Abstract
Mechanisms underlying the opposite effects of transmembrane 6 superfamily member 2 (TM6SF2) rs58542926 C>T polymorphism on liver injury and cardiometabolic risk in nonalcoholic fatty liver disease (NAFLD) are unclear. We assessed the impact of this polymorphism on postprandial lipoprotein metabolism, glucose homeostasis, and nutrient oxidation in NAFLD. Sixty nonobese nondiabetic normolipidemic biopsy-proven NAFLD patients and 60 matched controls genotyped for TM6SF2 C>T polymorphism underwent: indirect calorimetry; an oral fat tolerance test with measurement of plasma lipoprotein subfractions, adipokines, and incretin glucose-dependent insulinotropic polypeptide (GIP); and an oral glucose tolerance test with minimal model analysis of glucose homeostasis. The TM6SF2 T-allele was associated with higher hepatic and adipose insulin resistance, impaired pancreatic β-cell function and incretin effect, and higher muscle insulin sensitivity and whole-body fat oxidation rate. Compared with the TM6SF2 C-allele, the T-allele entailed lower postprandial lipemia and nefaemia, a less atherogenic lipoprotein profile, and a postprandial cholesterol (Chol) redistribution from smaller atherogenic lipoprotein subfractions to larger intestinal and hepatic VLDL1 subfractions. Postprandial plasma VLDL1-Chol response independently predicted the severity of liver histology. In conclusion, the TM6SF2 C>T polymorphism affects nutrient oxidation, glucose homeostasis, and postprandial lipoprotein, adipokine, and GIP responses to fat ingestion independently of fasting values. These differences may contribute to the dual and opposite effect of this polymorphism on liver injury and cardiometabolic risk in NAFLD.
Keywords: nonalcoholic steatohepatitis, cholesterol, lipoprotein subfractions, lipemia, nonalcoholic fatty liver disease, transmembrane 6 superfamily member 2
Nonalcoholic fatty liver disease (NAFLD) confers an increased risk of liver-related complications [largely limited to its progressive form, nonalcoholic steatohepatitis (NASH)], type 2 diabetes (T2DM), and CVD (1, 2). The wide inter-individual variability in the risk of hepatic and extra-hepatic complications in NAFLD may reflect the interplay between genetic and environmental factors. While in the general population an association between the type and amount of dietary fat and the development of obesity, CVD, and T2DM has been demonstrated (3), data linking dietary fat to the presence and severity of NAFLD are controversial (4, 5). We hypothesized that a genetically determined susceptibility to dietary fat lipotoxicity modulates liver injury and cardiometabolic risk in NAFLD.
The SNP, rs58542926 C>T, in the transmembrane 6 superfamily member 2 (TM6SF2) gene has recently been linked to the severity of NAFLD in genome-wide association studies (6, 7): the TM6SF2 T-allele, encoding the E167K amino acidic substitution, results in reduced transcript levels of its product protein, which is expressed in humans in the liver, intestine, adipose tissue, and pancreatic β-cells and has unclear biological function (8, 9).
The TM6SF2 C>T variant has been linked to a reduced LDL-cholesterol (LDL-C) level and cardiovascular risk and to an increased risk of T2DM (10, 11). Mechanisms connecting the TM6SF2 C>T polymorphism to liver injury and cardiometabolic risk are unclear. The impaired hepatic VLDL secretion associated with the TM6SF2 T-allele (8, 9) may not be the main mechanism mediating NASH, as enhanced lipid storage into neutral triglycerides (Tgs) protects against liver injury (12). Furthermore, the reduced CVD risk associated with the TM6SF2 T-allele is not fully explained by lower fasting cholesterol (Chol) levels (13). Postprandial lipemia is an emerging cardiometabolic risk factor, independently of fasting lipid levels (14), and dietary fat lipotoxicity has been implicated in liver injury in NASH (3–5): Hypothesizing that dietary fat lipotoxicity may mediate the impact of TM6SF2 on liver disease and cardiometabolic risk in NAFLD, we assessed the effect of the TM6SF2 C>T variant on postprandial lipoprotein metabolism and on glucose homeostasis in biopsy-proven NAFLD patients and healthy controls.
METHODS
Participants
There are no data on the impact of the TM6SF2 C>T variant on postprandial lipoprotein metabolism and glucose homeostasis. Based on available data on the impact of the TM6SF2 C>T variant on fasting lipid levels (6–8, 10) and on the impact of NAFLD on lipoprotein and glucose metabolism (12, 15), considering a type I error of 0.05 and a type II error of 0.20, at least 18 T-allele carriers per arm were needed to detect a significant difference in parameters related to lipoprotein metabolism [incremental area under the curve (IAUC) Tg and LDL-C] and glucose homeostasis (whole-body and tissue insulin sensitivity, β-cell function) within different TM6SF2 genotypes in NAFLD patients.
As obesity, dyslipidemia, and diabetes may modify the effect of the TM6SF2 C>T variant on glucose/lipid metabolism, adipokines, and liver disease, subjects with obesity (BMI ≥30 kg/m2), diabetes [fasting plasma glucose ≥126 mg/dl or plasma glucose ≥200 mg/dl at +2 h on oral glucose tolerance test (OGTT) or antidiabetic drugs], overt dyslipidemia (fasting serum Chol ≥200 mg/dl or plasma Tg ≥200 mg/dl), or clinical signs/symptoms of CVD were excluded.
Sixty nonobese nondiabetic normolipidemic biopsy-proven NAFLD patients referred to two hepato-metabolic clinics were included (criteria for diagnosis of NAFLD are detailed in the supplemental Appendix). Each pathological feature of liver biopsy was read by a single pathologist (Renato Parente, HUMANITAS Gradenigo) blinded to the patients’ clinical-biochemical characteristics and scored according to the NASH Clinical Research Network criteria; NASH was defined according to current recommendations (1).
Sixty randomly identified healthy controls, i.e., nondiabetic nonobese normolipidemic individuals without evidence of CVD, randomly selected from a population-based cohort study, matched for TM6SF2 C>T genotype, age, gender, BMI, and waist circumference were included (12). Criteria to rule out NAFLD in controls are detailed in the supplemental Appendix.
Patients and controls were characterized for lifestyle habits, routine biochemistry, adipokine profile, markers of inflammation, and endothelial dysfunction, as detailed below. The homeostatic model assessment of insulin resistance (HOMA-IR) index was calculated as the product of the fasting glucose and insulin concentration divided by 22.5 (16). Participants gave their consent to the study, which was conducted according to the Helsinki Declaration and was approved by the Institutional Review Board of San Giovanni Battista Hospital, Turin, Italy.
Genetic analyses.
Genotyping for the TM6SF2 rs58542926 C/T SNP utilized the real-time allele discrimination method, using the TaqMan allelic discrimination assay (Applied Biosystems, Foster City, CA). The TaqMan genotyping reaction was run on a 7300HT fast real-time PCR (Applied Biosystems). We also genotyped our population for the PNPLA3 SNP, rs738409 C/G, and for the apoE genotype, which have been previously linked to both NAFLD and lipid metabolism (17), to assess their interference with outcome variables (detailed in the supplemental Appendix).
Dietary and physical activity record.
Participants filled in the validated European Prospective Investigation into Cancer and Nutrition (EPIC) 7 day alimentary questionnaire and the Minnesota-Leisure-Time-Physical-Activity questionnaire, and data were analyzed as described in the supplemental Appendix.
