Abstract
Interleukin (IL)-1β is a potent pro-inflammatory cytokine of innate immunity involved in host defense. High systemic IL-1β levels, however, cause life-threatening inflammatory diseases, including systemic inflammatory response syndrome. In response to various danger signals, the pro-form of IL-1β is synthesized and stays in the cytoplasm unless a second signal, such as extracellular ATP, activates the inflammasome, which enables processing and release of mature IL-1β. As pulmonary surfactant is known for its anti-inflammatory properties, we hypothesize that surfactant inhibits ATP-induced release of IL-1β. Lipopolysaccharide-primed monocytic U937 cells were stimulated with an ATP analog in the presence of natural or synthetic surfactant composed of recombinant surfactant protein (rSP)-C, palmitoylphosphatidylglycerol, and dipalmitoylphosphatidylcholine (DPPC). Both surfactant preparations dose-dependently inhibited IL-1β release from U937 cells. DPPC was the active constituent of surfactant, whereas rSP-C and palmitoylphosphatidylglycerol were inactive. DPPC was also effective in primary mononuclear leukocytes isolated from human blood. Experiments with nicotinic antagonists, siRNA technology, and patch-clamp experiments suggested that stimulation of nicotinic acetylcholine receptors (nAChRs) containing subunit α9 results in a complete inhibition of the ion channel function of ATP receptor, P2X7. In conclusion, the surfactant constituent, DPPC, efficiently inhibits ATP-induced inflammasome activation and maturation of IL-1β in human monocytes by a mechanism involving nAChRs.
Keywords: CHRNA7, CHRNA9, CHRNA10, dipalmitoylphosphatidylcholine, inflammasome, monocyte, purinergic receptor P2X 7, phosphatidylcholine
In order to enable effective gas exchange, the blood-air barrier of the mammalian lung is extremely thin-walled and fragile. At the same time, lungs are exposed to a considerable intake of foreign matter, including allergens, particulates, and potentially pathogenic microorganisms. Hence, healthy lungs should efficiently clear invading pathogens without inducing excessive inflammation and impairment of the blood-air barrier. This is achieved by a whole arsenal of potent factors involved in host defense against infection and by numerous anti-inflammatory mediators, which are present in lung tissue and in the pulmonary immune system (1–4).
Interleukin (IL)-1β is a potent pro-inflammatory cytokine that is predominantly produced by activated monocytes/macrophages, but also by numerous other cells, such as pulmonary epithelia and endothelial cells (5–7). On the one hand, IL-1β plays a central role in host defense against viral and bacterial infections. On the other hand, IL-1β is an important pathogenic factor in acute lung injury, acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease, silicosis, and other pulmonary diseases (8–14). High systemic levels of IL-1β, which, among others, may originate from an inflamed lung, can cause a life-threatening systemic inflammatory response syndrome (6).
Synthesis and release of IL-1β are tightly controlled under steady-state conditions (15, 16). Lipopolysaccharide (LPS) originating from gram-negative bacteria is a classic stimulus inducing the biosynthesis of the inactive cytoplasmic pro-form of IL-1β (pro-IL-1β) in monocytes/macrophages. Release of mature IL-1β normally depends on a second danger signal, such as extracellular ATP, that induces the assembly of the Nod-like receptor protein 3 (NLRP3)-containing inflammasome via P2X7 receptors. Inflammasomes, in turn, activate the protease, caspase-1, that cleaves pro-IL-1β and enables release of bioactive mature IL-1β (15, 16). LPS and ATP are relevant stimuli to mimic sterile and infectious pulmonary inflammation. LPS is a cell wall component of gram-negative bacteria and serves as a model ligand of Toll-like receptor 4 (TLR4). Endogenous danger-associated molecular patterns, such as biglycan, HMGB1, or heat shock proteins, are also known agonists of TLR4 (17). An important source of extracellular ATP is traumatic cell injury. In the lung, this can be direct trauma caused by accidents, but can also be ventilator-induced lung injury and therapeutic alveolar recruitment maneuvers. In addition, during sterile and infectious inflammation, ATP can be released in significant amounts by different immune cells (18).
Recently, we discovered that activation of nicotinic acetylcholine receptors (nAChRs), composed of subunits α7, α9, and/or α10, efficiently control ATP-induced activation of P2X7 receptors and, thereby, release of IL-1β by human monocytes (19). These nAChR subunits are expressed by both human and rat monocytes under steady-state conditions and during severe inflammation (20–22). In this context, we identified free phosphocholine (PC), PC-modified protein, and PC-modified LPS as novel agonists of nAChR and as potent inhibitors of monocytic IL-1β release (19, 23). These findings suggested that other compounds bearing PC moieties might also trigger this noncanonical function of nAChR.
Pulmonary surfactant lines the surface of alveoli and is composed of a lipid fraction, which represents about 90%, and four surfactant proteins (SPs), named SP-A, SP-B, SP-C, and SP-D. The main constituents of the lipid fraction are phospholipids with phosphatidylcholine and phosphatidylglycerol as the main classes. Dipalmitoylated phosphatidylcholine [1,2-dipalmitoyl-sn-glycero-3-phosphocholine (dipalmitoylphosphatidylcholine) (DPPC)] is the most abundant single constituent, representing approximately 40% of all phospholipid molecules (24, 25). Surfactant exerts a dual function in the lung. First, it exerts a biophysical activity that lowers the surface tension within the alveoli and, thus, contributes to their stability and enables gas exchange. Lipids, in particular DPPC, and the hydrophobic proteins, SP-B and SP-C, are involved in this mechanical function. Second, surfactant is a core part of pulmonary innate immunity (26). A wide variety of pathogens are bound by SP-A and SP-D, microbial growth is inhibited, pathogens are opsonized, and phagocytosis by alveolar macrophages is mediated (26). In addition, a variety of anti-inflammatory effects have been described for SP-B and SP-C and for surfactant lipids (26, 27). For example, surfactant lipids suppress inflammation by interfering with sensing of LPS (28–30), by suppression of respiratory burst (31), and by regulating cyclooxygenase-2 expression (32).
Here, we test and confirm the hypothesis that pulmonary surfactant inhibits ATP-induced release of monocytic IL-1β. DPPC is identified as the active constituent of surfactant and we provide evidence that it signals via unconventional nAChR containing subunits α9, α7, and/or α10.
MATERIALS AND METHODS
U937 cells
U937 cells, a human histiocytic lymphoma cell line, were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) and cultured in RPMI 1640 (Gibco by Life Technologies GmbH, Darmstadt, Germany) supplemented with 10% fetal calf serum (Biochrome, Berlin, Germany) and 2 mM L-glutamine (Gibco). In the exponential phase of cell growth, 1 × 106 U937 cells were seeded in 24-well plates and primed with 1 μg/ml LPS from Escherichia coli (L2654; Sigma-Aldrich, Steinheim, Germany) for 5 h. Thereafter, cells were stimulated with 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt (BzATP; Sigma-Aldrich) at a concentration of 100 μM. In some experiments Ac-YVAD-cmk, an inhibitor of caspase-1 (50 μM; Sigma-Aldrich), was applied together with BzATP. Cells were spun down 30 min thereafter, and cell culture supernatant was stored at −20°C until measurement of IL-1β and lactate dehydrogenase (LDH).
A natural bovine surfactant extract (Alveofact®; Lyomark Pharma, Oberhaching, Germay), synthetic surfactant (Venticute®; Nycomed, Konstanz, Germany), human recombinant SP-C (rSP-C; Nycomed), palmitoyl-oleyl-phosphatidylglycerol [POPG, 2-oleoyl-1-palmitoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt; Sigma-Aldrich], 1,2-diacyl-sn-glycero-3-phospho-L-serine (PS; Sigma-Aldrich), DPPC (Sigma-Aldrich), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE; Sigma-Aldrich), or 1,2-dipalmitoyl-sn-glycerol (DPG; Sigma-Aldrich) were added at different concentrations shortly before stimulation with BzATP in the presence or absence of different nicotinic antagonists. All surfactant preparations and lipids were sonicated shortly before use. In some experiments, apyrase (0.5 U/ml, A6410; Sigma-Aldrich) was added to degrade endogenous extracellular ATP and cells were stimulated with the pore-forming bacterial toxin, nigericin (50 μM; Sigma-Aldrich), for 30 min. Leu-Leu methyl ester hydrobromide (LLME, 1 mM; Sigma-Aldrich) was also applied for 30 min as another ATP-independent stimulus for inflammasome activation. Nicotine hydrogen tartrate (100 μM; Sigma-Aldrich) served as a positive inhibitory control in most experiments. The following nicotinic antagonists were used: mecamylamine hydrochloride (100 μM; Sigma-Aldrich), α-bungarotoxin (1 μM; Tocris Bioscience, Bristol, UK), strychnine hydrochloride (10 μM; Sigma-Aldrich), as well as α-conotoxins, ArIB[V11L,V16D] (500 nM), and RgIA4 (200 nM).
