Abstract
The nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 (NLRP3) inflammasome has been implicated in podocyte injury and glomerular sclerosis during hyperhomocysteinemia (hHcys). However, it remains unclear whether the NLRP3 inflammasome can be a therapeutic target for treatment of hHcys-induced kidney injury. Given that DHA metabolites-resolvins have potent anti-inflammatory effects, the present study tested whether the prototype, resolvin D1 (RvD1), and 17S-hydroxy DHA (17S-HDHA), an intermediate product, abrogate hHcys-induced podocyte injury by targeting the NLRP3 inflammasome. In vitro, confocal microscopy demonstrated that 17S-HDHA (100 nM) and RvD1 (60 nM) prevented Hcys-induced formation of NLRP3 inflammasomes, as shown by reduced colocalization of NLRP3 with apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) or caspase-1. Both DHA metabolites inhibited Hcys-induced caspase-1 activation and interleukin-1β production. However, DHA had no significant effect on these Hcys-induced changes in podocytes. In vivo, DHA lipoxygenase metabolites substantially inhibited podocyte NLRP3 inflammasome formation and activation and consequent glomerular sclerosis in mice with hHcys. Mechanistically, RvD1 and 17S-HDHA were shown to suppress Hcys-induced formation of lipid raft redox signaling platforms and subsequent O2·− production in podocytes. It is concluded that inhibition of NLRP3 inflammasome activation is one of the important mechanisms mediating the beneficial action of RvD1 and 17S-HDHA on Hcys-induced podocyte injury and glomerular sclerosis
Keywords: inflammation, lipid mediators, ω-3 fatty acid, lipoxygenase, kidney, podocyte, lipid rafts, nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3
Elevated levels of plasma homocysteine (Hcys), namely, hyperhomocysteinemia (hHcys), have been reported as an important independent risk factor in the development of numerous chronic diseases, including metabolic disorders, cardiovascular dysfunction, neurological diseases, and many complications associated with aging (1–3). Increasing evidence also indicates a crucial role of hHcys in the progression of end-stage renal disease (ESRD) associated with various systemic pathological processes or local kidney diseases, such as hypertension, diabetic mellitus, and other chronic kidney diseases (4). Over the last 20 years, many studies have shown that the pathogenic effects of hHcys to cause ESRD may be associated with local inflammation, oxidative stress, altered cell metabolism or function, and consequent fibrogenesis (5–10). Recent studies have indicated that the inflammasome of the nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 (NLRP3) is activated in podocytes during hHcys, which is a central mechanism sensing and amplifying redox signals to produce podocyte injury and ultimate glomerular sclerosis. NLRP3 in podocytes serves as a sensor in response to different detrimental factors, such as Hcys and redox signals, to activate inflammasomes. Activated NLRP3 inflammasomes in podocytes can switch on inflammatory response, leading to recruitment or aggregation of inflammatory cells in glomeruli, such as macrophages or T-cells, as well as resulting in large production of various cytokines or chemokines. In addition to instigation of such classical sterile inflammatory response, activation of this inflammasome by hHcys also led to interference with the synthesis of podocyte-specific proteins, increased production of damage-associated molecular patterns, and triggering of pyroptosis, which is often referred to as a noncanonical effect of NLRP3 inflammasomes. Such noncanonical effects during inflammasome activation answered a long-lasting question of why many classic anti-inflammatory medicines, such as commonly used indole and arylpropionic acid derivatives, could not efficiently prevent or reverse glomerular sclerosis under many pathological conditions, such as hHcys, hypertension, and diabetes mellitus. It is because these classical anti-inflammatory strategies may not target the noninflammatory or noncanonical effects of inflammasome activation (11, 12). Therefore, it is imperative to develop novel therapeutic strategies to target the NLRP3 inflammasome, the root of both inflammatory response and direct cell injury, for treatment or prevention of glomerular injury and sclerosis, and ultimately ESRD.
DHA, a well-known ω-3 polyunsaturated fatty acid, especially at high dose of supplementation, has been reported to suppress inflammation and exert a beneficial action in numerous inflammatory human diseases (13, 14). Although DHA (in a form of fish oil) is widely used for prevention and treatment of chronic inflammatory disorders, its anti-inflammatory mechanisms remain poorly understood. Recent studies have indicated that a series of DHA metabolites, via lipoxygenase (LOX), such as resolvins and protectins, mediate the major role of DHA in the protection of tissue or organs against inflammation (15–18). Although these metabolites of DHA have originally been found to increase the resolution of inflammation, there is increasing evidence that they also prevent the activation of inflammatory responses (19, 20). However, it remains unknown how these DHA metabolites inhibit the activation of inflammatory response and whether they can be used to prevent hHcys-induced glomerular inflammation and sclerosis.
The present study was designed to investigate whether the prototype of DHA metabolites via LOX, resolvin D1 (RvD1), and 17S-hydroxy DHA (17S-HDHA), an intermediate product, abrogates hHcys-induced podocyte injury by inhibition of the NLRP3 inflammasome activation. In cultured podocytes, we first examined the effects of RvD1 and 17S-HDHA on Hcys-induced NLRP3 inflammasome activation and cell injury. Then, we went on to determine the beneficial action of DHA metabolites by LOX to prevent podocyte NLRP3 inflammasome activation and glomerular sclerosis in mice with hHcys. We also explored the possible mechanism by which these DHA metabolites suppress NLRP3 inflammasome activation with a focus on their inhibition of lipid raft (LR) redox signaling platforms. We demonstrate that DHA metabolites via LOX indeed exert their beneficial actions on hHcys-induced podocyte injury and glomerular sclerosis by inhibition of NLRP3 inflammasome activation.
MATERIALS AND METHODS
Cell culture
Conditionally immortalized mouse podocytes, kindly provided by Dr. P. E. Klotman (Division of Nephrology, Department of Medicine, Mount Sinai School of Medicine, New York, NY), were cultured and maintained as described previously (7). Briefly, they were grown at the permissive temperature (33°C) on collagen I-coated flasks or plates in RPMI 1640 medium supplemented with 10% fetal bovine serum and 10 U/ml recombinant mouse interferon-γ. The podocytes were then passaged and allowed to differentiate at 37°C for 10–14 days without interferon-γ before use in the experiments described below. Podocytes were treated with L-Hcys (40 μM, prepared in water) for 24 h and the dose and treatment time were optimized in earlier studies (7). The in vitro treatment doses of DHA, RvD1, and 17S-HDHA were based on previous studies (21–23).
Animals
Eight-week-old male C57BL/6J WT and NLRP3 KO mice were used in the present study. To speed up the damaging effects of hHcys on glomeruli, all mice were uninephrectomized, as we described previously (24). This model has been demonstrated to induce glomerular damage unrelated to the uninephrectomy and arterial blood pressure, but specific to hHcys. After a 1 week recovery period from uninephrectomy, mice were fed either normal chow or folate-free (FF) diet (Dyets Inc., Bethlahem, PA) for 6 weeks. At the same time, different groups of mice received DHA at 50 mg/kg/day by oral gavage (25) and cinnamyl-3,4-dihydroxy-cyanocinnamate (CDC), a LOX inhibitor, at 6 mg/kg/day by daily intraperitoneal injection (26). All protocols were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
Isolation of glomeruli from mouse kidneys
In another series of experiments, we treated mice as described above. Then, these mice were used to isolate their glomeruli of the kidney for measurements of interleukin (IL)-1β and IL-18, as described previously (27, 28). In brief, the mice were anesthetized with 2% isoflurane and then the kidney was perfused with ice-cold PBS and harvested. After blood samples were taken, the mice were euthanized. The harvested kidneys were hemisected on a sagittal plane and the renal cortex was separated from the medulla, chopped into fine pieces, and passed through filters with decreasing pore sizes from 150 to 106 μm (USA standard sieve numbers 100 and 140, respectively; Thermo Fisher Scientific, Waltham, MA) into a petri dish. The glomeruli were captured on a 70 μm cell strainer (BD Biosciences, San Jose, CA), then washed off from the sieve with ice-cold Hank’s solution containing 6% BSA, and pelleted for later use to measure IL-1β and IL-18 levels.