Anthropometry.
Percent body fat was estimated by the bioelectrical impedance analysis method (TBF-202; Tanita, Tokyo, Japan), closely correlating with dual X-ray absorption (18). Abdominal visceral fat area (square centimeters) was estimated using Stanforth equations validated against computed tomography in blacks and Caucasians (19).
Indirect calorimetry and substrate oxidation rates.
After an overnight (12 h) fast, participants underwent indirect calorimetry measurement of oxygen consumption (VO2) and carbon dioxide production (VCO2) using an open circuit indirect calorimeter with a ventilated-hood system (Deltatrac™ II; Datex Instrumentarium Corp., Helsinki, Finland) (see supplemental Appendix). Whole-body respiratory quotient (RQ) and nonproteic RQ (npRQ) were calculated as VCO2/VO2. Resting energy expenditure (REE) and whole-body carbohydrate (CHO) oxidation (CHOox) and fat oxidation (Fatox) rates were calculated from VO2 and VCO2 by using stoichiometric equations and appropriate energy equivalents (20). REE and substrate oxidation rates were corrected for fat-free mass (FFM).
Markers of cardiovascular risk/endothelial dysfunction and adipokines.
Serum C-reactive protein (CRP) and soluble adhesion molecules, E-selectin and intercellular adhesion molecule (ICAM)-1, were measured as validated markers of CVD risk, endothelial dysfunction, and subclinical atherosclerosis (21, 22) (detailed in the supplemental Appendix). Circulating adipokines, adiponectin, TNF-α, resistin, and leptin, were measured by immunoenzymatic methods (see the supplemental Appendix).
OGTT-derived indexes of glucose homeostasis.
Participants underwent a standard 75 g OGTT and indexes of glucose homeostasis were calculated (detailed in the supplemental Appendix). Whole-body oral glucose insulin sensitivity index (OGIS) and hepatic and muscle insulin resistance (IR) indexes were calculated as previously proposed and validated against clamp in nondiabetic subjects (23, 24). The adipose tissue IR index was calculated as fasting NEFAs × fasting insulin (15). The minimal model technique was used to calculate the following indexes of β-cell function: the insulinogenic index (IGI), the CP-genic index (CGI), and the two integrated indexes of β-cell function, the disposition index (DI) and adaptation index (AI), which relate β-cell insulin secretion to IR. The DI and AI were previously validated against the frequently sampled intravenous glucose tolerance test in NAFLD and nondiabetic subjects (21, 25), and reliably predict T2DM development (26).
Incretin effect
To assess whether differences in β-cell function were related to a reduced incretin stimulatory effect on β-cells, a frequently sampled intravenous glucose tolerance test was performed and the incretin effect, i.e., the effectiveness of ingested glucose in stimulating β-cell insulin secretion compared with intravenous glucose, was assessed (see the supplemental Appendix).
Oral fat tolerance test.
Participants underwent a 10 h oral fat tolerance test (OFTT) (14) with measurement of the following parameters (methods detailed in the supplemental Appendix): 1) Plasma total Chol, Tg, NEFA, and HDL-cholesterol (HDL-C). 2) Tg-rich lipoprotein (TRLP) subfractions and LDL. TRLPs were isolated through preparative ultracentrifugation and their total Tg and Chol content was subsequently measured as described in the supplemental Appendix. Two VLDL subfractions with decreasing Sf values (VLDL1: Sf >100; VLDL2: Sf = 20–100) were separated and their Chol and Tg content was determined (see supplemental Appendix). VLDL apoB48 and apoB100 were separated by SDS-polyacrylamide gel electrophoresis using 3.9% gel (detailed in supplemental Appendix). LDL-C content was measured with a standardized homogeneous enzymatic colorimetric method in order to avoid Tg effects on LDL determination (Sentinel) (see supplemental Appendix). 3) Lipid-induced oxidative stress: oxidized LDLs (oxLDLs). LDL conjugated dienes, validated markers of oxLDLs, were determined by capillary electrophoresis (detailed in supplemental Appendix). 4) Glucose-dependent insulinotropic polypeptide (GIP), adiponectin, and resistin. GIP is an emerging modulator of lipid metabolism independently of its incretin effect on pancreatic β-cell function. Dietary fat is the most potent stimulator of GIP secretion (27) and TM6SF2 protein is expressed by human intestinal cells (12); furthermore, acute and chronic administration of GIP, but not of glucagon-like peptide-1, reduces Fatox and energy expenditure (28), induces adipocyte dysfunction and proinflammatory adipokine secretion (29), and promotes development of obesity-associated metabolic disorders (30), including NAFLD, which were all reversed by GIP antagonists (28). Plasma GIP, as well as resistin and adiponectin, which have been linked to both liver disease severity and lipoprotein metabolism in NAFLD, were measured as detailed in the supplemental Appendix.
Statistical analysis
Differences across groups were analyzed by ANOVA followed by Bonferroni correction when variables were normally distributed; otherwise, the Kruskal-Wallis test, followed by the post hoc Dunn test, was used. Normality was evaluated by the Shapiro-Wilk test. The Fisher or chi-square test was used to compare categorical variables, as appropriate. Hardy-Weinberg equilibrium was assessed using the χ2 test. To adjust for multiple comparison testing, the Benjamini-Hochberg false discovery rate correction was applied to raw P values in all comparisons; significance was set at an adjusted P value threshold of 0.05 (31). The area under the curve (AUC) and IAUC of parameters measured during the OFTT and the OGTT were computed by the trapezoid method. Due to the low prevalence of TM6SF2 TT homozygotes and to the overlapping clinical characteristics with heterozygous CT carriers, TM6SF2 TT carriers were combined with CT heterozygotes for group comparisons. Differences were considered statistically significant at P < 0.05.
Analysis of dietary, anthropometric, and metabolic parameters and of genetic polymorphisms was made using the Spearman correlation test to assess correlation among different variables. Based on available evidence (6–8, 10), the TM6SF2 C>T variant was modeled as a dominant model of inheritance, that is, quantitative predictor variables reflecting the number of risk alleles (0, 1, or 2).
When a relation was found on univariate analysis, multivariate logistic regression was used to identify independent predictors of selected outcome variables of interest, namely: 1) for liver histology, the presence of NASH and of advanced (stage 3) fibrosis; 2) for CVD risk, serum CRP and endothelial adhesion molecules, E-selectin and ICAM-1; 3) for whole-body nutrient oxidation rates, CHOox and Fatox; 4) for glucose homeostasis, OGTT-derived parameters of whole-body/tissue IR and of β-cell function; and 5) for postprandial lipid metabolism, the IAUC of Tg, LDL-C, oxLDL, and of the main TRLP subfractions. For this analysis, continuous variables were divided into quartiles and independent predictors of the highest quartile of outcome variables were assessed after log transformation of skewed data. The independent predictors were those variables found to be related to the outcome variables on univariate analysis. Data are expressed as mean ± SEM, unless otherwise specified (STATISTICA software, 5.1; Statsoft Italia, Padua, Italy).
