Abstract
In mammals, ether lipids exert a wide spectrum of signaling and structural functions, such as stimulation of immune responses, anti-tumor activities, and enhancement of sperm functions. Abnormal accumulation of monoalkyl-diacylglycerol (MADAG) was found in Wolman’s disease, a human genetic disorder defined by a deficiency in lysosomal acid lipase. In the current study, we found that among the nine recombinant human lipid acyltransferases examined, acyl-CoA:diacylglycerol acyltransferase (DGAT)1, DGAT2, acyl-CoA:monoacylglycerol acyltransferase (MGAT)2, MGAT3, acyl-CoA:wax-alcohol acyltransferase 2/MFAT, and DGAT candidate 3 were able to use 1-monoalkylglycerol (1-MAkG) as an acyl acceptor for the synthesis of monoalkyl-monoacylglycerol (MAMAG). These enzymes demonstrated different enzymatic turnover rates and relative efficiencies for the first and second acylation steps leading to the synthesis of MAMAG and MADAG, respectively. They also exhibited different degrees of substrate preference when presented with 1-monooleoylglycerol versus 1-MAkG. In CHO-K1 cells, treatment with DGAT1 selective inhibitor, XP-620, completely blocked the synthesis of MADAG, indicating that DGAT1 is the predominant enzyme responsible for the intracellular synthesis of MADAG in this model system. The levels of MADAG in the adrenal gland of DGAT1 KO mice were reduced as compared with those of the WT mice, suggesting that DGAT1 is a major enzyme for the synthesis of MADAG in this tissue. Our findings indicate that several of these lipid acyltransferases may be able to synthesize neutral ether lipids in mammals.
Keywords: Wolman’s disease • alkylglycerol • membrane bound O-acyltransferase
Ether lipids constitute a lipid class structurally similar to glycerolipids, but containing ether linkages instead of ester bonds on the glycerol backbone. Ether lipids are widely distributed in nature and have been found in bacteria (1), deep-sea holothurians (2), fish (3), and mammals, including humans (4).
In mammals, alkylglycerols are found in high levels in bone marrow and spleen tissue (4). Injection of exogenous alkylglycerols in vivo and treatment of macrophages in vitro were shown to stimulate immune responses (5). Alkylglycerols were also suggested to be useful as adjuvants for boosting the production of vaccines (6).
Natural and synthetic alkylglycerols have been shown to have anti-tumor and anti-metastatic activities (7, 8). Paradoxically, ether lipids are synthesized at high levels across many aggressive human cancer cells and primary tumor types through the upregulation of alkylglycerone phosphate synthase, a critical enzyme for the synthesis of ether lipids (9). These seemingly confounding effects of exogenously added versus endogenously synthesized alkylglycerols suggest the importance of conversions between different ether lipid species.
In 1976, Lin, Lie Ken Jie, and Ho (10) reported the accumulation of neutral ether lipids in the adrenal, liver, and spleen of a male Chinese infant with Wolman’s disease, a rare genetic disorder caused by a deficiency in lysosomal acid lipase. Specifically, increases were found in monoalkyl-diacylglycerol (MADAG) levels, but not in ether lipid species containing phosphorus. In contrast, in Niemann-Pick disease, another lysosomal storage disease, ether lipid levels were found to be normal, suggesting that accumulation of neutral ether lipids might be a characteristic feature of Wolman’s disease (10). More recently, Bartz et al. (11) quantitatively determined that, in lipid droplets isolated from a line of cultured cells, MADAG accounts for approximately 10–20% of the total neutral lipid pool.
Despite emerging evidence that MADAG may play a (as yet undefined) role in various disease states, enzymes catalyzing their synthesis are not known. Acyltransferases of the ACAT family and acyl-CoA:diacylglycerol acyltransferase (DGAT)2 family use lipid molecules such as cholesterol, monoacylglycerol (MAG), diacylglycerol (DAG), and fatty alcohol as acyl acceptors to produce, respectively, cholesteryl esters (CEs), DAG, triacylglycerol (TAG), and wax esters with varying degrees of specificity and selectivity (12–21). With the exception of DGAT candidate (DC)3, all recombinant enzyme activities have been reported. Although ACAT enzymes have been shown to acylate oxysterols in addition to cholesterol, they appear to have no recognition of substrates with long alkyl chains as acyl acceptors (14, 22). In contrast, DGAT1, and acyl-CoA:wax-alcohol acyltransferase (AWAT)2, a member of DGAT2 family, appear to recognize a wide range of substrates, including MAG, DAG, fatty alcohol, and retinol (21, 23, 24). The structural resemblance between acylglycerols and alkylglycerols suggests a potential shared metabolic route of synthesis by members of the ACAT and DGAT2 gene family. The current studies are designed to explore this hypothesis.
MATERIALS AND METHODS
Materials
Recombinant Flag epitope-tagged human lipid acyltransferases were expressed in Sf9 or in High5 insect cells using a baculovirus system. The recombinant enzymes were prepared as previously described (18). The 1-monoalkylglycerol (1-MAkG) was synthesized in-house (see below). All other chemicals and materials were obtained from commercial sources: [14C]oleoyl-CoA and [14C]oleic acid (Perkin-Elmer); 1-monooleoylglycerol (1-MOG) (Sigma); cell culture-tested albumin conjugated oleic acid (Sigma); electrophoresis reagents (Life Technologies); monoclonal anti-Flag M2 antibody (Sigma); IRDye 800 goat anti-mouse IgG (Rockland); and silica gel TLC plates (Whatman 150A).
