Abstract
Objective
To evaluate the safety of combination antiretroviral therapy (ART) in conception and pregnancy in different health systems.
Design
A pilot ART registry to measure the prevalence of birth defects and adverse pregnancy outcomes in South Africa and Zambia.
Methods
HIV-infected pregnant women on ART prior to conception were enrolled until delivery, and their infants were followed until 1 year old.
Results
Between October 2010 and April 2011, 600 women were enrolled. The median CD4+ cell count at study enrollment was lower in South Africa than Zambia (320 vs. 430 cells/μl; P < 0.01). The most common antiretroviral drugs at the time of conception included stavudine, lamivudine, and nevirapine. There were 16 abortions (2.7%), one ectopic pregnancy (0.2%), 12 (2.0%) stillbirths, and 571 (95.2%) live infants. Deliveries were more often preterm (29.7 vs. 18.4%; P = 0.01) and the infants had lower birth weights (2900 vs. 2995 g; P = 0.11) in Zambia compared to South Africa. Thirty-six infants had birth defects: 13 major and 23 minor. There were more major anomalies detected in South Africa and more minor ones in Zambia. No neonatal deaths were attributed to congenital birth defects.
Conclusions
An Africa-specific, multi-site antiretroviral drug safety registry for pregnant women is feasible. Different prevalence for preterm delivery, delivery mode, and birth defect types between women on preconception ART in South Africa and Zambia highlight the potential impact of health systems on pregnancy outcomes. As countries establish ART drug safety registries, documenting health facility limitations may be as essential as the specific ART details.
Keywords: antiretroviral therapy, birth defects, drug safety, HIV, pregnancy
Introduction
With the widespread availability of affordable combination antiretroviral therapy (ART) in sub-Saharan Africa, HIV-infected womenare living longer and becoming pregnant while on ART [1–3]. Furthermore, women in developing countries are often exposed to multiple risk factors for adverse pregnancy outcomes, such as poor nutrition, anemia, and untreated hypertension [4–6]. Whereas individual-level variables areoften considered in ART drug safety studies, very few analyses look at the influence of health systems on pregnancy outcomes in women on ART [7,8]. Population-level characteristics, clinical guidelines and policies, and health systems vary within sub-Saharan Africa and may impact the detection and occurrence of adverse maternal, fetal, and newborn outcomes.
Data regarding antenatal ART and risk of stillbirths, preterm delivery (PTD), and other adverse outcomes are mixed. A district-wide Zambian obstetric database comprised approximately 70% of delivery data from primary urban health centers and 30% from the tertiary hospital, and found that antenatal ART compared to none led to decreased odds of stillbirth [9]. However, a study in Botswana based in six government hospitals, two of which were tertiary referral centers, demonstrated an increased risk of PTD, small for gestational age (SGA), and stillbirth in women on prepregnancy ART [10]. The difference in findings may be due to the possible increased proportion of complicated pregnancies in the hospital-based population and higher skilled health care workers’ ability to document clinical details. Different settings may be an unaccounted variable in analyses looking at ART exposure and pregnancy outcomes.
The WHO recommends establishing pregnancy registries for ART surveillance when feasible [11]. The diverse structure of health systems found in developing countries may affect the implementation of such registries and the ability to detect adverse outcomes, especially congenital anomalies. The scope of technology, human resources, and clinical expertise needs to be considered. The Maternal Events and Pregnancy Outcomes in a Cohort of HIV-Infected Women Receiving ART in sub-Saharan Africa (MEP) study measured the congenital anomalies and pregnancy outcomes reported in women receiving ART at conception and during pregnancy through a pilot program in two unique health systems. Sharing the baseline population characteristics and study outcomes between our distinct settings may inform patients, health care workers, and policy-makers on the limitations and strengths of drug safety monitoring in sub-Saharan Africa.
Methods
Study settings
Similar to a multi-country drug registry, the study was conducted in two different health systems: referral hospitals with specialists (South Africa) and primary health facilities with midwives (Zambia). South Africa and Zambia rank among the top 10 countries worldwide affected by the HIV epidemic [12].
In South Africa, study activities from enrollment to exit took place at the Dr George Mukhari Hospital (DGMH), a tertiary hospital affiliated with the University of Limpopo, which is located north of Pretoria. It conducts over 10 000 deliveries per year, composed of routine obstetrics from nearby communities and high-risk patients in the region [13]. Due to low numbers of enrollment, additional recruitment occurred at two affiliated primary health centers with obstetric and HIV services. In Zambia, five primary health centers in the capital city of Lusaka were selected; these sites are part of a larger public-sector network that conducts approximately 60 000 total deliveries annually [14]. Chosen for their high patient volume, the Zambian sites provide low-risk obstetric and HIV services to approximately 20 000 pregnant women annually. Most pregnant women in the public sector receive their antenatal care from nurse-midwives at primary health centers; HIV infection is not a reason for hospital referral. There is no capacity to perform cesarean deliveries or provide advanced clinical care for mother or newborn at these centers.
Study procedures
Inclusion criteria were HIV-infected pregnant women on combination ART at conception and age at least 18 years. Exclusion criteria included a history of mental illness; any condition that would make participation in the study unsafe; and inability to provide informed consent. Maternal participants were recruited from the antenatal or HIV care clinic. Women were followed prospectively until delivery and their infants until 1 year old.
Maternal study visits were scheduled on the same day as antenatal care visits. Gestational age at enrollment was determined by the last menstrual period in Zambia, whereas in South Africa, most participants underwent a dating ultrasound. Laboratory values, HIV test date, and ART regimens were extracted from the antenatal card or clinical file. There were no study-specific laboratory tests and clinical studies beyond routine care in either health system. Details regarding hospitalizations and deaths were recorded by verbal report and/or medical chart extraction. Autopsies for infant participants were offered in South Africa only.
