Abstract
SUMMARY
OBJECTIVES
To determine the yield of undetected active tuberculosis (TB), TB and human immunodeficiency virus (HIV) coinfection and the number needed to screen (NNS) to detect a case using active case finding (ACF) in an urban community in Kampala, Uganda.
METHODS
In a door-to-door survey conducted in Rubaga community from January 2008 to June 2009, residents aged ⩾15 years were screened for chronic cough (⩾2 weeks) and tested for TB disease using smear microscopy and/or culture. Rapid testing was used to screen for HIV infection. The NNS to detect one case was calculated based on population screened and undetected cases found.
RESULTS
Of 5102 participants, 3868 (75.8%) were females; the median age was 24 years (IQR 20–30). Of 199 (4%) with chronic cough, 160 (80.4%) submitted sputum, of whom 39 (24.4%, 95%CI 17.4–31.5) had undetected active TB and 13 (8.1%, 95%CI 6.7–22.9) were TB-HIV co-infected. The NNS to detect one TB case was 131 in the whole study population, but only five among the subgroup with chronic cough.
CONCLUSION
ACF obtained a high yield of previously undetected active TB and TB-HIV cases. The NNS in the general population was 131, but the number needed to test in persons with chronic cough was five. These findings suggest that boosting the identification of persons with chronic cough may increase the overall efficiency of TB case detection at a community level.
Keywords: case detection, tuberculosis, chronic cough, number needed to screen, TB-HIV co-infected
CASE DETECTION is the principal means of controlling transmission and reducing tuberculosis (TB) incidence.1 Globally, case detection has stagnated in recent years, while the rate of decline in estimated TB incidence has been slower than expected.2,3 Passive case finding (PCF), the detection of active TB or TB-HIV (human immunodeficiency virus) among symptomatic persons voluntarily presenting to the health system, is the standard approach adopted by most National TB Programmes (NTPs).4 Using PCF alone, however, leaves large pools of undetected prevalent TB cases who fail to seek care.5–7 Moreover, even with functional NTPs, health system delays occur in TB diagnosis or initiation of treatment. Patients also delay due to a lack of awareness of symptoms or lack of access to health services, particularly in sub-Saharan Africa.8–12 Many TB patients infect others before they are diagnosed and placed on effective treatment. Alternative strategies to overcome the detection gap should be geared towards shortening these delays and reducing the potential risk of transmission at community level.
Active case finding (ACF) is a known alternative strategy for case detection.4,13 It refers to provider-initiated efforts to find, evaluate and diagnose active TB among asymptomatic and symptomatic individuals who have not sought care.13 Ideally, ACF can interrupt the transmission of TB through early detection and prompt initiation of effective treatment.4,10,14 ACF can also reduce the risk of death due to TB, particularly among HIV-co-infected individuals.15 Mathematical models suggest that ACF is one of the most effective ways of reducing TB incidence and mortality.1,16 Recent randomised community trials14,17,18 and observational studies15,19,20 conducted in developing countries have shown that ACF identifies previously undetected TB cases.
Uganda has an estimated annual TB incidence of 234 per 100 000 population; however, only 57% of smear-positive cases were detected in 2011.2 The capital district, Kampala, accounts for nearly 25% of Uganda’s notified TB caseload.21
The purpose of the present study was to determine the yield and the number needed to screen (NNS) to detect a case of undetected TB and TB-HIV in Kampala. This expands on the existing evidence base that supports ACF as a supplementary strategy for TB case detection in Africa.
METHODS
Ethical considerations
The study received approval from the institutional review committees at the University Hospitals, Cleveland, OH; the University of Georgia, Athens, GA, USA; the Makerere University School of Public Health; and the Uganda National Council for Science and Technology, Kampala, Uganda. Written informed consent was obtained from all participants.
Study design, setting and population
We conducted a door-to-door cross-sectional survey of chronic cough in the Rubaga community located in Kampala City, Uganda, from January 2008 to June 2009. The division is subdivided into 13 parishes and 128 villages, with approximately 75 485 households and 400 000 people. About 50% of the population are adults aged ⩾15 years.22 Individuals can access TB and HIV diagnostic services from two public health centres free of charge or two tertiary private hospitals for a fee. Free treatment is provided in both private and public facilities.
Eligible residents were those aged ⩾15 years who lived in Rubaga during the survey period. Participants were excluded if there was a language barrier, declined to consent, were not at home on three separate attempts or did not plan to stay in the study area for 2 weeks after the survey date.