RESULTS
Subjects’ characteristics
The main features of patients and controls grouped according to the TM6SF2 C>T genotype are reported in Table 1. In study participants, the prevalence of TM6SF2 CC homozygotes was 64%, of CT heterozygotes was 34%, and of TT carriers was 2%. The distribution of the TM6SF2 CT genotype was in Hardy-Weinberg equilibrium (6, 7, 8). NAFLD, as a group, had higher HOMA, serum CRP, and endothelial adhesion molecules, E-selectin and ICAM-1, and lower HDL-C and adiponectin than controls. Within NAFLD patients and controls, TM6SF2 CT/TT carriers showed lower serum CRP and endothelial adhesion molecules than TM6SF2 CC genotype carriers (Table 1). Among NAFLD patients, 42% had NASH and 16% had advanced fibrosis. The TM6SF2 T-allele carriers had more severe liver histology than their counterpart genotype (Table 1).
TABLE 1.
Main clinical, biochemical, and histological parameters of biopsy-proven NAFLD patients and controls grouped according to TM6SF2 C/T polymorphism (n = 120)
| Controls | NAFLD | |||||
| TCM6F2 CC (n = 40) | TCM6F2 CT/TT (n = 20) | P | TM6SF2 CC (n = 40) | TM6SF2 CT/TT (n = 20) | P | |
| Age (years) | 42 ± 2 | 42 ± 2 | 0.851 | 42 ± 2 | 40 ± 2 | 0.851 |
| Sex (% males) | 68 | 65 | 0.693 | 68 | 65 | 0.693 |
| BMI (kg/m2) | 25.6 ± 0.5 | 25.9 ± 0.6 | 0.731 | 25.6 ± 0.5 | 25.8 ± 0.6 | 0.690 |
| Fat mass (%) | 22 ± 2 | 22 ± 2 | 0.872 | 23 ± 2 | 22 ± 2 | 0.232 |
| Waist (cm) | 89 ± 3 | 90 ± 4 | 0.482 | 89 ± 2 | 90 ± 2 | 0.426 |
| WHR | 0.91 ± 0.02 | 0.91 ± 0.03 | 0.756 | 0.92 ± 0.03 | 0.92 ± 0.03 | 0.731 |
| AVF (cm2) | 99 ± 5 | 103 ± 6 | 0.731 | 101 ± 5 | 97 ± 6 | 0.832 |
| Smokers (%) | 31 | 30 | 0.410 | 33 | 31 | 0.390 |
| Systolic BP (mm Hg) | 118 ± 3 | 123 ± 2 | 0.291 | 121 ± 2 | 127 ± 2 | 0.280 |
| Diastolic BP (mm Hg) | 80 ± 2 | 84 ± 2 | 0.130 | 83 ± 2 | 87 ± 5 | 0.122 |
| AST (U/l) | 15 ± 1 | 16 ± 2 | 0.591 | 32 ± 2 | 41 ± 4c | 0.131 |
| ALT (U/l) | 19 ± 2 | 2312 | 0.678 | 70 ± 5 | 88 ± 6c | 0.111 |
| GGT (U/l) | 35 ± 5 | 43 ± 4 | 0.702 | 89 ± 16 | 108 ± 18 | 0.089 |
| Tg (mg/dl) | 98 ± 211 | 86 ± 10 | 0.879 | 94 ± 17 | 85 ± 13 | 0.561 |
| Total Chol (mg/dl) | 179 ± 9 | 168 ± 7 | 0.311 | 187 ± 11 | 173 ± 12 | 0.132 |
| HDL-C (mg/dl) | 54 ± 2 | 55 ± 2 | 0.210 | 52 ± 2b | 54 ± 2c | 0.118 |
| LDL-C (mg/dl) | 103 ± 6 | 94 ± 6 | 0.131 | 107 ± 5 | 95 ± 10 | 0.210 |
| Glucose (mg/dl) | 99 ± 3 | 90 ± 3 | 0.394 | 100 ± 10 | 90 ± 7 | 0.273 |
| Insulin (μU/ml) | 7.2 ± 1.8 | 6.3 ± 1.2 | 0.569 | 13.7 ± 3.8 | 15.9 ± 6.4 | 0.543 |
| HOMA-IR | 1.9 ± 0.9 | 1.3 ± 0.8 | 0.298 | 3.55 ± 1.1 | 2.9 ± 0.90 | 0.220 |
| METS (h/week) | 21.2 ± 1.0 | 22.2 ± 1.7 | 0.413 | 22.7 ± 1.5 | 21.9 ± 1.4 | 0.639 |
| RQ | 0.81 ± 0.01 | 0.77 ± 0.01 | 0.001 | 0.81 ± 0.01 | 0.78 ± 0.01 | 0.003 |
| npRQ | 0.81 ± 0.02 | 0.76 ± 0.01 | 0.001 | 0.82 ± 0.01 | 0.77 ± 0.01 | 0.0009 |
| REE (kcal/24 h/kg/FFM) | 29.5 ± 1.8 | 29.9 ± 2.0 | 0.711 | 29.7 ± 1.5 | 28.4 ± 1.7 | 0.302 |
| Fatox (mg/kg/FFM/min) | 1.23 ± 0.05 | 1.54 ± 0.05 | 0.0009 | 1.22 ± 0.06 | 1.50 ± 0.08 | 0.002 |
| CHOox (mg/kg/FFM/min) | 2.00 ± 0.10 | 1.42 ± 0.11 | 0.001 | 1.99 ± 0.11 | 1.45 ± 0.10 | 0.002 |
| Hs-CRP (mg/l) | 1.9 ± 0.2 | 1.1 ± 0.4 | 0.009 | 3.1 ± 02a | 2.0 ± 02b | 0.001 |
| E-selectin (ng/ml) | 31.1 ± 3.1 | 20.1 ± 4.6 | 0.010 | 51.3 ± 4.8a | 28.9 ± 3.1b | 0.002 |
| ICAM-1(ng/ml) | 239.1 ± 4.6 | 191.8 ± 5.3 | 0.028 | 285.1 ± 5.2a | 228.6 ± 6.0b | 0.009 |
| TNF-α (pg/ml) | 1.20 ± 0.18 | 1.08 ± 0.21 | 0.512 | 1.18 ± 0.17 | 0.99 ± 0.25 | 0.471 |
| Leptin (pg/ml) | 1,830 ± 399 | 1,793 ± 224 | 0.430 | 1,746 ± 275 | 1,914 ± 201 | 0.711 |
| ApoE genotype (%) | ||||||
| 2-3 | 16 | 14 | 0.573 | 14 | 16 | 0.689 |
| 3-3 | 66 | 67 | 0.312 | 67 | 67 | 0.911 |
| 3-4 | 18 | 19 | 0.690 | 19 | 17 | 0.892 |
| PNPLA3 (%) | ||||||
| CC | 41 | 55 | 0.671 | 41 | 55 | 0.671 |
| CG | 41 | 33 | 0.312 | 41 | 33 | 0.312 |
| GG | 8 | 12 | 0.218 | 8 | 12 | 0.218 |
| Abdominal obesity (%) | 17 | 20 | 0.691 | 17 | 20 | 0.691 |
| IGR (%) | 19 | 8 | 0.231 | 21 | 10 | 0.289 |
| Hypertension (%) | 30 | 27 | 0.379 | 51 | 49 | 0.592 |
| Low HDL-C (%) | 13 | 9 | 0.398 | 16 | 9 | 0.401 |
| High Tg (%) | 13 | 9 | 0.412 | 14 | 8 | 0.379 |
| Met sy (%) | 37 | 29 | 0.311 | 40§ | 31c | 0.297 |
| Steatosis (% hep.) | — | — | — | 25 ± 3 | 32 ± 4 | 0.168 |
| NAFLD activity score | — | 2.0 ± 0.2 | 4.0 ± 0.3 | 0.0001 | ||
| Fibrosis stage | — | — | — | 0.2 ± 0.1 | 1.0 ± 0.2 | 0.0001 |
| NASH (%) | — | — | — | 31 | 61 | 0.045 |
Data are presented as mean ± SEM, unless otherwise specified. Statistically significant P values are in bold. AVF, abdominal visceral fat area; BP, blood pressure; hs-CRP, highly sensitive CRP; WHR, waist-on-hip ratio; IGR, impaired glucose regulation; METS, metabolic equivalent of activity; Met sy, metabolic syndrome (according to the joint statement of the American Diabetes Association, the International Diabetes Federation, and the National Heart, Lung, and Blood Institute); MTP, microsomal Tg transfer protein; SREBF, sterol regulatory element-binding factor. Met sy requires the presence of three or more of the following criteria: 1) abdominal obesity, waist circumference ≥102 cm (males) and ≥88 cm (females); 2) high Tgs, ≥150 mg/dl (1.7 mmol/l) or on drug treatment for elevated Tgs; 3) low HDL-C, <40 mg/dl (1.0 mmol/l) (males) or <50 mg/dl (1.3 mmol/l) (females) or on drug treatment for reduced HDL-C; 4) hypertension, systolic BP ≥130 mm Hg and/or diastolic BP ≥85 mm Hg or on drug treatment; 5) high fasting plasma glucose (FPG): FPG ≥100 mg/dl (5.6 mmol/l) or on drug treatment for elevated glucose.