The infants born in the study were enrolled at birth or soon after. For multiple gestations, both infants were enrolled. Infant participants underwent a physical examination at every study visit (birth, 6 weeks, 3 months, 6 months and 1 year old).
In South Africa, the participants were examined by specialists and infants with suspected congenital anomalies by the clinical geneticist. The specialists also provided routine clinical care to participants. In Zambia, the study-specific midwives were specially trained on physical examinations to look for congenital anomalies.
In-country specialists and the South African geneticist (via electronic consultation) were available upon request.
The study team telephoned participants who missed visits. If a participant failed to return, at least one home visit was conducted. Lost to follow-up was originally defined as no contact for 6 months from the last study visit and was revised to 6 months from the date of study restart when the study was briefly suspended due to changes in funding mechanisms. Data were included in the analysis from participants who re-enrolled on their own accord even though they were absent for 6 or more months.
Data analysis
The outcomes of interest included abortion, stillbirth, PTD, neonatal death, and congenital anomalies. ‘Abortion’ was defined as a pregnancy loss at less than 28 weeks gestation and birth weight below 1000 g [15]. The only exception was an infant less than 28 weeks’ gestation and who weighed 900 g, but was classified as a live birth to match the twin, who weighed 1000 g. ‘Stillbirth’ was a fetal death occurring prior to delivery of at least 28 weeks’ gestation or at a birth weight of at least 1000 g. ‘Term’ referred to deliveries of at least 37 weeks’ gestation, whereas ‘preterm’ comprised viable deliveries of less than 37 weeks’ gestation. ‘Neonatal deaths’ occurred within the first 28 days of life.
Physical examinations were performed at infant study visits to identify ‘congenital anomalies’. Minor and major birth defects were reviewed and classified by the clinical geneticist. A major congenital anomaly has an adverse outcome on either the function or the social acceptability of the individual, and a minor congenital anomaly has no medical or cosmetic importance [16]. When a live or stillbirth infant had more than one anomaly, he/she was categorized by the anomaly with higher severity.
Analyses were conducted via SAS version 9.2 (SAS Institute, Cary, North Carolina, USA). Maternal characteristics and pregnancy and infant outcomes were analyzed for differences between countries via Pearson chi-square test, Fisher’s exact test, and Wilcoxon rank-sum test.
The study was approved by the ethical review committees at the Harvard School of Public Health (Boston, Massachusetts, USA), Medical University of Southern Africa (Pretoria, South Africa), University of Zambia (Lusaka, Zambia), University of Alabama at Birmingham (Birmingham, Alabama, USA), and University of North Carolina at Chapel Hill (Chapel Hill, North Carolina, USA).
Results
From October 2010 to April 2011, 697 women were recruited and 636 were screened. Of the 600 women who were enrolled (300 in South Africa, 300 in Zambia), three were never pregnant and one had initiated ART after conception. Whereas one woman withdrew prior to delivery, seven (one in South Africa, six in Zambia) participants were lost to follow-up. Due to twin gestations, 588 women contributed 600 pregnancy outcomes (Fig. 1).
Fig. 1. Flow diagram of maternal participant recruitment, enrollment, and retention and pregnancy outcomes.
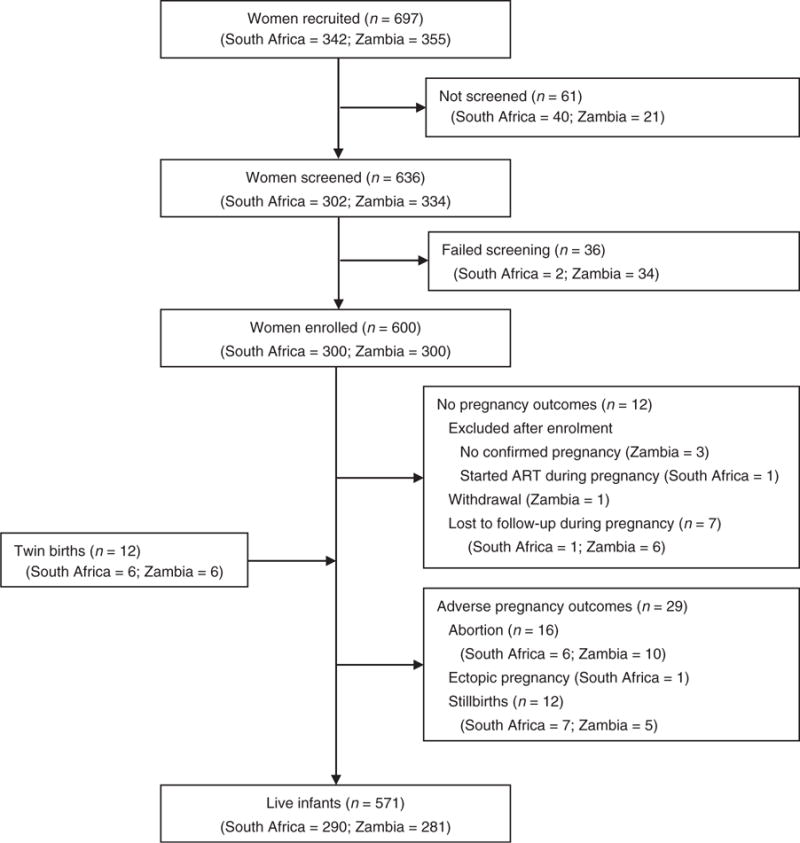
Maternal Events and Pregnancy Outcomes Study, 2010–2012.
The mean number of study visits, including enrollment and exit, was 3.4 (SD 1.6) in South Africa and 2.4 (SD 1.3) in Zambia. There was one postnatal maternal death in Zambia due to severe anemia following home delivery, complicated by bleeding. The last infants exited the study in October 2012 in Zambia and in December 2012 in South Africa.
