Abstract
In addition to functioning as detergents that aid digestion of dietary lipids in the intestine, some bile acids have been shown to exhibit antimicrobial activity. However, detailed information on the bactericidal activities of the diverse molecular species of bile acid in humans and rodents is largely unknown. Here, we investigated the toxicity of 14 typical human and rodent free bile acids (FBAs) by monitoring intracellular pH, membrane integrity, and viability of a human intestinal bacterium, Bifidobacterium breve Japan Collection of Microorganisms (JCM) 1192T, upon exposure to these FBAs. Of all FBAs evaluated, deoxycholic acid (DCA) and chenodeoxycholic acid displayed the highest toxicities. Nine FBAs common to humans and rodents demonstrated that α-hydroxy-type bile acids are more toxic than their oxo-derivatives and β-hydroxy-type epimers. In five rodent-specific FBAs, β-muricholic acid and hyodeoxycholic acid showed comparable toxicities at a level close to DCA. Similar trends were observed for the membrane-damaging effects and bactericidal activities to Blautia coccoides JCM 1395T and Bacteroides thetaiotaomicron DSM 2079T, commonly represented in the human and rodent gut microbiota. These findings will help us to determine the fundamental properties of FBAs and better understand the role of FBAs in the regulation of gut microbiota composition.
Keywords: bile acids and salts, bile acids and salts/metabolism, bile acids and salts/physical chemistry, obesity, deoxycholic acid, β-muricholic acid, hyodeoxycholic acid, gut microbiota
Bile salts, which are glycine or taurine conjugates of free bile acids (FBAs), play an essential role as detergents in the emulsification of dietary fats and nutrients to facilitate their digestion and absorption. In humans, the primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA) (Table 1), are synthesized from cholesterol in the liver as conjugates and secreted into the duodenum through the bile duct (1). During intestinal transit, the majority of bile acids are deconjugated into FBAs by the bile salt hydrolase activity of many intestinal bacteria and are further modified by the indigenous gut microbes to produce secondary bile acids, such as deoxycholic acid (DCA), lithocholic acid (LCA), and oxo- and β-hydroxy-type bile acids (2) (Table 1). In mice and rats, in addition to CA and CDCA, there are two primary bile acids that are not present in humans, namely, α-muricholic acid (MCA) and β-MCA. These bile acids are also transformed into rodent secondary bile acids by the indigenous intestinal microbes: ω-MCA from β-MCA (3) and hyodeoxycholic acid (HDCA) from α-MCA, β-MCA, λ-MCA, and ω-MCA (4).
TABLE 1.
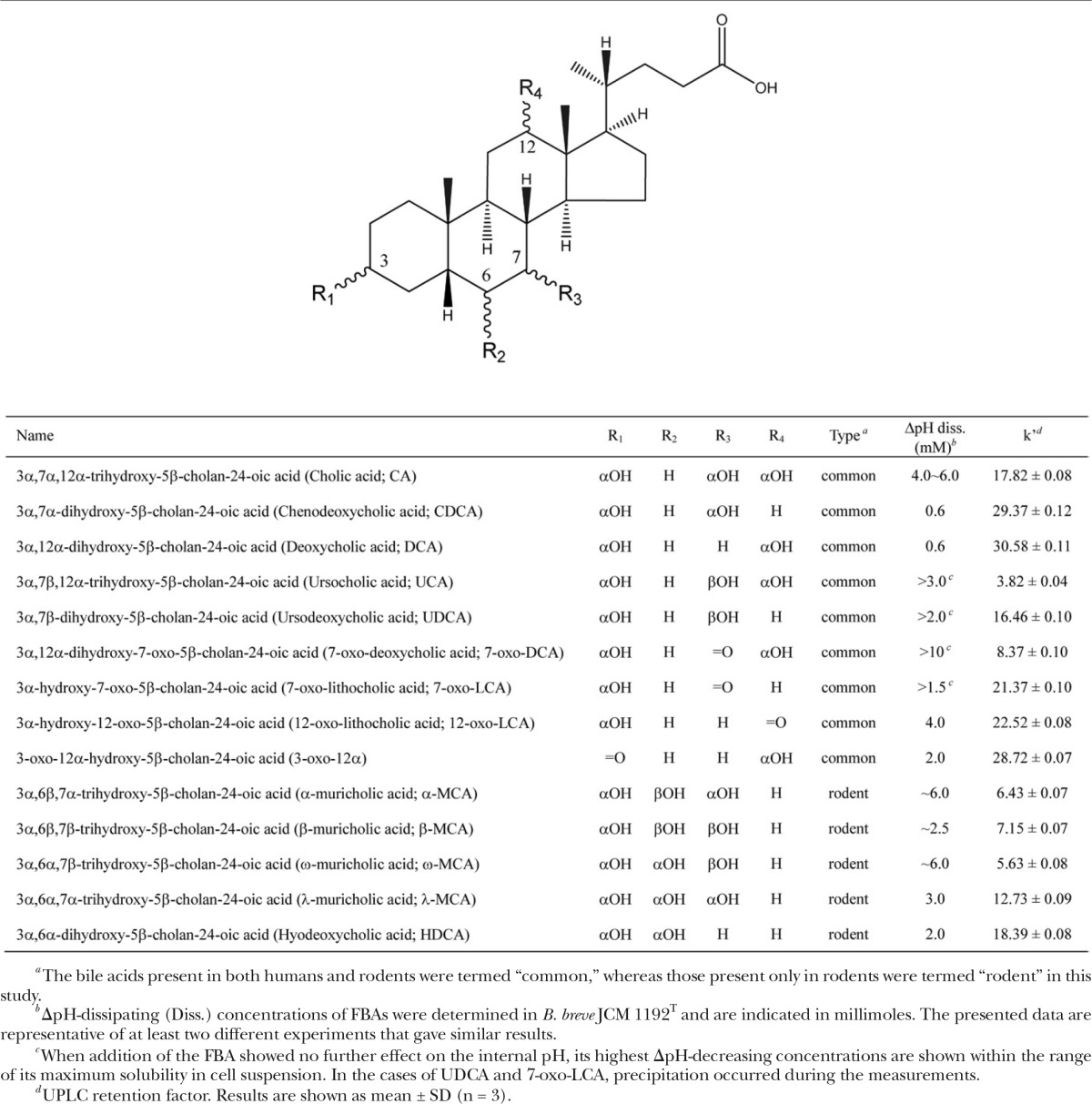
Structures, ΔpH-dissipating concentrations, and UPLC retention factors of the FBAs evaluated
In addition to digestive functions, some FBAs exhibit growth inhibition/bactericidal activities. Although these functions have been known since early in the last century (5), few FBAs have been investigated in detail, including the growth inhibition properties of CA, DCA, and 7-oxo-DCA against Staphylococcus aureus (5) and the bactericidal activities of CA, DCA, and CDCA on several intestinal bacterial strains (6) and many lactobacilli and bifidobacteria (7). Recently, a growth inhibition assay for 3β-DCA was conducted against selected intestinal microbes (8). In terms of rodent-specific FBAs, their bactericidal activities have not been investigated, with the exception of one report on the inhibition of spore germination and vegetative cell growth of Clostridium difficile by α-, β-, and ω-MCAs (9).