Synthesis of 1-MAkG
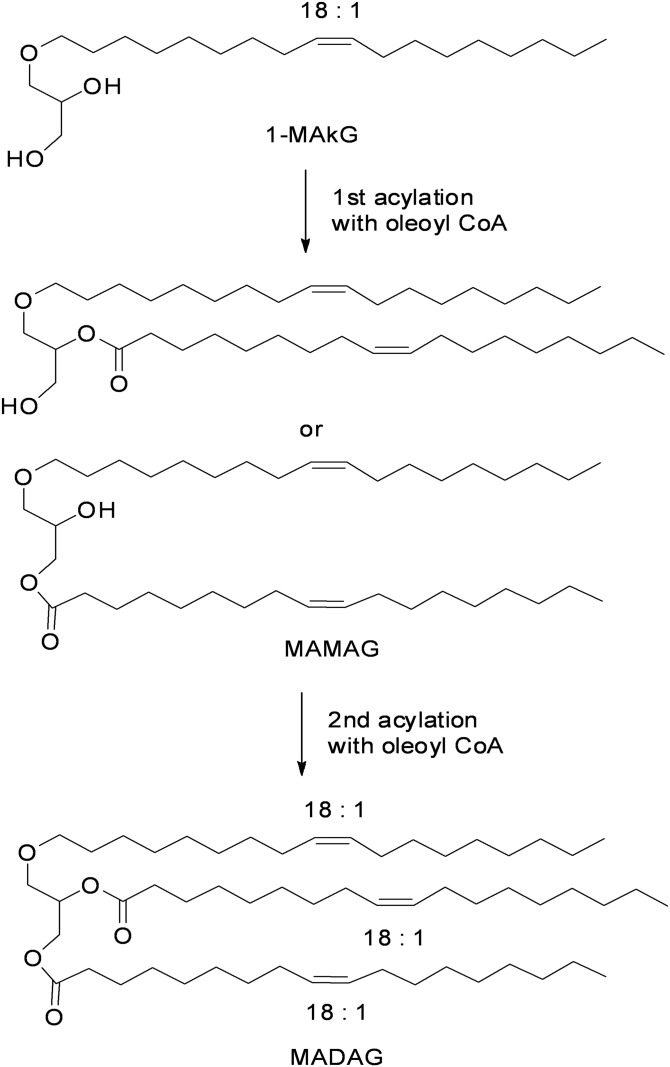
(Z)-1-(octadec-9-enyl)glycerol (Fig. 1) , a representative 1-MAkG, was prepared by alkylation of racemic solketal (Sigma) with cis-9-octadecenyl methanesulfonate (Aldrich) in the presence of sodium hydride in N,N-dimethylformamide, followed by acid-catalyzed hydrolysis (see supplemental Fig. S1 for the synthetic details).
Fig. 1.
Schematic diagram of two step acylations of 1-MAkG catalyzed by acyltransferases.
LC/MS characterization of MAMAG and MADAG
High-resolution MS was performed on a Thermo-Fisher Q-Exactive mass spectrometer fitted with a HESI source and coupled to an Accela uHPLC pump and DLW Pal autosampler. LC was performed on a Waters Symmetry C8 30 × 2 mm column heated to 65°C. Mobile phase A consisted of 5 mM ammonium acetate in 95:5 water:methanol, mobile phase B was 5 mM ammonium acetate in 5:95 water:methanol. Gradient LC was performed at a flow rate of 0.8 ml/min starting at 80% B to 100% B in 1 min then holding for 10 min. MS was performed in positive ionization mode, monitoring primarily the ammonium adducts of the alkylglycerides. MS parameters were as follows: resolution, 35,000; source voltage, 4,200 V; capillary temperature, 275°C; sheath gas, 60; auxiliary gas, 30; spare gas, 5; probe heater, 450°C; S-lens RF, 25. MS/MS spectra were acquired using step-collision energy of 10, 20, and 30. Software control was through Xcalibur.
Reactions with naïve High5 or acyl-CoA:monoacylglycerol acyltransferase (MGAT)3 microsomal membranes using 1-MAkG and oleoyl-CoA as substrates were conducted as described in “Acyltransferase enzyme reaction” below, except [14C]oleoyl-CoA was excluded from the reaction mix. Liquid-liquid extracts were reconstituted in 200 μl of methanol:isopropylalcohol:mobile phase A (60:20:20) and analyzed by LC/MS, as described above.
Quantitative immunoblot blot analyses of recombinant acyltransferase expression levels
Aliquots of 1 μg recombinant acyltransferase microsomal membranes used in the current study were mixed with reducing Laemmli sample buffer and subjected to SDS-PAGE electrophoresis in a 4–12% Bis-Tris gradient gel. Proteins were transferred to nitrocellulose membrane using iBlot dry blotting system. The blot was probed with mouse monoclonal anti-Flag M2 antibody and IRDye 800 goat anti-mouse IgG secondary antibody. The immunoblot signal was detected and quantified using an Odyssey infrared imaging system. The anti-Flag signal against Flag-DGAT1 was designated as 1 protein unit. The relative expression levels of all other acyltransferases were quantified using Flag-DGAT1 as the reference.
Acyltransferase enzyme reaction
All acyltransferase enzyme reactions were carried out in 16 × 100 screw cap glass tubes (VWR) in 150 mM KHxPO4 (pH 7.4) buffer. For each 200 μl reaction, 180 μl of 0.11 mM 1-MOG or 1-MAkG (final concentration 0.1 mM in the assay) and 10 μl of 3 mg/ml recombinant acyltransferase membranes (30 μg/assay) were added. Enzyme reactions were initiated by adding 10 μl of 1 mM [14C]oleoyl-CoA (final concentration 0.05 mM, specific activity 2 μCi/μmol). The reaction tubes were vortexed quickly and incubated in a 37°C water bath for 15 min. The reactions were quenched with 4 ml of stop solution [chloroform:methanol, 2:1 (v:v)] and 1 ml water. The tubes were capped, vortexed, and centrifuged at 800 g for 15 min at room temperature to facilitate phase separation (Sorvall RT6000). The aqueous upper phase was aspirated and the lipid products in the lower phase were dried under nitrogen, reconstituted with 70 μl hexane, and applied for TLC analysis. A radioactive marker lipid standard mix ([14C]CE, [14C]TG, and [14C]palmitic acid) was also loaded. TLC was developed in a pre-equilibrated tank using running solution [hexane:ether:acetic acid, 136:24:0.8 (v:v:v)] for 40 min. Air-dried TLC plates were exposed overnight onto a Phosphor Imager screen and the products were analyzed by the Phosphor Imager (GE Healthcare). Membranes prepared from Sf9 cells expressing human MGAT1 did not exhibit the expected MGAT activity using 1-MOG as substrate and, thus, were excluded from further analyses.