Characteristics of the study population
Maternal characteristics at enrollment are shown in Table 1. More participants in Zambia compared to South Africa were married (87.9 vs. 19.4%; P < 0.0001), whereas more in South Africa compared to Zambia enrolled into the study in the first trimester (11.7 vs. 6.4%; P = 0.017). The median parity in Zambia was higher than those in South Africa (three vs. two; P < 0.0001). More participants in Zambia had syphilis (6.4 vs. 1.0%; P < 0.0001), whereas more in South Africa had anemia (27.4 vs. 10.8%; P < 0.0001). Overall, two (0.3%) maternal participants reported having a previous pregnancy with an anomaly, and 73 (12.2%) women had one or more prior PTD with a median gestational age of 29.5 weeks [interquartile range (IQR) 28–32]. All 31 (5.2%) women who reported recreational drug use during the index pregnancy were in Zambia, of whom 30 drank alcohol (P < 0.0001).
Table 1.
Characteristics of maternal participants at study enrollment and/or during pregnancy in a cohort of HIV-infected women on combination antiretroviral therapy at the time of conception by country.
| Characteristic | Overall [N = 596 (%)] |
South Africa [N = 299 (%)] |
Zambia [N = 297 (%)] |
P value |
|---|---|---|---|---|
| Marital status | ||||
| Single or never married | 253 (42.4) | 239 (79.9) | 14 (4.7) | <0.0001 |
| Married or cohabitinga | 319 (53.5) | 58 (19.4) | 261 (87.9) | |
| Divorced, separated, or widowed | 24 (4.0) | 2 (0.7) | 22 (7.4) | |
| Education (highest level completed) | ||||
| None | 67 (11.2) | 32 (10.7) | 35 (11.8) | 0.087 |
| Primary | 306 (51.3) | 169 (56.5) | 137 (46.1) | |
| Secondary | 192 (32.2) | 83 (27.8) | 109 (36.7) | |
| Tertiary | 30 (5.0) | 14 (4.7) | 16 (5.4) | |
| Missing | 1 (0.2) | 1 (0.3) | 0 (0) | |
| Age at study enrollment, median years (IQR) | 31 (28, 35) | 31 (28, 35) | 31 (28, 35) | 0.854** |
| <20 | 3 (0.5) | 0 (0) | 3 (1.0) | 0.038* |
| 20–24 | 49 (8.2) | 17 (5.7) | 32 (10.8) | |
| 25–29 | 158 (26.5) | 88 (29.4) | 70 (23.6) | |
| 30–34 | 223 (37.4) | 115 (38.5) | 108 (36.4) | |
| ≥35 | 163 (27.3) | 79 (26.4) | 84 (28.3) | |
| Gestational age at study enrollment, median weeks (IQR) | 24 (18, 31) | 25 (17, 31) | 24 (19, 31) | |
| ≤12 weeks gestation | 54 (9.1) | 35 (11.7) | 19 (6.4) | 0.017 |
| 13–26 weeks gestation | 294 (49.3) | 133 (44.5) | 161 (54.2) | |
| >26 weeks gestation | 248 (41.6) | 131 (43.8) | 117 (39.4) | |
| Gravidity, median | 3 (2, 4) | 3 (2, 4) | 4 (3, 5) | |
| 1 | 34 (5.7) | 22 (7.4) | 12 (4.0) | <0.0001 |
| 2–3 | 313 (52.5) | 188 (62.8) | 125 (42.1) | |
| 4–5 | 200 (33.6) | 78 (26.1) | 122 (41.1) | |
| ≥6 | 49 (8.2) | 11 (3.7) | 38 (12.8) | |
| Parity, median | 2 (1, 3) | 2 (1, 3) | 3 (2, 4) | |
| 0 | 45 (7.6) | 27 (9.0) | 18 (6.1) | <0.0001 |
| 1–2 | 324 (54.4) | 197 (65.9) | 127 (42.8) | |
| 3–4 | 185 (31.0) | 68 (22.7) | 117 (39.4) | |
| ≥5 | 42 (7.0) | 7 (2.3) | 35 (11.8) | |
| Syphilis status during this pregnancy | ||||
| Nonreactive | 467 (78.4) | 285 (95.3) | 182 (61.3) | <0.0001 |
| Reactive | 22 (3.7) | 3 (1.0) | 19 (6.4) | |
| Not done | 107 (18.0) | 11 (3.7) | 96 (32.3) | |
| Hemoglobin during this pregnancy, median mg/dl (IQR) | 11.3 (10.4, 12.1) | 11.2 (10.3, 12.1) | 11.6 (10.4, 12.1) | 0.216** |
| ≥11 | 167 (28.0) | 107 (35.8) | 60 (20.2) | <0.0001* |
| 7–10.9 | 113 (19.0) | 81 (27.1) | 32 (10.8) | |
| <7 | 1 (0.2) | 1 (0.3) | 0 (0) | |
| Not done | 315 (52.9) | 110 (36.8) | 205 (69.0) | |
| History of congenital anomaly | ||||
| No | 591 (99.2) | 298 (99.7) | 293 (98.7) | 0.341* |
| Yes, one prior pregnancy with congenital anomaly | 2 (0.3) | 0 (0) | 2 (0.7) | |
| Unknown | 3 (0.5) | 1 (0.3) | 2 (0.7) | |
| History of preterm delivery | ||||
| No | 523 (87.8) | 262 (87.6) | 261 (87.9) | 0.0842 |
| Yes, one previous preterm delivery | 61 (10.2) | 30 (10.0) | 31 (10.4) | |
| Yes, >1 previous preterm delivery | 12 (2.0) | 7 (2.3) | 5 (1.7) | |
| Gestational age of last preterm delivery if applicable, median weeks (IQR) | 29.5 (28, 32) | 29 (26, 32) | 30 (28, 32) | 0.417** |