Transfection of siRNA
As previously described (19, 23), the expression of nAChR subunits α7, α9, and α10 (CHRNA7, CHRNA9, CHRNA10) was reduced in U937 cells using siRNA SMARTpool (Thermo Fisher Scientific, Schwerte, Germany). ON-TARGETplus nontargeting pool (Thermo Fisher Scientific) was used as a control for nonspecific effects. In addition to single knock-down, double knock-down experiments were performed.
Primary human mononuclear cells
Experiments on human blood cells were performed in accordance with the principles of the Declaration of Helsinki, as well as to Title 45, US Code of Federal Regulations, Part 46, Protection of Human Subjects, revised January 15, 2009, effective July 14, 2009. The ethics committee of the University of Giessen approved these studies (No. 81/13). Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood of healthy male nonsmoking volunteers by density gradient centrifugation using Leukosep gradients (Greiner Bio-One, Frickenhausen, Germany). In some experiments, heparinized blood was pulsed with 5 ng/ml LPS before separation. PBMCs were incubated in 24-well plates for 3 h in RPMI 1640 supplemented with 10% fetal bovine serum and 2 mM L-glutamine (Gibco) at a density of 5 × 105 PBMCs/0.5 ml per well. Nonadherent cells were removed, cell culture medium was replaced, and cells were stimulated for 30 min with BzATP (100 μM) in the presence or absence of DPPC (100 μM).
Immunocytochemistry
LPS-pulsed PBMCs were cultivated and stimulated as described in CellviewTM cell culture slides (Greiner Bio-One), fixed in Cytofix/CytopermTM (BD Biosciences, Heidelberg, Germany) for 20 min on ice, washed with Perm/WashTM (BD Biosciences), air-dried, and stored at 4°C until staining. Endogenous peroxidase activity was inactivated by incubation in 1% H2O2, unspecific protein binding sites were blocked with 1% BSA (Serva, Heidelberg, Germany), primary rabbit anti-ASC antibodies (Santa Cruz sc-22514-R; Santa Cruz, CA) were diluted 1:50 in Perm/WashTM, 1% BSA, and 5% heat-inactivated human serum and incubated for 1 h at room temperature. Bound antibodies were detected by horseradish peroxidase-labeled goat anti-rabbit Ig antibodies (DAKO, Hamburg, Germany) and 3,3′-diaminobenzidine (Sigma-Aldrich). Absence of unspecific background staining caused by secondary antibodies was tested by omission of primary antibodies. Slides were lightly counterstained with hemalum, covered with Glycergel (Dako), and evaluated with an Olympus (Hamburg, Germany) BX51 microscope and the analySIS software (Olympus).
Measurement of cytokines
Human quantikine immunoassays (R&D Systems, Minneapolis, MN) were used to determine IL-1β, IL-6, and IL-18 concentrations in cell culture supernatants according to the instructions of the supplier. A high sensitivity kit for the measurement of IL-1β was mandatory because U937 cells release low amounts of IL-1β in response to BzATP (19, 23).
Cell viability
LDH was measured in cell culture supernatants and lysed cells by nonradioactive cytotoxicity assay (Promega, Madison, WI) according to the protocol indicated by the supplier. The total content of LDH measured in a batch of lysed untreated control cells was set to 100% and values obtained for individual cell culture supernatants were calculated accordingly.
Western blots
Western blot analysis of IL-1β and caspase-1, as well as their pro-forms, was performed as described before (19). In short, human PBMCs were stimulated with BzATP in serum-free medium and supernatants were concentrated by a factor of 10 using Amicon® Ultra centrifugal filters (UltracelTM 10K; Merck Millipore, Darmstadt, Germany). Cell lysates and concentrated supernatants were separated along with prestained molecular weight standards (Precision Plus protein standards, dual color; Bio Rad, Hercules, CA) on 15% reducing SDS-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA). Mouse monoclonal antibodies to IL-1β (kindly supplied by the National Cancer Institute, Frederick, MD), polyclonal rabbit antibodies to caspase-1 (#2225; Cell Signaling Technology, Danvers, MA), and mouse monoclonal antibodies to β-actin (A2228; Sigma-Aldrich) were used as primary antibodies that were detected by horseradish peroxidase-conjugated secondary antibodies (Dako Cytomation, Glostrup, Denmark) and chemoluminescent substrates. Documentation and densitometry were performed using a digital gel documentation system (Biozym, Hessisch Oldendorf, Germany).
Real-time RT-PCR
Extraction of RNA, reverse transcription, and real-time RT-PCR for pro-IL-1β (IL1B), subunits α9 (CHRNA9) and α10 (CHRNA10), and the housekeeping gene, porphobilinogen deaminase (HMBS), were performed as described previously (19). In negative control experiments, where the cDNA was replaced by water, no DNA was amplified. Agarose gel electrophoresis and sequencing (SeqLab, Göttingen, Germany) of the PCR products confirmed the specificity of the PCR reaction. Gene expression was analyzed using the 2−ΔCt method and the mean values of the controls were set to one arbitrary unit.
Whole-cell patch-clamp recordings on U937 cells
Electrophysiological recordings on LPS-primed U937 cells were essentially performed as described before (19). Briefly, U937 cells were transferred to poly-L-lysine-coated cell culture dishes containing bath solution (in millimoles: 5.4 KCl, 120 NaCl, 2 CaCl2, 1 MgCl2, 25 glucose, and 10 HEPES [4-(2-hydroxyethyl)-piperazine-1-ethanesulfonic acid (pH 7.4)] and primed with LPS for 5 h. Thereafter, whole-cell patch-clamp recordings were performed at room temperature. BzATP (100 μM) was always applied as a first stimulus via a pressure-driven microperfusion system followed by DPPC (100 μM) and a second BzATP stimulus. Control experiments in which BzATP was applied twice in the absence of DPPC were performed at least once per day to confirm reversibility and repeatability of the BzATP-induced changes in ion currents.
Statistical analyses
SPSS software (Munich, Germany) was used to analyze data by nonparametric Kruskal-Wallis test followed by Mann-Whitney rank sum test. Wilcoxon sign rank test was used to analyze data obtained from PBMCs. P ≤ 0.05 was considered as statistically significant.
