Abstract
Sputum induction can aid tuberculosis (TB) diagnosis, but adult data from HIV-endemic environments are limited, and it is unclear how performance varies depending on the clinical context (inpatient versus outpatient), HIV status and whether patients are smear-negative or sputum-scarce.
696 adults with suspected smear-negative or sputum-scarce TB from Cape Town (South Africa) were referred for routine sputum induction. Liquid culture for Mycobacterium tuberculosis served as the reference standard.
82% (573 out of 696) of patients provided a specimen ⩾ 1 mL, 83% (231 out of 278) of which were of adequate quality. 15% (96 out of 652) of sputum induction specimens were culture-positive, and this yield was higher among inpatients versus outpatients (17% (71 out of 408) versus 10% (25 out of 244), p=0.01) and HIV-infected versus uninfected patients (17% (51 out of 294) versus 9% (16 out of 173), p=0.02), but similar for CD4 (>200 versus ≤ 200 cells·μL−1) and patient (smear-negative versus sputum-scarce) subcategories. Overall sensitivity (95% CI) of smear-microscopy was 49% (39–59%), higher among inpatients versus outpatients (55% (43–67%) versus 32% (14–50%), p=0.05), but unaffected by HIV co-infection, CD4 count or patient type. 29% (203 out of 696) of patients commenced anti-TB treatment and sputum induction offered microbiological confirmation and susceptibility testing in only 47% (96 out of 203).
Under programmatic conditions in an HIV-endemic environment although the yield of culture was approximately two-fold higher amongst HIV-infected patients and inpatients, a fifth of all patients were unable to provide a specimen following sputum induction. Same-day microbiological diagnosis was only possible in ~50% of patients.
Introduction
The diagnosis of patients suspected of tuberculosis (TB) who are sputum smear-negative for acid-fast bacilli or who are unable to produce sputum (sputum scarce) is a daily challenge for clinicians in HIV-endemic settings [1]. In developing countries facing the dual epidemics of TB and HIV, the burden of smear-negative or sputum-scarce TB is large and accounts for approximately every second notified TB case [2]. Failure to confirm a TB diagnosis negatively impacts both patients and TB control by: 1) increasing morbidity and mortality [3–5]; 2) fuelling the transmission of multidrug resistant (MDR)-TB by ineffectively treating undiagnosed disease [6, 7]; and 3) exposing patients that are inappropriately given empiric TB treatment to unnecessary, toxic and prolonged drug therapy [8]. Strategies to improve and decentralise the diagnosis of adults with suspected smear-negative and sputum-scarce TB are needed, with focus not only on improved diagnostic test efficacy but also on the optimisation of sputum specimen acquisition methods [9]. The World Health Organization endorsement [10] and roll-out of the novel MTB/RIF (Mycobacterium tuberculosis/rifampicin) assay (Cepheid, Sunnyvale, CA, USA) looks set to offer rapid diagnostic yields close to those of solid culture techniques, even in primary care settings [11–14], thus making the need to address the diagnostic bottleneck of sputum specimen acquisition urgent.
Sputum induction, performed using the ultrasonic nebulisation of hypertonic saline, is a relatively simple and safe procedure suitable for use in resource-limited decentralised settings [15–20]. Induction has been shown to offer similar TB case detection rates to more invasive techiques such as bronchoscopy as an aid to TB diagnosis [21, 22]. It has shown particular utility for diagnostic sampling in children [23, 24] and for TB screening in asymptomatic patients prior to the initiation of antiretrovirals [25]. Consequently, advocacy for the roll-out and widespread use of sputum induction in HIV-endemic, resource-limited primary care settings is increasing. However, data are limited for smear-negative or sputum-scarce adult TB suspects in HIV-endemic settings [15–18]. Available studies are small with large variability in diagnostic performance measures and wide confidence intervals [15–18]. Studies are heterogenous due to differences that include: 1) the clinical context of patients undergoing sputum induction (e.g. hospital inpatient versus respiratory clinic outpatient) [19]; 2) the preceding diagnostic workup of patients prior to sampling (e.g. chest radiography versus none) [19]; 3) HIV prevalence [19]; 4) sputum induction procedure (e.g.3% versus 5% hypertonic saline concentration) [20]; and 5) type of diagnostic testing on induced specimens (e.g. conventional light versus fluoresence microscopy) [15–18]. These differences prevent useful meta-analysis [19, 20] and limit generalisability. Larger studies that provide a more robust evidence base to guide national TB programme policy are overdue. Furthermore, the absence of direct comparative performance data between HIV-infected and uninfected (and stratified by CD4 cell count), outpatients and inpatients, and smear-negative and sputum-scarce TB suspects from a single study is a major research gap, and we hypothesised that between-group differences would be lower than could be expected from a simple comparison of pre-sampling TB prevalences.