| Recreational drug use (including illegal substances) during this pregnancy | ||||
| No | 565 (94.8) | 299 (100.0) | 266 (89.6) | <0.0001 |
| Yes, alcohol | 30 (5.0) | 0 (0) | 30 (10.1) | |
| Yes, traditional or herbal medicine | 1 (0.2) | 0 (0) | 1 (0.3) | |
| HIV infection diagnosis, months (IQR) (timing prior to conceptionb) | 32.0 (16.4, 52.4) | 29.3 (13.3, 52.0) | 35.6 (18.6, 53.3) | 0.010** |
| <6 months | 49 (8.2) | 38 (12.7) | 11 (3.7) | 0.002 |
| 6–12 months | 55 (9.2) | 31 (10.4) | 24 (8.1) | |
| 12–23 months | 117 (19.6) | 49 (16.4) | 68 (22.9) | |
| 2–4 years | 192 (32.2) | 93 (31.1) | 99 (33.3) | |
| 5–10 years | 179 (30.0) | 86 (28.8) | 93 (31.3) | |
| >10 years | 4 (0.7) | 2 (0.7) | 2 (0.7) | |
| HAART start date, months (IQR) (timing prior to conceptionb) | 22.0 (10.2, 39.3) | 17.2 (7.5, 32.5) | 27.4 (14.9, 45.3) | <0.0001** |
| <6 months | 81 (13.6) | 59 (19.7) | 22 (7.4) | <0.0001 |
| 6–12 months | 91 (15.3) | 61 (20.4) | 30 (10.1) | |
| 12–23 months | 141 (23.7) | 63 (21.1) | 78 (26.3) | |
| 2–4 years | 175 (29.4) | 74 (24.7) | 101 (34.0) | |
| 5–10 years | 108 (18.1) | 42 (14.0) | 66 (22.2) | |
| WHO stage at enrollment | ||||
| Stage 1 or 2 | 590 (99.0) | 297 (99.3) | 293 (98.7) | 0.407 |
| Stage 3 or 4 | 6 (1.0) | 2 (0.7) | 4 (1.3) | |
| Last CD4+ cell count prior to enrollment, median cells/μl (IQR) | 385 (254, 519) | 320 (205, 465) | 430 (296, 604) | |
| 0–199 | 90 (15.1) | 68 (22.7) | 22 (7.4) | <0.0001 |
| 200–349 | 165 (27.7) | 87 (29.1) | 78 (26.3) | |
| 350–499 | 169 (28.4) | 75 (25.1) | 94 (31.6) | |
| ≥500 | 161 (27.0) | 59 (19.7) | 102 (34.3) | |
| Missing | 11 (1.8) | 10 (3.3) | 1 (0.3) | |
| Antiretroviral drug regimen at time of conceptionb | ||||
| Nucleoside reverse transcriptase inhibitor | ||||
| Abacavir (ABC) | 9 (1.5) | 1 (0.3) | 8 (2.7) | 0.020* |
| Didanosine (ddI) | 2 (0.3) | 2 (0.7) | 0 (0) | 0.499* |
| Emtricitabine (FTC) | 87 (14.6) | 0 (0) | 87 (29.3) | <0.0001 |
| Lamivudine (3TC) | 505 (84.7) | 295 (98.7) | 210 (70.7) | <0.0001 |
| Stavudine (d4T) | 257 (43.1) | 167 (55.9) | 90 (30.3) | <0.0001 |
| Tenofovir (TDF) | 205 (34.4) | 74 (24.7) | 131 (44.1) | <0.0001 |
| Zidovudine (ZDV) | 128 (21.5) | 59 (19.7) | 69 (23.2) | 0.298 |
| Non-nucleoside reverse transcriptase inhibitor | ||||
| Efavirenz (EFV) | 233 (39.1) | 157 (52.5) | 76 (25.6) | <0.0001 |
| Nevirapine (NVP) | 332 (55.7) | 117 (39.1) | 215 (72.4) | <0.0001 |
| Protease inhibitor | ||||
| Indinavir (IDV) | 2 (0.3) | 2 (0.7) | 0 (0) | 0.499* |
| Lopinavir/ritonavir (LPV/r) | 25 (4.2) | 20 (6.7) | 5 (1.7) | <0.003* |
| Saquinavir (SQV) | 3 (0.5) | 3 (1.0) | 0 (0) | 0.249* |
Maternal Events and Pregnancy Outcomes Study, 2010–2012 (N = 596). IQR, interquartile range.
In Zambia most women view their main partner as their husband; 254 women considered themselves married, while seven responded as cohabiting. In South Africa, no participants reported cohabiting as their marital status.
Time of conception refers to 2 weeks after last menstrual period per verbal report, assuming a 28-day menstrual cycle.
P value is for Fisher’s exact test (others are Chi-square).
P value is for Wilcoxon’s test.
Maternal participants in Zambia were diagnosed with HIV earlier (35.6 vs. 29.3 months; P = 0.010) and had been on triple-drug ART longer (27.4=vs. 17.2 months; P < 0.0001) than those in South Africa. Almost all participants were WHO stage 1 or 2 at study enrollment. The median CD4+ cell count at study enrollment was lower in South Africa than Zambia (320 vs. 430 cells/μl; P < 0.0001).1
The most common antiretroviral drugs at the time of conception included stavudine (d4T), lamivudine (3TC), and nevirapine (NVP). Exposure to d4T was higher in South Africa than Zambia (55.9 vs. 30.3%; P < 0.0001), whereas tenofovir (TDF) use in Zambia was almost double that in South Africa (44.1 vs. 24.7%; P < 0.0001). More women in South Africa compared to Zambia were exposed to efavirenz (EFV) (52.5 vs. 25.6%; P < 0.0001) or protease inhibitor (8.4vs. 1.7%; P = 0.0002), but protease inhibitor use was uncommon overall.