Cell-based neutral lipid synthesis in CHO-K1 cells
On day 0, CHO-K1 cells were plated in a 6-well cell culture plate at a density of approximately 300,000 cells/well. After overnight incubation at 37°C, 5% CO2, on day 1, the cells were starved in serum-free medium (SFM) for 3 h. SFM was then replaced by 1.5 ml of reaction mix (0.4 mM oleic acid-albumin made in SFM) and incubated at 37°C, 5% CO2 for 30 min. Subsequently, 75 μl of label mix (0.5 mM [14C]oleic acid made in reaction mix with specific activity at 200 μCi/μmol; final concentration 0.4 mM [14C]oleic acid in assay with specific activity at 10 μCi/μmol) were added into each well and incubated for 1 h. The labeling materials were then aspirated, cells were washed twice with 2 ml PBS, and lipid products were extracted twice with 1.5 ml of extraction buffer [hexane:isopropanol, 3:2 (v:v)] for 5 min each. The pooled lipid extracts were dried under nitrogen, reconstituted with 70 μl of hexane, and developed with TLC procedure, as described above.
Tissue lipid analysis from WT and DGAT1 (−/−) mice
Mouse husbandry and tissue collection procedures were approved by the University of Wisconsin-Madison Animal Care and Use Committee and were conducted in conformity with the Public Health Service Policy on Humane Care and Use of Laboratory Animals. Mouse jejunum and liver tissues (16 mg) or whole adrenal gland (∼2.5–5 mg) were homogenized in 200 μl of ice cold 0.1% ammonium acetate and then 1.5 ml of methanol and 5 ml of methyl-tert-butyl-ether (MTBE) were added subsequently. The mixture was incubated at room temperature for 1 h with vigorous shaking. The lipid/water phases were separated by adding 1.25 ml of water followed by vortexing, incubation at room temperature for 10 min, and centrifugation for 10 min at 1,000 g. The upper lipid phase was transferred into a fresh glass tube. The lower phase was re-extracted with MTBE:methanol:water at 10:3:2.5 (v:v:v). The upper lipid phases were pooled and dried under nitrogen gas, reconstituted with 40 μl hexane, and used for TLC. To identify the migration position of MADAG, the acylation reaction product of 1-MAkG and oleoyl-CoA using MGAT3 recombinant membranes was used as a TLC reference standard. TLC plates were developed in a pre-equilibrated tank using running solution [hexane:ether:acetic acid, 136:24:0.8 (v:v:v)] for 50 min. The air dried TLC plates were soaked in the staining solution (10% CuSO4, 8% phosphoric acid) for 2 min and air dried for 5 min. The plates were then baked in an oven at 130°C for 50 min. The image of lipid bands on the TLC plate was captured using the G Box (Syngene).
RESULTS
Figure 1 illustrates the schematic diagram of acylation reactions of ether lipid, 1-MAkG, for the synthesis of MADAG. (Z)-1-(octadec-9-enyl)glycerol was synthesized as the representative 1-MAkG for all in vitro enzyme reactions, as an 18:1 alkyl chain at the 1-position is the most prominent form isolated from tissue and cellular systems (11). The first acylation at either the 2- or 3-position of the hydroxyl groups of 1-MAkG led to the products, 1-monoalkyl-2-monoacylglycerol or 1-monoalkyl-3-monoacylglycerol, respectively. Neither LC/MS nor TLC differentiates these two isoforms (Figs. 2, 3). Consequently, we collectively designated these two products, monoalkyl-monoacylglycerol (MAMAG). In the subsequent second acylation, the remaining hydroxyl group of MAMAG is further acylated to form the final product, MADAG.
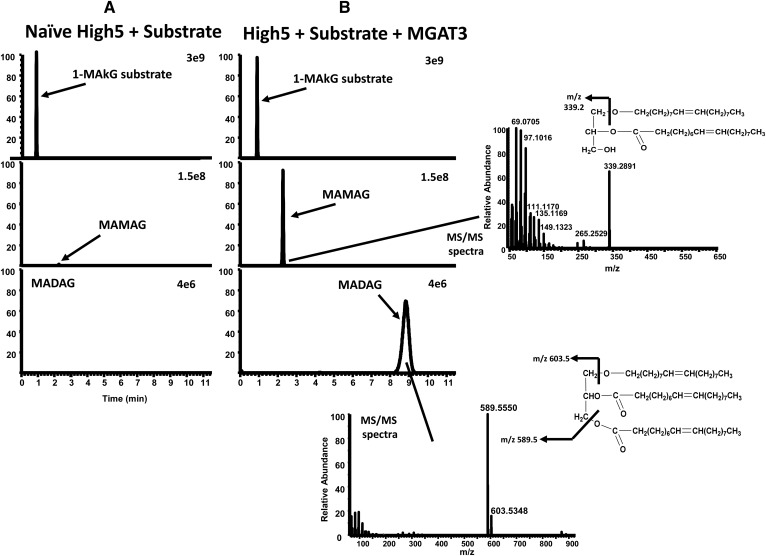
Fig. 2.
High-resolution LC/MS extracted ion chromatograms of MAMAG and MADAG in High5/MGAT3 cell reactions. Lipid products extracted from naïve High5 (A) or MGAT3 (B) reactions using 1-MAkG as substrate were analyzed by high-resolution LC/MS, as described in the Materials and Methods. Shown are the extracted ion chromatograms for the substrate, 1-MAkG (top), and products, MAMAG (middle) and MADAG (bottom). Also shown are the high-resolution MS/MS spectra for the products, MAMAG and MADAG.