HPLC analysis
Plasma and renal tissue Hcys levels were measured by HPLC method as described previously (9). One hundred microliters of plasma, samples, or standard solution mixed with 10 μl of internal standard, thioglycolic acid (2.0 mmol/l) solution, were treated with 10 μl of 10% tri-n-butylphosphine solution in dimethylformamide at 4°C for 30 min. Then, 80 μl of ice-cold 10% TCA in 1 mmol/l EDTA were added and centrifuged to remove proteins in the sample. One hundred microliters of the supernatant were transferred into the mixture of 20 μl of 1.55 mol/l sodium hydroxide, 250 μl of 0.125 mol/l borate buffer (pH 9.5), and 100 μl of 1.0 mg/ml ABD-F solution. The resulting mixture was incubated at 60°C for 30 min to accomplish derivatization of thiols. HPLC was performed with a HP 1100 series equipped with a binary pump, a vacuum degasser, a thermostatted column compartment, and an autosampler (Agilent Technologies, Waldbronn, Germany). Separation was carried out at an ambient temperature on an analytical column, Supelco LC-18-DB (1,504.6 mm ID, 5 m) with a Supelcosil LC-18 guard column (204.6 mm ID, 5 m). Fluorescence intensities were measured with an excitation wavelength of 385 nm and an emission wavelength of 515 nm by a Hewlett-Packard model 1046A fluorescence spectrophotometer. The peak area of the chromatographs was quantified with a Hewlett-Packard 3392 integrator. The analytical column was eluted with 0.1 mol/l potassium dihydrogen phosphate buffer (pH 2.1) containing 6% acetonitrile (v/v) as the mobile phase with a flow rate of 2.0 ml/min.
Immunofluorescence microscopy
Double-immunofluorescence staining was performed using cultured podocytes grown on collagen-coated glass cover slips and frozen mouse kidney sections, as described previously (8). Briefly, after fixation, the cells were incubated with goat anti-NLRP3 (Abcam, Cambridge, MA), followed by incubation with Alexa 488-labeled donkey anti-goat secondary antibody (1:200; Life Technologies, Carlsbad, CA). Then, rabbit anti-apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) or rabbit anti-caspase-1 (1:100; Santa Cruz Biotechnology Inc., Santa Cruz, CA) was added to the cell slides and then incubated overnight at 4°C. To examine the distribution of F-actin fibers in podocytes, F-actin was stained with rhodamine-phalloidin (Invitrogen, Carlsbad, CA) for 15 min at room temperature, as described previously (7). Frozen mouse kidneys were also fixed in acetone, blocked, and then incubated with the same aforementioned primary antibodies overnight at 4°C. Some coverslips with podocytes and frozen kidney sections were only stained for podocyte markers, podocin (1:100; Sigma) or desmin (1:100; BD Biosciences). Double immunofluorescent staining was performed by Alexa Fluor 488- or Alexa Fluor 555-labeled secondary antibody (1:200; Invitrogen) incubation for 1 h at room temperature. Slides were then washed, mounted, and observed using a confocal laser scanning microscope (Fluoview FV1000, Olympus, Japan) and Image Pro Plus software (version 6.0; Media Cybernetics, Bethesda, MD) was used to analyze colocalization, which was expressed as the Pearson correlation coefficient.
ELISA assay
Caspase-1 activity (Biovision, Mountain View, CA) and RvD1 level (Cayman Chemical Co., Ann Arbor, MI) were measured by commercially available colorimetric assay kits, while the levels of IL-1β (R&D Systems, Minneapolis, MN), IL-18 (Thermo Fisher Scientific), and vascular endothelial growth factor (VEGF)-A (R&D Systems) were measured in the supernatant of cultured podocytes or homogenates of isolated glomeruli from WT mice using an enzyme-linked immunosorbent assay kit according to manufacturer’s instructions.
Immunohistochemistry
Kidneys were embedded with paraffin and 5 mm sections were cut from the embedded blocks. After heat-induced antigen retrieval, washing with 3% H2O2, and 30 min blocking with fetal bovine serum, slides were incubated with primary antibody diluted in PBS with 4% fetal bovine serum. Anti-IL-1β antibody (Abcam) and anti-IL-18 antibody (Santa Cruz Biotechnology, Dallas, TX) were used as primary antibodies in this study. After incubation with primary antibody overnight, the sections were washed in PBS and incubated with biotinylated IgG (1:200) for 1 h and then with streptavidin-HRP for 30 min at room temperature. Fifty microliters of DAB were added to each kidney section and stained for 1 min. After washing, the slides were counterstained with hematoxylin for 5 min. The slides were then mounted and observed under a microscope in which photos were taken (29).
Urinary protein and albumin measurements
Total urinary protein concentration was determined spectrophotometrically using the Bradford assay (Sigma) and urinary albumin concentration was measured using a commercially available mouse albumin ELISA kit (Bethyl Laboratories, Montgomery, TX) (29).
Glomerular morphological examinations
Fixed kidney tissues were paraffin-embedded, sectioned, and stained with periodic acid-Schiff and picrosirius red. Using a light microscope, glomerular morphology was observed and assessed semiquantitatively, as described in detail previously (6, 30).
Statistical analysis
All of the values are expressed as mean ± SEM. Significant differences among multiple groups were examined using ANOVA followed by a Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
RESULTS
Effects of DHA metabolites on inflammasome formation in podocytes
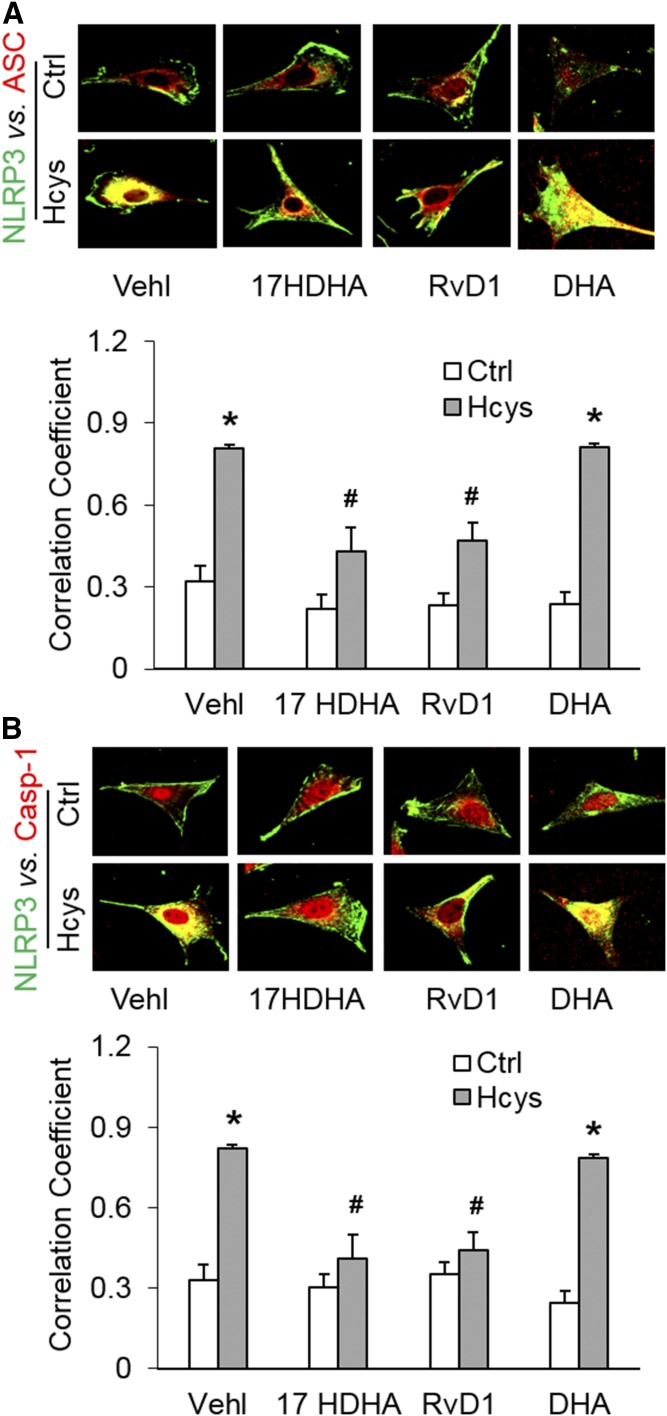
We first tested to determine whether DHA LOX metabolites, 17S-HDHA or RvD1, blocked Hcys-induced inflammasome formation in podocytes. By using confocal microscopy, we demonstrated that colocalization of NLRP3 (green) with ASC (red) or caspase-1 (red) was much higher in Hcys-treated podocytes compared with vehicle-treated podocytes, indicating enhanced formation of NLRP3 inflammasomes. However, prior treatment of podocytes with 17S-HDHA or RvD1 significantly attenuated or totally blocked Hcys-induced colocalization of NLRP3 with ASC or caspase-1. In contrast, DHA only had a slight effect to diminish the inflammasome formation induced by Hcys. The colocalization coefficient analyzed by a computer program was summarized and is shown below the representative confocal microscopic images (Fig. 1).
Fig. 1.