Pregnancy outcomes and congenital anomalies
Pregnancy outcomes are shown in Table 2. There were 17 (2.9%) nonviable pregnancies: 16 abortions (six in South Africa, 10 in Zambia) and one ectopic pregnancy. Of the 12 stillbirths, 75% were preterm and 25% were term. There were more preterm births in Zambia than in South Africa (29.7 vs. 18.4%; P = 0.010). More infants were born by cesarean delivery in South Africa than in Zambia (18.2 vs. 7.0%; P < 0.0001). Infants in Zambia compared to South Africa had lower mean birth weight (2900 vs. 2995 g; P = 0.106) and shorter mean lengths (48 vs. 50 cm; P < 0.0001). In addition, there were seven neonatal deaths in Zambia and one in South Africa (P = 0.079).
Table 2.
Pregnancy outcomes for all fetuses and viable pregnancies in a cohort of HIV-infected women on combination antiretroviral therapy at the time of conception by country.
| Outcomes | Overall [N = 600 (%)] |
South Africa [N = 304 (%)] |
Zambia [N = 296 (%)] |
P value |
|---|---|---|---|---|
| Pregnancy outcome | ||||
| Live birth, term | 427 (71.2) | 234 (77.0) | 193 (65.2) | 0.010 |
| Live birth, preterm | 144 (24.0) | 56 (18.4) | 88 (29.7) | |
| Stillbirth, term | 3 (0.5) | 2 (0.7) | 1 (0.3) | |
| Stillbirth, preterm | 9 (1.5) | 5 (1.6) | 4 (1.4) | |
| Abortiona | 16 (2.7) | 6 (2.0) | 10 (3.4) | |
| Ectopic pregnancy | 1 (0.2) | 1 (0.3) | 0 (0) | |
| Gestational age at delivery | ||||
| <28 weeks gestation | 19 (3.2) | 6 (2.0) | 13 (4.4) | <0.0001 |
| 28–33 weeks gestation | 42 (7.0) | 6 (2.0) | 36 (12.2) | |
| 34–36 weeks gestation | 107 (17.8) | 54 (17.8) | 53 (17.9) | |
| 37–41 weeks gestation | 403 (67.2) | 234 (77.0) | 169 (57.1) | |
| ≥42 weeks gestation | 26 (4.3) | 1 (0.3) | 25 (8.5) | |
| Missing specific gestational age | 3 (0.5) | 3 (1.0) | 0 (0) | |
| Characteristics of viable pregnancy outcomesb | Overall [N = 583 (%)] | South Africa [N = 297 (%)] | Zambia [N = 286 (%)] | P value |
| Number of fetuses | ||||
| Singleton | 559 (95.9) | 285 (96.0) | 274 (95.8) | 0.925 |
| Of a set of twins | 24 (4.1) | 12 (4.0) | 12 (4.2) | |
| Gestational age at delivery, median weeks (IQR) | 38 (36, 40) | 38 (37, 39) | 38 (36, 40) | 0.552** |
| Sex of infant | ||||
| Male | 301 (51.6) | 167 (56.2) | 134 (46.9) | 0.023* |
| Female | 281 (48.2) | 129 (43.4) | 152 (53.1) | |
| Not determined | 1 (0.2) | 1 (0.3) | 0 (0) | |
| Birth weight, median grams (%) | 2960 (2600, 3240) | 2995 (2655, 3265) | 2900 (2500, 3200) | 0.106** |
| <500 | 1 (0.2) | 1 (0.3) | 0 (0) | 0.866 |
| 500–1499 | 10 (1.7) | 5 (1.7) | 5 (1.7) | |
| 1500–2499 | 88 (15.1) | 41 (13.8) | 47 (16.4) | |
| 2500–3999 | 466 (79.9) | 240 (80.8) | 226 (79.0) | |
| ≥4000 | 16 (2.7) | 9 (3.0) | 7 (2.4) | |
| Missing | 2 (0.3) | 1 (0.3) | 1 (0.3) | |
| Length of newborn at delivery, median cm (IQR) | 49 (47,51) | 50 (48, 51) | 48 (45, 50) | <0.0001** |
| Missing | 102 (17.5) | 4 (1.3) | 98 (34.3) | |
| Characteristics of viable pregnancy outcomes | Overall [N = 583 (%)] | South Africa [N = 297 (%)] | Zambia [N = 286 (%)] | P value |
| Mode of delivery | ||||
| Vaginal delivery | 509 (87.3) | 243 (81.8) | 266 (93.0) | <0.0001 |
| Cesarean delivery (total) | 74 (12.7) | 54 (18.2) | 20 (7.0) | |
| Nonelective cesarean delivery | 41 (55.4) | 28 (51.9) | 13 (65.0) | 0.022* |
| Elective cesarean delivery | 31 (41.9) | 26 (48.1) | 5 (25.0) | |
| Unknown type of cesarean delivery | 2 (2.7) | 0 (0) | 2 (10.0) | |
| Congenital anomaly at delivery | 36 (6.2) | 20 (6.4) | 16 (5.6) | 0.545 |
| Infant with major congenital anomaly | 13 (36.1) | 11 (55.0) | 2 (12.5) | |
| Infant with minor congenital anomaly | 23 (63.9) | 9 (45.0) | 14 (87.5) | |
| Neonatal deathc | ||||
| Term | 3 (0.5) | 0 (0) | 3 (1.0) | 0.079* |
| Preterm | 5 (0.9) | 1 (0.3) | 4 (1.4) |
Maternal Events and Pregnancy Outcomes Study, 2010–2012 (N = 600). IQR, interquartile range.
Abortion is defined as estimated gestational age (EGA) less than 28=weeks’ gestation and birth weight below 1000 g. Using the alternate WHO definition of EGA less than 22 weeks’ gestation and birth weight below 500 g, South Africa has eight preterm stillbirths and three abortions, and Zambia seven preterm stillbirths and seven abortions.
Viable pregnancies are defined as EGA at least 28 weeks’ gestation or birth weight at least 1000 g.