To address these gaps and evaluate our hypothesis, we conducted a large, cross-sectional study of sputum induction to evaluate the procedural side-effects, sampling efficacy, specimen quality, culture-based diagnostic yield and diagnostic accuracy of smear microscopy stratified by clinical context (inpatient versus outpatient), HIV status and CD4 count, and TB suspect reason for induction (sputum scarce versus smear negative). In addition, we evaluated the ability of sputum induction sampling to allow for microbiological TB confirmation and susceptibility in all patients commencing anti-TB treatment.
Methods
Study population
The study was conducted at the Groote Schuur Hospital respiratory clinic in Cape Town, South Africa. Routine sputum induction facilities are available to hospital inpatients (including ward and emergency room admissions) and outpatients (including specialist and general medical clinics) on doctors’ request. Patients (⩾ 16 years old) referred for induction between February 12, 2008 and May 30, 2009 were eligible for inclusion in the study. Basic demographics, HIV status and reason for referral for induction were recorded by nursing staff. Only patients referred for induction with suspected smear-negative or sputum-scarce TB were included, and patients referred for any other indications, e.g. possible Pneumocystis jiroveci infection or malignancy were excluded. The study was approved by the University of Cape Town human research ethics committee.
Diagnostic workup and treatment
As per routine practice, all patients referred for induction with suspected smear-negative or sputum-scarce TB had received a doctor’s assessment and chest radiography, as well as an attempted collection of a self-expectorated early morning sputum sample prior to referral. Patients with two smear-negative sputum samples within 4 weeks of referral for induction were considered smear-negative. Data on the exact timing between attempted self-expectoration and induction was not documented for all patients; however, for sputum-scarce inpatients, self-expectoration was attempted on admission. Using the laboratory and hospital pharmacy record systems, the commencement of anti-TB treatment for study patients within a month of enrolment was noted. Any patient commencing treatment without a positive TB culture result on a recent (± 4 weeks from enrolment) specimen (induced sputum or other) was considered to have received empiric treatment.
Sputum induction procedure
Sputum induction was performed by respiratory clinic nursing staff in an enclosed negative-pressure induction booth as previously described [26]. Briefly, ~20 mL of sterile 5% hypertonic saline (Sabax; Adcock Ingram, Midrand, South Africa) was delivered via a Wilson’s 402A ultrasonic nebuliser (Medimark, Cape Town, South Africa) over 15–20 min or until 2–4 mL of induced sputm could be collected. No pro-expectorating manoeuvres were employed. Research nurses monitored for side-effects and induction was terminated if side-effects developed.
Laboratory methods
Induced sputa were processed by the National Health Laboratory Service reference laboratory (Groote Schuur Hospital, Cape Town, South Africa). Specimens were decontaminated with N-acetyl-L-cysteine/sodium hydroxide and then centrifuged. Thereafter, an auramine O-stained smear underwent fluoresence microscopy and 0.5 mL of the deposit was inoculated into a Mycobacterial Growth Indicator Tube 960 (Becton Dickinson Diagnostics, Franklin Lakes, NJ, USA). Approximately half of samples received Gram staining prior to decontamination and sputum quality was determined using the Bartlett score [27]. Culture-positive acid-fast bacilli were identified as M. tuberculosis complex using either an in-house PCR method [28] or the GenoType MTBDRplus assay (version 1; Hain LifeSciences, Nehren, Germany) if drug susceptibility testing had been requested. MTBDRplus assay testing was introduced for routine drug susceptibility testing only in the latter part of the study period (fig. 1).
FIGURE 1.
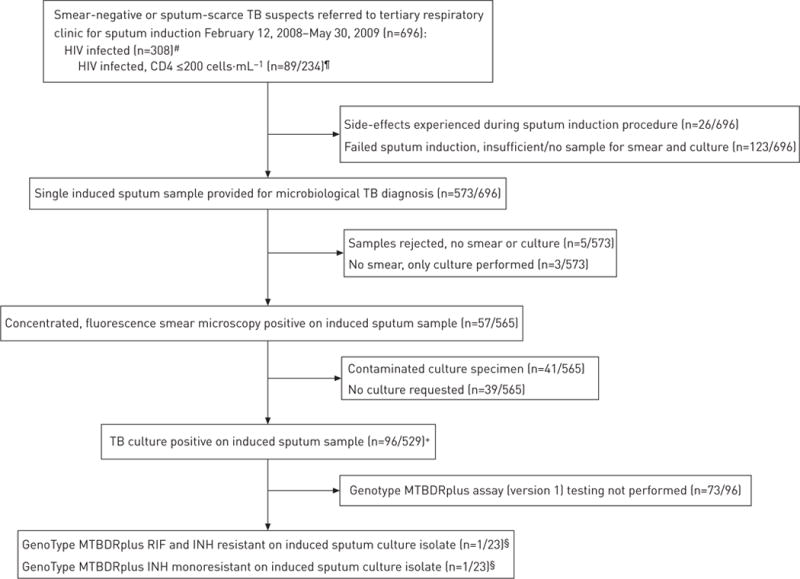
Study flow diagram of patients undergoing sputum induction and their smear and culture results. TB: tuberculosis; RIF: rifampicin; INH: isoniazid. #: 202 out of 696 patients had an unknown HIV status and 186 out of 696 were HIV-uninfected; ¶: no recent CD4 cell count results were available for 74 out of 308 HIV-infected patients; +: only patients that had a induced sputum culture performed are included as the denominator;§: routine genotypic drug susceptibility testing using the GenoType MTBDRplus assay (Hain LifeSciences, Nehren, Germany) was not begun until the last few months of the study enrolment period.
