the effect of WIN55,212-2 on memory retrieval in inhibitory avoidance task. Neurosci Lett. 2011;489:69–73. doi: 10.1016/j.neulet.2010.07.079. [DOI] [PubMed] [Google Scholar]
- Muntoni AL, Pillolla G, Melis M, Perra S, Gessa GL, Pistis M. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2006;23:2385–2394. doi: 10.1111/j.1460-9568.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Mackie K, Van Bockstaele EJ. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007;1127:36–44. doi: 10.1016/j.brainres.2006.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza VC, Page ME, Van Bockstaele EJ. Systemic administration of WIN 55,2122 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046:45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav. 2007;86:162–168. doi: 10.1016/j.pbb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Van Bockstaele EJ. Local administration of a cannabinoid agonist alters norepinephrine efflux in the rat frontal cortex. Neurosci Lett. 2008;431:1–5. doi: 10.1016/j.neulet.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, Justinova Z. Cannabinoid abuse and addiction: Clinical and preclinical findings. Clin Pharmacol Ther. 2015;97:616–627. doi: 10.1002/cpt.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Wiskerke J, Schoffelmeer AN. Cannabinoid modulation of executive functions. Eur J Pharmacol. 2008;585:458–463. doi: 10.1016/j.ejphar.2008.02.099. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Orlando, FL: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster, HdF . The Fine Structure of the Nervous System. New York: Oxford University Press; 1991. [Google Scholar]
- Pfitzer T, Niederhoffer N, Szabo B. Search for an endogenous cannabinoid-mediated effect in the sympathetic nervous system. Naunyn-Schmiedebergs ArchPharmacol. 2005;371:9–17. doi: 10.1007/s00210-004-1003-9. [DOI] [PubMed] [Google Scholar]
- Poddar MK, Dewey WL. Effects of cannabinoids on catecholamine uptake and release in hypothalamic and striatal synaptosomes. J Pharmacol Exp Ther. 1980;214:63–67. [PubMed] [Google Scholar]
- Pratt GD, Bowery NG. Repeated administration of desipramine and a GABAB receptor antagonist, CGP 36742, discretely up-regulates GABAB receptor binding sites in rat frontal cortex. Br J Pharmacol. 1993;110:724–735. doi: 10.1111/j.1476-5381.1993.tb13872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst A, Cortes R, Palacios JM. Distribution of alpha 2-adrenergic receptors in the human brainstem: an autoradiographic study using [3H]p-aminoclonidine. Eur J Pharmacol. 1984;106:477–488. doi: 10.1016/0014-2999(84)90051-7. [DOI] [PubMed] [Google Scholar]
- Pudovkina OL, Kawahara Y, de Vries J, Westerink BH. The release of noradrenaline in the locus coeruleus and prefrontal cortex studied with dual-probe microdialysis. Brain Res. 2001;906:38–45. doi: 10.1016/s0006-8993(01)02553-7. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BAS, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Reyes BAS, Heldt NA, Mackie K, Van Bockstaele EJ. Ultrastructural evidence for synaptic contacts between cortical noradrenergic afferents and endocannabinoid-synthesizing post-synaptic neurons. Neuroscience. 2015;303:323–337. doi: 10.1016/j.neuroscience.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BAS, Rosario JC, Piana PM, Van Bockstaele EJ. Cannabinoid modulation of cortical adrenergic receptors and transporters. J Neuroscience Res. 2009;87:3671–3678. doi: 10.1002/jnr.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BAS, Szot P, Sikkema C, Cathel AM, Kirby LG, Van Bockstaele EJ. Stress-induced sensitization of cortical adrenergic receptors following a history of cannabinoid exposure. Exp Neurol. 2012;236:327–335. doi: 10.1016/j.expneurol.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter H, Teixeira FM, Ferreira SG, Kittel A, Kofalvi A, Sperlagh B. Presynaptic alpha(2)-adrenoceptors control the inhibitory action of presynaptic CB(1) cannabinoid receptors on prefrontocortical norepinephrine release in the rat. Neuropharmacology. 2012;63:784–797. doi: 10.1016/j.neuropharm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Fernandez-Ruiz JJ, Murphy L, Eldridge JC, Steger RW, Bartke A. Effects of delta-9-tetrahydrocannabinol exposure on adrenal medullary function: evidence of an acute effect and development of tolerance in chronic treatments. Pharmacol Biochem Behav. 1991;40:593–598. doi: 10.1016/0091-3057(91)90368-c. [DOI] [PubMed] [Google Scholar]
- Rudoy CA, Reyes AR, Van Bockstaele EJ. Evidence for beta1-adrenergic receptor involvement in amygdalar corticotropin-releasing factor gene expression: implications for cocaine withdrawal. Neuropsychopharmacology. 2009;34:1135–1148. doi: 10.1038/npp.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi B, Matsumura M, Kubota K. Catecholaminergic effects on neuronal activity related to a delayed response task in monkey prefrontal cortex. J Neurophysiol. 1990;63:1385–1400. doi: 10.1152/jn.1990.63.6.1385. [DOI] [PubMed] [Google Scholar]