Effects of DHA metabolites, via LOX, on NRLP3 inflammasome formation in podocytes. Podocytes were cultured for 24 h with Hcys (40 μM) in the absence or presence of 17S-HDHA (17HDHA; 100 nM), RvD1 (60 nM), or DHA (20 μM) as indicated. A: Representative images and summarized data of the colocalization between NLRP3 (green) and ASC (red) in different podocytes, which were cultured and treated on different days (n = 6). B: Representative images and summarized data of the colocalization between NLRP3 (green) and caspase-1 (red) in different podocytes which were cultured and treated on different days (n = 6). *P < 0.05 versus Ctrl-Vehl group, #P < 0.05 versus Hcys-Vehl group. Ctrl, control; Vehl, vehicle; 17S-HDHA, 17-hydroxy docosahexaenoic acid; DHA, ω-3 fatty acid docosahexaenoic acid.
The lack of DHA effects on Hcys-induced inflammasome activation may be due to the inability of podocytes to convert DHA into RvD1. It was shown that even increasing DHA concentrations to 40 and 80 μM did not have significant effects on Hcys-induced caspase-1 activation (supplemental Fig. S1A). By ELISA assay, we found that podocytes could not produce RvD1 when they were incubated with as much as 80 μM DHA. Using the same assay, however, white blood cells isolated from C57BL/6J WT mouse blood produced a large amount of RvD1, 15-fold more than that in podocytes (supplemental Fig. S1B). These results suggest that podocytes may not metabolize DHA into RvD1 and other related metabolites.
Blockade of NLRP3 inflammasome activation by DHA metabolites in podocytes
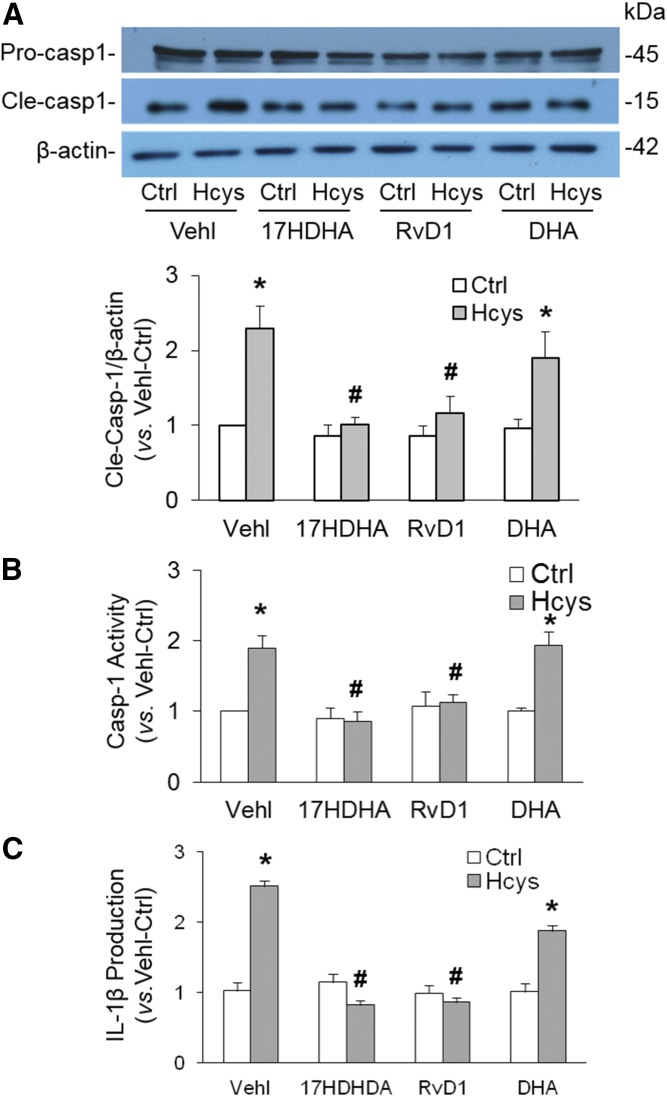
Previous studies have indicated that activation of NLRP3 inflammasomes is reflected as caspase-1 activation and IL-1β production. In the present study, we determined caspase-1 activity and IL-1β production in podocytes with or without prior treatment with 17S-HDHA or RvD1. As shown in Fig. 2A, Hcys treatment significantly increased the level of active caspase-1 (15 kDa), but not pro-caspase-1, indicating increased cleavage of pro-caspase-1 into active caspase-1, an important parameter of inflammasome activation. We also found that prior treatment of podocytes with either 17S-HDHA or DHA markedly attenuated Hcys-induced increases in the active caspase-1 level. Consistently, biochemical analysis showed that caspase-1 activity (Fig. 2B) and IL-1β production (Fig. 2C) significantly increased in Hcys-treated podocytes compared with vehicle-treated podocytes, which was significantly attenuated by prior treatment of these cells with either 17S-HDHA or RvD1. Additionally, we demonstrated that DHA only had slight effects on Hcys-induced NLRP3 inflammasome activation in podocytes, as shown by all measurements described above.
Fig. 2.
Blockade of NLRP3 inflammasome activation by DHA metabolites in podocytes. A: Representative Western blot gel document and summarized data showing the protein expression of pro-caspase-1 and active caspase-1 in different podocytes that were cultured and treated on different days (n = 7). B: Caspase-1 activity in different podocytes that were cultured and treated on different days (n = 8). C: IL-1β production in different podocytes that were cultured and treated on different days (n = 9). *P < 0.05 versus Ctrl-Vehl group, #P < 0.05 versus Hcys-Vehl group. Ctrl, control; Vehl, vehicle.
DHA metabolites prevented Hcys-induced podocyte injury
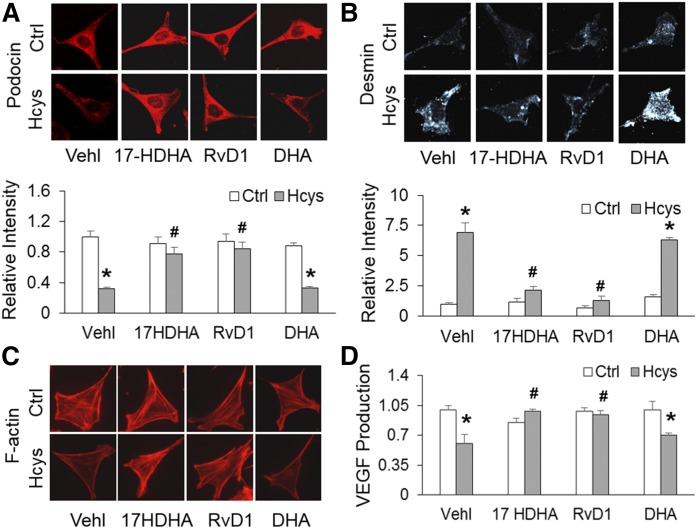
To assess the extent of podocyte damage associated with activation of inflammasomes, podocyte-specific marker, podocin, and its damaging marker, desmin, were examined. Immunofluorescence analysis demonstrated that Hcys-treated podocytes displayed a significant decrease in podocin staining and an increase in desmin staining, indicating podocyte damage (Fig. 3A, B). However, RvD1 and 17S-HDHA were found to restore podocin and diminished desmin to control levels, which was similar to the effects of inflammasome inhibition. Quantification of fluorescence from podocin and desmin staining is summarized below the representative images. Moreover, the actin cytoskeleton organization with fibroblast-like stress fibers extending into the foot processes was analyzed because such stress fibers in cultured podocytes correspond to the filamentous actin in podocyte foot processes in vivo, thus representing differentiation of podocytes. Hcys induced a dramatic disarrangement of F-actin, which was substantially blocked by prior treatment of DHA metabolites (Fig. 3C). Additionally, VEGF secretion is considered as an indicator of podocyte functionality because glomerular VEGF is primarily produced in podocytes. It was found that Hcys-treated podocytes displayed significantly impaired secretion of VEGF, which was restored by prior treatment of these cells with RvD1 and 17S-HDHA. In contrast, DHA did not have a significant effect on Hcys-induced podocyte injuries (Fig. 3D).
Fig. 3.
DHA LOX metabolites prevented Hcys-induced podocyte injury. A: Representative images and summarized data showing the expression of podocin in different podocytes that were cultured and treated on different days (n = 6). B: Representative images and summarized data showing the expression of desmin in different podocytes that were cultured and treated on different days (n = 6). C: Images showing the expression of F-actin fiber in different podocytes that were cultured and treated on different days (n = 5). D: Summarized data showing production of VEGF in different podocytes that were cultured and treated on different days (n = 4). *P < 0.05 versus Ctrl-Vehl group, #P < 0.05 versus Hcys-Vehl group. Ctrl, control; Vehl, vehicle.
DHA metabolites by LOX blocked NLRP3 inflammasome formation and activation in podocytes of hyperhomocysteinemic mice
To further determine whether DHA LOX metabolites display a potential effect to block the NLRP3 inflammasome formation and activation in vivo, LOX inhibitor, CDC, combined with a high dose of DHA were used to treat mice with hHcys. Ten groups of experiments were conducted, including WT mice on the normal diet (ND) or folate free (FF) diet treated with or without DHA and/or CDC, as well as NLRP3 KO mice on the ND or FF diet with or without DHA for 6 weeks.