RESULTS
Surfactant inhibits the release of IL-1β
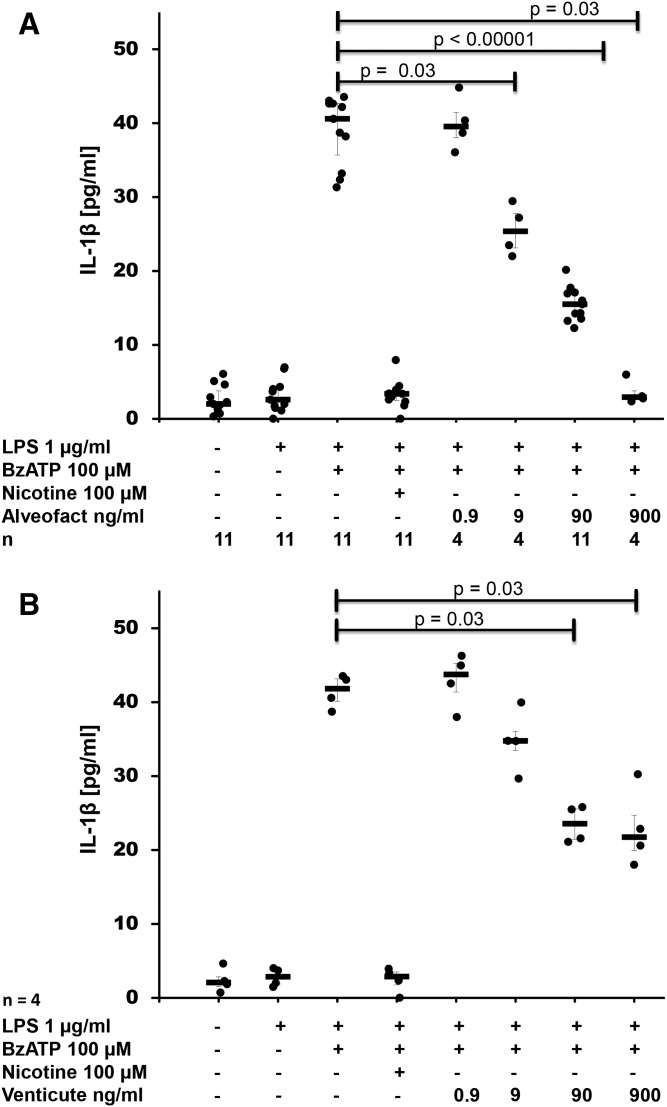
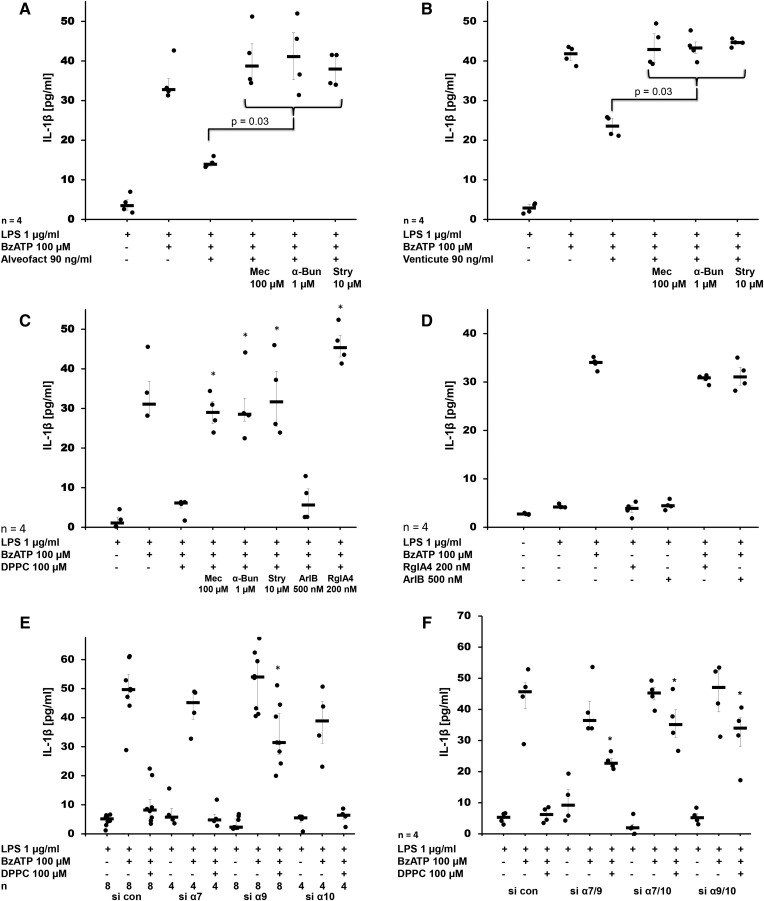
To test the hypothesis that pulmonary surfactant inhibits ATP-induced release of IL-1β, human monocytic U937 cells were primed with LPS for 5 h followed by stimulation with BzATP, a specific agonist of ATP receptor, P2X7. As expected, IL-1β was released into the cell culture supernatant (Fig. 1A, B), whereas IL-18 was not detected. Maturation and release of IL-1β depended on activated caspase-1 (supplemental Fig. S1). The natural bovine surfactant, Alveofact®, dose-dependently and efficiently inhibited BzATP-induced IL-1β release (P ≤ 0.00001, n = 11 at a concentration of 90 ng/ml) with an IC50 of about 9 ng/ml (Fig. 1A). Nicotine (100 μM) that was included in each experiment as a positive control also significantly inhibited BzATP-induced IL-1β release (P ≤ 0.00001, n = 25), as described before (19). Essentially the same results were obtained when the synthetic surfactant preparation, Venticute®, was used, which is composed of rSP-C, POPG, and DPPC (Fig. 1B). To estimate cell death, LDH was measured in the cell culture supernatant at the end of each experiment. Elevated LDH levels were not detected in any of the experimental settings (supplemental Fig. S2A, B).
Fig. 1.
Surfactant dose-dependently inhibits BzATP-mediated release of IL-1β. Different concentrations of the natural surfactant preparation, Alveofact® (A), and the synthetic surfactant, Venticute® (B), were added to LPS-primed U937 cells together with BzATP. IL-1β levels were measured 30 min thereafter in cell culture supernatants. Nicotine was included as a known inhibitor of BzATP-dependent IL-1β release. A Kruskal-Wallis test was followed by Mann-Whitney rank-sum test; data are presented as individual data points; bars represent median; whiskers represent percentiles 25 and 75.
The inhibitory function of surfactant is mediated by DPPC
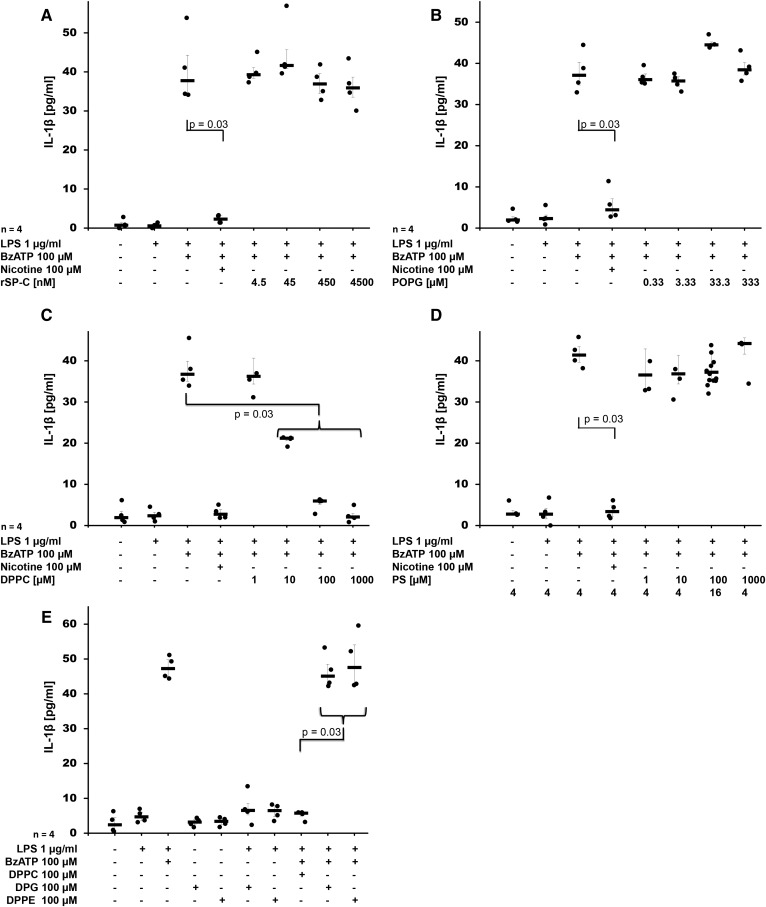
To identify the active compound that inhibits BzATP-induced release of IL-1β by U937 cells, we investigated the effect of the different constituents of Venticute®, rSP-C (Fig. 2A), POPG (Fig. 2B), and DPPC (Fig. 2C) at concentrations that reflected their relative concentration in Venticute® (33). As an additional control, we included PS, a constituent of natural surfactant (Fig. 2D). rSP-C, POPG, and PS did not inhibit BzATP-induced release of IL-1β from LPS-primed U937 cells, although nicotine that served as positive control was effective in the same experiments. In contrast, application of DPPC resulted in a dose-dependent and efficient inhibition of IL-1β release at concentrations of 10, 100, and 1,000 μM (P = 0.03, n = 4, each) with an IC50 of about 10 μM (Fig. 2D), which was in line with the data obtained for surfactant. When DPPC was added to LPS-primed U937 cells in the absence of BzATP, virtually no IL-1β was detected in the cell culture supernatant (n = 25; Fig. 2C). To test whether other dipalmitoylated compounds devoid of a PC group also inhibit BzATP-induced release of IL-1β, we tested DPPE (100 μM) and DPG (100 μM). Both compounds did not impair IL-1β release (Fig. 2E). None of the compounds tested resulted in an increased LDH content of cell culture supernatants (supplemental Fig. S3A–E).