P < 0.01 versus controls.
P < 0.05 versus controls bearing the same genotype.
P < 0.01 versus controls bearing the same genotype.
Alimentary record
There was no difference in daily total energy, macro- and micro-nutrients, types of fat, and antioxidant vitamin intake between patients with NAFLD and controls and among different TM6SF2 genotypes (not shown).
Indirect calorimetry
While the TM6SF2 C>T variant did not affect REE, the proportion of energy derived from Fatox and CHOox differed between TM6SF2 genotypes: TM6SF2 T-allele carriers had lower RQ and npRQ, indicating that they oxidized more fat and less CHO than CC homozygotes (Table 1).
OGTT-derived indexes of glucose homeostasis
The time course of plasma glucose and serum insulin during the OGTT is reported in supplemental Fig. S1. In patients and controls, TM6SF2 T-allele carriers showed higher hepatic and adipose IR and enhanced muscle insulin sensitivity compared to CC homozygotes. The TM6SF2 CT/TT genotype also displayed impaired pancreatic β-cell function and incretin effect compared to CC homozygotes (Table 2).
TABLE 2.
OGTT-derived indexes of glucose homeostasis in patients with biopsy-proven NAFLD and controls, grouped according to TM6SF2 rs58542926 C/T genotype (n = 120)
| TM6SF2 C/T Genotype | ||||||
| Controls | NAFLD | |||||
| CC (n = 40) | CT/TT (n = 20) | P | CC (n = 40) | CT/TT (n = 20) | P | |
| OGIS (ml min−1 m−2) | 427.9 ± 13.5 | 442.6 ± 15.1 | 0.318 | 385.5 ± 7.4a | 392.2 ± 11.0a | 0.810 |
| Hepatic IR (g/dl glucose·μU/mlIns·min−2) | 2,615.7 ± 126.4 | 3,298.4 ± 173.5 | 0.001 | 4,180.1 ± 107.4a | 4,779.7 ± 182.1b | 0.002 |
| Muscle IS | 0.014 ± 0.002 | 0.021 ± 0.001 | 0.028 | 0.012 ± 0.001 | 0.018 ± 0.002 | 0.002 |
| Adipose IR (mmol/l/pmol/l) | 21.2 ± 2.0 | 30.1 ± 1.4 | 0.0004 | 48.6 ± 4.2b | 88.4 ± 6.8b | 0.0001 |
| Hepatic extraction (%) | 74 ± 3 | 72 ± 4 | 0.414 | 73 ± 5 | 69 ± 7 | 0.582 |
| IGI (μUinsulin g−1glucose) | 187 ± 11 | 112 ± 14 | 0.009 | 171 ± 19c | 106 ± 13b | 0.001 |
| CGI (ngC-pep g −1glucose) | 511 ± 12 | 401 ± 11 | 0.0009 | 502 ± 13c | 394 ± 16b | 0.001 |
| DI (μUinsulin g−1glucose ml−1 m−2) | 80,124 ± 4,318 | 48,615 ± 4,379 | 0.001 | 52,136 ± 3,615c | 37,639 ± 1,713b | 0.0001 |
| AI (ngC-pep g−1glucose ml-1 m−2) | 220,709 ± 12,138 | 175,241 ± 8,136 | 0.009 | 189,420 ± 8,372c | 142,671 ± 9,139b | 0.001 |
| Incretin effect (%) | 72.6 ± 3.2 | 47.3 ± 2.9 | 0.0002 | 70.9 ± 3.1 | 45.2 ± 4.1 | 0.0001 |
Data are presented as mean ± SEM, unless otherwise specified. Statistically significant P values are in bold. IS, insulin sensitivity.
P < 0.05 versus controls.
P < 0.01 versus. controls.
P < 0.05 versus controls bearing the same genotype.
OFTT
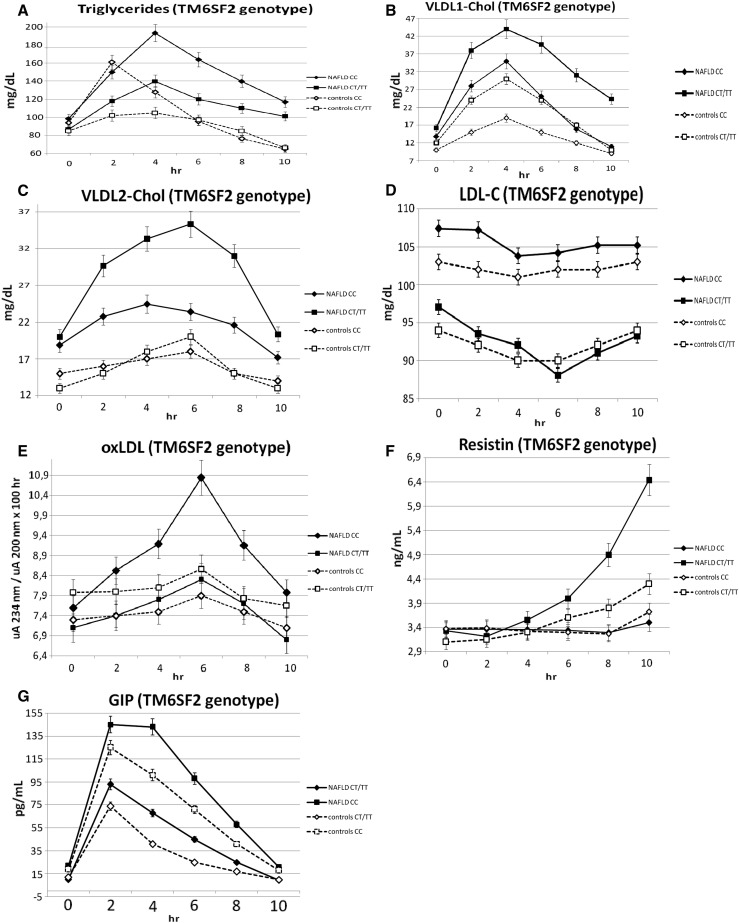
Within patients and controls, the TM6SF2 CT/TT genotype showed lower postprandial Tg, VLDL1-Tg, NEFA, and oxLDL responses, a higher increase in postprandial Chol content in the VLDL1 and VLDL2 subfractions of intestinal and hepatic origin, and a slight, but statistically significant, postprandial LDL-C decrease as compared with the TM6SF2 CC genotype (Table 3, Fig. 1A–D, supplemental Fig. S2). The TM6SF2 CT/TT genotype also showed lower postprandial GIP and higher resistin responses than homozygous CC carriers (Table 3, Fig. 1F, G).