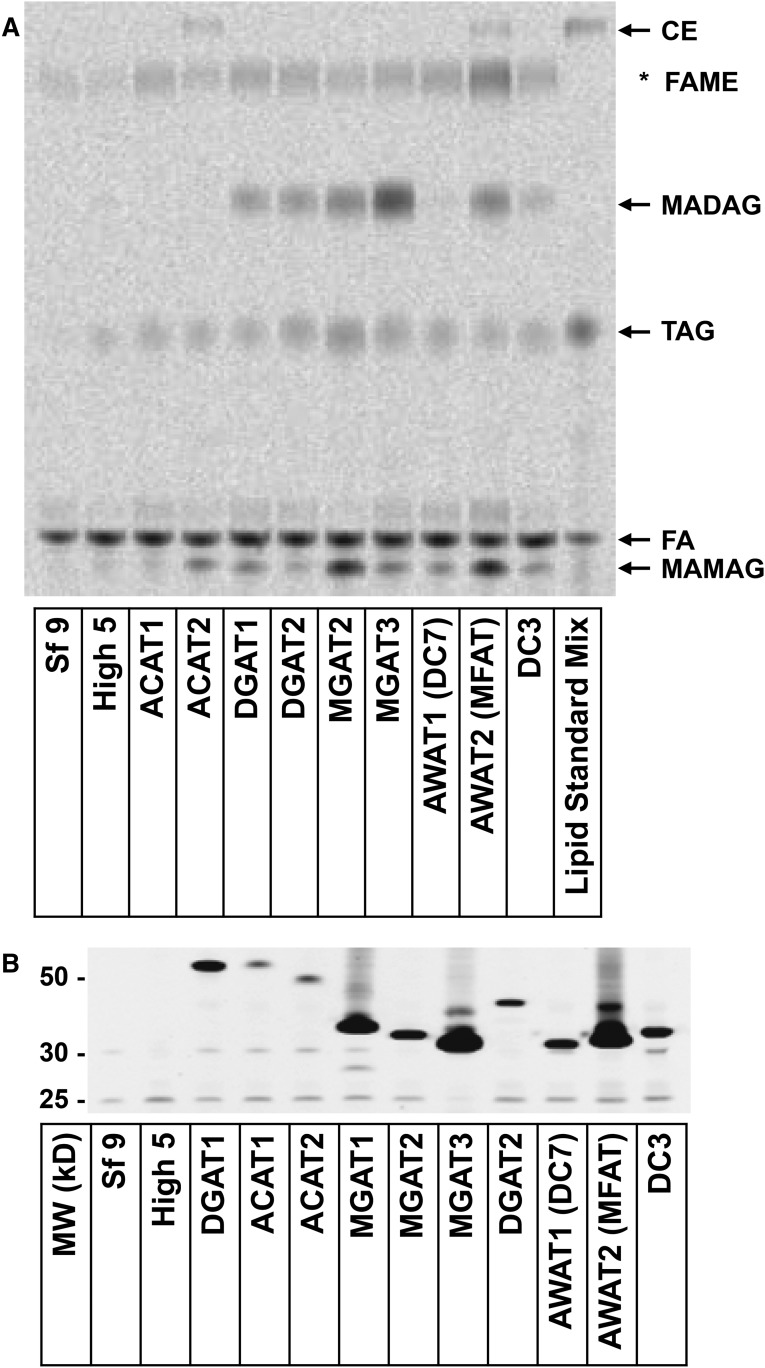
Fig. 3.
TLC analysis of various recombinant acyltransferase acylation products using 1-MAkG as substrate and their relative protein expressions. Aliquots of 30 μg microsomal membranes with recombinant human acyltransferase expression, naïve Sf9 or High5 microsomal membranes were used for acyltransferase reactions using 0.1 mM 1-MAkG and 0.05 mM [14C]oleoyl-CoA (specific activity at 2 μCi/μmol) in 150 mM KHxPO4 (pH 7.4) buffer at 37°C for 15 min, as described in the Materials and Methods, and the resulting enzyme products were analyzed by TLC. Aliquots of 1 μg acyltransferase microsomal membranes were subjected to quantitative immunoblot analysis, as described in the Materials and Methods. The relative protein expression level of each acyltransferase was reflected by the Flag band intensity and normalized against that of DGAT1, which was designated as 1 protein unit. A: TLC profile of lipid products. *Denotes fatty acid methyl ester (FAME), a nonspecific product formed during lipid extraction and analysis. B: Anti-Flag immunoblot for various acyltransferases.
High resolution LC/MS was performed on products extracted from naïve High5 or MGAT3 enzyme reaction, as described in the Materials and Methods. As commercial standards for the alkylglyceride products were unavailable, mass spectra were compared with available purchased mono-, di-, and tri-acylglyceride standards. Similar to purchased acylglyceride standards, the MAMAG and MADAG products were detected as primarily their ammonium adduct ions. Measured parent ion masses were within ±1.2 ppm of theoretical. Figure 2 shows the chromatograms for naïve High5 (Fig. 2A) and MGAT3 (Fig. 2B) microsomal membrane reaction extracts, demonstrating that formation of MADAG only occurred in the presence of MGAT3. Trace levels of MAMAG were detected in the naïve High5 extract (Fig. 2A); MAMAG in the presence of MGAT3 was qualitatively increased >60-fold over baseline naïve High5 levels. Further confirmation of structure was determined using high resolution MS/MS fragmentation. The MS/MS fragmentation patterns observed for MAMAG and MADAG were similar to the MS/MS fragmentation patterns of di- and triacylglyceride standards. The measured fragment masses were within ±2 ppm of theoretical.
To quantitatively compare the ability of lipid acyltransferases to use 1-MAkG as an acyl acceptor, we conducted acyltransferase reactions and quantified products using TLC analysis. As shown in Fig. 3A, compared with naïve Sf9 and High5 membrane controls, DGAT1, DGAT2, MGAT2, MGAT3, AWAT2/MFAT, and DC3 carried out both acylation steps resulting in synthesis of the final product, MADAG. ACAT2 and AWAT1/DC7 exhibited no ability to synthesize MADAG, but showed ability to synthesize MAMAG. ACAT1 failed to carry out both the first and second acylation reactions. We do not know whether human MGAT1 uses 1-MAkG as a substrate because the recombinant membrane prepared from Sf9 cells expressing human MGAT1 did not exhibit the expected MGAT activity using 1-MOG as substrate (data not shown).
Because the acyltransferases used here were not purified membrane proteins, we performed quantitative immunoblot analysis to assess relative protein expression levels for the estimation of their relative catalytic activities (Fig. 3B). Anti-Flag-DGAT1 immunoblot signal was arbitrarily designated as 1 protein unit and the rest of the Flag-tagged acyltransferases were compared with Flag-DGAT1 as the reference. After normalizing protein expression levels, the relative catalytic activity was expressed as nanomoles of products per minute per protein unit (Table 1).
TABLE 1.