Fig. 2.
DPPC is the active component of surfactant that dose-dependently inhibits BzATP-mediated release of IL-1β. Different concentrations of rSP-C (A), POPG (B), DPPC (C), PS (D), DPPE (E), or DPG (E) were added to LPS-primed U937 cells together with BzATP. IL-1β levels were measured 30 min thereafter in cell culture supernatants. Nicotine was included as a known inhibitor of BzATP-dependent IL-1β release. A Kruskal-Wallis test was followed by Mann-Whitney rank-sum test; data are presented as individual data points; bars represent median; whiskers represent percentiles 25 and 75.
When DPPC was added together with LPS during priming of U937 cells, no change in the mRNA expression of pro-IL-1β was seen (supplemental Fig. S4). Of note, release of IL-6, an inflammasome-independent cytokine, was induced by priming with LPS, but remained unchanged irrespective of the presence of BzATP, DPPC, or nicotine (supplemental Fig. S5).
DPPC inhibits release of IL-1β by primary PBMCs
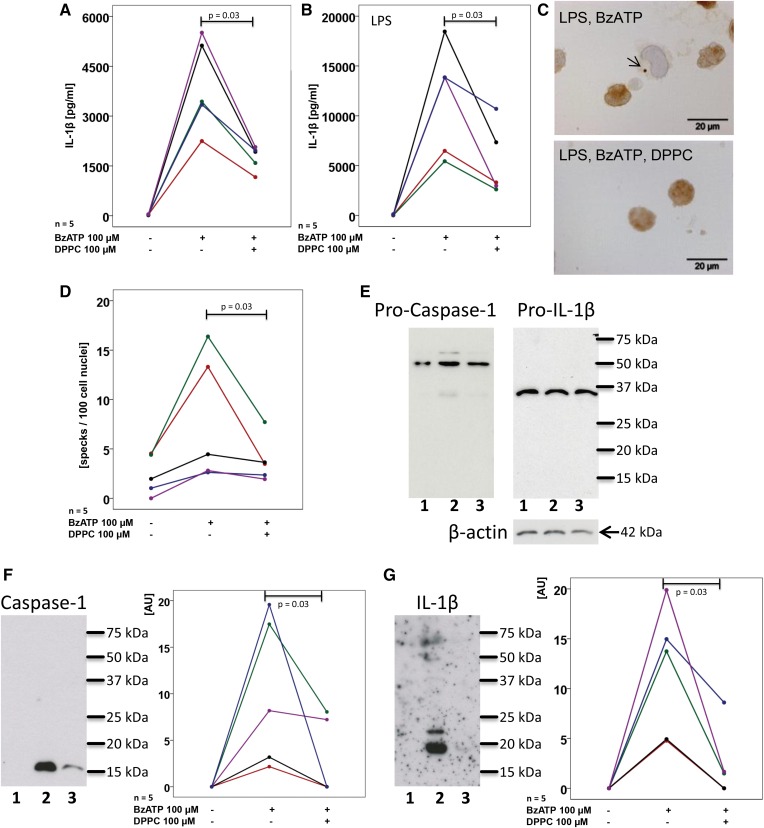
Next, we tested whether DPPC is also effective in primary human PBMCs isolated from the peripheral blood of healthy human donors. Unprimed PBMCs released a considerable amount of IL-1β upon stimulation with BzATP (Fig. 3A), whereas, at best, traces of IL-18 were detected. Priming of PBMCs with LPS led to even higher concentrations of IL-1β in cell culture supernatants (Fig. 3B). In both experimental settings, addition of 100 μM DPPC significantly (P = 0.03, n = 5, each) reduced the BzATP-induced release of IL-1β into the cell culture medium (Fig. 3A, B) without changing the release of LDH (supplemental Fig. S6).
Fig. 3.
DPPC inhibits BzATP-mediated inflammasome activation in human PBMCs. PBMCs were isolated from healthy human donors, left untreated (A) or pulsed with LPS (B), cultured for 3 h and stimulated with BzATP in the presence or absence of DPPC. IL-1β levels were measured 30 min thereafter in cell culture supernatants. C, D: ASC specks were detected by immunocytochemistry in LPS-pulsed PBMC and stimulated with BzATP in the presence or absence of DPPC. C: Micrographs of PBMC: ASC immunopositive material is stained in brown, cell nuclei were lightly counterstained with hemalum, and the arrow is pointing to an ASC speck. D: The number of specks per 100 cell nuclei is depicted. E–G: PBMCs were pulsed with LPS (lane 1) and stimulated with BzATP in the absence (lane 2) or presence (lane 3) of DPPC and Western blots were performed with antibodies directed to human caspase-1, IL-1β, and β-actin. E: In cell lysates, exclusively, the pro-forms, pro-caspase-1 and pro-IL-1β, were detected; whereas, in concentrated cell culture supernatants, mature caspase-1 (F) and IL-1β (G) were detected. F, G: Immunopositive signals were quantified by densitometry and expressed as arbitrary units (AU). Data points obtained from individual blood donors are depicted and connected by lines. Data from the same individuals are coded by the same color in (A) and (B) as well as in (F) and (G), but in (A) and (B) a cohort of volunteers was investigated that was different than in (F) and (G). Wilcoxon sign rank test.
Next, we used antibodies to ASC to detect ASC speck formation by immunohistochemistry. In unstimulated cells, a diffuse cytoplasmic and nuclear immunopositivity was seen in most cells and almost no ASC specks were detected. Upon stimulation with BzATP, the frequency of ASC specks per 100 nuclei increased and the presence of DPPC significantly (P = 0.03, n = 5) reduced speck formation (Fig. 3C, D). In controls, where the primary antibody was omitted, virtually no immunopositivity was visible. The specificity of the antibody to ASC was controlled by Western blotting, where a single band of the expected molecular mass was detected (data not shown).
In Western blots of cell lysates only pro-IL-1β and pro-caspase-1 were detected, suggesting that DPPC does not inhibit the release of mature IL-1β (Fig. 3E). Western blots of concentrated cell culture supernatants confirmed that, in response to BzATP, activated caspase-1 is released to cell culture supernatants along with mature IL-1β (Fig. 3F, G). In the presence DPPC, this release was significantly reduced (Fig. 3F, G).