Percentages are based on live births.
P value is for Fisher’s exact test (others are chi-square).
P value is for Wilcoxon’s test.
We identified 38 congenital anomalies in 36 individual infants (Fig. 2). One infant with multiple anomalies had a major birth defect, whereas the other had two minor birth defects. Thus, 23 infants had minor anomalies and 13 had major anomalies, giving a major congenital anomaly prevalence of 2.2% among women who conceived on combination ART. The most common congenital anomalies were minor – umbilical hernia and polydactyly – affecting 18 infants. More major than minor congenital anomalies were detected in South Africa, whereas in Zambia, more minor than major congenital anomalies were detected. One stillbirth had a major anomaly, ambiguous genitalia; no neonatal deaths were attributed to congenital birth defects.
Fig. 2. Congenital anomalies in infant participants by major and minor categories in a cohort of HIV-infected women on combination antiretroviral therapy at the time of conception by country.

Maternal Events and Pregnancy Outcomes Study, 2010–2012 (N = 36).
Indications for nonelective cesarean deliveries are shown in Table 3. Fetal distress, poor progress of labor, and severe preeclampsia remote from delivery were the most common indications.
Table 3.
Indications for nonelective cesarean delivery in a cohort of HIV-infected women on combination antiretroviral therapy at the time of conception by country.
| Indication for cesarean delivery | Overall [N = 41 (%)] | South Africa [N = 28 (%)] | Zambia [N = 13 (%)] | P value* |
|---|---|---|---|---|
| Antepartum hemorrhage | 1 (2.4) | 1 (3.6) | 0 (0) | 0.0659 |
| Fetal distress | 23 (56.1) | 19 (67.9) | 4 (30.8) | |
| Malpresentation | 1 (2.4) | 1 (3.6) | 0 (0) | |
| Poor progress of labor | 6 (14.6) | 3 (10.7) | 3 (23.1) | |
| Placenta previa | 4 (9.8) | 1 (3.6) | 3 (23.1) | |
| Severe preeclampsia remote from delivery | 5 (12.2) | 2 (7.1) | 3 (23.1) | |
| Other | 1 (2.4) | 1 (3.6) | 0 (0) |
Maternal Events and Pregnancy Outcomes Study, 2010–2012 (N = 41).
P value is for Fisher’s exact test.
Discussion
Pregnancy outcomes differed significantly between our prospective cohorts of women who conceived on combination ART in South Africa and Zambia: PTD, low birth weight (LBW) infants, mode of delivery, major and minor congenital anomalies, and neonatal deaths. Some baseline characteristics varied significantly between the countries, highlighting the diversity of the HIV-infected pregnant population and resource availability in sub-Saharan Africa. Whereas the higher risk of PTD in Zambia may be attributable to differences in maternal characteristics, such as higher parity [17], higher prevalence of syphilis [18], and higher rate of alcohol use [19], the disparity between the settings may also contribute. Syphilis and hemoglobin screening were often not done in Zambia. On the contrary, participants in South Africa received hospital-based antenatal care from an obstetrician and were more likely to undergo routine antenatal testing. Screening may have led to treatment of infections and consequently less incidence of PTD compared to Zambia. In addition, the non-significant mean birth weight difference of 95 g between the two countries may demonstrate the beneficial accuracy of gestational age ascertainment by ultrasound or clinical estimate over using last menstrual period [20]. The addition of dating ultrasounds in Zambian participants may have mitigated the PTD incidence [21].
The strengths of our study are numerous. The lost to follow-up rate was only 1.2% prior to delivery with an overall 2.0% of participants excluded in the analysis. Study sites spanned two countries in sub-Saharan Africa at different levels of the health system. Participants were recruited from government clinics, reflecting common drug exposures [22]. In addition, the study procedure to detect congenital anomalies was a simple, nonlabor-intensive physical examination of the infant. With adequate training and mentorship, the midwives performed infant examinations and confirmed findings with specialists as needed. Finally, our cohort continued preconception ART throughout pregnancy, representing a group we expect to encounter commonly in the future as combination ART eligibility criteria broaden worldwide [11].
The higher rate of cesarean deliveries (with higher proportion of elective indications), higher detection of major congenital anomalies, and lower incidence of neonatal deaths likely reflect bolstered clinical resources in South Africa compared to those in Zambia. Lack of appropriate-sized endotracheal tubes, limited incubators, and inability to diagnose definitively the major causes of neonatal deaths result in a poor survival rate for newborns admitted to the neonatal ICU in Zambia. A study in the United States showed significantly fewer in-hospital deaths among infants delivered at a hospital with a high-level neonatal ICU (NICU) than other hospitals [23]. Moreover, the study participants in South Africa were evaluated by a pediatrician and clinical geneticist as needed. The national incidences of neonatal encephalopathy and neonatal deaths decrease with increased access to skilled care at delivery [24]. As drug safety studies look at the risk of PTD, LBW, congenital anomalies, and perinatal mortality with ART exposure at conception and during pregnancy, aspects of health systems should be considered.
The significantly lower gestational age at study enrollment, utilization of autopsies, and higher participant retention in the study likely signify either better health-seeking behavior or more developed health care infrastructure or both in South Africa compared to Zambia. Two or more antenatal care visits compared to fewer has been associated with decreased risk of stillbirth [25]. However, because participants generally enrolled in the study in the late second trimester, first-trimester abortions and second-trimester losses are likely underrepresented in this cohort of HIV-infected pregnant women on pre-conception ART. The Development of Antiretroviral Therapy in Africa (DART) trial in Zimbabwe found 21.5% of outcomes were spontaneous abortions and intrauterine deaths less than 22 weeks’ gestation [26], whereas nonelective abortions comprised of 36.6% of pregnancy outcomes in the Tshepo study [27].