TABLE 3.
OFTT parameters in patients with NAFLD and controls grouped according to TCM6F2 rs58542926 C/T genotype (n = 120)
| Parameter | Controls | NAFLD | ||||
| TCM6F2 CC (n = 40) | TCM6F2 CT/TT (n = 20) | P | TCM6F2 CC (n = 40) | TCM6F2 CT/TT (n = 20) | P | |
| Fasting Tg (mg/dl) | 98 ± 11 | 86 ± 10 | 0.812 | 94 ± 15 | 85 ± 18 | 0.513 |
| IAUC Tg (mg/dl × h) | 141 ± 12 | 79 ± 10 | 0.001 | 525 ± 21b | 297 ± 20b | 0.00001 |
| Fasting NEFA (mmol/l) | 0.35 ± 0.23 | 0.47 ± 0.28 | 0.712 | 0.50 ± 0.29 | 0.63 ± 0.31 | 0.711 |
| IAUC NEFA (mmol/l × h) | 1.93 ± 0.27 | 0.82 ± 0.15 | 0.00009 | 5.24 ± 0.22b | 2.31 ± 0.28c | 0.0001 |
| Fasting VLDL1-Tg (mg/dl) | 42 ± 9 | 40 ± 10 | 0.812 | 52 ± 12 | 36 ± 10 | 0.201 |
| IAUC VLDL1-Tg (mg/dl × h) | 408 ± 29 | 123 ± 14 | 0.0001 | 922 ± 37b | 497 ± 31c | 0.00002 |
| Fasting VLDL2-Tg (mg/dl) | 30 ± 7 | 31 ± 7 | 0.813 | 36 ± 8 | 42 ± 9 | 0.312 |
| IAUC VLDL2-Tg (mg/dl × h) | 56 ± 10 | 89 ± 13 | 0.301 | 137 ± 14 | 131 ± 19 | 0.611 |
| Fasting VLDL1-Chol (mg/dl) | 10 ± 2 | 12 ± 2 | 0.812 | 14 ± 4 | 16 ± 4 | 0.713 |
| IAUC VLDL1-Chol (mg/dl × h) | 41 ± 4 | 92 ± 7 | 0.00009 | 97 ± 9c | 199 ± 11b | 0.000001 |
| Fasting VLDL2-Chol (mg/dl) | 15 ± 3 | 13 ± 3 | 0.712 | 18 ± 3 | 20 ± 4 | 0.611 |
| IAUC VLDL2-Chol (mg/dl × h) | 11 ± 1 | 32 ± 2 | 0.00009 | 37 ± 2c | 108 ± 4c | 0.000001 |
| Fasting LDL-C (mg/dl) | 103 ± 6 | 94 ± 6 | 0.131 | 107 ± 5 | 95 ± 10 | 0.210 |
| IAUC LDL-C (mg/dl × h) | −10 ± 2 | −24 ± 2 | 0.003 | −20 ± 3c,d | −51 ± 3b | 0.0001 |
| Fasting VLDL1 apoB48 (mg/dl) | 2.1 ± 0.4 | 2..0 ± 0.5 | 0.812 | 2.7 ± 0.9 | 2.4 ± 0.9 | 0.511 |
| IAUC VLDL1 apoB48 (mg/dl × h) | 4.5 ± 0.9 | 1.9 ± 0.5 | 0.0002 | 8.7 ± 1.4b | 4.3 ± 1.0c | 0.00001 |
| Fasting VLDL2 apoB48 (mg/dl) | 1.8 ± 0.4 | 1.5 ± 0.4 | 0.509 | 2.3 ± 0.6 | 2.1 ± 0.7 | 0.421 |
| IAUC VLDL2 apoB48 (mg/dl × h) | 1.5 ± 0.3 | 2.9 ± 0.5 | 0.008 | 1.6 ± 0.3 | 5.8 ± 0.6b | 0.0001 |
| Fasting VLDL1 apoB100 (mg/dl) | 3.7 ± 1.0 | 3.5 ± 1.1 | 0.712 | 4.5 ± 1.6 | 4.2 ± 1.7 | 0.913 |
| IAUC VLDL1 apoB100 (mg/dl × h) | 10.0 ± 1.5 | 3.9 ± 0.9 | 0.00009 | 22.4 ± 3.5b | 11.7 ± 2.9c | 0.00001 |
| Fasting VLDL2 apoB100 (mg/dl) | 3.7 ± 0.7 | 3.2 ± 0.9 | 0.802 | 5.2 ± 0.9 | 4.8 ± 1.1 | 0.611 |
| IAUC VLDL2 apoB100 (mg/dl × h) | 4.6 ± 0.9 | 8.3 ± 1.0 | 0.015 | 13.8 ± 1.9b | 24.5 ± 2.6b | 0.00001 |
| Fasting LDL C.D. (uA 234 nm/uA 200 nm × 100) | 7.3 ± 1.6 | 7.9 ± 1.8 | 0.902 | 7.5 ± 1.8 | 7.1 ± 1.6 | 0.616 |
| IAUC LDL C.D. (uA 234 nm/uA 200 nm × 100 × h) | 2.1 ± 0.1 | 0.8 ± 0.2 | 0.0009 | 15.1 ± 1.0b | 5.2 ± 1.2a | 0.00001 |
| Fasting HDL-C (mg/dl) | 54 ± 2 | 55 ± 2 | 0.210 | 52 ± 2 | 54 ± 2 | 0,212 |
| IAUC HDL-C (mg/dl × h) | −14 ± 2 | 2 ± 1 | 0.0001 | −56 ± 4b | −18 ± 2c | 0.00009 |
| Fasting GIP (pg/ml) | 18.8 ± 6.4 | 16.5 ± 6.1 | 0.712 | 22.1 ± 9.5 | 11.9 ± 5.2 | 0.211 |
| IAUC GIP (pg/ml × h) | 571.9 ± 18.5 | 266.4 ± 20.1 | 0.000008 | 703.9 ± 20.1b | 379.6 ± 24.4 | 0.000002 |
| Fasting adiponectin (ng/ml) | 8,631 ± 782 | 9,515 ± 812 | 0.412 | 6,161 ± 572 | 5,575 ± 650 | 0.713 |
| IAUC adiponectin (ng/ml × h) | 11,071 ± 912 | 12,916 ± 926 | 0.513 | 1,768 ± 246 | 1,536 ± 494 | 0.423 |
| Fasting resistin (ng/ml) | 3.4 ± 0.9 | 3.1 ± 1.0 | 0.912 | 3.8 ± 0.9 | 3.3 ± 0.9 | 0.301 |
| IAUC resistin (ng/ml × h) | 0.1 ± 0.1 | 1.5 ± 0.3 | 0.008 | 2.8 ± 1.1a | 6.4 ± 11.9b | 0.0000001 |
Oral fat load parameters of patients with NAFLD and controls according to TM6SF2 genotype. Data are presented as mean ± SEM. Statistically significant P values are in bold. C.D., conjugated dienes.