DPPC does not inhibit ATP-independent release of IL-1β
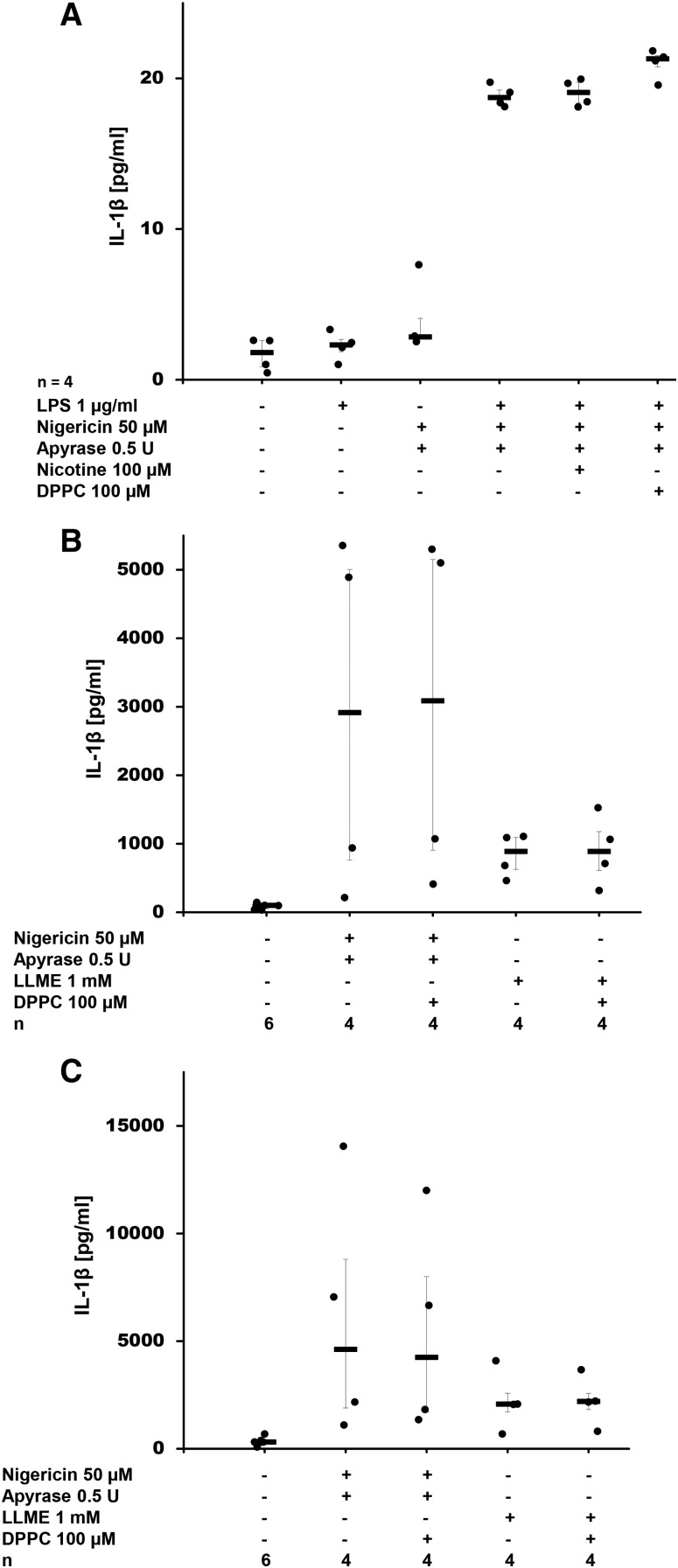
In order to investigate whether DPPC also affects ATP-independent IL-1β release, we added apyrase to degrade any ATP present in the cell culture medium and stimulated LPS-primed U937 cells with the bacterial pore-forming toxin, nigericin, which induced release of IL-1β (Fig. 4A), but not of LDH (supplemental Fig. S7A). DPPC did not inhibit nigericin-induced release of IL-1β (Fig. 4A). In the same experimental setting, nicotine was also ineffective, which is in line with published data (19). In LPS-primed U937 cells, LLME did not stimulate IL-1β secretion, which is in contrast to naïve and LPS-primed PBMCs. In these cells, nigericin and LLME triggered the release of IL-1β and LDH and, again, addition of DPPC did not change IL-1β levels in cell culture supernatants (Fig. 4B, C; supplemental Fig. S7B, C).
Fig. 4.
DPPC does not inhibit nigericin- or LLME-induced release of IL-1β. A: DPPC was added to LPS-primed U937 cells together with nigericin. IL-1β levels were measured 30 min after application of nigericin in cell culture supernatants. Nicotine was included as a known inhibitor of BzATP-dependent IL-1β release. B, C: Human PBMCs were isolated from healthy human donors, left untreated (B) or pulsed with LPS (C), cultured for 3 h and stimulated with nigericin or LLME in the presence or absence of DPPC. IL-1β levels were measured 30 min thereafter in cell culture supernatants. Apyrase was included (A–C) to hydrolyze ATP that might have been released by U937 cells. A Kruskal-Wallis test was followed by Mann-Whitney rank-sum test; data are presented as individual data points; bars represent median; whiskers represent percentiles 25 and 75.
The effect of surfactant and DPPC is mediated via nAChRs
To test the hypothesis that the inhibitory effects of Alveofact®, Venticute®, and DPPC are mediated via nAChRs, we used the general nicotinic antagonist, mecamylamine (100 μM), which blocked the inhibition of BzATP-induced release of IL-1β in U937 cells (P = 0.03, n = 4) (Fig. 5A–C). The same effects were seen when α-bungarotoxin (1 μM) and strychnine (10 μM) were used (P = 0.03, n = 4, each), suggesting that nAChRs composed of subunits α7, α9, and/or α10 are involved (Fig. 5A–C). RgIA4 (200 nM), an α-conotoxin specific for nAChRs containing subunit α9 (23, 34), also completely blocked the effect of DPPC (P = 0.03, n = 4, each), whereas ArIB[V11L,V16D] (500 nM), an α-conotoxin specifically antagonizing α7 nAChRs (35, 36), was ineffective (Fig. 5C). In all experimental settings, no increase in LDH release was seen (supplemental Fig. S5A–C). Treatment of U937 cells with LPS and BzATP did not change the mRNA expression of pro-IL-1β and nAChR subunits α9 and α10 (supplemental Fig. S8A–C). The mRNA of nAChR subunit α7 is detectable in U937 cells, but expression levels are too low for quantification by real-time RT-PCR (19). We demonstrated before that addition of mecamylamine, α-bungarotoxin, and strychnine did not induce the release of IL-1β in the absence of BzATP (19). Similarly, α-conotoxins, ArIB[V11L,V16D] and RgIA4, neither induced nor impaired BzATP-induced mRNA levels or the release of IL-β from LPS-primed U937 cells (supplemental Fig. S8A, Fig. 5D). None of the nicotinic antagonists caused a significant increase in LDH concentration in cell culture supernatants (supplemental Fig. S9A–C)
Fig. 5.
The inhibitory effect of surfactant and DPPC on the BzATP-mediated release of IL-1β by LPS-primed U937 cells is mediated by nAChR subunits α7, α9, and α10. Natural surfactant Alveofact® (A), synthetic surfactant Venticute® (B), or DPPC (C) were added to LPS-primed U937 cells together with BzATP in the presence and absence of the nicotinic antagonists mecamylamine (Mec), α-bungarotoxin (α-Bun), strychnine (Stry), RgIA4, or ArIB[V11L,V16D] (ArIB). IL-1β levels were measured 30 min thereafter in cell culture supernatants. In LPS-primed U937 cells that were transfected with control siRNA (si con) the BzATP-stimulated IL-1β release was inhibited by DPPC Conotoxins RgIA4 or ArIB did neither induce the release of IL-1beta by LPS-primed U937 cells nor impair the effects of BzATP. (E, F). E: After transfection of siRNA to nAChR subunit α9, the effect of DPPC was blunted, whereas silencing of subunit α7 and α10 or the use of control siRNA (si con) did not provoke any effect. F: When double knock-down experiments were performed, the inhibitory effect of DPPC was impaired in all siRNA combinations investigated (α7/9, α7/10, α9/10). A Kruskal-Wallis test was followed by a Mann-Whitney rank-sum test; data are presented as individual data points; bars represent median; whiskers represent percentiles 25 and 75; *P ≥ 0.03 compared with respective experiments performed on cells treated with si con.
To further elucidate the role of nAChR subunits α7, α9, and α10 in signal transduction of DPPC, we used siRNA technology to silence the expression of each individual gene separately. The siRNA-mediated downregulation of the target gene mRNA of subunits α9 and α10 was shown by real-time RT-PCR, whereas the mRNA expression of subunit α7 was too low for quantification (supplemental Fig. S10A–D). It is, however, not yet possible to investigate nAChR expression on the protein level because no specific antibodies are available (37, 38). Silencing of α7 or α10 alone, as well as transfection of U937 cells with irrelevant control siRNA, did not impair the inhibitory effect of DPPC on BzATP-induced release of IL-1β (Fig. 5E). Only treatment of U937 cells with siRNA to the α9 nAChR subunit blunted the effect of DPPC (P = 0.03, n = 4). Next, we performed double knock-down experiments (α7/α9, α7/α10, and α9/α10) in U937 cells. Silencing of each gene combination blunted the response to DPPC (P = 0.03, n = 4, each) (Fig. 5F). Transfection of U937 cells with siRNA caused the release of variable amounts of LDH into the cell culture medium, but no differences were seen between control siRNA and siRNA targeting nAChR subunits (supplemental Fig. S9D, E).