With regard to HIV care and treatment, women in Zambia initiated ART for longer durations before the index pregnancy and had higher CD4+ cell counts than women in South Africa. In addition, antiretroviral drug exposure between the two countries was significantly different, with many women in Zambia being exposed to TDF and NVP, whereas d4T and EFV were commonly used in South Africa. In 2010, the Zambian national guidelines recommended d4T for use in second-line regimens only due to its toxicity profile and CD4+ cell count threshold of 350 cells/μl for ART eligibility in pregnant women [28]. The difference in national policy and guidelines may result in higher numbers of ART-exposed pregnancies in Zambia compared to South Africa among healthier HIV-infected women who are willing to initiate treatment. The capacity to provide broader combination ART coverage to the HIV-infected population may imply stronger support for HIV care and treatment programs in Zambia than South Africa. Such inequalities within health systems may also impact associations among specific ART exposures and pregnancy outcomes.
Study limitations
Several limitations are acknowledged. First, most participants enrolled in the study after 20 weeks’ gestation, a common time for pregnant women to book antenatal care [14]; so abortions were likely underrepresented. Second, we might have missed nonviable anomalies as autopsies for any deaths, including abortions and stillbirths, werenot offered as part of the study in Zambia and not uniformly accepted in South Africa. Third, because we did not add resources beyond the local standards of care, there is heterogeneity in estimated gestational age (EGA). Finally, we did not validate infant examinations by midwives for proficiency to detect congenital anomalies, and so birth defects may be underestimated in Zambia. The study team in Zambia, though, did detect a number of congenital anomalies, demonstrating promise that such skills can be transferred to nonspecialists.
In conclusion, pharmacovigilance of combination ART prior to conception and during the antenatal period is a critical undertaking in sub-Saharan Africa where the burden of disease is high [2]. The potential impact of health systems on the detection of congenital anomalies and incidences of stillbirths, preterm deliveries, and neonatal deaths highlights the importance of comparing facility-level, as well as individual-level variables. Patients should be encouraged to establish antenatal care early to lessen the risk of adverse pregnancy outcomes, if any, with ART exposure. Health care workers should be trained in newborn examination and low-technology measures to not only detect congenital anomalies, but also diminish the incidences of perinatal mortality. Standard definitions and high-quality data collection are currently lacking in resource-limited settings [29]. Policy-makers should strive to incorporate health system measurements in monitoring and evaluation, especially as investments improve clinical standards of care. International sentinel sites for ART surveillance may be a preferred alternative to routine registries in developing countries. Sentinel sites should be equipped with a minimum package of resources to ascertain outcomes accurately, such as PTD. Ultrasound studies to determine EGA would require the ultrasound machine, continuous supplies, and a trained health care worker with months of mentorship [30]. Although gestational age estimation would improve, health care costs in resource-limited settings would rise with unlikely reduction of adverse pregnancy outcomes [31]. Findings from sentinel sites may then not be generalizable to the wider HIV-infected pregnant population. As countries establish ART drug safety registries, documenting the limitations of health facility levels, including technology and human resources, may be as essential as the specific ART drug regimen and length of exposure.
Acknowledgments
We would like to thank the South African National Department of Health, Zambian Ministry of Health, Lusaka District Health Management Team, MEP study team members, and study participants and their families for their support of public health evaluations and research. We also would like to thank Dr Lulu Mwangi for her help with early protocol and study development.
K.C.L. and M.F. supported interpretation of results and were responsible for editing the final manuscript. K.C.L., T.M., M.M. and N.C. were in-country investigators and integrally involved in study implementation. E.H. reviewed all congenital anomalies. R.M. and E.M.S. conceived the study, guided the analysis, and interpreted the data. M.F. and J.J. managed the data and performed the statistical analysis. M.G., N.V.S. and W.J. assisted with study implementation and closure, as well as manuscript development. All authors contributed to subsequent drafts and approved of the final version.
The study was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreements U62/CCU123541, 3U2GGH000175–01W1, and 3U2GPS001421.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC. Trainee support was provided by the National Institutes of Health through the International Clinical Research Fellows Program at Vanderbilt University (R24 TW007988).
Footnotes
At the time of the study, maternal HIV RNA PCR was not measured as part of standard of care for HIV care and treatment during pregnancy in either country.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ray M, Logan R, Sterne JA, Hernandez-Diaz S, Robins JM, Sabin C, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–137. doi: 10.1097/QAD.0b013e3283324283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Progress report. Geneva: WHO; 2011. Global HIV/AIDS response: epidemic update and health sector progress towards universal access. [Google Scholar]
- 3.Myer L, Carter RJ, Katyal M, Toro P, El-Sadr WM, Abrams EJ. Impact of antiretroviral therapy on incidence of pregnancy among HIV-infected women in Sub-Saharan Africa: a cohort study. PLoS Med. 2010;7:e1000229. doi: 10.1371/journal.pmed.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekouevi DK, Coffie PA, Becquet R, Tonwe-Gold B, Horo A, Thiebaut R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Cote d’Ivoire. AIDS. 2008;22:1815–1820. doi: 10.1097/QAD.0b013e32830b8ab9. [DOI] [PubMed] [Google Scholar]