Statistical analysis
All patients (or patients in a subgroup) referred for induction with suspected smear-negative or sputum-scarce TB were used as the denominator when calculating the culture-based TB case detection rates of sputum induction. M. tuberculosis culture was used as the reference standard for evaluating the diagnostic accuracy of induced sputum smear microscopy and only included patients with a valid culture result. Sensitivity, specificity, and positive and negative predictive values are presented with 95% confidence intervals. Univariate and multivariate logistic regression analysis was used to investigate the predictors of induced sputum: sputum sampling, culture positivity and smear positivity. Basic demographic and sputum induction characteristics, as well as diagnostic accuracy measures, of different patient groups were compared using the Chi-squared, Wilcoxon rank-sum and Kruskal—Wallis tests, as appropriate, and all statistical tests were two-sided at α=0.05. Stata IC (version 10; Stata Corp., College Station, TX, USA) was used for all statistical analyses.
Results
Demographics, patient setting and indication for sputum induction
Of the patients referred for sputum induction during the 15-month study period, 696 patients had suspected smear-negative or sputum-scarce TB (fig. 1). 62% (434 out of 696) and 74% (517 out of 696) of patients referred for induction were inpatients and had suspected sputum-scarce TB, respectively. Table 1 shows basic patient demographics and the reason for sputum induction referral stratifed by patient setting and HIV status. A greater proportion (95% CI) of outpatients compared to inpatients were sputum scarce (79% (74–84%) versus 71% (67–74%), p=0.02). The median (interquartile range (IQR)) age of patients was 40 (32–53) years, with a younger median age among inpatients versus outpatients (38 (29–48) years versus 45 (34–57) years, p<0.001), and HIV-infected versus-uninfected patients (35 (30–42) years versus 46 (36–56) years, p<0.001). Among HIV-infected patients, the median (IQR) CD4 cell count was 155 (65–269) cells·μL−1, with no difference in median CD4 cell count noted between inpatients and outpatients. 24% (74 out of 308) of HIV-infected patients had missing CD4 cell count data.
TABLE 1.
Demographics and indication for referral in patients undergoing sputum induction stratified by patient setting and HIV status
| All | Outpatients | Inpatients | p-value# | HIV infected | HIV uninfected | HIV status unknown | p-value# | |
|---|---|---|---|---|---|---|---|---|
| Subjects | 696 | 262 | 434 | 308 | 186 | 202 | ||
| Age years | 40 (32–53) | 45 (34–57) | 38 (29–48) | <0.001 | 35 (30–42) | 46 (36–56) | 49 (33–61) | <0.001 |
| Females | 408 (58) | 152 (58) | 256 (59) | 192 (62) | 98 (53) | 118 (58) | NS | |
| CD4 cell count¶ cells·μL−1 | 155 (65–269) | 185 (74–290) | 146 (60–262) | 155 (65–269) | NA | NA | NS | |
| Reason for referral to undergo sputum induction | ||||||||
| Sputum scarce+ | 517 (74) | 208 (79) | 309 (71) | 0.02 | 228 (74) | 133 (72) | 156 (77) | |
| Two smear-negative samples | 179 (26) | 54 (21) | 125 (29) | 0.02 | 80 (26) | 53 (28) | 46 (23) |
Data are presented as n, median (interquartile range] or n (%), unless otherwise stated. NA: not applicable; NS: not significant (p>0.05).
: significant differences between different patient groups;
: 74 HIV-infected patients had missing CD4 cell count data;
: unable to produce sputum.
Sputum induction sampling efficacy, specimen quality and side-effects
Table 2 outlines sputum induction sampling efficacy, specimen quality and side-effects stratifed by patient setting, HIV status and reason for referral. 82% (573 out of 696) of referred patients successfully provided a sputum sample ⩾ 1 mL. The success of sampling was the same irrespective of patient setting, HIV status and reason for referral for induction. A random selection of 49% (278 out of 573) of sputum samples of ⩾1 mL had a Bartlett score calculated to determine sputum quality; 83% (231 out of 278) of these tested samples were found to be of adequate quality. Thus, 32% (220 out of 696) of patients’ sputum induction was either unsuccessful or produced a sample of suboptimal quality. However, no association was found between sputum quality and smear or culture positivity (culture positive/all adequate sputum quality samples 18% (12–24%) (33 out of 185) versus culture-positive/all inadequate sputum quality samples 22% (8–36%) (7 out of 32), p=0.6). Overall, only 4% (26 out of 696) of induced patients experienced any side-effects, of which the commonest was nausea and vomiting in nine patients.