DPPC inhibits BzATP-induced ion currents
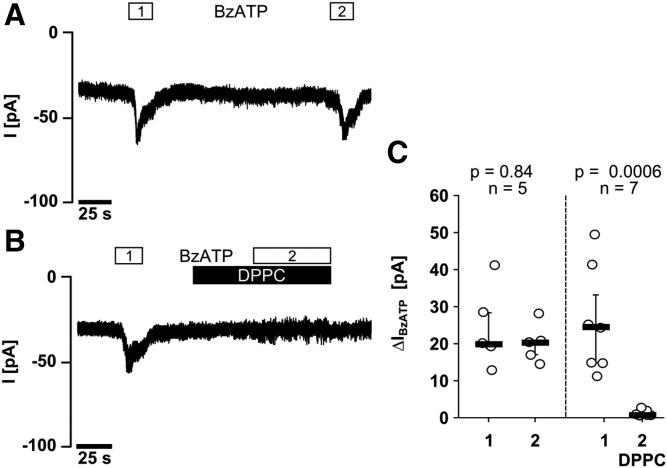
Patch-clamp experiments were performed in LPS-primed U937 cells to investigate the effect of DPPC on BzATP-induced ion currents in LPS-primed U937 cells. Application of BzATP reproducibly induced ion currents (Fig. 6A), which were fully inhibited in the presence of 100 μM DPPC (Fig. 6B, C).
Fig. 6.
DPPC inhibits BzATP-induced ion currents in LPS-primed U937 cells. Whole cell patch-clamp recordings of U937 cells are depicted in (A, B), and changes in ion currents in response to BzATP (ΔIBzATP) are summarized in (C). Two consecutive BzATP applications (1 and 2) resulted in repeatable ion current changes that were abolished in the presence of DPPC (100 μM). Data are presented as individual data points; bars indicate median; whiskers represent percentiles 25 and 75.
DISCUSSION
The results of this study confirm our hypothesis that natural and artificial pulmonary surfactants inhibit ATP-mediated release of IL-1β by human monocytic cells. We investigated all constituents of artificial surfactant separately and identified DPPC as the active substance. Furthermore, we provide evidence that DPPC acts via noncanonical nAChRs.
DPPC is the main lipid constituent of natural and synthetic surfactant (24, 25). It is a prototypical phosphatidylcholine, a class of zwitterionic phospholipids that incorporate choline as a head group, glycerophosphoric acid, and a variety of fatty acids. Apart from DPPC, natural surfactant also contains high concentrations of unsaturated phosphatidylcholines (24, 25), which might also inhibit IL-1β release and deserve further investigation. Phosphatidylcholines are important constituents of biomembranes, but are also found in lining fluids of inner surfaces, such as the intestinal mucus (39, 40).
Patients suffering from acute lung injury or ARDS typically have reduced surfactant DPPC levels (41), together with increased activation of the NLRP3-inflammasome and increased levels of inflammasome-dependent cytokines, such as IL-1β and IL-18 (8, 10, 11). Animal studies suggest that NLRP3-inflammasome activation is further enhanced by mechanical damage caused by artificial ventilation (42). DPPC and other phosphatidylcholines have been shown before to inhibit the response of human macrophage cell lines to various pro-inflammatory stimuli (28, 30, 31, 43) and they also seem to play a protective role in diverse inflammatory diseases (44–47). These effects were shown to be, at least in part, due to incorporation of phosphatidylcholine into the cell membrane and to alterations of the functionally critical localization of TLR4 in lipid rafts (30, 31, 43, 47).
In this study, we provide evidence that DPPC triggers a different mechanism of action in human monocytic cells, which closely resembles a cholinergic mechanism recently discovered by our group (19, 23). DPPC, much like PC and conventional nicotinic agonists (19), inhibited ATP-dependent activation of IL-1β release, but DPPC was ineffective when the activation of the NLRP3 inflammasome was triggered by the pore-forming toxin, nigericin. We demonstrated before that nicotine and PC completely inhibit BzATP-induced ion channel functions at ATP receptor, P2X7, in human monocytic cells (19, 23). Our experiments revealed that DPPC also efficiently inhibits BzATP-induced ion currents and, consequently, inflammasome assembly, activation of caspase-1, and maturation of IL-1β. In contrast to DPPC, other related lipids devoid of a PC group did not impair IL-1β release, suggesting that the efficiency of DPPC depends on the presence of the PC head group.
The inhibitory effect of DPPC on ATP-mediated IL-1β release by U937 cells was sensitive to the nicotinic antagonists, mecamylamine, α-bungarotoxin, and strychnine, suggesting that one or more members of the evolutionarily conserved nAChR family comprising subunits α7, α9, and α10 are involved (48). α-Conotoxin RgIA4, which is a specific antagonist of human α9-containing nAChR, also reverted the effect of DPPC, whereas the α7-specific α-conotoxin, ArIB[V11L,V16D], was ineffective. These results prompted us to silence nAChR expression by siRNA technology. When subunits α7, α9, and α10 were silenced individually, only silencing of α9 blunted the effect of DPPC. In contrast, whenever two of these subunits were silenced concomitantly, the inhibitory effect of DPPC was impaired. We conclude from the pharmacological and the siRNA data that nAChR subunit α9 is essential for signaling of DPPC, whereas subunits α7 and α10 seem to be functionally interchangeable.
The functional combination of nAChR subunits α7 and α9 is unexpected, as subunits α7 and α9 are known to form homopentamers (49). In addition, α9 forms heteropentamers together with α10, a subunit that requires additional subunits to form a functional receptor (50, 51). Rather exotic combinations of α7 and α10 have also been described (52), but no functional combination of subunits α7 and α9. In monocytic cells and in leukocytes in general, nAChRs do not function as ligand-gated ion channels, but exert different metabotropic functions (19–22, 53, 54). Accordingly, DPPC also did not provoke ion current changes in U937 cells.
The structure of these noncanonical nAChRs is still enigmatic and we do not know if they form multimers at all. In contrast to classic nicotinic agonists, PC does not provoke ion channel functions at conventional nAChRs (19, 23). Considering the structural similarity between the head group of DPPC and PC, we speculate that DPPC also exclusively activates metabotropic functions at nAChRs expressed by immune cells, but does not activate canonical ionotropic functions. However, the physicochemical properties of the larger amphiphilic DPPC are strikingly distinct from PC and from classical agonists of nAChRs.
U937 cells, like every cell line, do not necessarily represent the properties of the cells from which they were derived. Therefore, we investigated freshly isolated PBMCs from healthy nonsmoking male donors. In these cells, the process of cell isolation and cell culturing was sufficient to induce the expression of pro-IL-1β and IL-1β was released in response to BzATP, as described previously (19, 55). As expected, treatment of these cells with LPS further increased IL-1β levels. In these primary cells, DPPC efficiently inhibited BzATP-induced inflammasome activation and maturation of caspase-1 and IL-1β, but not IL-1β secretion, as mature IL-1β was not detected in lysates of PBMCs that were stimulated with BzATP in the presence of DPPC.
DPPC or other phosphatidylcholines might be effective for the treatment of inflammatory diseases involving ATP-induced inflammasome activation. Numerous preclinical and clinical studies have investigated the use of various surfactant formulations in the context of ARDS. As the results were promising, but overall very diverse (56), this treatment never reached the clinical arena. Recently, however, a surfactant preparation rich in DPPC was highly protective in pediatric ARDS patients when combined with an alveolar recruitment maneuver (57). Hence, the use of surfactant containing high concentrations of DPPC deserves further investigation in a subpopulation of ARDS patients that are exposed to additional mechanical lung damage that results in ATP-dependent inflammasome activation. In addition, phosphatidylcholines were very efficient in a multi-center study for the treatment of ulcerative colitis, an inflammatory gut disease (58). We speculate that beneficial effects of phosphatidylcholines are at least in part due to the anti-inflammatory mechanism discovered in this study.
In conclusion, we describe a novel anti-inflammatory function of DPPC, the main lipid constituent of pulmonary surfactant that efficiently inhibits ATP receptor signaling and, hence, ATP-mediated release of IL-1β by monocytic cells. We discovered a novel anti-inflammatory mechanism of surfactant and suggest that DPPC might be effective for the treatment of various inflammatory diseases. In addition, we describe for the first time that the phosphatidylcholine, DPPC, functions as an agonist of noncanonical nAChRs expressed by immune cells.
Supplementary Material
Acknowledgments
The authors thank Sabine Stumpf (Department of General and Thoracic Surgery, Laboratory of Experimental Surgery, Justus-Liebig-University Giessen) for excellent experimental support and Prof. Dr. W. Kummer (Institute of Anatomy and Cell Biology, Justus-Liebig-University of Giessen) for thoughtful discussions.