Summary of products of various acyltransferases using 1-MAkG or 1-MOG as substrates
| Enzyme | Product of 1-MAkG (nmol/min/protein unit) | Product of 1-MOG (nmol/min/protein unit) | (TAG + DAG)/(MADAG + MAMAG) | ||||
| MAMAG | MADAG | MADAG/MAMAG | DAG | TAG | TAG/DAG | ||
| ACAT2 | 3.879 ± 0.100 | 0.064 ± 0.013 | 0.02 | ||||
| DGAT1 | 0.326 ± 0.051 | 0.248 ± 0.035 | 0.76 | 0.649 ± 0.018 | 1.776 ± 0.069 | 2.7 | 4.2 |
| DGAT2 | 0.202 ± 0.043 | 0.981 ± 0.165 | 4.86 | 0.013 ± 0.013 | 41.434 ± 3.604 | 3137.4 | 35 |
| MGAT2 | 4.740 ± 0.271 | 0.733 ± 0.046 | 0.15 | 7.778 ± 0.213 | 2.084 ± 0.010 | 0.3 | 1.8 |
| MGAT3 | 0.115 ± 0.011 | 0.925 ± 0.103 | 8.04 | 0.069 ± 0.009 | 3.042 ± 0.006 | 43.9 | 3.0 |
| AWAT1/DC7 | 0.383 ± 0.004 | 0.026 ± 0.004 | 0.07 | ||||
| AWAT2/MFAT | 0.893 ± 0.082 | 0.131 ± 0.013 | 0.15 | 1.082 ± 0.044 | 0.124 ± 0.005 | 0.1 | 1.2 |
| DC3 | 0.247 ± 0.024 | 0.105 ± 0.014 | 0.43 | ||||
Data presented are mean ± SEM from two to four experiments.
To compare substrate selectivity, we measured the activities of these enzymes toward 1-MAkG versus the structurally similar acylglycerol, 1-MOG. The results shown in Table 1 were generated using the same batch of respective enzyme source and the same lot of [14C]oleoyl-CoA; all reactions were carried out in parallel for comparison with 1-MOG substrate. AWAT2/MFAT and MGAT2 appeared to be the least selective enzymes in that the levels of TAG/DAG formation and MAMAG/MADAG formation were very similar, using the two substrates, respectively. DGAT1 and MGAT3 were moderately selective for 1-MOG (ratios of TAG + DAG formation vs. MAMAG + MADAG formation were 4.2- and 3.0-fold for DGAT1 and MGAT3, respectively). DGAT2 appeared to have the highest selectivity for 1-MOG; the level of TAG + DAG formation was approximately 35-fold higher than the formation of MAMAG + MADAG (Table 1).
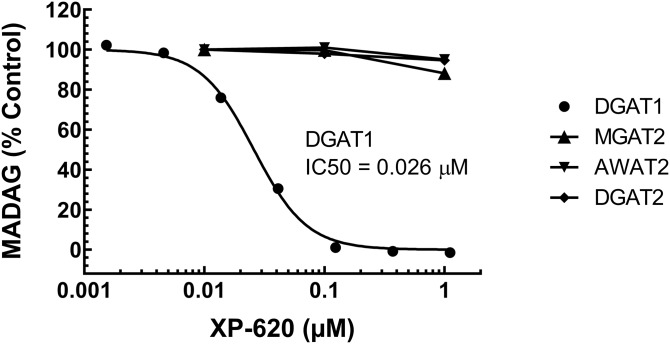
XP-620 is a selective DGAT1 inhibitor that has no inhibitory activities against DGAT2 gene family members when using monoacylglycerol or DAG as substrates (23, 25). When 2-MOG was used as a substrate, the XP-620 IC50 values for human, rat, and mouse DGAT1 were determined to be 0.017 ± 0.004 μM, 0.003 ± 0.001 μM, and 0.003 ± 0.001 μM, respectively (25). Figure 4 illustrates an example of a dose-dependent inhibition of XP-620 against human DGAT1 activity using 1-MAkG as a substrate; the IC50 value was determined to be 0.026 ± 0.002 μM (n = 2). As expected, no inhibitory activity of XP-620 was observed for MGAT2, AWAT2/MFAT, or DGAT2 at the concentrations that showed complete inhibition for DGAT1 (Fig. 4).
Fig. 4.
XP-620 concentration-dependent effect on 1-MAkG acylation by DGAT1, MGAT2, AWAT2, and DGAT2. Recombinant DGAT1, MGAT2, AWAT2, or DGAT2 were incubated with varying concentrations of XP-620, substrates 0.05 mM 1-MAkG and 0.05 mM of [14C]oleoyl-CoA (specific activity at 2 μCi/μmol) in 150 mM KHxPO4 (pH 7.4) at 37°C for 15 min. For each enzyme, the percent of products in the presence of each concentration of XP-620 was calculated compared with that of DMSO (100% control). The dose response curve was plotted and IC50 values were calculated using the “dose-response variable slope” model in the GraphPad Prism program. XP-620 up to 1 μM showed no inhibition against MGAT2, AWAT2, and DGAT2. In contrast, it inhibited DGAT1 with an IC50 of 0.026 μM.
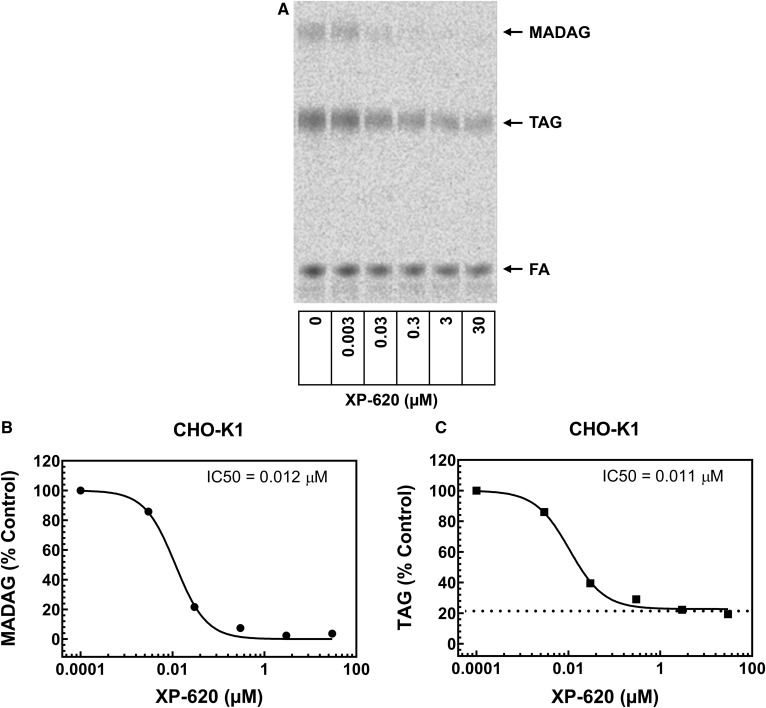
CHO-K1 cells are known to contain high levels of ether lipids (11). Hence, we used a CHO-K1 whole cell system to investigate intracellular ether lipid synthesis by monitoring the incorporation of [14C]oleic acid into MADAG. In the absence of XP620, in a 1 h time period, confluent CHO-K1 cells produced approximately 0.10 nmol of MADAG and 0.54 nmol of TAG per well of a 6-well plate. XP-620 dose-dependently inhibited the synthesis of both MADAG and TAG. At high doses, XP-620 completely inhibited MADAG production, indicating that DGAT1 is the predominant enzyme catalyzing the synthesis of MADAG in CHO-K1 cells. In contrast, XP-620 inhibition of TAG synthesis plateaued at ∼80%. The remaining ∼20% likely occurs through an alternate mechanism, such as DGAT2. From the dose response curve, the IC50 values for XP-620 were determined to be 0.012 ± 0.0002 μM (n = 2) for MADAG production and 0.011 ± 0.0002 μM (n = 2) for TAG production, respectively (an example is shown in Fig. 5. )
Fig. 5.