Recently, we discovered using CA-feeding experiments that bile acids are host factors that regulate the cecal microbiota composition in rats (10). The CA feeding induced a dramatic decrease in the Bacteroidetes/Firmicutes ratio, which is similar to gut microbiota alterations on a high-fat diet (HFD) in a mouse model (11). As increased intestinal bile acid flow occurs in response to the administration of a HFD, bile acids with strong antimicrobial activity may exert selective pressure to alter the gut microbiota composition in response to a HFD, which we have termed the bile acid hypothesis (11). In sharp contrast, it has been reported that low bile acid input into the gut in patients with cirrhosis resulted in increased Bacteroidetes/Firmicutes ratio (12, 13). Therefore, to evaluate the contribution of each FBA to alteration of the gut microbiota composition in both humans and rodents, a detailed characterization of the bactericidal activities of the diverse FBA molecules found in humans and rodents is required.
Previously, in many lactobacilli and bifidobacteria, we evaluated the membrane-damaging effects of FBAs, such as CA and DCA, as a growth inhibition mechanism (7). In that study, the bactericidal activity of DCA, which contains two hydroxy groups, was approximately 10-fold higher than that of CA, which contains three hydroxy groups. These results suggest that the bactericidal activity of FBAs is associated with their hydrophobicity, which increases the affinity of FBA molecules to the phospholipid bilayer of the bacterial cell membrane, where they exert their membrane-damaging activities. However, the bactericidal activities of other FBAs such as oxo- and β-hydroxy-type bile acids in humans and rodents, as well as their correlation with the hydrophobicities of FBA molecules, are still largely unknown. Particularly, detailed information on the bactericidal activities of rodent MCAs is scarce. This is partly due to the high cost of the MCA reagents [approximately $300 (US) per 10 mg], which prevents us from conducting growth experiments using different concentrations of MCAs and limits the number of target bacteria for evaluating their bactericidal activities in detail.
We previously established an efficient method to evaluate the membrane-damaging effects of FBAs, based on monitoring the transmembrane proton gradient (ΔpH, alkaline interior) via measurement of the intracellular pH using a fluorescence method and Bifidobacterium breve Japan Collection of Microorganisms (JCM) 1192T (7). This strain was selected from many bifidobacteria and lactobacilli because of its rapid and reproducible generation of a ΔpH in response to energization by glucose. This method allowed precise estimation of the toxic concentration ranges of the FBAs in a small-scale experiment. Thus, in this study, we first predicted the membrane-damaging effects of 14 human and rodent FBAs in B. breve JCM 1192T cells using this method. Thereafter, we conducted a comprehensive study on the toxicity of these FBAs using several intestinal bacteria including B. breve JCM 1192T.
Although the molecular species of bile acids in vertebrates are numerous and complex (14), conjugated bile acids do not exist in the large intestine due to the bile salt hydrolase activity of gut microbes (15). Thus, we excluded the conjugated bile acids from our evaluation and concentrated only on the FBAs. The 14 FBAs evaluated in this study comprise ∼75% and ∼95% of the bile acid pool in the large intestines of humans and rodents, respectively, which are good representations of the molecular species found in the large intestine (2, 16). Based on the appropriate concentration ranges determined, we then monitored the membrane-damaging effects and bactericidal activities of B. breve JCM 1192T by fluorescence measurements and plate-out methods, respectively.
B. breve is an actinobacterium abundant in breastfed infants and a minority species in the gut microbiota of adult humans (17). Meanwhile, in adult humans and rodents, members of the phyla, Firmicutes and Bacteroidetes, account for more than 90% of fecal bacteria (18, 19). Thus, we also evaluated two additional strains, Blautia coccoides JCM 1395T of Firmicutes and Bacteroides thetaiotaomicron DSM 2079T of Bacteroidetes, as typical bacterial species among the human and rodent gut microbiota (20–23). Although B. coccoides JCM 1395T and B. thetaiotaomicron DSM 2079T were isolated from mice and humans, respectively, these species are common in both human and rodent intestines; thus, it is reasonable to employ these bacterial strains in comprehensive studies of FBA toxicities.