Footnotes
Abbreviations:
- ARDS
- acute respiratory distress syndrome
- BzATP
- 2´(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt
- DPG
- 1,2-dipalmitoyl-sn-glycerol
- DPPC
- 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (dipalmitoylphosphatidylcholine)
- DPPE
- 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine
- IL
- interleukin
- LDH
- lactate dehydrogenase
- LLME
- Leu-Leu methyl ester hydrobromide
- LPS
- lipopolysaccharide
- nAChR
- nicotinic acetylcholine receptor
- NLRP3
- Nod-like receptor protein 3
- PBMC
- peripheral blood mononuclear cell
- PC
- phosphocholine
- POPG
- palmitoyl-oleyl-phosphatidylglycerol
- pro-
- pro-form
- PS
- 1,2-diacyl-sn-glycero-3-phospho-L-serine (phosphatidylserine)
- rSP
- recombinant surfactant protein
- SP
- surfactant protein
- TLR4
- Toll-like receptor 4
This work was supported by a German Centre for Lung Research (DZL) grant (V.G.), German Research Foundation Grant GR 1094/7-1 (V.G.), and National Institutes of Health Grants P01-GM48677 and R01-GM103801 (J.M.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Hiemstra P. S. 2007. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Exp. Lung Res. 33: 537–542. [DOI] [PubMed] [Google Scholar]
- 2.Alkhouri H., Poppinga W. J., Tania N. P., Ammit A., and Schuliga M.. 2014. Regulation of pulmonary inflammation by mesenchymal cells. Pulm. Pharmacol. Ther. 29: 156–165. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya J., and Westphalen K.. 2016. Macrophage-epithelial interactions in pulmonary alveoli. Semin. Immunopathol. 38: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robb C. T., Regan K. H., Dorward D. A., and Rossi A. G.. 2016. Key mechanisms governing resolution of lung inflammation. Semin. Immunopathol. 38: 425–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G. Y., and Nuñez G.. 2010. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello C. A., Simon A., and van der Meer J. W.. 2012. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11: 633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vladimer G. I., Marty-Roix R., Ghosh S., Weng D., and Lien E.. 2013. Inflammasomes and host defenses against bacterial infections. Curr. Opin. Microbiol. 16: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meduri G. U., Headley S., Kohler G., Stentz F., Tolley E., Umberger R., and Leeper K.. 1995. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 107: 1062–1073. [DOI] [PubMed] [Google Scholar]
- 9.Ganter M. T., Roux J., Miyazawa B., Howard M., Frank J. A., Su G., Sheppard D., Violette S. M., Weinreb P. H., Horan G. S., et al. 2008. Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circ. Res. 102: 804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolinay T., Kim Y. S., Howrylak J., Hunninghake G. M., An C. H., Fredenburgh L., Massaro A. F., Rogers A., Gazourian L., Nakahira K., et al. 2012. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am. J. Respir. Crit. Care Med. 185: 1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grailer J. J., Canning B. A., Kalbitz M., Haggadone M. D., Dhond R. M., Andjelkovic A. V., Zetoune F. S., and Ward P. A.. 2014. Critical role for the NLRP3 inflammasome during acute lung injury. J. Immunol. 192: 5974–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luna-Gomes T., Santana P. T., and Coutinho-Silva R.. 2015. Silica-induced inflammasome activation in macrophages: role of ATP and P2X7 receptor. Immunobiology. 220: 1101–1106. [DOI] [PubMed] [Google Scholar]
- 13.Borthwick L. A. 2016. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin. Immunopathol. 38: 517–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S., Suh G. Y., Ryter S. W., and Choi A. M.. 2016. Regulation and function of the nucleotide binding domain leucine-rich repeat-containing receptor, pyrin domain-containing-3 inflammasome in lung disease. Am. J. Respir. Cell Mol. Biol. 54: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross O., Thomas C. J., Guarda G., and Tschopp J.. 2011. The inflammasome: an integrated view. Immunol. Rev. 243: 136–151. [DOI] [PubMed] [Google Scholar]
- 16.Rathinam V. A., Vanaja S. K., and Fitzgerald K. A.. 2012. Regulation of inflammasome signaling. Nat. Immunol. 13: 333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer L. 2014. Complexity of danger: the diverse nature of damage-associated molecular patterns. J. Biol. Chem. 289: 35237–35245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cekic C., and Linden J.. 2016. Purinergic regulation of the immune system. Nat. Rev. Immunol. 16: 177–192. [DOI] [PubMed] [Google Scholar]
- 19.Hecker A., Küllmar M., Wilker S., Richter K., Zakrzewicz A., Atanasova S., Mathes V., Timm T., Lerner S., Klein J., et al. 2015. Phosphocholine-modified macromolecules and canonical nicotinic agonists inhibit ATP-induced IL-1β release. J. Immunol. 195: 2325–2334. [DOI] [PubMed] [Google Scholar]
- 20.Hecker A., Lips K. S., Pfeil U., Zakrzewicz A., Wilker S., Padberg W., Wessler I., Kummer W., and Grau V.. 2009. Pivotal Advance: up-regulation of acetylcholine synthesis and paracrine cholinergic signaling in intravascular transplant leukocytes during rejection of rat renal allografts. J. Leukoc. Biol. 86: 13–22. [DOI] [PubMed] [Google Scholar]
- 21.Mikulski Z., Hartmann P., Jositsch G., Zasłona Z., Lips K. S., Pfeil U., Kurzen H., Lohmeyer J., Clauss W. G., Grau V., et al. 2010. Nicotinic receptors on rat alveolar macrophages dampen ATP-induced increase in cytosolic calcium concentration. Respir. Res. 11: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawashima K., Fujii T., Moriwaki Y., Misawa H., and Horiguchi K.. 2015. Non-neuronal cholinergic system in regulation of immune function with a focus on α7 nAChRs. Int. Immunopharmacol. 29: 127–134. [DOI] [PubMed] [Google Scholar]
- 23.Richter K., Mathes V., Fronius M., Althaus M., Hecker A., Krasteva-Christ G., Padberg W., Hone A. J., McIntosh J. M., Zakrzewicz A., et al. 2016. Phosphocholine – an agonist of metabotropic but not of ionotropic functions of α9-containing nicotinic acetylcholine receptors. Sci. Rep. 6: 28660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Rodriguez E., and Pérez-Gil J.. 2014. Structure-function relationships in pulmonary surfactant membranes: from biophysics to therapy. Biochim. Biophys. Acta. 1838: 1568–1585. [DOI] [PubMed] [Google Scholar]
- 25.Lang C. J., Postle A. D., Orgeig S., Possmayer F., Bernhard W., Panda A. K., Jürgens K. D., Milsom W. K., Nag K., and Daniels C. B.. 2005. Dipalmitoylphosphatidylcholine is not the major surfactant phospholipid species in all mammals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289: R1426–R1439. [DOI] [PubMed] [Google Scholar]
- 26.Chroneos Z. C., Sever-Chroneos Z., and Shepherd V. L.. 2010. Pulmonary surfactant: an immunological perspective. Cell. Physiol. Biochem. 25: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glasser S. W., Witt T. L., Senft A. P., Baatz J. E., Folger D., Maxfield M. D., Akinbi H. T., Newton D. A., Prows D. R., and Korfhagen T. R.. 2009. Surfactant protein C-deficient mice are susceptible to respiratory syncytial virus infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 297: L64–L72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gille C., Spring B., Bernhard W., Gebhard C., Basile D., Lauber K., Poets C. F., and Orlikowsky T. W.. 2007. Differential effect of surfactant and its saturated phosphatidylcholines on human blood macrophages. J. Lipid Res. 48: 307–317. [DOI] [PubMed] [Google Scholar]
- 29.Kuronuma K., Mitsuzawa H., Takeda K., Nishitani C., Chan E. D., Kuroki Y., Nakamura M., and Voelker D. R.. 2009. Anionic pulmonary surfactant phospholipids inhibit inflammatory responses from alveolar macrophages and U937 cells by binding the lipopolysaccharide-interacting proteins CD14 and MD-2. J. Biol. Chem. 284: 25488–25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abate W., Alghaithy A. A., Parton J., Jones K. P., and Jackson S. K.. 2010. Surfactant lipids regulate LPS-induced interleukin-8 production in A549 lung epithelial cells by inhibiting translocation of TLR4 into lipid raft domains. J. Lipid Res. 51: 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonks A., Parton J., Tonks A. J., Morris R. H., Finall A., Jones K. P., and Jackson S. K.. 2005. Surfactant phospholipid DPPC downregulates monocyte respiratory burst via modulation of PKC. Am. J. Physiol. Lung Cell. Mol. Physiol. 288: L1070–L1080. [DOI] [PubMed] [Google Scholar]
- 32.Morris R. H., Tonks A. J., Jones K. P., Ahluwalia M. K., Thomas A. W., Tonks A., and Jackson S. K.. 2008. DPPC regulates COX-2 expression in monocytes via phosphorylation of CREB. Biochem. Biophys. Res. Commun. 370: 174–178. [DOI] [PubMed] [Google Scholar]
- 33.Spragg R. G., Lewis J. F., Wurst W., Häfner D., Baughman R. P., Wewers M. D., and Marsh J. J.. 2003. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am. J. Respir. Crit. Care Med. 167: 1562–1566. [DOI] [PubMed] [Google Scholar]
- 34.McIntosh J. M., inventor. Conotoxin peptides, pharmaceutical compositions and uses thereof. Patent WO2014194284A1. December 4, 2014. (Google Patents, 2014). https://www.google.com/patents/WO2014194284A1?cl=en.