P < 0.05 versus controls.
P < 0.01 versus controls.
P < 0.05 versus controls bearing the same genotype.
P < 0.01 versus controls bearing the counterpart genotype.
Fig. 1.
OFTT: postprandial responses in plasma Tgs (A), VLDL1-Chol (B), VLDL2-Chol (C), LDL-C (D), oxLDL (E), resistin (F), and GIP (G). Patients and controls were grouped according to TM6SF2 genotype. Data are presented as mean ± SEM (n = 120).
Independent predictors of outcome variables on multiple logistic regression analysis
Liver histology.
NASH was independently predicted by IAUC VLDL1-Chol [odds ratio (OR) = 1.60; 95% CI, 1.1–2.2; P = 0.009], while advanced (stage 3) fibrosis was predicted by IAUC adiponectin (OR = 1.41; 95% CI, 1.1–2.0; P = 0.021) and IAUC VLDL1-Chol (OR = 1.53; 95% CI, 1.1–2.2; P = 0.010).
Circulating markers of CVD risk.
IAUC Tg and IAUC oxLDLs independently predicted CRP (OR = 1.51; 95% CI, 1.05–2.65; P = 0.006 and β = 1.48; 95% CI, 1.08–2.54; P = 0.005, respectively), E-selectin (OR = 1.56; 95% CI, 1.11–2.61; P = 0.002 and OR = 1.54; 95% CI, 1.19–2.63; P = 0.0009, respectively), and ICAM-1 (OR = 1.54; 95% CI, 1.18–2.78; P = 0.009 and OR = 1.52; 95% CI, 1.07–2.77; P = 0.010, respectively). Whole-body Fatox was independently predicted by IAUC adiponectin (OR = 1.49; 95% CI, 1.14–2.59; P = 0.002) and IAUC GIP (β = 0.49; 95% CI, 0.18–0.88; P = 0.012). The independent determinants of OGTT-related glucose homeostasis parameters and of posptrandial lipoprotein and adipokine responses during the OFTT are reported in Table 4.
TABLE 4.
Independent predictors of parameters related to glucose and lipid metabolism in biopsy-proven NAFLD subjects and matched controls on multivariate logistic regression analysis (n = 120)
| Outcome Variable | Independent Predictor | OR (95% CI) | P |
| OGTT-related parameters of glucose homeostasis | |||
| OGIS | IAUC adiponectin | 1.50 (1.15–2.51) | 0.001 |
| Hepatic IR | IAUC adiponectin | 0.54 (0.16–0.86) | 0.001 |
| IAUC resistin | 1.58 (1.12–2.63) | 0.006 | |
| Adipose tissue IR | PNPLA3 | 1.52 (1.06–2.76) | 0.008 |
| IAUC VLDL1-Chol | 1.45 (1.05–2.59) | 0.002 | |
| Muscle IS | IAUC adiponectin | 1.47 (1.07–2.46) | 0.011 |
| IAUC GIP | 0.49 (0.18–0.91) | 0.012 | |
| IGI | TM6SF2 | 0.49 (0.04–0.83) | 0.009 |
| IAUC adiponectin | 1.49 (1.04–2.50) | 0.004 | |
| DI | TM6SF2 | 0.51 (0.16–0.86) | 0.001 |
| IAUC adiponectin | 1.49 (1.12–2.55) | 0.009 | |
| CGI | TM6SF2 | 0.46 (0.11–0.81) | 0.001 |
| IAUC adiponectin | 1.68 (1.04–2.50) | 0.003 | |
| AI | TM6SF2 | 0.43 (0.10–0.70) | 0.001 |
| IAUC adiponectin | 1.79 (1.23–2.84) | 0.002 | |
| Incretin effect | TM6SF2 | 0.45 (0.11–0.80) | 0.009 |
| IAUC GIP | 0.51 (0.16–0.86) | 0.007 | |
| OFTT parameters | |||
| IAUC Tgs | IAUC adiponectin | 0.50 (0.14–0.87) | 0.003 |
| TM6SF2 | 0.47 (0.02–0.82) | 0.001 | |
| IAUC VLDL1-Tg | IAUC adiponectin | 0.49 (0.13–0.84) | 0.001 |
| TM6SF2 | 0.43 (0.08–0.78) | 0.0009 | |
| IAUC VLDL1-Chol | TM6SF2 | 1.69 (1.11–2.81) | 0.00002 |
| IAUC VLDL2-Chol | TM6SF2 | 1.55 (1.15–2.60) | 0.0009 |
| IAUC VLDL1-apoB100 | TM6SF2 | 0.49 (0.13 –0.83) | 0.002 |
| IAUC VLDL2-apoB100 | TM6SF2 | 0.45 (0.10–0.81) | 0.004 |
| IAUC VLDL1-apoB48 | TM6SF2 | 0.44 (0.02–0.80) | 0.0001 |
| IAUC VLDL2-apoB48 | TM6SF2 | 0.51 (0.06–0.91) | 0.023 |
| IAUC LDL-C | TM6SF2 | 0.50 (0.15–0.85) | 0.003 |
| Fasting LDL-C | 1.91 (0.36–3.11) | 0.0008 | |
| IAUC LDL conjugated dienes | IAUC VLDL1-Tg | 1.89 (1.23–2.95) | 0.0001 |
| IAUC HDL-C | IAUC VLDL1-Tg | 0.52 (0.17–0.87) | 0.009 |
| IAUC GIP | TM6SF2 | 1.88 (1.21–3.01) | 0.001 |
| IAUC resistin | TM6SF2 | 1.58 (1.13–2.92) | 0.012 |
IS, insulin sensitivity.
DISCUSSION
The main findings of our study are the following: 1) The TM6SF2 C>T variant modulated postprandial lipid metabolism: despite similar fasting lipid levels, TM6SF2 CT/TT carriers showed lower postprandial Tg, NEFA, and oxLDL responses, higher HDL-C levels, and a Chol redistribution from LDL to larger intestinal and hepatic TRLP subfractions. TM6SF2 T-allele carriers also had higher incretin GIP and resistin elevations after fat ingestion. 2) Postprandial plasma VLDL1-Chol elevation independently predicted the severity of liver histology in NAFLD, while Tg and oxLDL responses were independently associated with markers of CVD risk. 3) The TM6SF2 C>T variant affected tissue IR, pancreatic β-cell function, and whole-body substrate oxidation rate, the latter possibly through modulation of the GIP response to dietary fat.