MATERIALS AND METHODS
Chemicals
Sodium cholate (CA), sodium deoxycholate (DCA), and CDCA were purchased from Sigma-Aldrich (St. Louis, MO). Sodium ursodeoxycholate [ursodeoxycholic acid (UDCA)] and 7-oxo-LCA were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Ursocholic acid (UCA) was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). α-MCA, β-MCA, ω-MCA, λ-MCA, HDCA, 7-oxo-DCA, 12-oxo-LCA, and 3-oxo-12α-hydroxy-5β-cholanoic acid (3-oxo-12α) were purchased from Steraloids, Inc. (Newport, RI). Fluorescent dye 5(6)-carboxyfluorescein diacetate succinimidyl ester (cFDASE) was purchased from Thermo Fisher Scientific Inc. (Waltham, MA). The chemical structures of the bile acids are shown in Table 1. FBAs commonly found in both humans and rodents are classified as common FBAs (CA, UCA, UDCA, DCA, CDCA, 7-oxo-DCA, 7-oxo-LCA, 12-oxo-LCA, and 3-oxo-12α), while those specific to rodents are termed rodent FBAs (α-MCA, β-MCA, ω-MCA, λ-MCA, and HDCA) (Table 1).
Bacterial strains and culture conditions
B. breve JCM 1192T and B. coccoides JCM 1395T were obtained from the JCM (Tsukuba, Ibaraki, Japan). B. thetaiotaomicron DSM 2079T was obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). B. breve JCM 1192T was grown in half-strength (1/2) MRS medium (Becton, Dickinson and Co., Franklin Lakes, NJ) containing 0.025% l-cysteine hydrochloride at 37°C under anaerobic conditions (mixed gas, N2:CO2:H2 = 8:1:1). B. coccoides JCM 1395T and B. thetaiotaomicron DSM 2079T were grown in Gifu anaerobic medium (GAM; Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) containing 0.1 M MOPS buffer at 37°C under anaerobic conditions.
Intracellular pH measurement
We previously demonstrated that the primary mechanism underlying the bactericidal activity of FBAs is membrane damage to bacterial cells (7). During that study, we established a method of intracellular pH measurement using fluorescent probe to evaluate the membrane-damaging effects of FBAs. Using this method, we can monitor the buildup of the ΔpH after energizing the bacterial cells with glucose. Next, the FBAs were exogenously added to the cell suspension. This resulted in a reduction in the internal pH, and at a certain cumulative FBA concentration, the internal pH became equal to the external pH, resulting in dissipation of the ΔpH. Under these conditions, not only protons, but also other intracellular ions and metabolites, leaked out across the damaged cell membrane, suggesting that measurement of the ΔpH is an efficient method to evaluate membrane barrier function after exposure to FBAs. Therefore, determination of the FBA concentration resulting in dissipation of the ΔpH was applied here to predict the concentration range of FBA toxicity in B. breve cells.
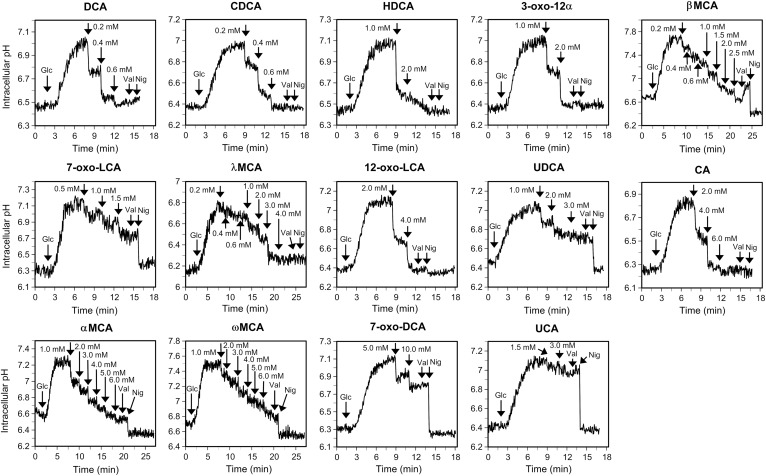
The internal pH of the B. breve JCM 1192T strain was monitored using the internally conjugated fluorescent pH probe 5(6)-carboxyfluorescein succinimidyl ester (cFSE) according to a previously described method (7). The cells were grown to mid-exponential phase and then washed twice with 50 mM potassium phosphate buffer (pH 6.5) containing 1 mM MgSO4 and 0.1 U/ml horseradish peroxidase (buffer 1). The cells were resuspended in 150 mM potassium phosphate buffer (pH 6.5) containing 1 mM MgSO4 and 1.0 U/ml horseradish peroxidase (buffer 2) to an OD660 of ∼0.5. The precursor probe, cFDASE, was added to the bacterial cell suspension to a final concentration of 4 μM at 37°C for 30 min. After centrifugation, the cells were resuspended in buffer 2 and incubated in the presence of 10 mM glucose at 37°C for 30 min to eliminate unbound probe. The mixtures were washed twice with buffer 2, resuspended in the same buffer to an OD660 of 0.5, dispensed into cuvettes, and stirred and maintained at 37°C using the cuvette holder of the LS50B fluorimeter (PerkinElmer, Waltham, MA). The internal pH of the bacteria was determined by measuring the fluorescence intensities of the cell suspension with excitation and emission wavelengths of 490 and 520 nm, respectively (slit widths of 2.5 nm). The cells were energized by adding glucose (10 mM final concentration) to generate the ΔpH. Then, FBAs were added as described in Fig. 1. Dissipation of the ΔpH was confirmed by the observation that no further effect on the internal pH of JCM 1192T was detected after adding 20 nM valinomycin and 200 nM nigericin, which dissipate transmembrane electrical potential (ΔΨ) and ΔpH, respectively. During premeasurement preparations and throughout the internal pH measurements in the cuvette, an anaerobic atmosphere was maintained using mixed gas as described in our previous report (7).