A sample size of 5000 participants was calculated to estimate the prevalence of TB with a 95% confidence interval (CI) of 5%, based on a previous ACF study of undiagnosed TB cases in Kampala.20 Participants were selected using a multistage sampling approach. A simple random sample was used to select five of the 13 parishes using a computer-based random number generator and sampling frame from the Uganda Bureau of Statistics, Kampala, Uganda.23 Weighted proportions-to-village population sizes were calculated to estimate the number of participants to be recruited from each village. This was done to account for the variability in crowding. We identified the first house from a defined central point such as road or drainage junctions in each village, and enrolled a convenience sample of persons at home.
A two-step approach was used to screen for active TB and TB-HIV: a cough interview administered to identify chronic coughers, and diagnostic testing for TB and HIV infection. Trained interviewers administered a cough questionnaire that assessed the presence, duration and frequency of cough. Chronic cough was defined as self-reported cough for ⩾2 weeks at the time of the survey. Information was collected on socio-demographics, TB history, TB-related symptoms (weight loss, evening fever, haemoptysis, excessive night sweats), health care seeking (assessed as ‘evaluation by a health provider since the start of your cough’), previous HIV testing and current treatment for TB or HIV/AIDS (acquired immune-deficiency syndrome). The main study outcomes were undetected active TB and TB-HIV. The secondary outcome was the NNS to identify a single case in the subgroup of interest.
Spot and early morning sputum samples were collected from the chronic coughers at their homes and transported in a cool box to the certified national reference TB laboratory in Kampala. Samples were processed using standard methods,24 and two technicians independently examined the sputum smear for acid-fast bacilli (AFB) and used Löwenstein-Jensen slants for culture. Smears were quantified and reported as negative, scanty, 1 +, 2 +, 3+ per International Union Against Tuberculosis and Lung Disease standards.25 Smear test results were reported within 48–72 h.
Rapid HIV testing was performed using the serial algorithm as recommended by the Ugandan Ministry of Health, Kampala, Uganda.26 Determine HIV-1/2 assay (Abbott Laboratories, Abbott Park, IL, USA) for screening and the HIV-1/2 STAT-PAK Dipstick assay (Chembio Diagnostic System Inc, New York, NY, USA) were used for confirmation. The Uni-Gold test (Trinity Biotech, Bray, Ireland) was used for confirmation in case of discordance. HIV Western Blot test was performed on a 10% random sample of blood specimens for quality control purposes.
A TB case was defined as a positive sputum smear or culture from one or more collected samples.27,28 An HIV case was defined as a positive rapid HIV test according to the Uganda Ministry of Health algorithm.26 A positive TB-HIV co-infected case was defined as a confirmed TB case with a concurrent positive HIV test. New cases identified during the survey were referred to public health centres for further care as appropriate.
Statistical methods
The yield of active TB and TB-HIV cases was calculated as proportions with 95%CIs of undetected cases in chronic coughers. The NNS to identify one case of chronic cough, active TB, TB-HIV and HIV infection was also calculated accordingly. Appropriate statistical tests were used to test for differences in groups at α = 0.05. Data were analysed using Stata version 11.0 (StataCorp, College Station, TX, USA).
RESULTS
Study participants
The study enrolled 5102 participants from 4494 households (Figure) and 28 villages visited. The study excluded 2289 people, including 2257 who were not at home (82.3% male) and 32 who declined to consent. Of those enrolled, 3868 (75.8%) were female (median age 24 years, IQR 20–30; Table 1). The prevalence of self-reported cough of any duration was nearly 10.1% (95%CI 9–11). More than half (50.8%) of the participants were aged 15–24 years. Half the sample had had at least 8–13 years of education; 44.2% were employed, and 54.4% had an average weekly income of not more than US$2. More than one third (38.2%) of the participants reported that they had never undergone HIV testing.
Figure.
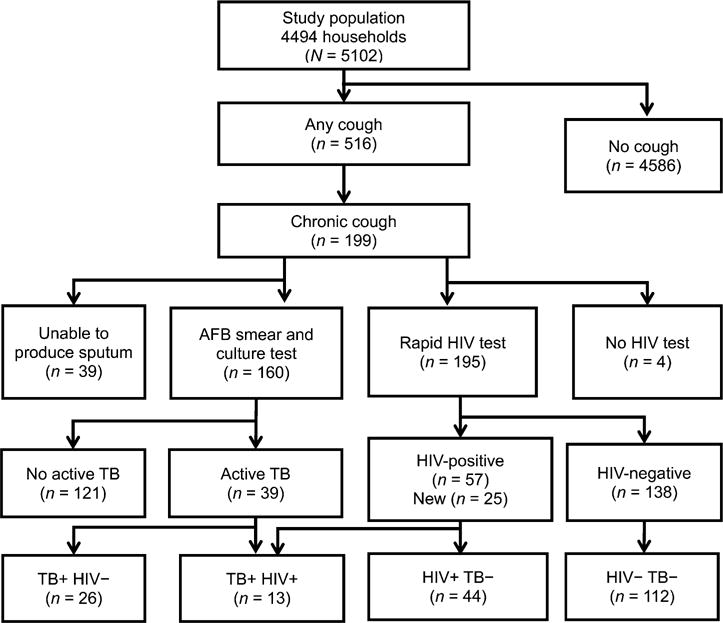
Flow diagram of study participants in Rubaga Division, Kampala, Uganda. AFB = acid-fast bacilli; HIV = human immunodeficiency virus; TB = tuberculosis; + = positive; − = negative.