Postprandial lipemia is an independent cardiometabolic risk factor in the Western world and, consistently, individuals spend most of the day in the postprandial phase rather than in fasting conditions (14). The effect of the TM6SF2 variant on dietary fat metabolism may contribute to the dual and opposite effect of this SNP on liver disease severity and on CVD risk in NAFLD (32): following fat ingestion, TM6SF2 T-allele carriers showed a shift in Chol content from LDL to larger intestinal and hepatic VLDL subfractions, which are preferentially taken-up by liver cells and adipocytes through the LDL receptor-related protein (33, 34) and the VLDL receptor (35), thereby triggering hepatocyte apoptosis and adipocyte dysfunction (33–35). The independent association of postprandial VLDL-Chol response with liver histology is consistent with recent data, demonstrating an important role for TRLP uptake in promoting high fat-induced liver injury (36) and linking Chol concentration in VLDL subclasses to hepatic Chol content, inflammation, and fibrosis (37). These findings suggest that the TM6SF2 T-allele-associated postprandial lipoprotein pattern may divert toxic Chol away from the vessel walls into the liver and adipose tissue, enhancing liver injury and adipose dysfunction and protecting from CVD.
The independent association of CVD risk markers with postprandial Tg and oxLDL responses, which were lower in TM6SF2 T-allele carriers, is also consistent with an important role for postprandial lipoprotein metabolism in mediating the cardioprotective role of the T-allele observed in large epidemiological studies (7, 10) The lower postprandial Tg response in TM6SF2 T-allele carriers may be due to lower fat absorption or greater chylomicron clearance. The lower increase in NEFA is not consistent with greater chylomicron clearance, which would have increased plasma NEFA through spillover. Additionally, a recent report showed that the TM6SF2 T-allele impairs Tg processing and secretion in enterocytes (38), confirming that reduced Tg absorption may underlie the lower postprandial lipemia observed in TM6SF2 T-allele carriers. If confirmed by larger studies, these findings may have therapeutic implications, as Chol-lowering interventions may reduce Chol hepatotoxicity in TM6SF2 T-allele carriers, irrespective of fasting Chol levels.
We also evaluated the impact of the TM6SF2 SNP on glucose homeostasis, as both NAFLD and the TM6SF2 C>T variant have been associated with an increased risk of T2DM (2, 11). The TM6SF2 gene variant affected tissue insulin sensitivity and pancreatic β-cell function: the TM6SF2 T-allele was associated with an impaired incretin effect and β-cell function, possibly via reduced incretin secretion or action on β-cells, which express TM6SF2 protein (13). These findings may help to select NAFLD carriers of the TM6SF2 at-risk genotype, who are also at higher risk of T2DM, for targeted preventive interventions improving β-cell dysfunction, including incretin mimetics. An intriguing finding was the impact of the TM6SF2 SNP on muscle insulin sensitivity and whole-body Fatox rates, both effects related to postprandial adiponectin and GIP responses to fat (Table 4).
Consistent with our data, adiponectin stimulates muscle Fatox and insulin sensitivity, while GIP potently reduces energy expenditure and Fatox (39). The link between TM6SF2 and incretins and the role of GIP antagonism to enhance Fatox and insulin sensitivity warrant future investigation. In the meantime, it should be noted that the GIP increase induced by dipeptidyl peptidase-IV inhibitors, currently evaluated in NAFLD, may attenuate the benefits of glucagon-like peptide-1 elevation (40).
In conclusion, a maladaptive response to a chronic daily repetitive metabolic challenge, like fat ingestion, may link the TM6SF2 C>T variant to liver injury and cardiometabolic disease in NAFLD. Future research should unravel the underlying molecular pathways in different tissues and organs, allowing therapeutic interventions tailored to individual risk profile and mechanism of injury (41–43). The strength of our study is the careful selection and thorough characterization of participants. The limitations are the small number of subjects and the cross-sectional design, which prevents any causal inference between the TM6SF2 variant and the abnormalities in lipid and glucose metabolism, and requires confirmation by larger follow-up studies.
A further caveat is that we did not directly measure hepatic and muscle insulin sensitivity, but rather estimated them from the time course of glucose and insulin during the OGTT. This method assumes a similar intestinal glucose absorption rate across TM6SF2 genotypes, as a faster glucose absorption rate in TM6SF2 T-allele carriers would cause a steeper increase and an earlier peak and fall in plasma glucose regardless of any actual differences in tissue insulin sensitivity. However, the visual inspection of the plasma glucose curve during the OGTT (supplemental Fig. S1) shows a similar slope in the 0–30 min ascending limb of the curve across the TM6SF2 genotypes and the same peak time (+60 min), making differences in glucose absorption very unlikely to occur.
Supplementary Material
Footnotes
Abbreviations:
- AI
- adaptation index
- AUC
- area under the curve
- CGI
- CP-genic index
- CHO
- carbohydrate
- Chol
- cholesterol
- CHOox
- carbohydrate oxidation
- CRP
- C-reactive protein
- DI
- disposition index
- Fatox
- fat oxidation
- FFM
- fat-free mass
- GIP
- glucose-dependent insulinotropic polypeptide
- HDL-C
- HDL-cholesterol
- HOMA-IR
- homeostatic model assessment of insulin resistance
- IAUC
- incremental area under the curve
- ICAM
- intercellular adhesion molecule
- IGI
- insulinogenic index
- IR
- insulin resistance
- LDL-C
- LDL-cholesterol
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- npRQ
- nonproteic respiratory quotient
- OFTT
- oral fat tolerance test
- OGIS
- oral glucose insulin sensitivity index
- OGTT
- oral glucose tolerance test
- OR
- odds ratio
- oxLDL
- oxidized LDL
- REE
- resting energy expenditure
- RQ
- respiratory quotient
- T2DM
- type 2 diabetes
- Tg
- triglyceride
- TM6SF2
- transmembrane 6 superfamily member 2
- TRLP
- triglyceride-rich lipoprotein
- VCO2
- carbon dioxide production
- VO2
- oxygen consumption
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Chalasani N., Younossi Z., and Lavine J. E.. 2012. The diagnosis and management of NAFLD: practice guidelines by the AASLD, ACG and the AGA. Hepatology. 55: 2005–2023. [DOI] [PubMed] [Google Scholar]
- 2.Musso G., Cassader M., Gambino R., and Pagano G. F.. 2011. Meta-analysis: natural history of NAFLD and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 43: 617–649. [DOI] [PubMed] [Google Scholar]
- 3.Schwab U., Lauritzen L., and Tholstrup T.. 2014. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: a systematic review. Food Nutr. Res. 58: 25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arsov T., Carter C. Z., and Nolan C. J.. 2006. Adaptive failure to high-fat diet characterizes steatohepatitis in Alms1 mutant mice. Biochem. Biophys. Res. Commun. 342: 1152–1159. [DOI] [PubMed] [Google Scholar]
- 5.Westerbacka J., Lammi K., and Hakkinen A. M.. 2005. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J. Clin. Endocrinol. Metab. 90: 2804–2809. [DOI] [PubMed] [Google Scholar]
- 6.Kozlitina J., and Smagris E.. 2014. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 46: 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dongiovanni P., Petta S., Maglio C., and Fracanzani A. L.. 2015. TM6SF2 gene variant disentangles NASH from cardiovascular disease. Hepatology. 61: 506–514. [DOI] [PubMed] [Google Scholar]
- 8.Mahdessian H., Taxiarchis A., and Popo S.. 2014. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proc. Natl. Acad. Sci. USA. 111: 8913–8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Center for Biotechnology Information. US National Library of Medicine website. Accessed December 25, 2016, at http://www.ncbi.nlm.nih.gov/geoprofiles.