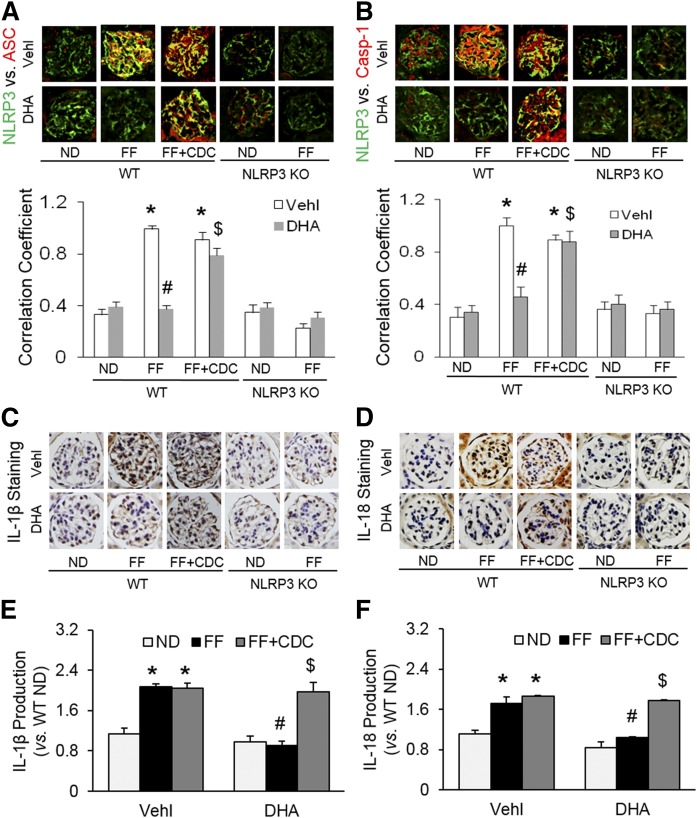
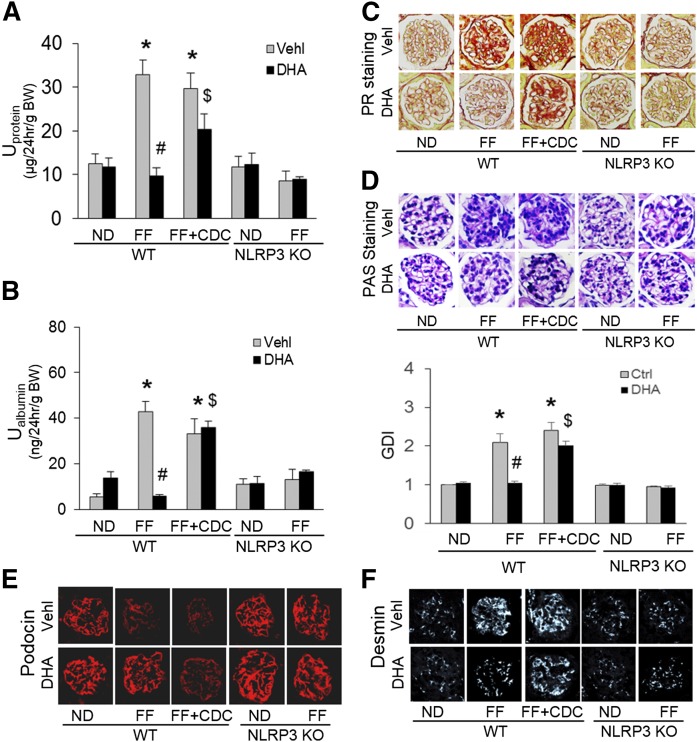
By HPLC analysis, mice on the FF diet for 6 weeks had significantly elevated plasma Hcys concentrations compared with those on the ND. Neither NLRP3 KO nor treatment with DHA or CDC altered the Hcys levels on either diet (supplemental Fig. S2). As depicted in Fig. 4A, B, the glomeruli of WT mice on the FF diet had significantly elevated colocalization of NLRP3 with ASC or caspase-1 compared with ND-fed WT mice, which could be blocked by a large dose of DHA (calculated blood concentration of DHA is about 2.54 mM). This DHA effect was abolished by LOX inhibitor, CDC. In NLRP3 gene KO mice, such inflammasome formation in glomeruli of mice on the FF diet failed. Quantitation of the NLRP3 colocalization by measurement of Pearson’s correlation coefficient is presented below representative confocal microscopic images, showing that NLRP3 inflammasome formation only occurred in mice on the FF diet, but not in mice on the ND. Because our previous studies have shown that NLRP3 molecules are most enriched in podocytes, these results indicate the formation of NLRP3 inflammasomes in glomerular podocytes of mice with hHcys (induced by the FF diet). Consistently, IL-1β production and IL-18 production were significantly increased in mice with hHcys (on the FF diet) compared with mice on the ND (WT), which was blocked by a large dose of DHA treatment (Fig. 4C, D). However, if mice received the LOX inhibitor, CDC, the beneficial effects of DHA on hHcys-induced inflammasome formation and activation were abolished. In NLRP3 KO mice, no increase in the IL-1β or IL-18 levels was detected in glomeruli, even during hHcys. In the homogenates of isolated glomeruli, moreover, the production of both IL-1β and IL-18 was significantly enhanced during hHcys, which was remarkably attenuated by treatment of mice with DHA. However, the effects of DHA were blocked by CDC. These findings further confirm that enhanced production of both IL-1β and IL-18 contribute to hHcys-induced podocyte injury and glomerular sclerosis (Fig. 4E, F).
Fig. 4.
DHA metabolites by LOX blocked NLRP3 inflammasome formation and activation in podocytes of hyperhomocysteinemic mice. A: Representative images and summarized data of the colocalization between NLRP3 (green) and ASC (red) in glomeruli from mice with different genotypes and treatments (n = 6). B: Representative images and summarized data of the colocalization between NLRP3 (green) and caspase-1 (red) in glomeruli from mice with different genotypes and treatments (n = 6). C: Immunohistochemical staining of IL-1β production in glomeruli from mice with different genotypes and treatments (n = 5). D: Immunohistochemical staining of IL-18 production in glomeruli from mice with different genotypes and treatments (n = 5). E: IL-1β production in isolated glomeruli from WT mice with different treatments (n = 4). F: IL-18 production in isolated glomeruli from WT mice with different treatments (n = 4). *P < 0.05 versus WT-ND-Vehl group, #P < 0.05 versus WT-FF-Vehl group, $P < 0.05 versus WT-FF-DHA group. Vehl, vehicle.
Correspondingly, the plasma RvD1 level was found to be markedly higher in mice treated with DHA compared with control mice. CDC cotreatment with DHA significantly reduced the mouse plasma RvD1 level (supplemental Fig. S3). These results further confirm that the beneficial effects of DHA result from the production of RvD1, which may be produced independently of podocytes because they did not convert DHA in in vitro experiments.
DHA metabolites by LOX protected mouse glomeruli from hHcys-induced dysfunction and injury
As shown in Fig. 5, FF diet-induced hHcys in WT mice resulted in marked increases in urinary protein (Fig. 5A) and urinary albumin (Fig. 5B) excretion compared with mice on the ND. Furthermore, these mice with hHcys exhibited abnormal glomerular morphology, characterized as accumulation of extra matrix, collagen deposition, capillary collapse, and mesangial cell expansion (Fig. 5C, D). The glomerular damage index was more than doubled in mice on the FF diet compared with mice on the ND. By confocal microscopy, hHcys was found to markedly alleviate the amount of podocin staining (Fig. 5E) and to increase the fluorescence intensity of desmin staining (Fig. 5F) in glomeruli. However, all these glomerular and podocyte injuries induced by hHcys were substantially blocked by administration of a high dose of DHA, and this protective effect of DHA was significantly suppressed when mice were pretreated with the LOX inhibitor, CDC, which blocks the conversion of DHA into hydroxy metabolites. Additionally, in NLRP3 KO mice, the FF diet-induced hHcys failed to induce podocyte injury and glomerular damage, as shown by no differences in all measured glomerular injurious parameters between mice on the FF diet and ND.
Fig. 5.
DHA metabolites by LOX protected mouse glomeruli from hHcys-induced dysfunction and injury. A: Summarized data showing the urinary protein from mice with different genotypes and treatments (n = 7). B: Summarized data showing the urinary albumin from mice with different genotypes and treatments (n = 7). C: Images showing the glomerular collagen deposition (picrosirius red staining) from mice with different genotypes and treatments (n = 6). D: Representative images and summarized data of the glomerular morphological changes (periodic acid-Schiff staining) from mice with different genotypes and treatments (n = 6). E: Representative images showing immunostained glomeruli for podocin from mice with different genotypes and treatments (n = 6). F: Representative images showing immunostained glomeruli for desmin from mice with different genotypes and treatments (n = 6). *P < 0.05 versus WT-ND-Vehl group, #P < 0.05 versus WT-FF-Vehl group, $P < 0.05 versus WT-FF-DHA group. Vehl, vehicle.