TABLE 2.
Sampling outcomes for sputum induction using a Wilson ultrasonic nebuliser and 5% hypertonic saline
| All# | Outpatients | Inpatients | HIV infected¶ | HIV uninfected¶ | p-value | Sputum-scarce | Two smear-negative samples | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Sputum sample ⩾ 1 mL produced after induction | 82 (573/696) | 85 (224/262) | 80 (349/434) | 79 (244/308) | 86 (160/186) | 81 (419/517) | 86 (154/179) | ||
| Side-effects experience during procedure | 4 (26/696) | 2 (6/262) | 5 (20/434) | 4 (13/308) | 4 (7/186) | 3 (16/517) | 6 (10/179) | ||
| Sputum sample of adequate quality# | 83 (231/278) | 78 (87/111) | 86 (144/167) | 92 (81/88) | 81 (80/99) | 0.02 | 80 (155/194) | 90 (76/84) | 0.02 |
Data are presented as % (n/N), unless otherwise stated, p-values indicate significant differences between patient groups for a particular diagnostic accuracy measure.
: Gram staining was performed and a Bartlett score determined for a random selection of ~50% (278 out of 573) of the induced sputum samples received by the National Health Laboratory Services; a Bartlett score of ⩾ 0 is considered to represent a sputum sample of adequate quality for bacteriology.
: only patients with available HIV results were included (202 patients refused test/HIV status unknown).
Culture-based diagnostic yield and drug susceptibility testing
The culture-based diagnostic yields of a single induced sputum sample for all patients and stratified by reason for induction, patient setting, HIV status and CD4 cell count is shown in figure 2. 6% (44 out of 696) of patients were excluded from this analysis as, despite provision of an adequate sputum sample, the sample was rejected by the laboratory (e.g. for leakage during transport or no liquid culture was requested). The overall TB culture yield of a single induced sputum was 15% (96 out of 652 patients). In a multivariate analysis, age (p=0.03), HIV positivity (p = 0.05) and inpatient setting (p=0.03) were associated with TB culture positivity (table 3). Median (IQR) culture time to positivity for induced sputum samples was 13 (10–19) days, with no differences noted between patient groups. The GenoType MTBDRplus assay, introduced for routine use late in the study in accordance with national policy, was performed on 24% (23 out of 96) of culture-positive induced sputum samples. 4% (one out of 23) of patients undergoing Genotypes MTBDRplus testing were diagnosed with MDR-TB (rifampicin and isoniazid resistance) and 4% (one out of 23 patients) were diagnosed isoniazid monoresistance.
FIGURE 2.
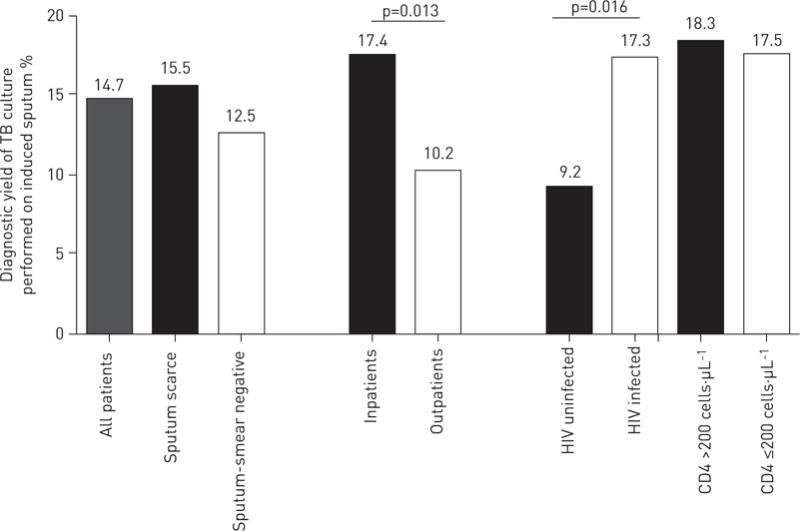
Overall diagnostic yield of tuberculosis (TB) culture and a single induced sputum stratified by reason for sputum induction, outpatient and inpatient setting, HIV status and CD4 cell count. The denominator incorporated all patients undergoing sputum induction (n=696), including those in whom sputum induction failed to produce a sample for diagnostic testing (n=123), but excluding those in whom no culture was requested or the sample was rejected for leakage (n=44). See figure 1 for further detail.
TABLE 3.
Predictors of induced sputum tuberculosis (TB) culture positivity in univariate and multivariate regression analysis
| OR (95% CI) | p-value | |
|---|---|---|
| Univariate analysis | ||
| Age years | 0.97 (0.95–0.99) | <0.001 |
| Female | 0.88 (0.56–1.4) | 0.6 |
| HIV positive# | 2.4 (1.3–4.4) | 0.005 |
| CD4 count (if HIV positive) | 0.999 (0.996–1.001) | 0.4 |
| Sputum scarce | 1.3 (0.8–2.3)) | 0.3 |
| Inpatient | 2.1 (1.3–3.5) | 0.003 |
| Adequate sputum quality | 0.78 (0.31–1.94) | 0.6 |
| Multivariate analysis¶ | ||
| Age years | 0.972 (0.947–0.997) | 0.03 |
| HIV positive | 1.88 (0.99–3.54) | 0.05 |
| Inpatient | 2.06 (1.07–3.93) | 0.03 |
All patients with either a TB culture-positive or-negative induced sputum result were included (n5488). Patients unable to produce an induced sputum sample or with a contaminated culture result were excluded.