Table 1.
Baseline characteristics of study participants in Kampala, Uganda, 2008–2009
| Characteristic | Total population (N = 5102) n (%) |
|---|---|
| Age, years, median [IQR] | 24 [20–30] |
| 15–24 | 2592 (50.8) |
| 25–34 | 1614 (31.7) |
| 35–44 | 533 (10.8) |
| 45–54 | 191 (3.7) |
| ⩾55 | 152 (3.0) |
| Sex | |
| Female | 3868 (75.8) |
| Male | 1234 (24.2) |
| Marital status | |
| Never married | 1737 (34.1) |
| Currently married | 2583 (50.6) |
| Previously married | 782 (15.6) |
| Religion | |
| Catholic | 1683 (33.0) |
| Protestant | 1387 (27.2) |
| Muslim | 1255 (24.6) |
| Others* | 777 (15.2) |
| Education level, years | |
| None | 214 (4.2) |
| 1–7 | 1872 (36.7) |
| 8–13 | 2563 (50.2) |
| >13 | 453 (8.9) |
| Employed | |
| Yes | 2325 (45.6) |
| No | 2777 (54.4) |
| Reported weekly income, UGX, median [IQR]† | 4000 [1000–10 000] |
| None | 862 (16.9) |
| 1–<1000 | 164 (3.2) |
| 1000–5000 | 2253 (44.2) |
| 5001–10000 | 797 (15.6) |
| >10000 | 1026 (20.1) |
| Ever tested for HIV | |
| Yes | 3151 (61.8) |
| No | 1951 (38.2) |
| Presence of cough | |
| Yes | 516 (10.1) |
| No | 4686 (89.9) |
Pentecostal, Seventh Day Adventist or religion not specified.
1USD ~ UGX 2500.
IQR = interquartile range; UGX = Ugandan shillings; HIV = human immunodeficiency virus.
Yield of chronic cough and other tuberculosis-related symptoms
Of the 5102 participants, 199 (4%, 95%CI 3.5–4.5) had cough of ⩾2 weeks (Table 2). Of the five most common TB-related symptoms, self-reported unintentional weight loss was the most prevalent (35.2%), followed by excessive night sweats (32.7%). Haemoptysis was the least common symptom (6.5%).
Table 2.
Characteristics of undetected TB cases among persons with chronic cough in Kampala, Uganda
| Characteristic | Total (n = 199) n (%)* |
No TB (n = 121) n (%) |
Undetected TB (n = 39) n (%) |
P value |
|---|---|---|---|---|
| Produced sputum | 160 (80.4) | 121 (75.6) | 39 (24.4) | |
| Unable to produce sputum | 39 (19.6) | |||
| Sex | ||||
| Female | 125 (62.8) | 80 (66.1) | 22 (56.4) | 0.273 |
| Male | 74 (37.2) | 41 (33.1) | 17 (43.6) | |
| Age, years | ||||
| 15–24 | 64 (32.2) | 43 (35.5) | 9 (23.1) | 0.263+ |
| 25–34 | 80 (40.2) | 45 (37.2) | 17 (43.6) | |
| 35–44 | 28 (14.1) | 17 (14.1) | 8 (20.0) | |
| 45–54 | 13 (6.5) | 6 (5.0) | 4 (10.3) | |
| ⩾55 | 14 (7) | 10 (8.2) | 1 (3.0) | |
| Marital status | ||||
| Never married | 40 (20.1) | 24 (19.8) | 8 (20.5) | 0.596 |
| Currently married | 99 (49.8) | 66 (54.5) | 18 (46.2) | |
| Previously married | 60 (30.2) | 31 (25.6) | 13 (33.3) | |
| Education level (years of school) | ||||
| None | 22 (11.1) | 15 (12.4) | 2 (5.1) | 0.524† |
| 1–7 | 106 (53.2) | 62 (51.2) | 19 (48.7) | |
| 8–13 | 61 (30.7) | 37 (30.6) | 16 (41.0) | |
| >13 | 10 (5) | 7 (5.8) | 2 (5.1) | |
| Cough duration, weeks‡ | ||||
| 2–3 | 71 (35.7) | 41 (34.8) | 13 (33.3) | 0.204§ |
| >3–8 | 52 (26.1) | 36 (30.5) | 6 (15.4) | |
| >8–12 | 19 (9.6) | 11 (9.3) | 6 (15.4) | |
| >12 | 57 (28.6) | 30 (25.4) | 14 (35.9) | |
| TB-related symptoms | ||||
| None | 90 (45.2) | 59 (48.8) | 15 (38.5) | 0.106§ |
| 1–2 | 58 (29.2) | 38 (31.4) | 11 (28.2) | |
| 3–5 | 51 (25.6) | 24 (19.8) | 13 (33.3) | |
| Ever undergone HIV testing | ||||
| No | 83 (41.7) | 51 (42.2) | 16 (41.0) | 0.902 |
| Yes | 116 (58.3) | 70 (57.9) | 23 (59.0) | |
| HIV status | ||||
| Negative | 138 (69.4) | 86 (72.9) | 25 (65.8) | 0.154 |
| Positive | 57 (28.6) | 32 (27.1) | 13 (34.2) | |
| Not done | 4 (2) | 3 | 1 | |
| Health care seeking | ||||
| No | 51 (28.7) | 31 (28.7) | 8 (23.5) | 0.556 |
| Yes | 127 (71.3) | 77 (71.3) | 26 (76.5) |
Other totals may not add up to 199 due to 39 missing AFB smear tests; of 160 only 4 did not produce two samples.
