Abstract
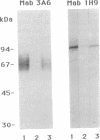
Autoantibodies in the sera of patients with pernicious anemia recognize, in addition to the alpha subunit of the gastric H+/(+)-ATPase, an abundant gastric microsomal glycoprotein of apparent Mr 60,000-90,000. Herein we have colocalized the glycoprotein and the alpha subunit of the gastric H+/K(+)-ATPase to the tubulovesicular membranes of the parietal cell by immunogold electron microscopy. Moreover, the glycoprotein and the alpha subunit were coimmunoprecipitated, and copurified by immunoaffinity chromatography, with an anti-glycoprotein monoclonal antibody. The pig glycoprotein was purified by chromatography on tomato lectin-Sepharose, and five tryptic peptides from the purified glycoprotein were partially sequenced. The complete amino acid sequence, deduced from the nucleotide sequence of overlapping cDNA clones, showed 33% similarity to the sequence of the beta subunit of the pig kidney Na+/K(+)-ATPase. We therefore propose that the 60- to 90-kDa glycoprotein autoantigen is the beta subunit of the gastric H+/K(+)-ATPase and that the alpha and beta subunits of the proton pump are major targets for autoimmunization in autoimmune gastritis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin G. S., Chandler R., Scanlon D. B., Weinstock J. Identification of a gastrin binding protein in porcine gastric mucosal membranes by covalent cross-linking with iodinated gastrin. J Biol Chem. 1986 Sep 15;261(26):12252–12257. [PubMed] [Google Scholar]
- Baldwin G. S., Chandler R., Seet K. L., Weinstock J., Grego B., Rubira M., Mortiz R. L., Simpson R. J. Structural studies on a 75-kDa glycoprotein isolated from porcine gastric mucosal membranes: close homology with the 78-kDa glucose-regulated family of proteins. Protein Seq Data Anal. 1987;1(1):7–12. [PubMed] [Google Scholar]
- Burman P., Mårdh S., Norberg L., Karlsson F. A. Parietal cell antibodies in pernicious anemia inhibit H+, K+-adenosine triphosphatase, the proton pump of the stomach. Gastroenterology. 1989 Jun;96(6):1434–1438. doi: 10.1016/0016-5085(89)90509-x. [DOI] [PubMed] [Google Scholar]
- Callaghan J. M., Toh B. H., Pettitt J. M., Humphris D. C., Gleeson P. A. Poly-N-acetyllactosamine-specific tomato lectin interacts with gastric parietal cells. Identification of a tomato-lectin binding 60-90 X 10(3) Mr membrane glycoprotein of tubulovesicles. J Cell Sci. 1990 Apr;95(Pt 4):563–576. doi: 10.1242/jcs.95.4.563. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dow C. A., De Aizpurua H. J., Pedersen J. S., Ungar B., Toh B. H. 65-70 kD protein identified by immunoblotting as the presumptive gastric microsomal autoantigen in pernicious anaemia. Clin Exp Immunol. 1985 Dec;62(3):732–737. [PMC free article] [PubMed] [Google Scholar]
- Faller L., Jackson R., Malinowska D., Mukidjam E., Rabon E., Saccomani G., Sachs G., Smolka A. Mechanistic aspects of gastric (H+ + K+)-ATPase. Ann N Y Acad Sci. 1982;402:146–163. doi: 10.1111/j.1749-6632.1982.tb25738.x. [DOI] [PubMed] [Google Scholar]
- Goldkorn I., Gleeson P. A., Toh B. H. Gastric parietal cell antigens of 60-90, 92, and 100-120 kDa associated with autoimmune gastritis and pernicious anemia. Role of N-glycans in the structure and antigenicity of the 60-90-kDa component. J Biol Chem. 1989 Nov 5;264(31):18768–18774. [PubMed] [Google Scholar]
- Hall K., Perez G., Anderson D., Gutierrez C., Munson K., Hersey S. J., Kaplan J. H., Sachs G. Location of the carbohydrates present in the HK-ATPase vesicles isolated from hog gastric mucosa. Biochemistry. 1990 Jan 23;29(3):701–706. doi: 10.1021/bi00455a016. [DOI] [PubMed] [Google Scholar]