: missing HIV results meant that n=348 for this univariate analysis and for the subsequent multivariate analyses;
: only variables with a significant p-value are shown for the multivariate analyses.
Smear microscopy diagnostic accuracy
Diagnostic accuracy measures of smear microscopy, using patients who were induced-sputum culture-positive or-negative in the analysis, for all patients and stratified by clinical context, HIV status and CD4 cell count are shown in table 4. Smear microscopy sensitivity (95% CI) was higher among inpatients versus outpatients (55% (43–67%) versus 32% (14–50%), p=0.05), but unaffected by reason for referral, HIV status or CD4 cell count. Three smear microscopy-positive patients were found to be culture-positive for a non-tuberculous mycobacterium.
TABLE 4.
Diagnostic accuracy of induced sputum smear microscopy stratified by patient setting, HIV status and CD4 cell count
| All# | Outpatients | Inpatients | HIV infected¶ | HIV uninfected¶ | CD4 >200 cells·μL−1§ | CD4 ≤ 200 cells·μL−1§ | |
|---|---|---|---|---|---|---|---|
| Subjects | 485 | 190 | 295 | 210 | 136 | 104 | 89 |
| Sensitivity | 49 (39–59) 47/96 |
32f (14–50) 8/25 |
55f (43–67) 39/71 |
53 (40–66) 27/51 |
50 (28–72) 8/16 |
58 (39–76) 14/24 |
50 (31–69) 12/24 |
| Specificity | 98 (97–100) 383/389 |
99 (97-100) 164/165 |
98 (95–99) 219/224 |
98 (94–99) 155/159 |
100 (97–100) 120/120 |
99 (93–100) 79/80 |
95 (87–98) 62/65 |
| PPV | 89 (77–95) 47/53 |
89 (57–98) 8/9 |
89 (76–95) 39/44 |
87 (71–95) 27/31 |
100 (68–100) 8/8 |
93 (70–99) 14/15 |
80 (55–93) 12/15 |
| NPV | 89 (85–91) 383/432 |
91 (86–94) 164/181 |
87 (83–91) 219/251 |
87 (81–92) 155/179 |
94 (88–97) 120/128 |
89 (81–94) 79/89 |
84 (74–91) 62/74 |
Data are presented as n or % (95% CI] n/N. Mycobacterium tuberculosis culture positivity was used as the reference standard. PPV: positive predictive value; NPV: negative predictive value.
: only patients with a valid induced sputum smear and culture result are included (fig. 1);
: only patients with available HIV results are included (202 patients refused test/HIV status unknown);
: only patients with available CD4 cell count data are included (74 HIV-infected patients missing recent CD4 cell count data);
:p = 0.05 between the two patient groups.
Microbiological confirmation of clinical TB using a single induced sputum
Of all patients referred for induction with suspected smear-negative or sputum-scarce TB 29% (203 out of 696) commenced anti-TB treatment within 1 month of study enrolment. A single induced sputum provided culture confirmation in 47% (96 out of 203) of cases. The proportion of culture-confirmed TB among those receiving treatment was similar irrespective of patient setting, reason for sputum induction referral and HIV status (data not shown).
Discussion
In HIV-prevalent settings, both in primary and hospital practice, the burden and diagnosis of suspected smear-negative and sputum-scarce TB continues to challenge both clinicians and national TB programmes. With the development and roll-out of novel TB diagnostic tests, such as the MTB/RIF assay, specimen acquisition has become an even more important diagnostic bottleneck. Given the simplicity, safety and performance data from large childhood TB studies, there is increasing advocacy for the widespread roll-out of sputum induction in primary care clinics in resource-limited, HIV-endemic settings. Yet, for adults with suspected smear-negative or sputum-scarce TB, data are limited and heterogenous. Thus, the main findings of our study are of relevance to both clinicians and national TB programmes, and include: 1) under programmatic conditions, sputum induction was unsuccessful or samples were of suboptimal quality in 32% of cases; 2) the overall culture-based diagnostic yield of a single induced sputum was 16%, and culture yield was almost two-fold higher among HIV-infected patients and inpatients; 3) the overall sensitivity of induced sputum smear microscopy is only 49%, but is higher among inpatients compared to outpatients; and 4) irrespective of clinical setting, HIV status or patient phenotype, a single induced sputum sample only microbiologically confirms TB, thus offering drug susceptibility testing in just under half of all patients receiving anti-TB treatment.