Fig. 1.
Effects of FBAs on the intracellular pH of B. breve JCM 1192T. Internal pH measurements were conducted by fluorescence measurement of cFSE. Cells were energized by 10 mM glucose (indicated as “Glc”) in buffer 2 (pH 6.5) to generate the ΔpH. The respective FBAs were then added at the indicated final concentrations. The bile acids evaluated are listed in Table 1. Precipitation of bile acids was observed using 7-oxo-LCA and UDCA, which prevented complete dissipation of the ΔpH. Valinomycin (Val, 20 nM) and nigericin (Nig, 200 nM) were added to completely dissipate the residual ΔΨ and ΔpH, respectively. The data are representative of at least two experiments that yielded similar results.
Measurement of membrane integrity upon exposure to FBAs
We investigated the membrane integrity of B. breve JCM 1192T, B. coccoides JCM 1395T, and B. thetaiotaomicron DSM 2079T in the presence of FBAs using a fluorescence method based on the membrane permeability of dead cells, as reported previously (7). This method can be used to detect the ratio of cells with intact membranes in the population. JCM 1192T cells were cultured until mid-exponential phase in 1/2 MRS medium containing 0.025% l-cysteine hydrochloride at 37°C under anaerobic conditions. JCM 1395T and DSM 2079T cells were cultured in GAM containing 0.1 M MOPS buffer at 37°C under anaerobic conditions. Then, bacterial cells were washed with buffer 2 and resuspended in the same buffer to an OD660 of 0.3. The JCM 1192T cell suspension was incubated at 37°C in the presence of 10 mM glucose and each bile acid at various concentrations for 3 h. The JCM 1395T and DSM 2079T cell suspensions were incubated at 37°C in the presence of 10 mM glucose and each bile acid at the concentrations determined in JCM 1192T cells to cause viability loss in an anaerobic chamber for 3 h. After incubation, the cells were subsequently incubated with a fluorescent dye mixture (component A plus component B) of the LIVE/DEAD BacLight bacterial viability kit (Thermo Fisher Scientific) according to the manufacture’s recommendations for 15 min at 37°C. The cell suspensions were excited by 480 nm light, and the emission spectra between 490 and 700 nm were measured using an LS50B fluorimeter with both the excitation and emission slits set at 3.0 nm. Calibration was based on 100% live (no treatment) and 100% dead (treated with 100% isopropanol for 1 h) cells. The ratio of the integrated intensity of the portion of each spectrum between 500 and 530 nm (green) to that between 620 and 650 nm (red) was calculated. To obtain a calibration curve, the ratio of integrated green/red fluorescence was plotted against the known percentage of live cells in the standard cell suspensions (10, 50, and 90% live cell suspensions, prepared using 100% live and 100% dead cells). The membrane integrities (percent) were calculated based on those of the untreated cell suspension (no exposure to bile acid, but incubated for 3 h) as 100%.
Measurement of viability upon exposure to FBAs
Changes in the viability of B. breve JCM 1192T were measured according to the pour-plate method, as described previously (7). Changes in the viability of B. coccoides JCM 1395T and B. thetaiotaomicron DSM 2079T were measured according to the spread-plate method. Cell suspensions were prepared and treated with FBAs under the same conditions as those used for the determination of membrane integrity. After treatment, dilutions of the cell suspensions were made using sterile 0.85% NaCl solution; JCM 1192T cells of the appropriate dilution were plated onto 1/2 MRS agar plates containing 0.025% l-cysteine hydrochloride, and JCM 1395T and DSM 2079T cells of the appropriate dilutions were plated onto GAM agar plates. The plates were incubated for 2 days at 37°C under anaerobic conditions using AnaeroPack (Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan). Colonies were counted and the viabilities (percent) calculated based on the untreated cell suspension (no exposure to bile acid, but incubated for 3 h) viability of 100%.
Estimation of FBA hydrophobicity
Previous reports have suggested that the hydrophobicity or hydrophobic-hydrophilic balance of a bile acid molecule can be evaluated indirectly by the retention factor (k′) (defined below), determined by C18 reverse-phase HPLC analysis (24, 25). Thus, this method was applied in this study to quantitatively estimate the hydrophobicity of each FBA using an ultra-performance (UP)LC/ESI-MS system (Waters Corporation, Milford, MA) (15). The retention factor (k′) was calculated from the peak retention times according to Armstrong and Carey (24): k′ = (tr − t0)/t0, where t0 is the retention time of the solvent front, and tr is the retention time of the bile acid elution.
Statistics
The mean ± SD was calculated for each retention factor (k′). Double logarithmic linear regression analyses were performed by the least squares method to determine the correlation between the retention factor (k′) and the FBA concentration that caused remarkable viability loss, with a viability <20% of that of the untreated cells.