- Horowitz B., Eakle K. A., Scheiner-Bobis G., Randolph G. R., Chen C. Y., Hitzeman R. A., Farley R. A. Synthesis and assembly of functional mammalian Na,K-ATPase in yeast. J Biol Chem. 1990 Mar 15;265(8):4189–4192. [PubMed] [Google Scholar]
- Jørgensen P. L. Mechanism of the Na+, K+ pump. Protein structure and conformations of the pure (Na+ +K+)-ATPase. Biochim Biophys Acta. 1982 Aug 11;694(1):27–68. doi: 10.1016/0304-4157(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Karlsson F. A., Burman P., Löf L., Mårdh S. Major parietal cell antigen in autoimmune gastritis with pernicious anemia is the acid-producing H+,K+-adenosine triphosphatase of the stomach. J Clin Invest. 1988 Feb;81(2):475–479. doi: 10.1172/JCI113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirley T. L. Determination of three disulfide bonds and one free sulfhydryl in the beta subunit of (Na,K)-ATPase. J Biol Chem. 1989 May 5;264(13):7185–7192. [PubMed] [Google Scholar]
- Kyte J. Molecular considerations relevant to the mechanism of active transport. Nature. 1981 Jul 16;292(5820):201–204. doi: 10.1038/292201a0. [DOI] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Martin-Vasallo P., Dackowski W., Emanuel J. R., Levenson R. Identification of a putative isoform of the Na,K-ATPase beta subunit. Primary structure and tissue-specific expression. J Biol Chem. 1989 Mar 15;264(8):4613–4618. [PubMed] [Google Scholar]
- Mori Y., Fukuma K., Adachi Y., Shigeta K., Kannagi R., Tanaka H., Sakai M., Kuribayashi K., Uchino H., Masuda T. Parietal cell autoantigens involved in neonatal thymectomy-induced murine autoimmune gastritis. Studies using monoclonal autoantibodies. Gastroenterology. 1989 Aug;97(2):364–375. doi: 10.1016/0016-5085(89)90072-3. [DOI] [PubMed] [Google Scholar]
- Okamoto C. T., Karpilow J. M., Smolka A., Forte J. G. Isolation and characterization of gastric microsomal glycoproteins. Evidence for a glycosylated beta-subunit of the H+/K(+)-ATPase. Biochim Biophys Acta. 1990 Mar 1;1037(3):360–372. doi: 10.1016/0167-4838(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Modyanov N. N., Broude N. E., Petrukhin K. E., Grishin A. V., Arzamazova N. M., Aldanova N. A., Monastyrskaya G. S., Sverdlov E. D. Pig kidney Na+,K+-ATPase. Primary structure and spatial organization. FEBS Lett. 1986 Jun 9;201(2):237–245. doi: 10.1016/0014-5793(86)80616-0. [DOI] [PubMed] [Google Scholar]
- Plummer T. H., Jr, Elder J. H., Alexander S., Phelan A. W., Tarentino A. L. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984 Sep 10;259(17):10700–10704. [PubMed] [Google Scholar]
- Saccomani G., Stewart H. B., Shaw D., Lewin M., Sachs G. Characterization of gastric mucosal membranes. IX. Fractionation and purification of K+-ATPase-containing vesicles by zonal centrifugation and free-flow electrophoresis technique. Biochim Biophys Acta. 1977 Mar 1;465(2):311–330. doi: 10.1016/0005-2736(77)90081-5. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Lingrel J. B. Molecular cloning of the rat stomach (H+ + K+)-ATPase. J Biol Chem. 1986 Dec 25;261(36):16788–16791. [PubMed] [Google Scholar]
- Strickland R. G., Mackay I. R. A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Dig Dis. 1973 May;18(5):426–440. doi: 10.1007/BF01071995. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Berman C. H., Dull T. J., Gray A., Lee J. M. Isolation of the human insulin-like growth factor I gene using a single synthetic DNA probe. EMBO J. 1984 Feb;3(2):361–364. doi: 10.1002/j.1460-2075.1984.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- White T. J., Arnheim N., Erlich H. A. The polymerase chain reaction. Trends Genet. 1989 Jun;5(6):185–189. doi: 10.1016/0168-9525(89)90073-5. [DOI] [PubMed] [Google Scholar]