Small studies conducted under strict research conditions overestimate procedural success rates and diagnostic accuracy and are prone to reporting bias [29]. Thus, data from this large programmatic cohort has important implications for clinicians and TB services managing smear-negative and sputum-scarce TB suspects in HIV-endemic settings. Notably, the main outcome measures of diagnostic utility, including procedural success rate, culture-based yield, smear microscopy sensitivity and the ability to offer a microbiologically confirmed TB diagnosis for drug susceptibility testing were all substantially lower, or at the lower end, compared to those previously reported [15–19]. Furthermore, we have unpublished data that suggests that the sensitivity of the Xpert MTB/RIF assay is reduced in induced sputum specimens from adults with smear-negative and sputum-scarce TB, which is plausible given the paucibacillary nature of TB disease in these patients. These programmatic findings also have relevance to low-burden TB settings where MTB/RIF testing is increasingly routine and sputum sampling is thus the major diagnostic challenge. This highlights the limitations of sputum induction and emphasises the need for ongoing research to improve sampling methodology and to investigate and utilise non-sputum-based biological fluids in the diagnosis of adult smear-negative and sputum-scarce TB in both HIV-endemic and low TB burden settings.
How does the performance of smear and culture differ in induced versus self-expectorated sputum? Although this study did not directly compare performance in the same hospital setting, data from large recent studies of TB suspects presenting to outpatient settings in Cape Town find culture-based diagnostic yields of 24–28% [11, 12], approximately three-fold higher than the induced sputum samples from outpatients in this study. Smear microscopy sensitivity in induced sputum specimens is also, unsurprisingly, reduced compared to in self-expectorated sputum specimens, although in HIV-infected patients sensitivity appears equivalent at ~50% [11]. This highlights the particular utility of sputum induction for HIV-infected patients with suspected TB irrespective of self-expectorated smear status. In fact, the incremental culture-based yield of induced sputum, even in HIV-infected patients able to self-expectorate, has been demonstrated recently [30].
Does induction offer greater diagnostic utility in certain patient subgroups, and is the only important determinate of these differences related to the expected differences in pre-sampling TB prevalence between groups? Our study finding of an almost two-fold increased culture yield in HIV-infected compared to uninfected patients and inpatients compared to outpatients confirms that induction offers particular utility in these two patient subgroups. However, as hypothesised, the between-group differences found were lower than expected, given that studies from a similar setting have shown a four- to five-fold higher TB prevalence amongst HIV-infected compared to-uninfected patients [31]. The lower than expected differences in culture yield are not explained by procedure-related factors, such as sampling success and side-effects, which we found to be unaffected by HIV status or clinical context. Thus, these differences probably simply reflect the limitations of using a single sputum sample for TB diagnosis in HIV-infected patients with high rates of disseminated, extrapulmonary TB and paucibacillary pulmonary disease. In addition, it is worth noting that despite the increased culture yield in the above patient groups, we found that in all patient groups, sputum induction could only microbiologically confirm TB allowing for drug susceptibility testing in under 50% of patients commencing anti-TB treatment. Undoubtedly, further large prospective studies in HIV-endemic settings with high rates of empiric treatment are required.
Increased TB case detection at primary care level offers a number of potential public health benefits including: 1) reduction in diagnostic delay and, consequently, TB-related transmission, morbidity and even mortality benefits [1, 32]; 2) early and increased diagnosis of MDR-TB; and 3) reduction in empiric treatment rates and, hence, inappropriate toxic drug exposure. However, should sputum induction facilities be decentralised and rolled-out to primary care facilities to aid the diagnosis of adult smear-negative and sputum-scarce TB? Unfortunately, no impact data evaluating the effect of sampling with sputum induction on patient-important outcomes, such as time to treatment and rates of treatment initiation, are available for any patient group. However, data from large cohorts of children with suspected TB and from antiretroviral therapy initiation clinics support the use of induction [23–25]. However, few data are available for adults presenting with suspected smear-negative or sputum-scarce TB to primary care clinics. Outcome data from the outpatient group in our study raise concern that the benefits of induction in primary clinics may be limited, as not only was the culture-based detection rate < 10%, but the sensitivity of smear microscopy was significantly lower in outpatients compared to inpatients. Furthermore, a study from Malawi found that a healthcare worker-observed self-expectorated sputum sample offered the greatest initial diagnostic yield with minimal additional benefit from the use of sputum induction [15]. Pragmatic studies evaluating the impact of sputum induction on patient-important outcomes such as treatment initiation, studies directly comparing induction to other simple sputum sampling strategies (e.g. healthcare-worker instruction) and studies evaluating the performance of the novel MTB/RIF assay on induced sputum samples are urgently needed from primary care clinic settings.