Fisher’s exact test.
Mean and median difference in cough duration not significant.
Test for trend.
TB = tuberculosis; HIV = human immunodeficiency virus; AFB = acid-fast bacilli.
Yield of undetected active TB, TB-HIV coinfection and HIV among chronic coughers
Of 199 chronic coughers, 160 (80.4%) submitted sputum for examination, of whom 39 met the criteria for active TB (24.4%, 95%CI 17.8–31.1; Table 2); 26 were HIV-negative or had unknown HIV status (16.3%, 95%CI 2.1–30.5) and 13 (8.1%, 95%CI 6.7–22.9) were found to be HIV-infected. Chronic coughers with and without active TB were similar in socio-demographic and clinical characteristics and health-seeking behaviour. There were 57 (28.6%, 95%CI 22.6–35.5) HIV-positive cases among the chronic coughers. Of these, 25 (44%) were newly diagnosed (Figure).
Sputum smear grade of TB cases detected by ACF
Of the 39 active TB cases, the majority (66.7%) had negative or low AFB sputum smear grade (scanty or + 1). There was no statistically significant difference in levels of smear grades and other characteristics between TB cases with and those without HIV infection (Table 3).
Table 3.
Characteristics of 39 previously undetected active tuberculosis cases by HIV status
| Characteristic | Total (n = 39) n (%) |
HIV-negative (n = 25) n (%) |
HIV-positive (n = 13) n (%) |
P value |
|---|---|---|---|---|
| Age, years | ||||
| Mean ± SD | 33 ± 11 | 33.5 ± 15.6 | 31.0 ± 8.9 | 0.560* |
| Median [IQR] | 29 [25–42] | 27 [22–39] | 29 [26–33] | 0.77† |
| Sex‡ | Not 100% | |||
| Female | 22 (56) | 15 (60) | 7 (53.9) | |
| Male | 17 (44) | 10 (40) | 6 (46.1) | 0.482 |
| AFB smear grade‡ | ||||
| Negative | 9 (23.1) | 5 (20) | 3 (23.1) | 0.690 |
| Scanty | 13 (33.3) | 8 (32) | 5 (38.5) | |
| 1+ | 4 (10.3) | 2 (8) | 2 (15.4) | |
| 2+ | 5 (12.8) | 3 (12) | 2 (15.4) | |
| 3+ | 8 (20.5) | 7 (28) | 1 (7.6) | |
| Smear and culture | ||||
| AFB +, culture + | 14 (36) | 4 (31) | 10 (38) | 0.713 |
| AFB +, culture− | 16 (41) | 6 (46) | 10 (38) | |
| AFB−, culture + | 9 (23) | 3 (23) | 6 (24) | |
| Cough duration, weeks | ||||
| Mean ± SD | 15.7 ± 26.9 | 11.0 ± 14.5 | 14.7 ± 25.3 | 0.80* |
| Median [IQR] | 8.7 [3–17.4] | 4.3 [2.9–8.7] | 4.3 [3–13] | 0.60† |
t-test.
Mann-Whitney test.
Stratified totals do not add up to 39 as 1 HIV result was missing.
HIV = human immunodeficiency virus; SD = standard deviation; IQR = interquartile range; AFB = acid-fast bacilli; + = positive; − = negative.