Contribution of LR redox signaling platform formation and activation to the protective effects of DHA metabolites
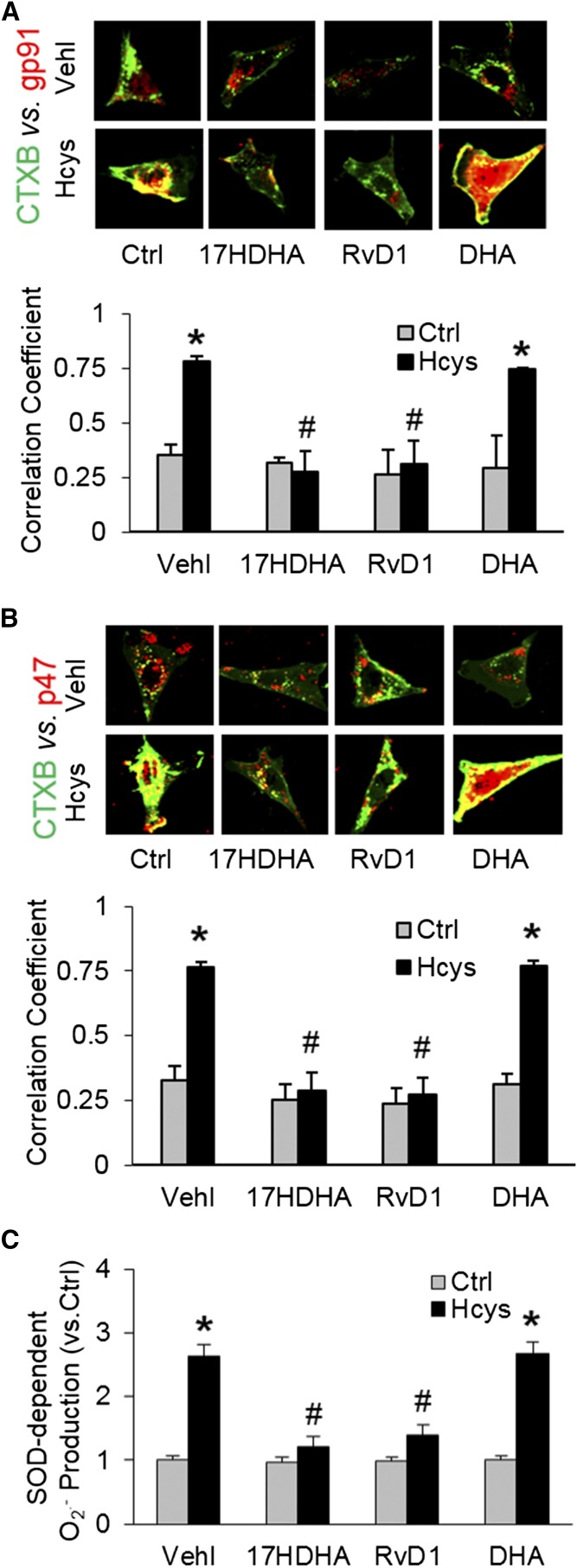
To explore the mechanisms by which DHA metabolites exert their protective action on Hcys-induced podocyte injury, we determined the contribution of LR redox signaling platform formation and activation, given that our previous studies showed that the LR redox signaling platforms are involved in inflammasome activation (7, 8). In these experiments, podocytes were stained with Alexa Fluor 488-labeled CTXB (a LR marker) and anti-gp91 or anti-p47 antibody [NADPH oxidase (NOX) subunits] to detect clustering of LRs with both NOX subunits, an indicator of LR redox signaling platform formation or activation. It was found that Hcys stimulation significantly increased LR clustering with gp91 (Fig. 6A) or p47 (Fig. 6B) NOX subunits, as shown by the yellow patch formation on the podocyte membrane. This LR redox platform formation led to the activation of NOX and subsequent generation of O2·−. As measured by ESR, O2·− production significantly increased when podocytes were treated with Hcys (Fig. 6C). When podocytes were pretreated with RvD1 and 17S-HDHA, the colocalization of these NOX subunits with LRs was blocked compared with podocytes only treated with Hcys. Correspondingly, Hcys-induced O2·− production, as measured by ESR, was also found to be blocked by two DHA metabolites. DHA had only minor effects on Hcys-induced LR clustering and O2·− production in these podocytes.
Fig. 6.
Contribution of LR redox signaling platform formation and activation to the protective effects of DHA metabolites. Podocytes were cultured as described above. A: Representative images and summarized data of the colocalization between LT marker, CTXB (green), with NOX subunit, gp91 (red), in different podocytes that were cultured and treated on different days (n = 6). B: Representative images and summarized data of the colocalization between CTXB (green) with NOX subunit, p47 (red), in different podocytes that were cultured and treated on different days (n = 6). C: Summarized data of SOD-dependent O2·− production in different podocytes that were cultured and treated on different days (n = 6). *P < 0.05 versus Ctrl-Vehl group, #P < 0.05 versus Hcys-Vehl group. Ctrl, control; Vehl, vehicle.
DISCUSSION
The major goal of the present study was to determine whether DHA and its LOX metabolites inhibit NLRP3 inflammasome activation and thereby prevent podocytes and glomeruli from dysfunction and damage during hHcys. In cultured podocytes, we demonstrated that DHA metabolites, via LOX, significantly blocked Hcys-induced NLRP3 inflammasome activation and cell dysfunction in podocytes, but DHA had no significant effect. In mice with hHcys, DHA metabolites from large doses of DHA via LOX were found to inhibit NLRP3 inflammasome activation and to protect glomeruli from hHcys. We also demonstrated that the beneficial effects of LOX-dependent DHA metabolites on NLRP3 inflammasome activation and glomerular injury were attributed to the inhibition of LR redox signaling platform formation and activation. These results together provide the first evidence that inhibition of NLRP3 inflammasome activation is one of the important mechanisms that mediates the beneficial action of DHA and its LOX metabolites.
In 1993, De Caterina et al. (31) reported that ω-3 fatty acids reduce proteinuria in patients with chronic glomerular disease, which may be the first evidence showing the protective role of ω-3 fatty acids in renal disease. In the following decades, many other research groups have found that DHA, as the effective component of fish oil, has protective effects against IgA nephropathy (32, 33), nephrotoxicity, renal tissue injury (34), glomerulonephritis (35), nephrotoxic serum nephritis (36), renal fibrosis (37), and ESRD (38). However, the precise mechanisms by which DHA and its metabolites produce beneficial action remain controversial. For example, there are many reported mechanisms mediating the protective effects of DHA against IgA nephropathy, which include inhibition of mesangial cell activation and proliferation (39), suppression of IL-6 expression as well as ERK 1/2 and JNK 1/2 activation (32, 40), and inhibition of mitogen-activated protein kinase phosphorylation (40). Afterwards, the therapeutic role of DHA in other renal diseases was also studied. There is evidence that DHA exerts antioxidative and anti-inflammatory actions by reducing the cellular levels of reactive oxygen species, pro-inflammatory mediators, and nitrite levels and by maintaining higher glutathione levels and antioxidative enzyme activities (34, 41). In addition, downregulation of the C-reactive protein level is also attributed to the beneficial effects of DHA in some renal diseases, such as nephrotoxicity, renal tissue injury (34), and ESRD (38). However, these proposed mechanisms could not fully explain the beneficial actions of DHA and its metabolites in chronic degenerative diseases, in particular, in chronic kidney disease, and the targets for the action of DHA and its metabolites have yet to be identified.
Recently, the NLRP3 inflammasome, as intracellular inflammatory machinery, has been shown to play a critical role in the development of various chronic degenerative diseases and related complications. These diseases or pathological processes include obesity (42), chronic heart failure (43), Alzheimer’s disease (44), liver cirrhosis (45), arteritis (46), atherosclerosis (47), diabetes mellitus (48), silicosis (49), and ESRD (5, 6, 29). In our recent studies, we have established that NLRP3 inflammasome activation importantly contributes to podocyte injury, glomerular damage, and consequent ESRD during hHcys, which is mainly due to its effects to instigate glomerular sterile inflammatory response and to directly suppress podocyte function (7, 8). These studies led us to wonder whether the NLRP3 inflammasome could be a target for DHA or its metabolites to produce beneficial action on the kidney during hHcys. In the present study, we first characterized the expression and activity of this NLRP3 inflammasome complex in mouse podocytes and determined whether DHA possessed inhibitory effects on Hcys-induced NLRP3 inflammasome activation and protective effects against podocyte injury initiated by NLRP3 inflammasome activation. We found that the formation of NLRP3 inflammasome complex in mouse podocytes was induced after Hcys stimulation and LOX metabolites of DHA, RvD1 and 17S-HDHA, remarkably attenuated this Hcys-induced activation of NLRP3 inflammasomes. To our knowledge, this is the first evidence that NLRP3 inflammasomes can be inhibited in podocytes by DHA metabolites, whereby these cells are protected from Hcys-induced injury.