This study has important limitations. Missing data was a problem given the programmatic nature of the study. Approximately a quarter of patients had an unknown HIV status or refused HIV testing and a similar proportion of HIV-infected patients had missing recent CD4 cell counts. However, induction data (reason for referral, sampling and side-effects) of the HIV-unknown group was similar to the HIV-uninfected group, and, despite missing data, large numbers of both HIV-infected and-uninfected patients were included in whom sputum induction performance could be evaluated. Limited clinical data were available for study patients, other than the reason for referral, and no radiological data or clinical follow-up data were available. This lack of more detailed data makes it difficult to further explore the reasons for failed induction, as well as to assess for the appropriateness of empiric treatment, treatment response or the development of MDR-TB. Lastly, the lack of a direct comparator method is a limitation and we are thus only able to compare our study findings with the yield of self-expectorated sputum from other studies performed in a similar setting.
In conclusion, this study provides robust outcome data on the routine diagnostic utility of sputum induction for adults with suspected smear-negative or sputum-scarce TB. Although sputum induction was safe and culture yield was almost two-fold higher among HIV-infected patients and inpatients, in almost a third of cases, sputum induction was unsuccessful or samples of suboptimal quality were obtained. Same-day diagnosis using smear microscopy was only possible in less than half of patients. Direct comparison between patient subgroups, with the low culture yield and same-day smear microscopy diagnosis in outpatients undergoing sputum induction, raises concerns about the use of sputum induction as the preferred intial sputum sampling strategy for adult outpatients with suspected smear-negative or sputum-scarce TB. Impact studies, and studies comparing different initial sputum sampling strategies to aid diagnosis in adults with suspected smear-negative and sputum-scarce TB presenting to primary care facilities in HIV-endemic settings are urgently required to inform policy.
Acknowledgments
We thank the respiratory clinic nursing staff who were involved in the recruitment of patients and the performance of sputum induction. We would like to acknowledge L. Roodt and T. Tebbutt (University of Cape Town, Cape Town, South Africa) for data capture. Their support was greatly appreciated.
Support statement: J.G. Peter was supported by the Fogarty International Clinical Research Scholars/Fellows Support Centre National Institutes of Health grant R24TW007988, South African Tuberculosis AIDS Training, the Discovery Foundation and the European and Developing Countries Clinical Trials Partnership (EDCTP) (grant number IP0932040009), for this project. G. Theron is supported by the EDCTP, the South African National Research Foundation (NRF) and the Claude Leon Foundation. K. Dheda is supported by the EDCTP (TBNeat and Trials of Excellence in Southern Africa) and the South African Department of Science and Technology and NRF (South African Research Chairs Initiative).
Footnotes
@ERSpublications
TB culture yield is approximately two-fold higher among HIV-infected patients and in patients http://ow.ly/qeJrx
Conflict of interest: None declared.
References
- 1.Whitehorn J, Ayles H, Godfrey-Faussett P. Extra-pulmonary and smear-negative forms of tuberculosis are associated with treatment delay and hospitalisation. Int J Tuberc Lung Dis. 2010;14:741–744. [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Control 2011. Geneva: World Heath Organisation; 2011. [Google Scholar]
- 3.Banda H, Kang’ombe C, Harries AD, et al. Mortality rates and recurrent rates of tuberculosis in patients with smear-negative pulmonary tuberculosis and tuberculous pleural effusion who have completed treatment. Int J Tuberc Lung Dis. 2000;4:968–974. [PubMed] [Google Scholar]
- 4.Harries AD, Nyirenda TE, Banerjee A, et al. Treatment outcome of patients with smear-negative and smear-positive pulmonary tuberculosis in the National Tuberculosis Control Programme, Malawi. Trans R Soc Trop Med Hyg. 1999;93:443–446. doi: 10.1016/s0035-9203(99)90153-0. [DOI] [PubMed] [Google Scholar]
- 5.Salaniponi FM, Gausi F, Kwanjana JH, et al. Time between sputum examination and treatment in patients with smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:581–583. [PubMed] [Google Scholar]
- 6.Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 7.Tostmann A, Kik SV, Kalisvaart NA, et al. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis. 2008;47:1135–1142. doi: 10.1086/591974. [DOI] [PubMed] [Google Scholar]
- 8.McIlleron H, Meintjes G, Burman WJ, et al. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis. 2007;196(Suppl. 1):S63–S75. doi: 10.1086/518655. [DOI] [PubMed] [Google Scholar]