- 10.Holmen O. L., Zhang H., and Fan Y.. 2014. Systematic evaluation of coding variation identifies a candidate causal variant in TM6SF2 influencing total cholesterol and myocardial infarction risk. Nat. Genet. 46: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris A. P., Voight B. F., and Teslovich T. M.. 2012. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 44: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musso G., Gambino R., and Cassader M.. 2013. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog. Lipid Res. 52: 175–191. [DOI] [PubMed] [Google Scholar]
- 13.Musso G., Paschetta E., Gambino R., Cassader M., and Molinaro F.. 2013. Interactions among bone, liver, and adipose tissue predisposing to diabesity and fatty liver. Trends Mol. Med. 19: 522–535. [DOI] [PubMed] [Google Scholar]
- 14.Pirillo A., Norata G. D., and Catapano A. L.. 2014. Postprandial lipemia as a cardiometabolic risk factor. Curr. Med. Res. Opin. 30: 1489–1503. [DOI] [PubMed] [Google Scholar]
- 15.Musso G., Cassader M., and Bo S.. 2013. SREBF-2 predicts 7-year NAFLD incidence and severity of liver disease and lipoprotein and glucose dysmetabolism. Diabetes. 62: 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsuda M., and R. A. DeFronzo. 1999. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 17.Anstee Q. M., Daly A. K., and Day C. P.. 2011. Genetic modifiers of non-alcoholic fatty liver disease progression. Biochim. Biophys. Acta. 1812: 1557–1566. [DOI] [PubMed] [Google Scholar]
- 18.Nuñez C., Gallagher D., Visser M., and Pi-Sunyer F. X.. 1997. Bioimpedance analysis: evaluation of leg-to-leg system based on pressare contact footpad electrodes. Med. Sci. Sports Exerc. 29: 524–531. [DOI] [PubMed] [Google Scholar]
- 19.Stanforth P. R., Jackson A. S., and Green J. S.. 2004. Generalized abdominal visceral fat prediction models for black and white adults aged 17-65 y: the HERITAGE family study. Int. J. Obes. Relat. Metab. Disord. 28: 925–932. [DOI] [PubMed] [Google Scholar]
- 20.Frayn K. N. 1983. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 55: 628–634. [DOI] [PubMed] [Google Scholar]
- 21.Ridker P. M., and Hennekens C. H.. 1998. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 351: 88–92. [DOI] [PubMed] [Google Scholar]
- 22.Vaidya D., Szklo M., and Cusman M.. 2011. Association of endothelial and oxidative stress with metabolic syndrome and subclinical atherosclerosis: multi-ethnic study of atherosclerosis. Eur. J. Clin. Nutr. 65: 818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobelli C., Toffolo G. M., Dalla Man C., Campioni M., Denti P., Caumo A., Butler P., and Rizza R.. 2007. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am. J. Physiol. Endocrinol. Metab. 293: E1–E15. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Ghani M. A., Matsuda M., and Balas B.. 2007. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 30: 89–94. [DOI] [PubMed] [Google Scholar]
- 25.Musso G., Gambino R., and Cassader M.. 2010. Lipoprotein metabolism mediates the association of MTP polymorphism with beta-cell dysfunction in healthy subjects and in nondiabetic normolipidemic patients with nonalcoholic steatohepatitis. J. Nutr. Biochem. 21: 834–840. [DOI] [PubMed] [Google Scholar]
- 26.Abdul-Ghani M. A., and Williams K.. 2007. What is the best predictor of future type 2 diabetes? Diabetes Care. 30: 1544–1548. [DOI] [PubMed] [Google Scholar]
- 27.Thomsen C., Rasmussen O., and Lousen T.. 1999. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am. J. Clin. Nutr. 69: 1135–1143. [DOI] [PubMed] [Google Scholar]
- 28.Daousi C., Wilding J. P., and Aditya S.. 2009. Effects of peripheral administration of synthetic human GIP on energy expenditure and subjective appetite sensations in healthy normal weight subjects and obese patients with type 2 diabetes. Clin. Endocrinol. (Oxf.). 71: 195–201. [DOI] [PubMed] [Google Scholar]
- 29.Hansotia T., Maida A., and Flock G.. 2007. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J. Clin. Invest. 117: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasteska D., Harada N., and Suzuki K.. 2014. Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes. 63: 2332–2343. [DOI] [PubMed] [Google Scholar]
- 31.Benjamini Y., and Hochberg Y.. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57: 289–300. [Google Scholar]
- 32.Musso G., Cassader M., Paschetta E., and Gambino R.. 2016. TM6SF2 may drive postprandial lipoprotein cholesterol toxicity away from the vessel walls to the liver in NAFLD. J. Hepatol. 64: 979–981. [DOI] [PubMed] [Google Scholar]
- 33.Pieper-Fürst U., and Lammert F.. 2013. LDL receptors in liver: old acquaintances and a newcomer. Biochim. Biophys. Acta. 1831: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 34.Llorente-Cortes V., Barbarigo V., and Badinon L.. 2012. LRP-1 modulates the proliferation and migration of human hepatic stellate cells. J. Cell. Physiol. 227: 3528–3533. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen A., Tao H., and Metrione M.. 2014. VLDLR expression is a determinant factor in adipose tissue inflammation and adipocyte-macrophage interaction. J. Biol. Chem. 289: 1688–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jo H., Choe S. s., Shin K. C., and Jang H.. 2013. Endoplasmic reticulum stress induces hepatic steatosis via increased expression of the hepatic VLDLR. Hepatology. 57: 1366–1377. [DOI] [PubMed] [Google Scholar]
- 37.Männistö V. T., Simonen M., and Soininen P.. 2014. Lipoprotein subclass metabolism in nonalcoholic steatohepatitis. J. Lipid Res. 55: 2676–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Hare E. A., Yang R., Yerges-Armstrong L., Sreenivasan U., McFarland R., Leitch C. C., Wilson M. H., Narina S., Gorden A., Ryan K., et al. TM6SF2 rs58542926 impacts lipid processing in liver and small intestine. Hepatology. Epub ahead of print. December 27, 2016; doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y., Turdi S., and Park T.. 2013. Adiponectin corrects high-fat diet-induced disturbances in muscle metabolomic profile and whole-body glucose homeostasis. Diabetes. 62: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamont B. J., and Drucker D. J.. 2008. Differential antidiabetic efficacy of incretin agonists versus DPP-4 inhibition in high fat fed mice. Diabetes. 57: 190–198. [DOI] [PubMed] [Google Scholar]
- 41.Musso G., Olivetti C., Cassader M., and Gambino R.. 2012. Obstructive sleep apnea-hypopnea syndrome and nonalcoholic fatty liver disease: emerging evidence and mechanisms. Semin. Liver Dis. 32: 49–64. [DOI] [PubMed] [Google Scholar]
- 42.Musso G., Cassader M., and Gambino R.. 2016. Non-alcoholic steatohepatitis: emerging molecular targets and therapeutic strategies. Nat. Rev. Drug Discov. 15: 249–274. [DOI] [PubMed] [Google Scholar]
- 43.Musso G., Cassader M., Paschetta E., and Gambino R.. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis of randomized trials. JAMA Intern. Med. Epub ahead of print. February 27, 2017; doi:10.1001/jamainternmed.2016.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.