RESULTS
Effect of FBAs on the ΔpH in B. breve JCM 1192T
Our previous study showed that DCA and CDCA dissipated the ΔpH of JCM 1192T at concentrations ∼10-fold lower than the necessary concentration of CA, indicating 10-fold greater membrane-damaging activity for DCA and CDCA compared with CA (7). In this study, the membrane-damaging activities of various common and rodent FBAs (Table 1), including DCA, CDCA, and CA, were monitored by the same method. Following the build-up of the ΔpH in cells energized using glucose, the stepwise reduction of internal pH occurred in response to the addition of all FBAs except UCA (Fig. 1). Almost complete dissipation of the ΔpH was observed at the following bile acid concentrations for 10 of the 14 FBAs: DCA (0.6 mM), CDCA (0.6 mM), HDCA (2.0 mM), 3-oxo-12α (2.0 mM), β-MCA (∼2.5 mM), λ-MCA (3.0 mM), 12-oxo-LCA (4.0 mM), CA (4.0–6.0 mM), α-MCA (∼6.0 mM), and ω-MCA (∼6.0 mM) (Fig. 1, Table 1). On the other hand, the ΔpH was not completely dissipated by the remaining FBAs (Fig. 1, Table 1). Precipitation occurred in the cell suspensions when 7-oxo-LCA or UDCA was added above the concentrations indicated in Table 1. In the cases of 7-oxo-DCA and UCA, they were soluble, but showed weak ΔpH-dissipating activities within the concentration ranges used in the experiments.
Judging from the overall pattern of the decrease in the internal pH in response to the FBAs (Fig. 1), DCA and CDCA, which are already recognized as highly bactericidal bile acids, displayed the highest toxicities of the FBAs. Among the other FBAs, HDCA, 3-oxo-12α, β-MCA, 7-oxo-LCA, λ-MCA, 12-oxo-LCA, and UDCA displayed toxicities intermediate to those of DCA/CDCA and CA, whereas α-MCA and ω-MCA showed toxicity similar to that of CA. The 7-oxo-DCA and UCA displayed weaker toxicity than that of CA. Oxo-type FBAs, 7-oxo-DCA, 3-oxo-12α, 12-oxo-LCA, and 7-oxo-LCA, showed weaker ΔpH-dissipating activities than those induced by their corresponding α-hydroxyl-type FBAs, CA (corresponding oxo-type FBA: 7-oxo-DCA), DCA (3-oxo-12α, 12-oxo-LCA), and CDCA (7-oxo-LCA) (Fig. 1). Among the 7β-hydroxy-type FBAs, UCA and UDCA, which are common to humans and rodents, showed lower ΔpH-dissipating activities than did their 7α-hydroxy epimer FBAs, CA and CDCA, respectively.
Effect of FBAs on the cell membrane integrity and viability of B. breve JCM 1192T, B. coccoides JCM 1395T, and B. thetaiotaomicron DSM 2079T
First, based on estimation of the toxicity of each FBA by intracellular pH measurements, we investigated the membrane integrity and viability of JCM 1192T upon exposure to FBAs (Table 2). Severe membrane damage and viability loss were observed with 12 of the 14 FBAs at their ΔpH-dissipating concentrations (DCA, 0.6 mM; CDCA, 0.6 mM; β-MCA, 1.0 mM; HDCA, 1.0 mM; 3-oxo-12α, 1.0 mM; λ-MCA, 3.0 mM; 12-oxo-LCA, 2.0 mM; CA, 6.0–8.0 mM; α-MCA, 2.0 mM; and ω-MCA, 4.0 mM) or at their maximum soluble concentrations, at which substantial dissipation of the ΔpH was observed (7-oxo-LCA, 1.5 mM; UDCA, 2 mM). In contrast, 7-oxo-DCA and UCA, which slightly reduced the internal pH (Fig. 1), exhibited no substantial membrane-damaging effect or viability loss at the indicated concentrations, except for a moderate reduction in viability (∼50%) observed in the case of 10 mM 7-oxo-DCA treatment (Table 2). The overall results strongly confirm the link between the predicted membrane-damaging effect of each FBA (Fig. 1, Table 1) and the actual membrane-damaging effect leading to cell death (Table 2). In addition, the observed coordinated reductions in both membrane integrity and viable cell count led us to conclude that the mechanism underlying the bactericidal activity of FBAs in humans and rodents can generally be extended to their damaging effect on bacterial cell membranes, as reported previously for CA and DCA (7).
TABLE 2.