In previous studies, DHA was shown to attenuate kidney disease via alteration of the IL-1β level (50). However, these studies did not examine the effects of DHA on the molecular machinery for IL-1β production and define corresponding mechanisms mediating the effects of DHA or its metabolites on the production of this cytokine. In other studies, the anti-inflammatory effects of DHA and its LOX products, resolvins, have been studied extensively (51–55) and were shown to not only interfere with production of inflammation, such as preventing the infiltration of neutrophils into sites of inflammation and inhibition of pro-inflammatory cytokine production, such as TNF, IL-1β, and IL-6, but also to promote inflammation resolution. It has been reported that renal production of resolvins and protectins plays an important role in resolution of bilateral ischemia/reperfusion renal injury (56). Administration of resolvins and protectin D1 reduced leukocyte infiltration, blocked TLR-mediated activation of macrophages, and produced an antifibrotic effect. For the first time, the present study reveals that the NLRP3 inflammasome may be a therapeutic target for the LOX-dependent DHA metabolites, RvD1 and 17S-HDHA, which can inhibit glomerular inflammation induced by pro-inflammatory cytokines, such as IL-1β, leading to improvement of degenerative pathology of glomeruli under this pathological condition.
It has been well-characterized that Hcys-induced NLRP3 inflammasome activation results in the activation of caspase-1 and the proteolytic cleavage of IL-1β and IL-18 into their biologically active form, which produces other damage-associated molecular patterns (5, 57–59). Inflammatory cells, such as macrophages and T-cells, may be recruited in glomeruli by these factors released by podocytes, contributing to O2·− and cytokine production associated with chronic sterile glomerular inflammation and leading to tissue injury and sclerosis (60, 61). However, more recent studies have demonstrated that, in addition to these canonical effects, IL-1β or other inflammasome products may directly act on podocytes to decrease functional protein expression and NO availability, further contributing to local tissue fibrosis (7, 8). An interesting finding of the present study is that Hcys-induced podocyte dysfunction in vitro, as shown by the decrease in podocin expression, the increase in desmin expression, and the disruption of actin cytoskeleton, was almost completely blocked by RvD1 and 17S-HDHA. This effect may not be due to classical inflammatory response, but to the direct effects on the podocyte, because such an in vitro condition has no inflammatory cell involvement. To our knowledge, this effect of DHA metabolites on noncanonical cell injury of NLRP3 inflammasome activation has not yet been reported. It appears that DHA metabolites not only target the inflammatory response, but also the noninflammatory effect induced by NRLP3 inflammasome activation, which is undoubtedly more effective than only targeting inflammation to produce beneficial action on glomerular injury or ESRD.
To further confirm the inhibitory role of DHA metabolites by LOX in Hcys-induced NLRP3 inflammasome activation and related glomerular injury in vivo, we produced an experimental model of hHcys by feeding mice with the FF diet. It is well-known that two major experimental models to produce hHcys are widely used (62–64). One is to use a methionine-rich diet, because methionine can be metabolized to produce Hcys. Another model is to use a FF diet to interfere with Hcys conversion into methionine. Because hHcys can be effectively reduced by treatment of folic acid in clinical studies and there is some concern about possible nonspecific effects of methionine diet in producing hHcys, we used dietary folate restriction to produce the mouse model of hHcys.
Using confocal microscopy and immunohistochemistry, we detected the formation and activation of NLRP3 inflammasomes in glomeruli of WT mice on the FF diet, but not in Nlrp3−/− mice. However, the results of FF diet-fed WT mice receiving a large dose of DHA demonstrated that NLRP3 inflammasome activation and IL-1β production were almost completely blocked. Moreover, hHcys-induced NLRP3 inflammasome activation resulted in podocyte injury and glomerular sclerosis in WT mice, but not in NLRP3 gene KO mice. DHA treatment markedly attenuated the podocyte damage and glomerular injury in WT mice on the FF diet, but had no further effect on NLRP3 KO mice. This in vivo effect of a large dose of DHA may be due to increased production of its LOX production of RvD1 and 17S-HDHA. Indeed, cotreatment with CDC substantially attenuated the anti-inflammatory and protective effects of DHA in WT mice on FF diet, as shown by enhanced NLRP3 inflammasome activation and podocyte injury in glomeruli during hHcys.
The present study also explored the mechanisms mediating the inhibitory effects of RvD1 and 17S-HDHA on hHcys-induced NLRP3 inflammasome activation in podocytes. In previous studies, one of the triggering mechanisms of NLRP3 inflammasome activation was due to O2·− production via LR clustering (7, 8) and the effects of DHA are related to decreased LR-associated membrane molecular changes (65–67). Therefore, we tested a hypothesis that the LOX products of DHA might inhibit NLRP3 inflammasome activation and prevent podocyte damage and glomerular injury via blocking LR clustering and associated O2·− production during hHcys. It was found that Hcys stimulation significantly increased LRs clustering with NOX subunits that led to the activation of NOX and subsequent generation of O2·−. The Hcys-induced LR clustering and O2·− production were blocked by RvD1 and 17S-HDHA, two LOX metabolites of DHA. These results indicate that LOX products of DHA indeed inhibit NLRP3 inflammasome activation and prevent podocyte damage and glomerular injury through blocking the formation of LR-redox signaling platforms in the podocyte membrane, which is one of important triggering mechanisms of NLRP3 inflammasome activation.
In this study, we have focused on the protective effect of DHA LOX metabolites by inhibiting NLRP3 inflammasome activation because previous studies have demonstrated its physiological relevance and related regulatory mechanisms in podocytes. Recently, a few studies also reported the beneficial effects of DHA or its metabolites on other types of inflammasome. For example, DHA was found to ameliorate palmitate-induced lipid accumulation and inflammation through repressing NLRC4 inflammasome activation in HepG2 cells (68). It has also been reported that DHA reduced IL-1β production by ligands that stimulate the NLRP3, AIM2, and NAIP5/NLRC4 inflammasomes in macrophages (69). However, there is no evidence showing that NLRC4 or AIM2 inflammasomes are functioning in podocytes. In the near future, we may need to characterize these uncanonical inflammasomes in renal cells and then test the beneficial effects of DHA or its metabolites in protecting podocytes and kidney from injuries.
In summary, the present study demonstrated a novel anti-inflammatory mechanism of DHA LOX metabolites, RvD1 and 17S-HDHA, which is associated with inhibition of hHcys-induced NLRP3 inflammasome activation. This inhibitory action of RvD1 and 17S-HDHA on NLRP3 inflammasomes contributes to the prevention of podocyte injury and glomerular damage during hHcys. It is concluded that DHA and its metabolites also target NLRP3 inflammasomes to exert beneficial action to protect podocytes or glomeruli from hHcys-induced injury or sclerosis.