- 9.Getahun H, Harrington M, O’Brien R, et al. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Automated Real-time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 11.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theron G, Peter J, van Zyl-Smit R, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med. 2011;184:132–140. doi: 10.1164/rccm.201101-0056OC. [DOI] [PubMed] [Google Scholar]
- 13.Weyer K, Mirzayev F, Migliori GB, et al. Rapid molecular TB diagnosis: evidence, policy-making and global implementation of Xpert MTB/RIF. Eur Respir J. 2013;42:252–271. doi: 10.1183/09031936.00157212. [DOI] [PubMed] [Google Scholar]
- 14.Pantoja A, Fitzpatrick C, Vassall A, et al. Xpert MTB/RIF for diagnosis of tuberculosis and drug-resistant tuberculosis: a cost and affordability analysis. Eur Respir J. 2013;42:708–720. doi: 10.1183/09031936.00147912. [DOI] [PubMed] [Google Scholar]
- 15.Bell DJ, Dacombe R, Graham SM, et al. Simple measures are as effective as invasive techniques in the diagnosis of pulmonary tuberculosis in Malawi. Int J Tuberc Lung Dis. 2009;13:99–104. [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson D, Nachega J, Morroni C, et al. Diagnosing smear-negative tuberculosis using case definitions and treatment response in HIV-infected adults. Int J Tuberc Lung Dis. 2006;10:31–38. [PubMed] [Google Scholar]
- 17.Morse M, Kessler J, Albrecht S, et al. Induced sputum improves the diagnosis of pulmonary tuberculosis in hospitalized patients in Gaborone, Botswana. Int J Tuberc Lung Dis. 2008;12:1279–1285. [PubMed] [Google Scholar]
- 18.Parry CM, Kamoto O, Harries AD, et al. The use of sputum induction for establishing a diagnosis in patients with suspected pulmonary tuberculosis in Malawi. Tuber Lung Dis. 1995;76:72–76. doi: 10.1016/0962-8479(95)90583-9. [DOI] [PubMed] [Google Scholar]
- 19.Hepple P, Ford N, McNerney R. Microscopy compared to culture for the diagnosis of tuberculosis in induced sputum samples: a systematic review. Int J Tuberc Lung Dis. 2012;16:579–588. doi: 10.5588/ijtld.11.0617. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Angulo Y, Wiysonge CS, Geldenhuys H, et al. Sputum induction for the diagnosis of pulmonary tuberculosis: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2012;31:1619–1630. doi: 10.1007/s10096-011-1485-6. [DOI] [PubMed] [Google Scholar]
- 21.Anderson C, Inhaber N, Menzies D. Comparison of sputum induction with fiber-optic bronchoscopy in the diagnosis of tuberculosis. Am J Respir Crit Care Med. 1995;152:1570–1574. doi: 10.1164/ajrccm.152.5.7582296. [DOI] [PubMed] [Google Scholar]
- 22.Conde MB, Soares SL, Mello FC, et al. Comparison of sputum induction with fiberoptic bronchoscopy in the diagnosis of tuberculosis: experience at an acquired immune deficiency syndrome reference center in Rio de Janeiro, Brazil. Am J Respir Crit Care Med. 2000;162:2238–2240. doi: 10.1164/ajrccm.162.6.2003125. [DOI] [PubMed] [Google Scholar]
- 23.Zar HJ, Hanslo D, Apolles P, et al. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet. 2005;365:130–134. doi: 10.1016/S0140-6736(05)17702-2. [DOI] [PubMed] [Google Scholar]
- 24.Hatherill M, Hawkridge T, Zar HJ, et al. Induced sputum or gastric lavage for community-based diagnosis of childhood pulmonary tuberculosis? Arch Dis Child. 2009;94:195–201. doi: 10.1136/adc.2007.136929. [DOI] [PubMed] [Google Scholar]
- 25.Lawn SD, Kerkhoff AD, Pahlana P, et al. Diagnostic yield of tuberculosis using sputum induction in HIV-positive patients before antiretroviral therapy. Int J Tuberc Lung Dis. 2012;16:1354–1357. doi: 10.5588/ijtld.12.0174. [DOI] [PubMed] [Google Scholar]
- 26.Cashmore TJ, Peter JG, van Zyl-Smit RN, et al. Feasibility and diagnostic utility of antigen-specific interferon-gamma responses for rapid immunodiagnosis of tuberculosis using induced sputum. PLoS One. 2010;5:e10389. doi: 10.1371/journal.pone.0010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartlett RC. Medical Microbiology: Quality, Cost and Clinical Relevance. New York: John Wiley and Sons; 1974. [Google Scholar]
- 28.De Wit D, Steyn L, Shoemaker S, et al. Direct detection of Mycobacterium tuberculosis in clinical specimens by DNA amplification. J Clin Microbiol. 1990;28:2437, 2341. doi: 10.1128/jcm.28.11.2437-2441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knottnerus JA, van Weel C, Muris JW. Evaluation of diagnostic procedures. BMJ. 2002;324:477–480. doi: 10.1136/bmj.324.7335.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawn SD, Kerkhoff AD, Pahlana P, et al. Diagnostic yield of tuberculosis using sputum induction in HIV-positive patients before antiretroviral therapy. Int J Tuberc Lung Dis. 2012;16:1354–1357. doi: 10.5588/ijtld.12.0174. [DOI] [PubMed] [Google Scholar]
- 31.Gupta A, Wood R, Kaplan R, et al. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7:e34156. doi: 10.1371/journal.pone.0034156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finnie RK, Khoza LB, van den Borne B, et al. Factors associated with patient and health care system delay in diagnosis and treatment for TB in sub-Saharan African countries with high burdens of TB and HIV. Trop Med Int Health. 2011;16:394–411. doi: 10.1111/j.1365-3156.2010.02718.x. [DOI] [PubMed] [Google Scholar]