Membrane integrity and viability of B. breve JCM 1192T, B. coccoides JCM 1395T, and B. thetaiotaomicron DSM 2079T upon exposure to FBAs
| FBA (mM) | B. breve JCM 1192T | B. coccoides JCM 1395T | B. thetaiotaomicron DSM 2079T | |||
| Membrane Integrity (%)a | Viability (%)a | Membrane Integrity (%)a | Viability (%)a | Membrane Integrity (%)a | Viability (%)a | |
| DCA | ||||||
| 0.1 | 100.00 | 96.60 | 96.86 | 100.00 | 87.67 | 99.87 |
| 0.2 | 87.75 | 81.84 | 74.20 | 63.00 | 32.00 | 11.07 |
| 0.4 | 66.14 | 31.24 | —b | — | — | — |
| 0.6 | 4.07 | 0.29 | 0 | 0.02 | 5.40 | 0 |
| 0.8 | 6.72 | 1.15 × 10−5 | — | — | — | — |
| CDCA | ||||||
| 0.01 | 97.60 | 100.00 | — | — | — | — |
| 0.6 | 4.33 | 0.02 | — | — | — | — |
| 1.2 | 7.50 | 0 | — | — | — | — |
| HDCA | ||||||
| 0.1 | 81.00 | 100.00 | 85.75 | 100.00 | 100.00 | 100.00 |
| 1 | 0.47 | 0.54 | 1.15 | 0 | 18.10 | 4.26 × 10−4 |
| 2 | 0 | 1.45 × 10−4 | — | — | — | — |
| 3-oxo-12α | ||||||
| 0.1 | 86.49 | 81.10 | — | — | — | — |
| 1 | 40.00 | 1.34 | — | — | — | — |
| 2 | 3.35 | 0.01 | — | — | — | — |
| β-MCA | ||||||
| 0.1 | 82.43 | 92.03 | 90.76 | 95.01 | — | — |
| 0.5 | 93.77 | 71.58 | — | — | — | — |
| 1 | 57.44 | 1.40 | 63.19 | 3.26 | 100.00 | 46.66 |
| 2 | — | — | — | — | 18.30 | 1.86 × 10−3 |
| 7-oxo-LCA | ||||||
| 1.5 | 11.78 | 3.44 | — | — | — | — |
| λ-MCA | ||||||
| 0.1 | 98.25 | 98.74 | — | — | — | — |
| 1 | 53.77 | 35.17 | — | — | — | — |
| 3 | 28.66 | 7.07 | — | — | — | — |
| 12-oxo-LCA | ||||||
| 0.2 | 100.00 | 100.00 | — | — | — | — |
| 2 | 41.05 | 2.19 | — | — | — | — |
| 4 | 0.18 | 0.04 | — | — | — | — |
| UDCA | ||||||
| 0.1 | 100.00 | 72.18 | — | — | — | — |
| 1 | 96.57 | 39.41 | — | — | — | — |
| 2 | 15.42 | 0.19 | — | — | — | — |
| CA | ||||||
| 0.1 | 100.00 | 100.00 | 94.96 | 100.00 | — | — |
| 1 | 92.27 | 91.65 | 87.14 | 70.75 | 100.00 | 71.68 |
| 2 | 63.60 | 53.80 | 15.00 | 0.75 | 100.00 | 73.06 |
| 4 | 43.46 | 26.55 | — | — | — | — |
| 6 | 23.59 | 18.22 | 15.40 | 0 | 6.20 | 0 |
| 8 | 9.62 | 6.20 | — | — | — | — |
| 10 | 4.52 | 0.84 | — | — | — | — |
| α-MCA | ||||||
| 0.1 | 80.54 | 100.00 | 100.00 | 100.00 | — | — |
| 1 | 41.37 | 39.45 | — | — | — | — |
| 2 | 16.70 | 2.25 | 22.93 | 4.72 | 100.00 | 56.73 |
| 4 | — | — | — | — | 62.65 | 0.49 |
| ω-MCA | ||||||
| 1 | 100.00 | 100.00 | 39.18 | 46.65 | 84.13 | 98.77 |
| 2 | 75.73 | 75.25 | — | — | — | — |
| 4 | 14.81 | 1.12 | 2.02 | 3.71 | 35.40 | 0.33 |
| 7-oxo-DCA | ||||||
| 3 | — | — | 85.91 | 59.63 | 100.00 | 91.85 |
| 5 | — | — | 85.79 | 25.00 | 100.00 | 98.54 |
| 10 | 100.00 | 52.72 | — | — | — | — |
| UCA | ||||||
| 3 | 93.53 | 90.57 | 99.00 | 69.92 | 100.00 | 92.04 |
Membrane integrity and viability of B. breve JCM 1192T, B. coccoides JCM 1395T, and B. thetaiotaomicron DSM 2079T cells were monitored by fluorescence measurements and the plate-out method, respectively. The cell suspensions were incubated with each FBA on a small scale (2.3 or 4.5 ml) due to the limited availability of FBAs such as MCA and UCA. The treated cells were used to measure both membrane integrity and viability. Data are the means of two independent experiments that yielded similar results.
Not determined.
To demonstrate the bactericidal activities of human and rodent FBAs toward bacteria belonging to the Firmicutes and Bacteroidetes phyla, we investigated the membrane integrity and viability of B. coccoides JCM 1395T and B. thetaiotaomicron DSM 2079T after exposure to rodent FBAs, as well as CA and DCA, at the viability loss-inducing concentrations determined for B. breve JCM 1192T. The 7-oxo-DCA and UCA were used as negative controls. Similar trends were observed for the toxicities of these FBAs to JCM 1395T and DSM 2079T (Table 2); DCA, HDCA, β-MCA, CA, α-MCA, and ω-MCA showed decreasing membrane integrity and viability at concentrations similar to those inducing loss of viability. In contrast, treatment with 7-oxo-DCA and UCA caused no serious membrane damage or viability loss at 3 mM. These results suggest that the bactericidal activities determined against B. breve JCM 1192T could be generalized to other bacterial species commonly represented in the human and rodent gut microbiota.