- 35.Whiteaker P., Christensen S., Yoshikami D., Dowell C., Watkins M., Gulyas J., Rivier J., Olivera B. M., and McIntosh J. M.. 2007. Discovery, synthesis, and structure activity of a highly selective alpha7 nicotinic acetylcholine receptor antagonist. Biochemistry. 46: 6628–6638. [DOI] [PubMed] [Google Scholar]
- 36.Innocent N., Livingstone P. D., Hone A., Kimura A., Young T., Whiteaker P., McIntosh J. M., and Wonnacott S.. 2008. Alpha-conotoxin arenatus IB[V11L,V16D] [corrected] is a potent and selective antagonist at rat and human native alpha7 nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 327: 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moser N., Mechawar N., Jones I., Gochberg-Sarver A., Orr-Urtreger A., Plomann M., Salas R., Molles B., Marubio L., Roth U., et al. 2007. Evaluating the suitability of nicotinic acetylcholine receptor antibodies for standard immunodetection procedures. J. Neurochem. 102: 479–492. [DOI] [PubMed] [Google Scholar]
- 38.Rommel F. R., Raghavan B., Paddenberg R., Kummer W., Tumala S., Lochnit G., Gieler U., and Peters E. M.. 2015. Suitability of nicotinic acetylcholine receptor α7 and muscarinic acetylcholine receptor 3 antibodies for immune detection: evaluation in murine skin. J. Histochem. Cytochem. 63: 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kao Y. C., and Lichtenberger L. M.. 1991. Phospholipid- and neutral lipid-containing organelles of rat gastroduodenal mucous cells. Possible origin of the hydrophobic mucosal lining. Gastroenterology. 101: 7–21. [DOI] [PubMed] [Google Scholar]
- 40.Lichtenberger L. M. 1995. The hydrophobic barrier properties of gastrointestinal mucus. Annu. Rev. Physiol. 57: 565–583. [DOI] [PubMed] [Google Scholar]
- 41.Dushianthan A., Cusack R., Goss V., Postle A. D., and Grocott M. P.. 2012. Clinical review: exogenous surfactant therapy for acute lung injury/acute respiratory distress syndrome–where do we go from here? Crit. Care. 16: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones H. D., Crother T. R., Gonzalez-Villalobos R. A., Jupelli M., Chen S., Dagvadorj J., Arditi M., and Shimada K.. 2014. The NLRP3 inflammasome is required for the development of hypoxemia in LPS/mechanical ventilation acute lung injury. Am. J. Respir. Cell Mol. Biol. 50: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treede I., Braun A., Sparla R., Kühnel M., Giese T., Turner J. R., Anes E., Kulaksiz H., Füllekrug J., Stremmel W., et al. 2007. Anti-inflammatory effects of phosphatidylcholine. J. Biol. Chem. 282: 27155–27164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erös G., Ibrahim S., Siebert N., Boros M., and Vollmar B.. 2009. Oral phosphatidylcholine pretreatment alleviates the signs of experimental rheumatoid arthritis. Arthritis Res. Ther. 11: R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider H., Braun A., Füllekrug J., Stremmel W., and Ehehalt R.. 2010. Lipid based therapy for ulcerative colitis—modulation of intestinal mucus membrane phospholipids as a tool to influence inflammation. Int. J. Mol. Sci. 11: 4149–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajaie S., and Esmaillzadeh A.. 2011. Dietary choline and betaine intakes and risk of cardiovascular diseases: review of epidemiological evidence. ARYA Atheroscler. 7: 78–86. [PMC free article] [PubMed] [Google Scholar]
- 47.Stremmel W., Ehehalt R., Staffer S., Stoffels S., Mohr A., Karner M., and Braun A.. 2012. Mucosal protection by phosphatidylcholine. Dig. Dis. 30 (Suppl. 3): 85–91. [DOI] [PubMed] [Google Scholar]
- 48.Baker E. R., Zwart R., Sher E., and Millar N. S.. 2004. Pharmacological properties of alpha 9 alpha 10 nicotinic acetylcholine receptors revealed by heterologous expression of subunit chimeras. Mol. Pharmacol. 65: 453–460. [DOI] [PubMed] [Google Scholar]
- 49.Elgoyhen A. B., Johnson D. S., Boulter J., Vetter D. E., and Heinemann S. F.. 1994. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 79: 705–715. [DOI] [PubMed] [Google Scholar]
- 50.Elgoyhen A. B., Vetter D. E., Katz E., Rothlin C. V., Heinemann S. F., and Boulter J.. 2001. Alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc. Natl. Acad. Sci. USA. 98: 3501–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vincler M., Wittenauer S., Parker R., Ellison M., Olivera B. M., and McIntosh J. M.. 2006. Molecular mechanism for analgesia involving specific antagonism of alpha9alpha10 nicotinic acetylcholine receptors. Proc. Natl. Acad. Sci. USA. 103: 17880–17884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lips K. S., König P., Schätzle K., Pfeil U., Krasteva G., Spies M., Haberberger R. V., Grando S. A., and Kummer W.. 2006. Coexpression and spatial association of nicotinic acetylcholine receptor subunits alpha7 and alpha10 in rat sympathetic neurons. J. Mol. Neurosci. 30: 15–16. [DOI] [PubMed] [Google Scholar]
- 53.Peng H., Ferris R. L., Matthews T., Hiel H., Lopez-Albaitero A., and Lustig L. R.. 2004. Characterization of the human nicotinic acetylcholine receptor subunit alpha (α) 9 (CHRNA9) and alpha (α) 10 (CHRNA10) in lymphocytes. Life Sci. 76: 263–280. [DOI] [PubMed] [Google Scholar]
- 54.Razani-Boroujerdi S., Boyd R. T., Dávila-García M. I., Nandi J. S., Mishra N. C., Singh S. P., Pena-Philippides J. C., Langley R., and Sopori M. L.. 2007. T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J. Immunol. 179: 2889–2898. [DOI] [PubMed] [Google Scholar]
- 55.Grahames C. B., Michel A. D., Chessell I. P., and Humphrey P. P.. 1999. Pharmacological characterization of ATP- and LPS-induced IL-1beta release in human monocytes. Br. J. Pharmacol. 127: 1915–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davidson W. J., Dorscheid D., Spragg R., Schulzer M., Mak E., and Ayas N. T.. 2006. Exogenous pulmonary surfactant for the treatment of adult patients with acute respiratory distress syndrome: results of a meta-analysis. Crit. Care. 10: R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodríguez-Moya V. S., Gallo-Borrero C. M., Santos-Áreas D., Prince-Martínez I. A., Díaz-Casañas E., and López-Herce Cid J.. Exogenous surfactant and alveolar recruitment in the treatment of the acute respiratory distress syndrome. Clin. Respir. J. Epub ahead of print. February 16, 2016; doi:10.1111/crj.12462. [DOI] [PubMed] [Google Scholar]
- 58.Karner M., Kocjan A., Stein J., Schreiber S., von Boyen G., Uebel P., Schmidt C., Kupcinskas L., Dina I., Zuelch F., et al. 2014. First multicenter study of modified release phosphatidylcholine “LT-02” in ulcerative colitis: a randomized, placebo-controlled trial in mesalazine-refractory courses. Am. J. Gastroenterol. 109: 1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.