Concentration dependent inhibition of intracellular synthesis of MADAG in CHO-K1 cells by XP-620. The lipid synthesis of CHO-K1 cells was analyzed in 6-well plates when cells were at ∼90% confluence. The cells were incubated with 0.5 mM [14C]oleic acid for 1 h in the absence or presence of a series of concentrations of XP-620. The lipid products were extracted and developed in TLC plates. To calculate IC50 values, percent of MADAG or TAG formed in the presence of each concentration of XP-620 was compared with DMSO (100% control). Dose response curves were plotted and XP-620 IC50 values were calculated for MADAG or TAG using the “dose-response variable slope” model in the GraphPad Prism program. A: Phospho Image profile of intracellular synthesis of MADAG and TAG by CHO-K1 cells. B: XP-620 concentration-dependent inhibition of intracellular synthesis of MADAG in CHO-K1 cells. C: XP-620 concentration dependent inhibition of intracellular synthesis of TAG in CHO-K1 cells.
To further confirm that the inhibition of MADAG synthesis is DGAT1 dependent, we tested compound A and compound B, two compounds with entirely distinct structural motifs comparing with XP-620 (supplemental Fig. S2). Compound A has potent inhibitory potency against human DGAT1 (IC50: 0.020 ± 0.012 μM), but with no inhibitory activity against human DGAT2. Compound B belongs to the same chemical series, but has no inhibitory activity to both human DGAT1 and DGAT2. When these compounds were tested in CHO-K1 cells, compound A demonstrated concentration-dependent inhibition of synthesis of MADAG (IC50: 9.5 μM), whereas compound B showed no inhibition. For the TAG synthesis, compound A exhibited partial inhibition, but compound B had no effect (supplemental Fig. S2).
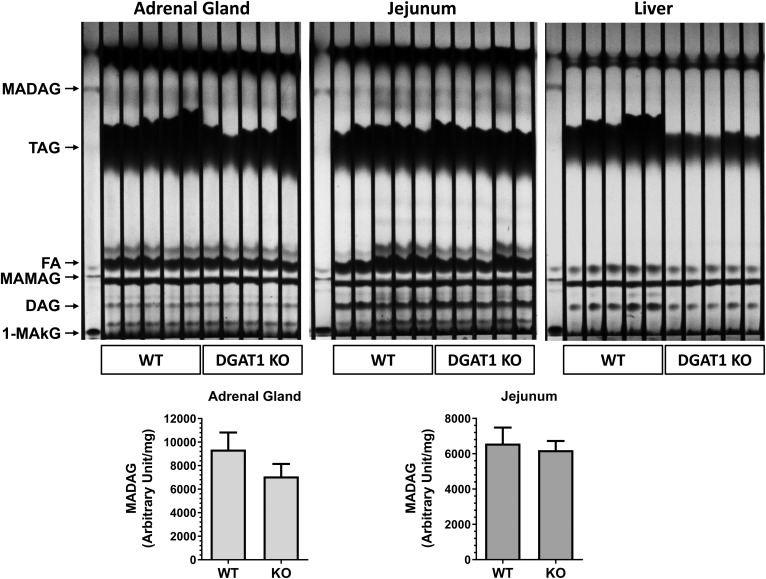
To investigate the importance of DGAT1 enzyme for the synthesis of MADAG in vivo, we analyzed lipid extracts from WT and DGAT1 KO mice from tissues of liver, spleen, jejunum, adrenal gland, and plasma. In initial studies, LC/MS, developed to monitor for the presence of MADAG18:1/18:1/18:1 in our in vitro assays, was used to assess MADAG levels in the tissue extracts. Given the lack of commercially available standards, this in vitro acylation reaction product of 1-MAkG and oleoyl-CoA was used as a reference standard. Unfortunately, the levels of MADAG18:1/18:1/18:1 in mouse tissues were below detection by LC/MS. This finding was not surprising given that more than 100 endogenous molecular species of MADAG were reported by Bartz et al. (11), with MADAG18:1/18:1/18:1 being a minor species. As monitoring for more than 100 various species of MADAG in the absence of standards by MS was not practical, we turned to TLC to qualitatively analyze total MADAG species. In Fig. 6, lipid extracts of adrenal gland and jejunum showed a cluster of MADAG bands that comigrated with MADAG18:1/18:1/18:1. The quantification of the bands in the TLC indicates that MADAGs in the adrenal gland of DGAT1 KO mice were ∼24% less than those in WT mice. In the jejunum, MADAG levels were similar in DGAT1 KO and WT mice. Stainable MADAG was not detected in the liver or spleen tissue (data not shown). These results suggest that DGAT1 might be a major enzyme for the synthesis of MADAG in the adrenal gland, while other acyltransferases, such as MGAT2 and DGAT2, might compensate and catalyze MADAG synthesis in DGAT1 KO mice.
Fig. 6.
TLC analysis of lipids in various mouse tissues in WT or DGAT1 KO mice. Lipids in whole adrenal gland or 16 mg of jejunum and liver were extracted using the MTBE method, as described in the Materials and Methods. The first lane shown on each plate contains the reaction products of MGAT3 microsomal membranes using 1-MAkG and oleoyl-CoA as substrates. TLC plates were developed in a pre-equilibrated tank using running solution [hexane:ether:acetic acid, 136:24:0.8 (v:v:v)] for 50 min. The air-dried TLC plates were soaked in the staining solution (10% CuSO4, 8% phosphoric acid) and baked in an oven at 130°C for 50 min. The image (upper panel) of lipid bands was captured and the MADAG band quantified (lower panel) using the G Box (Syngene).