Correlation between the hydrophobicities and bactericidal activities of FBAs
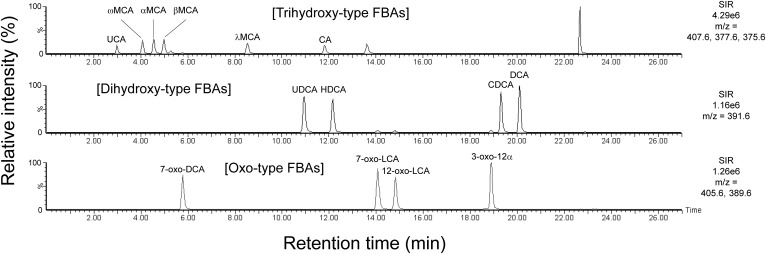
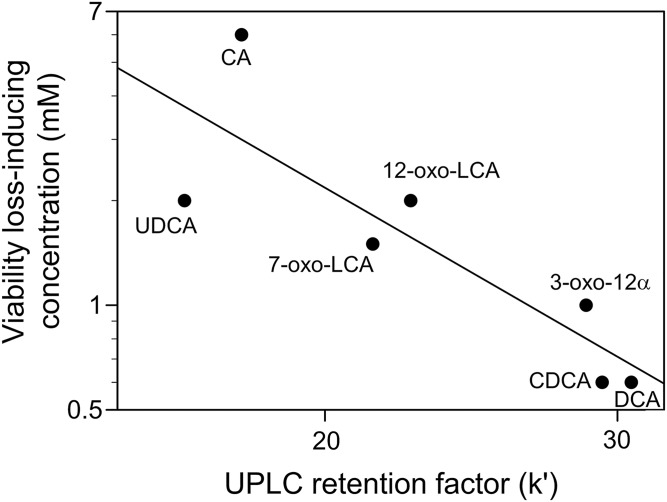
To estimate the hydrophobicity of each FBA molecule, UPLC/MS analysis was performed (Fig. 2). The retentions (mobility−1) of the common FBAs on the reverse-phase UPLC column followed the rank order UCA < 7-oxo-DCA < UDCA < CA < 7-oxo-LCA < 12-oxo-LCA < 3-oxo-12α < CDCA < DCA. The calculated specific retention factors (k′) varied from 3.82 ± 0.04 for UCA (most mobile) to 30.58 ± 0.11 for DCA (least mobile) (Table 1). Double logarithmic plots of k′ versus the viability loss-inducing concentrations (FBA concentration that reduced viability to <20% of that of the untreated cells under the experimental conditions) for the common FBAs revealed a significant inverse correlation (r2 = 0.67, P < 0.05) (Fig. 3). In other words, the lower the viability loss-inducing concentration of the bile acid (higher bactericidal activity), the longer it was retained on the UPLC column (more hydrophobic). These results suggest that the bactericidal activity of an FBA molecule is related to its hydrophobicity.
Fig. 2.
Selected ion-recording (SIR) chromatograms for the FBA standard mixture solution obtained from UPLC/ESI-MS analysis. See Table 1 for the abbreviated names of the FBAs. The standard mixture solution contains 50 μM of each FBA.
Fig. 3.
Double logarithmic plots for the UPLC retention factors versus the concentrations of FBAs common to humans and rodents required for inducing loss of viability. See Table 1 for the abbreviated names of the FBAs. k′, UPLC retention factor.
For the rodent FBAs, the rank order of UPLC retention was ω-MCA < α-MCA < β-MCA < λ-MCA < HDCA (Fig. 2), with k′ ranging from 5.63 ± 0.08 for ω-MCA to 18.39 ± 0.08 for HDCA (Table 1). In contrast to the common FBAs, rodent FBAs did not exhibit a correlation between k′ and bactericidal activity (data not shown). For example, while β-MCA exhibited very low hydrophobicity (Table 1, Fig. 2), it displayed the potent bactericidal activity of the 14 FBAs evaluated. In addition, α-MCA showed relatively strong bactericidal activity while showing a low hydrophobicity similar to that of β-MCA (Table 1). These results suggest that the bactericidal activity of a rodent FBA is not related to its hydrophobicity, in contrast to the common FBAs.
DISCUSSION
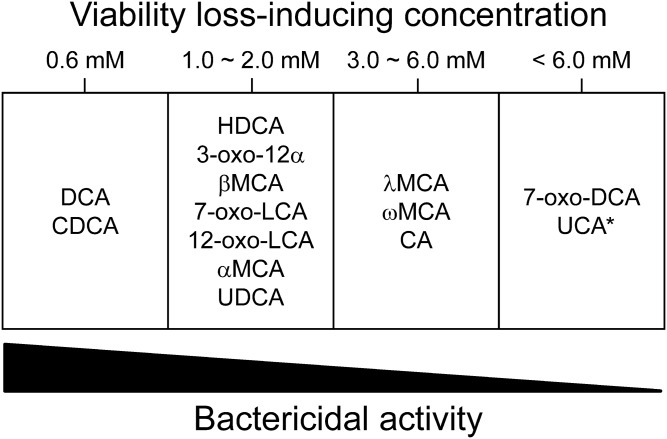
To date, the growth inhibition/bactericidal activities of only a few FBAs in humans and rodents have been reported. Here, we investigated the toxicities of 14 FBA molecules in humans and rodents using typical intestinal bacteria, B. breve JCM 1192T, B. coccoides JCM 1395T, and B. thetaiotaomicron DSM 2079T, as indicator strains. To the best of our knowledge, this is the first comprehensive study of the bactericidal activities of FBAs toward gut microbes. While B. breve, belonging to the phylum Actinobacteria, is abundant in the intestines of breastfed infants and thus a minor member of the gut microbiota in adult humans (17), B. coccoides and B. thetaiotaomicron, belonging to the Firmicutes and Bacteroidetes, respectively, are representative members of the gut microbiota in both adult humans and rodents (20–23). Furthermore, bifidobacteria may become major members of the gut microbiota if their growth is boosted by appropriate prebiotics (e.g., raffinose) in both adult humans (up to 37.2% of the total bacterial cells after a 4 week administration) (17) and rodents (indigenous Bifidobacterium animalis was boosted up to 20.5% of the total bacterial cells after a 3 week administration) (26). Thus, the indicator strains from among the three phyla, Actinobacteria, Firmicutes, and Bacteroidetes, are rational representatives of the gut microbiota for a comprehensive evaluation of the toxicity of FBAs found in humans and rodents. Based on the results, these FBAs were classified into four groups according to their bactericidal activities (Fig. 4). These results may be extended to other bacterial species belonging to these phyla and increase our understanding of the contribution of each FBA to the regulation of the gut microbiota in humans and rodents.