Supplementary Material
Footnotes
Abbreviations:
- ASC
- apoptosis-associated speck-like protein containing a caspase recruitment domain
- CDC
- cinnamyl-3,4-dihydroxy-cyanocinnamate
- ESRD
- end-stage renal disease
- FF
- folate-free
- Hcys
- homocysteine
- hHcys
- hyperhomocysteinemia
- IL
- interleukin
- LOX
- lipoxygenase
- LR
- lipid raft
- ND
- normal diet
- NLRP3
- nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3
- NOX
- NADPH oxidase
- RvD1
- resolvin D1
- 17S-HDHA
- 17S-hydroxy DHA
- VEGF
- vascular endothelial growth factor
This study was supported by National Institutes of Health Grants DK054927, HL057244, and HL075316. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Perła-Kaján J., and Jakubowski H.. 2012. Paraoxonase 1 and homocysteine metabolism. Amino Acids. 43: 1405–1417. [DOI] [PubMed] [Google Scholar]
- 2.Białecka M., Kurzawski M., Roszmann A., Robowski P., Sitek E. J., Honczarenko K., Gorzkowska A., Budrewicz S., Mak M., Jarosz M., et al. 2012. Association of COMT, MTHFR, and SLC19A1(RFC-1) polymorphisms with homocysteine blood levels and cognitive impairment in Parkinson’s disease. Pharmacogenet. Genomics. 22: 716–724. [DOI] [PubMed] [Google Scholar]
- 3.Yu M., Sturgill-Short G., Ganapathy P., Tawfik A., Peachey N. S., and Smith S. B.. 2012. Age-related changes in visual function in cystathionine-beta-synthase mutant mice, a model of hyperhomocysteinemia. Exp. Eye Res. 96: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C. C., Zheng C. M., Lin Y. F., Lo L., Liao M. T., and Lu K. C.. 2012. Role of homocysteine in end-stage renal disease. Clin. Biochem. 45: 1286–1294. [DOI] [PubMed] [Google Scholar]
- 5.Abais J. M., Xia M., Li G., Chen Y., Conley S. M., Gehr T. W., Boini K. M., and Li P. L.. 2014. Nod-like receptor protein 3 (NLRP3) inflammasome activation and podocyte injury via thioredoxin-interacting protein (TXNIP) during hyperhomocysteinemia. J. Biol. Chem. 289: 27159–27168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abais J. M., Xia M., Li G., Gehr T. W., Boini K. M., and Li P. L.. 2014. Contribution of endogenously produced reactive oxygen species to the activation of podocyte NLRP3 inflammasomes in hyperhomocysteinemia. Free Radic. Biol. Med. 67: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abais J. M., Zhang C., Xia M., Liu Q., Gehr T. W., Boini K. M., and Li P. L.. 2013. NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia. Antioxid. Redox Signal. 18: 1537–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C., Boini K. M., Xia M., Abais J. M., Li X., Liu Q., and Li P. L.. 2012. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension. 60: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C., Hu J. J., Xia M., Boini K. M., Brimson C. A., Laperle L. A., and Li P. L.. 2010. Protection of podocytes from hyperhomocysteinemia-induced injury by deletion of the gp91phox gene. Free Radic. Biol. Med. 48: 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang G., Woo C. W., Sung F. L., Siow Y. L., and O K.. 2002. Increased monocyte adhesion to aortic endothelium in rats with hyperhomocysteinemia: role of chemokine and adhesion molecules. Arterioscler. Thromb. Vasc. Biol. 22: 1777–1783. [DOI] [PubMed] [Google Scholar]
- 11.Bracey N. A., Duff H. J., and Muruve D. A.. 2015. Hierarchical regulation of wound healing by NOD-like receptors in cardiovascular disease. Antioxid. Redox Signal. 22: 1176–1187. [DOI] [PubMed] [Google Scholar]
- 12.Li P. L. 2015. Cardiovascular pathobiology of inflammasomes: inflammatory machinery and beyond. Antioxid. Redox Signal. 22: 1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiecolt-Glaser J. K., Belury M. A., Andridge R., Malarkey W. B., Hwang B. S., and Glaser R.. 2012. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav. Immun. 26: 988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ariel A., Li P. L., Wang W., Tang W. X., Fredman G., Hong S., Gotlinger K. H., and Serhan C. N.. 2005. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J. Biol. Chem. 280: 43079–43086. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M. J., Sansbury B. E., Hellmann J., Baker J. F., Guo L., Parmer C. M., Prenner J. C., Conklin D. J., Bhatnagar A., Creager M. A., et al. 2016. Resolvin D2 enhances postischemic revascularization while resolving inflammation. Circulation. 134: 666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rey C., Nadjar A., Buaud B., Vaysse C., Aubert A., Pallet V., Laye S., and Joffre C.. 2016. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav. Immun. 55: 249–259. [DOI] [PubMed] [Google Scholar]
- 17.Ramon S., Dalli J., Sanger J. M., Winkler J. W., Aursnes M., Tungen J. E., Hansen T. V., and Serhan C. N.. 2016. The protectin PCTR1 is produced by human M2 macrophages and enhances resolution of infectious inflammation. Am. J. Pathol. 186: 962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X., Li C., Liang W., Bi Y., Chen M., and Dong S.. 2014. Protectin D1 promotes resolution of inflammation in a murine model of lipopolysaccharide-induced acute lung injury via enhancing neutrophil apoptosis. Chin. Med. J. (Engl.). 127: 810–814. [PubMed] [Google Scholar]
- 19.Martínez-Micaelo N., Gonzalez-Abuin N., Pinent M., Ardevol A., and Blay M.. 2016. Dietary fatty acid composition is sensed by the NLRP3 inflammasome: omega-3 fatty acid (DHA) prevents NLRP3 activation in human macrophages. Food Funct. 7: 3480–3487. [DOI] [PubMed] [Google Scholar]
- 20.Snodgrass R. G., Huang S., Namgaladze D., Jandali O., Shao T., Sama S., Brune B., and Hwang D. H.. 2016. Docosahexaenoic acid and palmitic acid reciprocally modulate monocyte activation in part through endoplasmic reticulum stress. J. Nutr. Biochem. 32: 39–45. [DOI] [PubMed] [Google Scholar]
- 21.Sun H., Meng X., Han J., Zhang Z., Wang B., Bai X., and Zhang X.. 2013. Anti-cancer activity of DHA on gastric cancer–an in vitro and in vivo study. Tumour Biol. 34: 3791–3800. [DOI] [PubMed] [Google Scholar]
- 22.Erdinest N., Ovadia H., Kormas R., and Solomon A.. 2014. Anti-inflammatory effects of resolvin-D1 on human corneal epithelial cells: in vitro study. J. Inflamm. (Lond.). 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X., Hong S., Li P. L., and Zhang Y.. 2011. Docosahexanoic acid-induced coronary arterial dilation: actions of 17S-hydroxy docosahexanoic acid on K+ channel activity. J. Pharmacol. Exp. Ther. 336: 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boini K. M., Xia M., Li C., Zhang C., Payne L. P., Abais J. M., Poklis J. L., Hylemon P. B., and Li P. L.. 2011. Acid sphingomyelinase gene deficiency ameliorates the hyperhomocysteinemia-induced glomerular injury in mice. Am. J. Pathol. 179: 2210–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeMorrow S., Francis H., Gaudio E., Venter J., Franchitto A., Kopriva S., Onori P., Mancinelli R., Frampton G., Coufal M., et al. 2008. The endocannabinoid anandamide inhibits cholangiocarcinoma growth via activation of the noncanonical Wnt signaling pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 295: G1150–G1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho H., Ueda M., Tamaoka M., Hamaguchi M., Aisaka K., Kiso Y., Inoue T., Ogino R., Tatsuoka T., Ishihara T., et al. 1991. Novel caffeic acid derivatives: extremely potent inhibitors of 12-lipoxygenase. J. Med. Chem. 34: 1503–1505. [DOI] [PubMed] [Google Scholar]
- 27.Savin V. J., Sharma R., Lovell H. B., and Welling D. J.. 1992. Measurement of albumin reflection coefficient with isolated rat glomeruli. J. Am. Soc. Nephrol. 3: 1260–1269. [DOI] [PubMed] [Google Scholar]
- 28.Savin V. J., and Terreros D. A.. 1981. Filtration in single isolated mammalian glomeruli. Kidney Int. 20: 188–197. [DOI] [PubMed] [Google Scholar]
- 29.Xia M., Conley S. M., Li G., Li P. L., and Boini K. M.. 2014. Inhibition of hyperhomocysteinemia-induced inflammasome activation and glomerular sclerosis by NLRP3 gene deletion. Cell. Physiol. Biochem. 34: 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raij L., Azar S., and Keane W.. 1984. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 26: 137–143. [DOI] [PubMed] [Google Scholar]
- 31.De Caterina R., Caprioli R., Giannessi D., Sicari R., Galli C., Lazzerini G., Bernini W., Carr L., and Rindi P.. 1993. n-3 fatty acids reduce proteinuria in patients with chronic glomerular disease. Kidney Int. 44: 843–850. [DOI] [PubMed] [Google Scholar]
- 32.Moon Y., and Pestka J. J.. 2003. Deoxynivalenol-induced mitogen-activated protein kinase phosphorylation and IL-6 expression in mice suppressed by fish oil. J. Nutr. Biochem. 14: 717–726. [DOI] [PubMed] [Google Scholar]
- 33.Donadio J. V., and Grande J. P.. 2004. The role of fish oil/omega-3 fatty acids in the treatment of IgA nephropathy. Semin. Nephrol. 24: 225–243. [DOI] [PubMed] [Google Scholar]