DISCUSSION
Ether lipids have been shown to exert a wide range of physiological functions, such as stimulation of immune responses, anti-tumor activity, and enhancement of sperm functions. These physiological functions are associated with the structural and signaling roles played by ether lipids at the cellular level. MADAGs are ether lipids that can account for a significant portion of the total neutral lipid pool. However, metabolic enzymes responsible for the synthesis of MADAG have not been identified. In the current study, we found that at least six lipid acyltransferases from both the ACAT and DGAT2 gene families were able to use 1-MAkG as an acyl acceptor for the synthesis of MADAG in vitro. The existence of this redundant system of six enzymes that are able to catalyze similar acylation reactions suggests that synthesis of the neutral ether lipid, MADAG, is critical for mammals. Although mice lacking DGAT1 or MGAT2, two enzymes catalyzing MADAG synthesis in our study, have not been carefully examined for abnormalities in ether lipid metabolism, their pleiotropic phenotypes suggest that they may function beyond TAG synthesis (24, 26–30). In addition, the genetic deletion of each of these genes presumably can be compensated for by the presence of multiple alternate acyltransferases capable of carrying out similar reactions.
CHO-K1 cells contain high levels of MADAG in their lipid droplets (11). When [14C]oleate was used to trace the synthesis of neutral lipids, the generation of MADAG was readily detected (Fig. 5). The DGAT1 selective inhibitor, XP-620, inhibited intracellular MADAG synthesis in a dose-dependent manner; at levels above 0.3 μM, XP-620 completely blocked the synthesis of MADAG. In addition, the IC50 values derived from the inhibition curve of intracellular synthesis of MADAG were in good agreement with inhibitory curves of intracellular TAG synthesis and in vitro enzymatic inhibition curves. These data suggest that DGAT1 is the predominant acyltransferase responsible for the synthesis of MADAG in CHO-K1 cells.
The physiological functions of neutral ether lipid synthesis are currently unclear. The process is likely involved in the absorption and transportation of dietary ether lipids, as well as the regulation and storage of bioactive ether lipids. Marine species, such as certain sharks or rat fish, contain rich sources of alkylglycerol in their liver oil. Indeed, multiple beneficial effects have been reported from traditional medicine supplements of dietary shark liver oil in countries such as Japan, Norway, or Iceland (31). DGAT1, MGAT2, and MGAT3 are expressed at high levels in human small intestines. That there is little reduction of MADAG synthesis in the jejunum of DGAT1 KO mice compared with WT mice (Fig. 6) is consistent with the hypothesis that multiple acyltransferases may play important roles in the absorption of dietary alkylglycerols from sources such as shark liver oil. When there are excess amounts of ether lipids present (as when supplied from diet), MADAG may be synthesized and stored in lipid droplets for future use. When ether lipid levels are low, lipases (such as lysosomal acid lipase) may hydrolyze the ester bonds of MADAG and liberate alkylglycerols for signaling purposes or to serve as precursors for the synthesis of phospho ether lipids.
MADAG accumulation in the affected tissues lacking lysosomal acid lipase suggests that MADAG metabolism is involved in the pathogenesis of Wolman’s disease. Our current study identifies several enzymes that can catalyze MADAG synthesis. Their identification could facilitate further investigation in neutral ether lipid metabolism.
Supplementary Material
Acknowledgments
The authors thank Chongqing Sun for the synthesis of 1-MAkG and Ching-Hsuen Chu for providing several recombinant acyltransferase enzymes.
Footnotes
Abbreviations:
- AWAT
- acyl-CoA:wax-alcohol acyltransferase
- CE
- cholesteryl ester
- DAG
- diacylglycerol
- DC
- DGAT candidate
- DGAT
- acyl-CoA:diacylglycerol acyltransferase
- MADAG
- monoalkyl-diacylglycerol
- 1-MAkG
- 1-monoalkylglycerol
- MAMAG
- monoalkyl-monoacylglycerol
- MGAT
- acyl-CoA:monoacylglycerol acyltransferase
- 1-MOG
- 1-monooleoylglycerol
- MTBE
- methyl-tert-butyl-ether
- SFM
- serum-free medium
- TAG
- triacylglycerol
This work was supported, in part, by Office of Extramural Research, National Institutes of Health Grant R01DK088210 (to C-L.E.Y.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Grossi V., Mollex D., Vincon-Laugier A., Hakil F., Pacton M., and Cravo-Laureau C.. 2015. Mono- and dialkyl glycerol ether lipids in anaerobic bacteria: biosynthetic insights from the mesophilic sulfate-reducer Desulfatibacillum alkenivorans PF2803T. Appl. Environ. Microbiol. 81: 3157–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos V. L. C. S., Billett D. S. M., and Wolff G. A.. 2002. 1-O-alkylglyceryl ether lipids of the gut walls and contents of an abyssal holothurian (Oneirophanta mutabilis). J. Braz. Chem. Soc. 13: 653–657. [Google Scholar]
- 3.Bordier C. G., Sellier N., Foucault A. P., and Le Goffic F.. 1996. Purification and characterization of deep sea shark Centrophorus squamosus liver oil 1-O-alkylglycerol ether lipids. Lipids. 31: 521–528. [DOI] [PubMed] [Google Scholar]
- 4.Hallgren B., and Larsson S.. 1962. The glyceryl ethers in man and cow. J. Lipid Res. 3: 39–43. [Google Scholar]
- 5.Yamamoto N., St Claire D. A. Jr., Homma S., and Ngwenya B. Z.. 1988. Activation of mouse macrophages by alkylglycerols, inflammation products of cancerous tissues. Cancer Res. 48: 6044–6049. [PubMed] [Google Scholar]
- 6.Acevedo R., Gil D., del Campo J., Bracho G., Valdes Y., and Perez O.. 2006. The adjuvant potential of synthetic alkylglycerols. Vaccine. 24 (Suppl. 2): S2–32–S2–33. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto S., Olson A. C., Berdel W. E., and Vogler W. R.. 1988. Purging of acute myeloid leukemic cells by ether lipids and hyperthermia. Blood. 72: 1777–1783. [PubMed] [Google Scholar]