- 5.Traisathit P, Mary JY, Le Coeur S, Thantanarat S, Jungpichanvanich S, Pornkitprasarn W, et al. Risk factors of preterm delivery in HIV-infected pregnant women receiving zidovudine for the prevention of perinatal HIV. J Obstet Gynaecol Res. 2009;35:225–233. doi: 10.1111/j.1447-0756.2008.00925.x. [DOI] [PubMed] [Google Scholar]
- 6.Young S, Murray K, Mwesigwa J, Natureeba P, Osterbauer B, Achan J, et al. Maternal nutritional status predicts adverse birth outcomes among HIV-infected rural Ugandan women receiving combination antiretroviral therapy. PLoS One. 2012;7:e41934. doi: 10.1371/journal.pone.0041934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry International Interim Report for 1 January 1989 through 31 January 2012. Wilmington, NC: Registry Coordinating Center; 2012. [Google Scholar]
- 8.European Collaborative Study. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr. 2003;32:380–387. doi: 10.1097/00126334-200304010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Stringer EM, Vwalika B, Killam WP, Giganti MJ, Mbewe R, Chi BH, et al. Determinants of stillbirth in Zambia. Obstet Gynecol. 2011;117:1151–1159. doi: 10.1097/AOG.0b013e3182167627. [DOI] [PubMed] [Google Scholar]
- 10.Chen JY, Ribaudo HJ, Souda S, Parekh N, Ogwu A, Lockman S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206:1695–1705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 12.UNAIDS. AIDSinfo epidemiologystatus. New York City: 2012. http://www.unaids.org/en/dataanalysis/datatools/aidsinfo/. [Accessed 5 October 2013] [Google Scholar]
- 13.van Schaik N, Madale R, Day C, Cois A, Moodley I, Padayachee T. In-depth analysis of hospital efficiency indicators (2008/09 to 2012/13): Gauteng province. Durban, South Africa: Health Systems Trust; 2014. [Google Scholar]
- 14.Chi BH, Vwalika B, Killam WP, Wamalume C, Giganti MJ, Mbewe R, et al. Implementation of the Zambia electronic perinatal record system for comprehensive prenatal and delivery care. Int J Gynaecol Obstet. 2011;113:131–136. doi: 10.1016/j.ijgo.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. International statistical classification of diseases and related health problems: 10th Revision Version for 2007. Geneva: WHO; 2007. [Google Scholar]
- 16.Turnpenny PD, Ellard S. Emery’s elements of medical genetics. Philadelphia: Elsevier Churchill Livingstone; 2012. p. 14e. [Google Scholar]
- 17.Aliyu MH, Salihu HM, Keith LG, Ehiri JE, Islam MA, Jolly PE. High parity and fetal morbidity outcomes. Obstet Gynecol. 2005;105:1045–1051. doi: 10.1097/01.AOG.0000157444.74674.75. [DOI] [PubMed] [Google Scholar]
- 18.Watson-Jones D, Changalucha J, Gumodoka B, Weiss H, Rusizoka M, Ndeki L, et al. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. J Infect Dis. 2002;186:940–947. doi: 10.1086/342952. [DOI] [PubMed] [Google Scholar]
- 19.Aliyu MH, Lynch O, Belogolovkin V, Zoorob R, Salihu HM. Maternal alcohol use and medically indicated vs. spontaneous preterm birth outcomes: a population-based study. Eur J Public Health. 2010;20:582–587. doi: 10.1093/eurpub/ckq036. [DOI] [PubMed] [Google Scholar]
- 20.Lazariu V, Davis CF, McNutt LA. Comparison of two measures of gestational age among low income births. The potential impact on health studies, New York, 2005. Matern Child Health J. 2013;17:42–48. doi: 10.1007/s10995-012-0944-8. [DOI] [PubMed] [Google Scholar]
- 21.Geerts L, Poggenpoel E, Theron G. A comparison of pregnancy dating methods commonly used in South Africa: a prospective study. S Afr Med J. 2013;103:552–556. doi: 10.7196/samj.6751. [DOI] [PubMed] [Google Scholar]
- 22.Keiser O, Anastos K, Schechter M, Balestre E, Myer L, Boulle A, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13:870–879. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorch SA, Baiocchi M, Ahlberg CE, Small DS. The differential impact of delivery hospital on the outcomes of premature infants. Pediatrics. 2012;130:270–278. doi: 10.1542/peds.2011-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawn JE, Lee AC, Kinney M, Sibley L, Carlo WA, Paul VK, et al. Two million intrapartum-related stillbirths and neonatal deaths: where, why, and what can be done? Int J Gynaecol Obstet. 2009;107(Suppl 1):S5–S18. S19. doi: 10.1016/j.ijgo.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Brown CA, Sohani SB, Khan K, Lilford R, Mukhwana W. Antenatal care and perinatal outcomes in Kwale district, Kenya. BMC Pregnancy Childbirth. 2008;8:2. doi: 10.1186/1471-2393-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibb DM, Kizito H, Russell EC, Chidziva E, Zalwango E, Nalumenya R, et al. Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial. PLoS Med. 2012;9:e1001217. doi: 10.1371/journal.pmed.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bussmann H, Wester CW, Wester CN, Lekoko B, Okezie O, Thomas AM, et al. Pregnancy rates and birth outcomes among women on efavirenz-containing highly active antiretroviral therapy in Botswana. J Acquir Immune Defic Syndr. 2007;45:269–273. doi: 10.1097/QAI.0b013e318050d683. [DOI] [PubMed] [Google Scholar]
- 28.Government of the Republic of Zambia. Adult and adolescent antiretroviral therapy protocols. Health Mo; Lusaka: 2010. Edited by. [Google Scholar]
- 29.Lawn JE, OsrinF D, Adler A, CousensF S. Four million neonatal deaths: counting and attribution of cause of death. Paediatr Perinat Epidemiol. 2008;22:410–416. doi: 10.1111/j.1365-3016.2008.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wylie BJ, Kalilani-Phiri L, Madanitsa M, Membe G, Nyirenda O, Mawindo P, et al. Gestational age assessment in malaria pregnancy cohorts: a prospective ultrasound demonstration project in Malawi. Malar J. 2013;12:183. doi: 10.1186/1475-2875-12-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geerts LT, Brand EJ, Theron GB. Routine obstetric ultrasound examinations in South Africa: cost and effect on perinatal outcome: a prospective randomised controlled trial. Br J Obstet Gynaecol. 1996;103:501–507. doi: 10.1111/j.1471-0528.1996.tb09796.x. [DOI] [PubMed] [Google Scholar]