- 34.El-Mesery M., Al-Gayyar M., Salem H., Darweish M., and El-Mowafy A.. 2009. Chemopreventive and renal protective effects for docosahexaenoic acid (DHA): implications of CRP and lipid peroxides. Cell Div. 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halade G. V., Rahman M. M., Bhattacharya A., Barnes J. L., Chandrasekar B., and Fernandes G.. 2010. Docosahexaenoic acid-enriched fish oil attenuates kidney disease and prolongs median and maximal life span of autoimmune lupus-prone mice. J. Immunol. 184: 5280–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura N., Kumasaka R., Fu L. Y., Fujita T., Murakami R., Shimada M., Shimaya Y., Osawa H., Yamabe H., Okumura K., et al. 2011. Effects of tridocosahexaenoyl-glycerol emulsion on proteinuria in rats with nephrotoxic serum nephritis. Nephron Extra. 1: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Priante G., Musacchio E., Valvason C., Clari G., Bordin L., Sartori L., and Baggio B.. 2013. Further insights about the beneficial effects of n-3 fatty acids in the early molecular events of renal fibrosis in vitro. J. Nephrol. 26: 652–659. [DOI] [PubMed] [Google Scholar]
- 38.Bowden R. G., Wilson R. L., Deike E., and Gentile M.. 2009. Fish oil supplementation lowers C-reactive protein levels independent of triglyceride reduction in patients with end-stage renal disease. Nutr. Clin. Pract. 24: 508–512. [DOI] [PubMed] [Google Scholar]
- 39.Grande J. P., Walker H. J., Holub B. J., Warner G. M., Keller D. M., Haugen J. D., Donadio J. V. Jr., and Dousa T. P.. 2000. Suppressive effects of fish oil on mesangial cell proliferation in vitro and in vivo. Kidney Int. 57: 1027–1040. [DOI] [PubMed] [Google Scholar]
- 40.Jia Q., Zhou H. R., Bennink M., and Pestka J. J.. 2004. Docosahexaenoic acid attenuates mycotoxin-induced immunoglobulin a nephropathy, interleukin-6 transcription, and mitogen-activated protein kinase phosphorylation in mice. J. Nutr. 134: 3343–3349. [DOI] [PubMed] [Google Scholar]
- 41.Kim Y. J., and Chung H. Y.. 2007. Antioxidative and anti-inflammatory actions of docosahexaenoic acid and eicosapentaenoic acid in renal epithelial cells and macrophages. J. Med. Food. 10: 225–231. [DOI] [PubMed] [Google Scholar]
- 42.Wang X., Chrysovergis K., Kosak J., and Eling T. E.. 2014. Lower NLRP3 inflammasome activity in NAG-1 transgenic mice is linked to a resistance to obesity and increased insulin sensitivity. Obesity (Silver Spring). 22: 1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butts B., Gary R. A., Dunbar S. B., and Butler J.. 2015. The importance of NLRP3 inflammasome in heart failure. J. Card. Fail. 21: 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saresella M., La Rosa F., Piancone F., Zoppis M., Marventano I., Calabrese E., Rainone V., Nemni R., Mancuso R., and Clerici M.. 2016. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol. Neurodegener. 11: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wree A., Eguchi A., McGeough M. D., Pena C. A., Johnson C. D., Canbay A., Hoffman H. M., and Feldstein A. E.. 2014. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 59: 898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y., Li X., Boini K. M., Pitzer A. L., Gulbins E., Zhang Y., and Li P. L.. 2015. Endothelial Nlrp3 inflammasome activation associated with lysosomal destabilization during coronary arteritis. Biochim. Biophys. Acta. 1853: 396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tumurkhuu G., Shimada K., Dagvadorj J., Crother T. R., Zhang W., Luthringer D., Gottlieb R. A., Chen S., and Arditi M.. 2016. Ogg1-dependent DNA repair regulates NLRP3 inflammasome and prevents atherosclerosis. Circ. Res. 119: e76–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H. M., Kim J. J., Kim H. J., Shong M., Ku B. J., and Jo E. K.. 2013. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 62: 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peeters P. M., Eurlings I. M., Perkins T. N., Wouters E. F., Schins R. P., Borm P. J., Drommer W., Reynaert N. L., and Albrecht C.. 2014. Silica-induced NLRP3 inflammasome activation in vitro and in rat lungs. Part. Fibre Toxicol. 11: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halade G. V., Williams P. J., Veigas J. M., Barnes J. L., and Fernandes G.. 2013. Concentrated fish oil (Lovaza(R)) extends lifespan and attenuates kidney disease in lupus-prone short-lived (NZBxNZW)F1 mice. Exp. Biol. Med. (Maywood). 238: 610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bannenberg G., and Serhan C. N.. 2010. Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim. Biophys. Acta. 1801: 1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serhan C. N., Yacoubian S., and Yang R.. 2008. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 3: 279–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serhan C. N., Chiang N., and Van Dyke T. E.. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serhan C. N., and Chiang N.. 2013. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr. Opin. Pharmacol. 13: 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balas L., Guichardant M., Durand T., and Lagarde M.. 2014. Confusion between protectin D1 (PD1) and its isomer protectin DX (PDX). An overview on the dihydroxy-docosatrienes described to date. Biochimie. 99: 1–7. [DOI] [PubMed] [Google Scholar]
- 56.Duffield J. S., Hong S., Vaidya V. S., Lu Y., Fredman G., Serhan C. N., and Bonventre J. V.. 2006. Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 177: 5902–5911. [DOI] [PubMed] [Google Scholar]
- 57.Boini K. M., Xia M., Koka S., Gehr T. W., and Li P. L.. 2016. Instigation of NLRP3 inflammasome activation and glomerular injury in mice on the high fat diet: role of acid sphingomyelinase gene. Oncotarget. 7: 19031–19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li G., Xia M., Abais J. M., Boini K., Li P. L., and Ritter J. K.. 2016. Protective action of anandamide and its COX-2 metabolite against l-homocysteine-induced NLRP3 inflammasome activation and injury in podocytes. J. Pharmacol. Exp. Ther. 358: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao P., He F. F., Tang H., Lei C. T., Chen S., Meng X. F., Su H., and Zhang C.. 2015. NADPH oxidase-induced NALP3 inflammasome activation is driven by thioredoxin-interacting protein which contributes to podocyte injury in hyperglycemia. J. Diabetes Res. 2015: 504761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takano Y., Yamauchi K., Hayakawa K., Hiramatsu N., Kasai A., Okamura M., Yokouchi M., Shitamura A., Yao J., and Kitamura M.. 2007. Transcriptional suppression of nephrin in podocytes by macrophages: roles of inflammatory cytokines and involvement of the PI3K/Akt pathway. FEBS Lett. 581: 421–426. [DOI] [PubMed] [Google Scholar]
- 61.Han Y., Ma F. Y., Tesch G. H., Manthey C. L., and Nikolic-Paterson D. J.. 2011. c-fms blockade reverses glomerular macrophage infiltration and halts development of crescentic anti-GBM glomerulonephritis in the rat. Lab. Invest. 91: 978–991. [DOI] [PubMed] [Google Scholar]
- 62.Duthie S. J., Grant G., and Narayanan S.. 2000. Increased uracil misincorporation in lymphocytes from folate-deficient rats. Br. J. Cancer. 83: 1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller J. W., Nadeau M. R., Smith J., Smith D., and Selhub J.. 1994. Folate-deficiency-induced homocysteinaemia in rats: disruption of S-adenosylmethionine’s co-ordinate regulation of homocysteine metabolism. Biochem. J. 298: 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonaventura D., Tirapelli C. R., Haddad R., Hoehr N. F., Eberlin M. N., and de Oliveira A. M.. 2004. Chronic methionine load-induced hyperhomocysteinemia enhances rat carotid responsiveness for angiotensin II. Pharmacology. 70: 91–99. [DOI] [PubMed] [Google Scholar]
- 65.Lee E. J., Yun U. J., Koo K. H., Sung J. Y., Shim J., Ye S. K., Hong K. M., and Kim Y. N.. 2014. Down-regulation of lipid raft-associated onco-proteins via cholesterol-dependent lipid raft internalization in docosahexaenoic acid-induced apoptosis. Biochim. Biophys. Acta. 1841: 190–203. [DOI] [PubMed] [Google Scholar]
- 66.Huang C. Y., Sheu W. H., and Chiang A. N.. 2015. Docosahexaenoic acid and eicosapentaenoic acid suppress adhesion molecule expression in human aortic endothelial cells via differential mechanisms. Mol. Nutr. Food Res. 59: 751–762. [DOI] [PubMed] [Google Scholar]
- 67.Schaefer M. B., Schaefer C. A., Schifferings S., Kuhlmann C. R., Urban A., Benscheid U., Fischer T., Hecker M., Morty R. E., Vadasz I., et al. 2016. N-3 vs. n-6 fatty acids differentially influence calcium signalling and adhesion of inflammatory activated monocytes: impact of lipid rafts. Inflamm. Res. 65: 881–894. [DOI] [PubMed] [Google Scholar]
- 68.Luo X., Yang Y., Shen T., Tang X., Xiao Y., Zou T., Xia M., and Ling W.. 2012. Docosahexaenoic acid ameliorates palmitate-induced lipid accumulation and inflammation through repressing NLRC4 inflammasome activation in HepG2 cells. Nutr. Metab. (Lond.). 9: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams-Bey Y., Boularan C., Vural A., Huang N. N., Hwang I. Y., Shan-Shi C., and Kehrl J. H.. 2014. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-kappaB activation and enhancing autophagy. PLoS One. 9: e97957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.