The number needed to screen using ACF to detect TB, TB-HIV and HIV cases
Using chronic cough to screen the general population for TB suspects, the NNS to identify one chronic cougher was 26. To identify one active TB case using cough screening with smear and/or culture testing, the NNS was 131. However, when considering only smear results, the NNS was 170. The number needed to test (NNT) to find one case of TB among those with chronic cough was five (Table 4). When stratifying the subgroup of suspects by HIV status, the NNT was eight for TB-HIV-negative and 15 for TB-HIV co-infected persons.
Table 4.
NNS and NNT to detect a TB suspect, active TB or TB-HIV case using active case finding, Kampala, 2008–2009*
| Study population | Total n |
NNS (95%CI)† | NNT (95%CI)‡ |
|---|---|---|---|
| All participants enrolled | 5102 | ||
| Chronic cough (⩾2 weeks) | 199 | 26 (23–30) | NA |
| Previously undetected active TB (smear- and/or culture-positive) | 39 | 131 (96–179) | 5 (5.3–4.7) |
| Previously undetected active TB (smear only) | 30 | 170 (123–256) | 7 (7.1–6.3) |
| TB-HIV-negative | 26 | 196 (145–323) | 8 (7.2–8.3) |
| TB-HIV co-infected | 13 | 393 (222–667) | 15 (14–17) |
| Undetected HIV-positive in those with chronic cough | 25 | NA | 8 (7.4–8.6) |
The risk subgroup constitutes the denominators for NNS or NNT, and numerators are either overall population or those with chronic cough.
Numerator for NNS = 5102; the rest of the numbers in the column with totals constitute the denominators.
Numerator for NNT = 199; the rest of the numbers in the column with totals below chronic cough constitute the denominators.
NNS = number needed to screen; NNT = number needed to test; TB = tuberculosis; HIV = human immunodeficiency virus; NA = not available.
DISCUSSION
In this door-to-door cough survey of adults living in an African city, we determined the yield of undetected infectious TB using a two-step approach: screening for chronic cough with a questionnaire, followed by smear microscopy and culture for diagnosis. Chronic cough was uncommon in the community at large, with a yield of 4%. The NNS to find a single case of active TB was 131; however, operationally, it could be as high as 170 if only smear microscopy was used. Once it was known that the person had a chronic cough, however, the NNT was only five. This finding suggests that gaining access to residents with chronic cough is a critical step toward an efficient community TB ACF programme. Previous studies have used educational leaflets, flyers, mobile vans with loudspeakers, community awareness campaigns by trained lay workers and cell phone text messaging to enhance case finding among persons with chronic cough.14,17,29,30
The NNS is a useful metric for comparing efficiency across screening programmes in the context of resource allocation. In this study, the NNS of 131 to find a TB case is similar to the median NNS of 148 (range 29–5000) reported in a systematic review from population-based surveys of TB case finding in Africa.31 The NNS can vary depending on the prevalence of TB and HIV in the target population, the screening strategy, the sensitivity of the diagnostic tests used and the functionality of NTPs.32 A recent review demonstrated that the NNS to detect a TB case was lower when high-risk populations such as prisoners or the homeless in high-prevalence settings were screened.33 The NNT of five in persons with chronic cough in our study is consistent with the need to test on average seven suspects (range 3.3–20) to detect one smear-positive case in NTP clinics.25
The high prevalence of 24.4% undetected infectious TB cases among those with chronic cough in Kampala is consonant with another study from the same setting in 2009.20 Importantly, the low diagnostic accuracy of the tests used may have led to missed TB cases or overdiagnosis, particularly given the finding that 16 of 30 smear-positive cases were culturenegative. As this may have been due to either specimen contamination or other quality problems with culture, corrective measures should be taken. We do not know the true prevalence of undetected TB in this community subgroup of chronic cough. Nonetheless, the value of ACF in identifying pools of undetected infectious TB cases in high TB prevalence settings is well highlighted.
Although the primary goal of ACF is to detect undiagnosed active TB, our study shows that HIV-associated TB was also detected. A higher proportion of smear-positive cases detected were HIV-negative and not -positive. This difference may be explained by diagnostic inaccuracy, particularly when using smear microscopy in HIV-associated TB.34 In Kampala, where HIV prevalence is high and often remains undiagnosed,23,35 it is logical to couple HIV testing with TB screening. As indicated in the ‘3Is’ policy recommended by the World Health Organization, intensified TB case finding in HIV-infected persons should continue to be implemented in specialised HIV clinics as an effective way to find TB cases.31,33 Nevertheless, ACF for TB-HIV in the general population should not be neglected, as it is especially helpful in uncovering additional, previously unknown, HIV cases.19,31
Our findings support the idea that ACF detects cases much earlier based on smear grade; most detected cases had low smear grades. A similar study in Zambia found that the majority of detected cases had a scanty smear grade at diagnosis.19 In contrast, in a PCF setting in Uganda, 82% of the TB patients had high smear grades of 3 +, suggesting more severe disease at the time of diagnosis.36 Higher smear positivity has been associated with a greater level of TB transmission among close contacts.37,38 Theoretically, earlier detection implies shorter duration of infectiousness and a reduced likelihood of transmission. However, what remains unclear is the optimal timing of ACF to attain a meaningful reduction in duration of infectivity to other persons and thus incidence. A standard epidemiological measure that captures the impact of ACF on TB epidemiology is therefore urgently needed.