- Scavone JL, Mackie K, Van Bockstaele EJ. Characterization of cannabinoid-1 receptors in the locus coeruleus: relationship with mu-opioid receptors. Brain Res. 2010;1312:18–31. doi: 10.1016/j.brainres.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JY, Ahn KH, Kendall DA. Molecular basis of cannabinoid CB1 receptor coupling to the G protein heterotrimer Galphaibetagamma: identification of key CB1 contacts with the C-terminal helix alpha5 of Galphai. J Biol Chem. 2013;288:32449–32465. doi: 10.1074/jbc.M113.489153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkins TJ, Fried D, Parikh K, Galligan JJ, Goudreau JL, Lookingland KJ, Kaplan BL. Reduced Noradrenergic Signaling in the Spleen Capsule in the Absence of CB1 and CB2 Cannabinoid Receptors. J Neuroimmune Pharmacol. 2016;11(4):669–679. doi: 10.1007/s11481-016-9689-2. [DOI] [PubMed] [Google Scholar]
- Suarez J, Bermudez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, de Fonseca FR. Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol. 2008;509:400–421. doi: 10.1002/cne.21774. [DOI] [PubMed] [Google Scholar]
- Sulkowski A, Vachon L. Side effects of simultaneous alcohol and marijuana use. Am J Psychiatry. 1977;134:691–692. doi: 10.1176/ajp.134.6.691. [DOI] [PubMed] [Google Scholar]
- Szot P, Miguelez C, White SS, Franklin A, Sikkema C, Wilkinson CW, Ugedo L, Raskind MA. A comprehensive analysis of the effect of DSP4 on the locus coeruleus noradrenergic system in the rat. Neuroscience. 2010;166:279–291. doi: 10.1016/j.neuroscience.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci. 2006;26:467–478. doi: 10.1523/JNEUROSCI.4265-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Changes in adrenoreceptors in the prefrontal cortex of subjects with dementia: evidence of compensatory changes. Neuroscience. 2007;146:471–480. doi: 10.1016/j.neuroscience.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, White SS, Veith RC. Effect of pentylenetetrazol on the expression of tyrosine hydroxylase mRNA and norepinephrine and dopamine transporter mRNA. Brain Res Mol Brain Res. 1997;44:46–54. doi: 10.1016/s0169-328x(96)00217-3. [DOI] [PubMed] [Google Scholar]
- Thiemann G, Watt CA, Ledent C, Molleman A, Hasenohri RU. Modulation of anxiety by acute blockade and genetic deletion of the CB(1) cannabinoid receptor in mice together with biogenic amine changes in the forebrain. Behav Brain Res. 2009;200:60–67. doi: 10.1016/j.bbr.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, Witkin JM, Nomikos GG. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara ET, Perry KW, Rodriguez DE, Bymaster FP, Nomikos GG. The cannabinoid CB(1) receptor antagonist SR141716A increases norepinephrine outflow in the rat anterior hypothalamus. Eur J Pharmacol. 2001;426:R3–4. doi: 10.1016/s0014-2999(01)01228-6. [DOI] [PubMed] [Google Scholar]
- Uriguen L, Perez-Rial S, Ledent C, Palomo T, Manzanares J. Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB1 receptors. Neuropharmacology. 2004;46:966–973. doi: 10.1016/j.neuropharm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Valverde O, Torrens M. CB1 receptor-deficient mice as a model for depression. Neuroscience. 2012;204:193–206. doi: 10.1016/j.neuroscience.2011.09.031. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. Ultrastructure of serotonin-immunoreactive terminals in the core and shell of the rat nucleus accumbens: cellular substrates for interactions with catecholamine afferents. J Comp Neurol. 1993;334:603–617. doi: 10.1002/cne.903340408. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Vasquez C, Lewis DL. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan C, Song XZ, Go CG, Kurose H, Aoki C. Cellular and subcellular distribution of alpha 2A-adrenergic receptors in the visual cortex of neonatal and adult rats. J Comp Neurol. 1996;365:79–95. doi: 10.1002/(SICI)1096-9861(19960129)365:1<79::AID-CNE7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Villanueva A, Yilmaz SM, Millington WR, Cutrera RA, Stouffer DG, Parsons LH, Cheer JF, Feleder C. Central cannabinoid 1 receptor antagonist administration prevents endotoxic hypotension affecting norepinephrine release in the preoptic anterior hypothalamic area. Shock. 2009;32:614–620. doi: 10.1097/SHK.0b013e3181a4fd8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF. α2A-Adrenoreceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wyrofsky R, McGonigle P, Van Bockstaele EJ. Drug discovery strategies that focus on the endocannabinoid signaling system in psychiatric disease. Expert Opin Drug Discov. 2015;10:17–36. doi: 10.1517/17460441.2014.966680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yger P, Stimberg M, Brette R. Fast Learning with Weak Synaptic Plasticity. J Neurosci. 2015;35:13351–13362. doi: 10.1523/JNEUROSCI.0607-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate J, Churruca I, Echevarria E, Casis L, Lopez de Jesus M, Saenz del Burgo L, Salles J. Immunohistochemical localization of CB1 cannabinoid receptors in frontal cortex and related limbic areas in obese Zucker rats: effects of chronic fluoxetine treatment. Brain Res. 2008;1236:57–72. doi: 10.1016/j.brainres.2008.07.100. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Burkovskiy I, Yang H, Sardinha J, Lehmann C. CB2 and GPR55 Receptors as Therapeutic Targets for Systemic Immune Dysregulation. Frontiers Pharmacol. 2016;7:264. doi: 10.3389/fphar.2016.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]