- 8.Brohult A., Brohult J., and Brohult S.. 1978. Regression of tumour growth after administration of alkoxyglycerols. Acta Obstet. Gynecol. Scand. 57: 79–83. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin D. I., Cozzo A., Ji X., Roberts L. S., Louie S. M., Mulvihill M. M., Luo K., and Nomura D. K.. 2013. Ether lipid generating enzyme AGPS alters the balance of structural and signaling lipids to fuel cancer pathogenicity. Proc. Natl. Acad. Sci. USA. 110: 14912–14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin H. J., Lie Ken Jie M. S., and Ho F. C.. 1976. Accumulation of glyceryl ether lipids in Wolman’s disease. J. Lipid Res. 17: 53–56. [PubMed] [Google Scholar]
- 11.Bartz R., Li W. H., Venables B., Zehmer J. K., Roth M. R., Welti R., Anderson R. G., Liu P., and Chapman K. D.. 2007. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 48: 837–847. [DOI] [PubMed] [Google Scholar]
- 12.Chang C. C., Huh H. Y., Cadigan K. M., and Chang T. Y.. 1993. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J. Biol. Chem. 268: 20747–20755. [PubMed] [Google Scholar]
- 13.Cases S., Stone S. J., Zhou P., Yen E., Tow B., Lardizabal K. D., Voelker T., and Farese R. V. Jr. 2001. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 276: 38870–38876. [DOI] [PubMed] [Google Scholar]
- 14.Cases S., Novak S., Zheng Y. W., Myers H. M., Lear S. R., Sande E., Welch C. B., Lusis A. J., Spencer T. A., Krause B. R., et al. 1998. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem. 273: 26755–26764. [DOI] [PubMed] [Google Scholar]
- 15.Yen C. L., Stone S. J., Cases S., Zhou P., and Farese R. V. Jr. 2002. Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc. Natl. Acad. Sci. USA. 99: 8512–8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yen C. L., and Farese R. V. Jr. 2003. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 278: 18532–18537. [DOI] [PubMed] [Google Scholar]
- 17.Cao J., Lockwood J., Burn P., and Shi Y.. 2003. Cloning and functional characterization of a mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J. Biol. Chem. 278: 13860–13866. [DOI] [PubMed] [Google Scholar]
- 18.Cheng D., Nelson T. C., Chen J., Walker S. G., Wardwell-Swanson J., Meegalla R., Taub R., Billheimer J. T., Ramaker M., and Feder J. N.. 2003. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J. Biol. Chem. 278: 13611–13614. [DOI] [PubMed] [Google Scholar]
- 19.Turkish A. R., Henneberry A. L., Cromley D., Padamsee M., Oelkers P., Bazzi H., Christiano A. M., Billheimer J. T., and Sturley S. L.. 2005. Identification of two novel human acyl-CoA wax alcohol acyltransferases: members of the diacylglycerol acyltransferase 2 (DGAT2) gene superfamily. J. Biol. Chem. 280: 14755–14764. [DOI] [PubMed] [Google Scholar]
- 20.Cheng J. B., and Russell D. W.. 2004. Mammalian wax biosynthesis. II. Expression cloning of wax synthase cDNAs encoding a member of the acyltransferase enzyme family. J. Biol. Chem. 279: 37798–37807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yen C. L., Brown C. H. IV, Monetti M., and Farese R. V. Jr. 2005. A human skin multifunctional O-acyltransferase that catalyzes the synthesis of acylglycerols, waxes, and retinyl esters. J. Lipid Res. 46: 2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng D., Chang C. C., Qu X., and Chang T. Y.. 1995. Activation of acyl-coenzyme A:cholesterol acyltransferase by cholesterol or by oxysterol in a cell-free system. J. Biol. Chem. 270: 685–695. [DOI] [PubMed] [Google Scholar]
- 23.Orland M. D., Anwar K., Cromley D., Chu C. H., Chen L., Billheimer J. T., Hussain M. M., and Cheng D.. 2005. Acyl coenzyme A dependent retinol esterification by acyl coenzyme A: diacylglycerol acyltransferase 1. Biochim. Biophys. Acta. 1737: 76–82. [DOI] [PubMed] [Google Scholar]
- 24.Yen C. L., Monetti M., Burri B. J., and Farese R. V. Jr. 2005. The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J. Lipid Res. 46: 1502–1511. [DOI] [PubMed] [Google Scholar]
- 25.Cheng D., Iqbal J., Devenny J., Chu C. H., Chen L., Dong J., Seethala R., Keim W. J., Azzara A. V., Lawrence R. M., et al. 2008. Acylation of acylglycerols by acyl coenzyme A:diacylglycerol acyltransferase 1 (DGAT1). Functional importance of DGAT1 in the intestinal fat absorption. J. Biol. Chem. 283: 29802–29811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith S. J., Cases S., Jensen D. R., Chen H. C., Sande E., Tow B., Susan D. A., Raber J., Eckel R. H., and Farese R. V. Jr. 2000. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat. Genet. 25: 87–90. [DOI] [PubMed] [Google Scholar]
- 27.Chen H. C., Smith S. J., Tow B., Elias P. M., and Farese R. V. Jr. 2002. Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J. Clin. Invest. 109: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cases S., Zhou P., Shillingford J. M., Wiseman B. S., Fish J. D., Angle C. S., Hennighausen L., Werb Z., and Farese R. V. Jr. 2004. Development of the mammary gland requires DGAT1 expression in stromal and epithelial tissues. Development. 131: 3047–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson D. W., Gao Y., Spencer N. M., Banh T., and Yen C. L.. 2011. Deficiency of MGAT2 increases energy expenditure without high-fat feeding and protects genetically obese mice from excessive weight gain. J. Lipid Res. 52: 1723–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson D. W., Gao Y., Yen M. I., and Yen C. L.. 2014. Intestine-specific deletion of acyl-CoA:monoacylglycerol acyltransferase (MGAT) 2 protects mice from diet-induced obesity and glucose intolerance. J. Biol. Chem. 289: 17338–17349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deniau A. L., Mosset P., Pedrono F., Mitre R., Le Bot D., and Legrand A. B.. 2010. Multiple beneficial health effects of natural alkylglycerols from shark liver oil. Mar. Drugs. 8: 2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.