Our urban population-based study leads to findings that are consistent with existing evidence in support of implementing ACF strategies for case detection in high TB and HIV burden areas. However, the cross-sectional design limits the interpretation of the prevalence of undetected TB and TB-HIV beyond the study period. Possible underestimation of the prevalence may be due to the lack of sputum for evaluation from 19.6% of those with chronic cough, and further diagnostic evaluations should be conducted. Selection bias could arise from the heavily female-skewed sample and non-participation of persons who were not at home during the day. Previous door-to-door studies have shown similar recruitment patterns, as men mostly work outside the home.18,20 A study showed that men tended to seek care for TB-related symptoms earlier than females;39 however, ACF may help identify disease earlier among females.
High-quality TB diagnosis, treatment, management and support for patients should be in place before ACF is initiated. Under NTP conditions, barriers to case finding, such as the low sensitivity of smear microscopy, quality assurance problems that render culture testing less sensitive as well as diagnostic delays, could be overcome by the deployment of rapid, highly sensitive point-of-care diagnostics such as Xpert® MTB/RIF.40,41 Finally, ACF is more resourceintensive than PCF, irrespective of target population; more cost-effectiveness studies comparing ACF and PCF strategies42,43 are therefore needed to inform policy decisions for TB control programmes in Africa.
CONCLUSIONS
ACF obtained a high yield of previously undetected active TB and TB-HIV cases. The NNS in the general population was 131, while the NNT to detect a TB case among persons with chronic cough was five. These findings suggest that boosting the identification of persons with chronic cough may increase the overall efficiency of TB case detection at the community level.
Acknowledgments
The authors thank the participants and acknowledge the invaluable contribution made by the home health visitors in collecting the data: H Sempeera, J Nabisere, J Nalubowa, K Godffrey, E Nakayenga, J Nassuna, M Mubiru, S Nanyonga and K Muwanga (deceased). They acknowledge the contribution of the data managers: L Malone, M Mugerwa, Y Mulumba and the support of all the staff of Uganda Case Western Reserve Collaboration, particularly S Zalwango. The authors also thank the staff of TB Bacteriological Unit, Wandegeya, for their contributions to this study. Lastly, they thank F Adatu, the former manager of the Uganda National Tuberculosis and Leprosy Programme, for his expert advice and support of this project.
The authors acknowledge the funding support by the Operations Research on AIDS Care and Treatment in Africa grant, Doris Duke Charitable Foundation. This work was also supported by the National Institutes of Health Office of the Director, Fogarty International Center, Office of AIDS Research, National Cancer Center, Bethesda, MD; National Eye Institute, Bethesda, MD; National Heart, Blood, and Lung Institute, Bethesda, MD; National Institute of Dental and Craniofacial Research, Bethesda, MD; National Institute on Drug Abuse, Bethesda, MD; National Institute of Mental Health, Bethesda, MD; National Institute of Allergy and Infectious Diseases, Bethesda, MD; National Institutes of Health Office of Women’s Health and Research through the Fogarty International Clinical Research Scholars, Bethesda, MD; and Fellows Program at Vanderbilt University, Nashville, TN, USA (R24 TW007988) and the American Relief and Recovery Act.
Footnotes
Conflict of interest: none declared.
[A version in French of this article is available from the Editorial Office in Paris and from the Union website www.theunion.org]
References
- 1.De Cock KM, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis. 1999;3:457–465. [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2012. Geneva, Switzerland: WHO; 2012. WHO/HTM/TB/2012.6. [Google Scholar]
- 3.Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 4.Golub JE, Mohan CI, Comstock GW, Chaisson RE. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis. 2005;9:1183–1203. [PMC free article] [PubMed] [Google Scholar]
- 5.Tadesse T, Demissie M, Berhane Y, Kebede Y, Abebe M. Two-thirds of smear-positive tuberculosis cases in the community were undiagnosed in northwest Ethiopia: population based cross-sectional study. PLOS ONE. 2011;6:e28258. doi: 10.1371/journal.pone.0028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoa NB, Sy DN, Nhung NV, Tiemersma EW, Borgdorff MW, Cobelens FG. National survey of tuberculosis prevalence in Viet Nam. Bull World Health Organ. 2010;88:273–280. doi: 10.2471/BLT.09.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van’t Hoog AH, Laserson KF, Githui WA, et al. High prevalence of pulmonary tuberculosis and inadequate case finding in rural western Kenya. Am J Respir Crit Care Med. 2011;183:1245–1253. doi: 10.1164/rccm.201008-1269OC. [DOI] [PubMed] [Google Scholar]
- 8.Kiwuwa MS, Charles K, Harriet MK. Patient and health service delay in pulmonary tuberculosis patients attending a referral hospital: a cross-sectional study. BMC Public Health. 2005;5:122. doi: 10.1186/1471-2458-5-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golub JE, Bur S, Cronin WA, et al. Patient and health care system delays in pulmonary tuberculosis diagnosis in a low-incidence state. Int J Tuberc Lung Dis. 2005;9:992–998. [PubMed] [Google Scholar]
- 10.den Boon S, Verver S, Lombard CJ, et al. Comparison of symptoms and treatment outcomes between actively and passively detected tuberculosis cases: the additional value of active case finding. Epidemiol Infect. 2008;136:1342–1349. doi: 10.1017/S0950268807000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sendagire I, Schim Van der Loeff M, Mubiru M, Konde-Lule J, Cobelens F. Long delays and missed opportunities in diagnosing smear-positive pulmonary tuberculosis in Kampala, Uganda: a cross-sectional study. PLOS ONE. 2010;5:e14459. doi: 10.1371/journal.pone.0014459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey SL, Roper MH, Huayta M, Trejos N, López Alarcó V, Moore DAJ. Missed opportunities for tuberculosis diagnosis. Int J Tuberc Lung Dis. 2011;15:205–210. [PMC free article] [PubMed] [Google Scholar]
- 13.Lönnroth K, Corbett E, Golub J, et al. Systematic screening for active tuberculosis: rationale, definitions and key considerations. Int J Tuberc Lung Dis. 2013;17:289–298. doi: 10.5588/ijtld.12.0797. [DOI] [PubMed] [Google Scholar]
- 14.Corbett EL, Bandason T, Duong T, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a clusterrandomised trial. Lancet. 2010;376:1244–1253. doi: 10.1016/S0140-6736(10)61425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood R, Middelkoop K, Myer L, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Currie CS, Williams BG, Cheng RC, Dye C. Tuberculosis epidemics driven by HIV: is prevention better than cure? AIDS. 2003;17:2501–2508. doi: 10.1097/01.aids.0000096903.73209.ac. [DOI] [PubMed] [Google Scholar]
- 17.Miller AC, Golub JE, Cavalcante SC, et al. Controlled trial of active tuberculosis case finding in a Brazilian favela. Int J Tuberc Lung Dis. 2010;14:720–726. [PMC free article] [PubMed] [Google Scholar]
- 18.Datiko DG, Lindtjorn B. Health extension workers improve tuberculosis case detection and treatment success in southern Ethiopia: a community randomized trial. PLOS ONE. 2009;4:e5443. doi: 10.1371/journal.pone.0005443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayles H, Schaap A, Nota A, et al. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLOS ONE. 2009;4:e5602. doi: 10.1371/journal.pone.0005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekandi JN, Neuhauser D, Smyth K, Whalen CC. Active case finding of undetected tuberculosis among chronic coughers in a slum setting in Kampala, Uganda. Int J Tuberc Lung Dis. 2009;13:508–513. [PMC free article] [PubMed] [Google Scholar]
- 21.Uganda Ministry of Health. National Tuberculosis and Leprosy Control Programme report 2008. Kampala, Uganda: MoH; 2008. [Google Scholar]
- 22.Uganda Bureau of Statistics. Uganda national household survey, 2009/10. Kampala, Uganda: UBOS; 2010. [Google Scholar]
- 23.Uganda Bureau of Statistics. Uganda Demographic and Health Survey 2011. Kampala, Uganda: UBOS; 2011. [Google Scholar]
- 24.Asiimwe BB, Koivula T, Källenius G, et al. Mycobacterium tuberculosis Uganda genotype is the predominant cause of TB in Kampala, Uganda. Int J Tuberc Lung Dis. 2008;12:386–391. [PubMed] [Google Scholar]
- 25.Rieder H, Van Deun A, Kam KM, et al. Priorities for tuberculosis bacteriology services in low-income countries. 2. Paris, France: International Union Against Tuberculosis and Lung Disease; 2007. http://www.tbrieder.org/publications/books_english/red_book.pdf Accessed September 2013. [Google Scholar]
- 26.Ministry of Health. Manual of the National TB and Leprosy Programme. Kampala, Uganda: MoH; 2010. [Google Scholar]
- 27.Mabaera B, Lauritsen JM, Katamba A, Laticevschi D, Naranbat N, Rieder HL. Sputum smear-positive tuberculosis: empiric evidence challenges the need for confirmatory smears. Int J Tuberc Lung Dis. 2007;11:959–964. [PubMed] [Google Scholar]
- 28.World Health Organization. Global tuberculosis report 2011. Geneva, Switzerland: WHO; 2011. WHO/HTM/TB/2011.16. [Google Scholar]
- 29.Shargie EB, Morkve O, Lindtjorn B. Tuberculosis case-finding through a village outreach programme in a rural setting in southern Ethiopia: community randomized trial. Bull World Health Organ. 2006;84:112–119. doi: 10.2471/blt.05.024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan AJ, Khowaja S, Khan FS, et al. Engaging the private sector to increase tuberculosis case detection: an impact evaluation study. Lancet Infect Dis. 2012;12:608–616. doi: 10.1016/S1473-3099(12)70116-0. [DOI] [PubMed] [Google Scholar]
- 31.Kranzer K, Houben RM, Glynn JR, Bekker LG, Wood R, Lawn SD. Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:93–102. doi: 10.1016/S1473-3099(09)70326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kranzer K, Lawn SD, Meyer-Rath G, et al. Feasibility, yield, and cost of active tuberculosis case finding linked to a mobile HIV service in Cape Town, South Africa: a cross-sectional study. PLoS Med. 2012;9:e1001281. doi: 10.1371/journal.pmed.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro AE, Golub JE. Report to the WHO. Baltimore, MD, USA: Center for Tuberculosis Research, Johns Hopkins; 2012. A systematic review of active casefinding strategies in risk groups for tuberculosis and the relationship to number needed to screen. [Google Scholar]
- 34.Lalloo UG, Pillay S. Managing tuberculosis and HIV in subSaharan Africa. Curr HIV/AIDS Rep. 2008;5:132–139. doi: 10.1007/s11904-008-0021-5. [DOI] [PubMed] [Google Scholar]
- 35.Sekandi JN, Sempeera H, List J, et al. High acceptance of home-based HIV counseling and testing in an urban community setting in Uganda. BMC Public Health. 2011;11:730. doi: 10.1186/1471-2458-11-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guwatudde D, Nakakeeto M, Jones-Lopez EC, et al. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol. 2003;158:887–898. doi: 10.1093/aje/kwg227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whalen CC, Zalwango S, Chiunda A, et al. Secondary attack rate of tuberculosis in urban households in Kampala, Uganda. PLOS ONE. 2011;6:e16137. doi: 10.1371/journal.pone.0016137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lohmann EM, Koster BFPJ, le Cessie S, Kamst-van Agterveld MP, van Soolingen D, Arend SM. Grading of a positive sputum smear and the risk of Mycobacterium tuberculosis transmission. Int J Tuberc Lung Dis. 2012;16:1477–1484. doi: 10.5588/ijtld.12.0129. [DOI] [PubMed] [Google Scholar]
- 39.Ahsan G, Ahmed J, Singhasivanon P, et al. Gender difference in treatment seeking behaviors of tuberculosis cases in rural communities of Bangladesh. Southeast Asian J Trop Med Public Health. 2004;35:126–135. [PubMed] [Google Scholar]
- 40.Ntinginya EN, Squire SB, Millington KA, et al. Performance of the Xpert® MTB/RIF assay in an active case-finding strategy: a pilot study from Tanzania. Int J Tuberc Lung Dis. 2012;16:1468–1470. doi: 10.5588/ijtld.12.0127. [DOI] [PubMed] [Google Scholar]
- 41.Rachow A, Zumla A, Heinrich N, et al. Rapid and accurate detection of Mycobacterium tuberculosis in sputum samples by Cepheid Xpert MTB/RIF assay—a clinical validation study. PLOS ONE. 2011;6:e20458. doi: 10.1371/journal.pone.0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mupere E, Schiltz NK, Mulogo E, Katamba A, Nabbuye-Sekandi J, Singer ME. Effectiveness of active case-finding strategies in tuberculosis control in Kampala, Uganda. Int J Tuberc Lung Dis. 2013;17:207–213. doi: 10.5588/ijtld.12.0160. [DOI] [PubMed] [Google Scholar]
- 43.Datiko DG, Lindtjorn B. Cost and cost-effectiveness of smearpositive tuberculosis treatment by health extension workers in southern Ethiopia: a community randomized trial. PLOS ONE. 2010;5:e9158. doi: 10.1371/journal.pone.0009158. [DOI] [PMC free article] [PubMed] [Google Scholar]