Fig. 4.
Classification of human and rodent FBAs according to their bactericidal activities. See Table 1 for the abbreviated names of the FBAs. “Viability loss-inducing concentration (mM)” was defined as the FBA concentration that caused a reduction in viability to <20% of that of the untreated cells. The asterisk indicates that the bactericidal activities of UCA were not tested at concentrations above 6 mM (Table 2), but were predicted to be above 6 mM from their extremely high hydrophilicities (Table 1) and the decreasing internal pH profiles (Fig. 1).
It has been demonstrated, using B. breve JCM 1192T as the indicator strain, that membrane damage is the primary antimicrobial mechanism of FBAs such as CA, DCA, and CDCA (7). Here, we demonstrated that this mechanism can be generalized to the other FBAs and bacteria used in this study (Table 2). Intestinal bacteria develop various kinds of resistance mechanisms to counteract membrane damage induced by bile acid attack, which include exopolysaccharide production (27), extrusion of bile acids by multidrug resistance transporters (28, 29), membrane lipid alterations (30), among others. However, these mechanisms are generally induced during “bile adaptation,” in which exponentially growing cells are exposed to a sublethal concentration of FBAs (31). In this study, we monitored bile acid toxicity by direct attack of each FBA to exponentially growing cells of the indicator strains, which we call “bile shock”. Thus, our system seems to eliminate the effect of these resistance mechanisms, and therefore the bactericidal activity of each FBA (Fig. 4) can be interpreted to reflect the direct interaction of the FBA with the bacterial cell membrane.
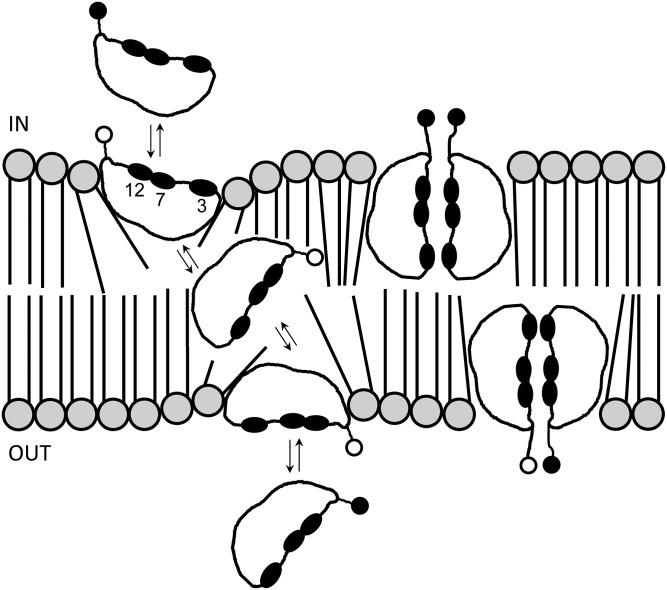
In the common FBAs, our data support our previous hypothesis that the bactericidal activity of an FBA molecule is related to its hydrophobicity, using the 10-fold difference observed in the bactericidal activities of CA and DCA as an example (7). The steroid skeleton of a bile acid molecule forms a hydrophilic side (α-face) and a hydrophobic side (β-face), rendering the molecule a biplanar amphiphile. The interaction of bile acid molecules with the lipid bilayer domains of biological membranes can be explained by two models. In the first model, the highly hydrophobic protonated form of the FBA molecule can freely diffuse across the lipid bilayer (32) (Fig. 5), while the dissociated FBA molecule with negative charge (polarity) cannot diffuse into the membrane. In this context, we have previously demonstrated that the energized bacterial cells accumulate FBA inside the cells according to the membrane ΔpH (interior alkaline) (33, 34). After being diffused into the cells, the protonated FBA dissociates under alkaline conditions according to the Henderson-Hasselbalch equation and the dissociated FBA molecule is internalized due to its polarity. This continues until concentrations of the protonated FBA in both sides of the membrane become equal. In another model, bile acid molecules sneak into the cell membrane as face-to-face dimers, with the hydrophilic α-face on the inside and the hydrophobic β-face on the outside (35) (Fig. 5). We speculate that this may happen during diffusion of the protonated FBAs across the cell membrane. In either case, bile acids can damage bacterial cell membranes at concentrations lower than their critical micelle concentrations, thus exhibiting bactericidal activity. According to these models, the greater the hydrophobicity of the bile acid molecule, the stronger the interaction between the bile acid molecule and the biological membrane. This explains the positive correlation observed among common FBAs between the hydrophobicity and bactericidal activity of the FBA molecule (Fig. 3).
Fig. 5.
Two models of the interaction between CA molecules and the phospholipid bilayer in the bacterial cell membrane. The closed ovals and open/closed circles represent the α-hydroxy groups and carboxyl group in a CA molecule, respectively. The open circle corresponds to the protonated form, while closed circles correspond to the dissociated form of the carboxyl group. The number of carbon atoms is indicated. See text for description. IN, inside of the cell; OUT, outside of the cell.
In contrast to the common FBAs, rodent FBAs did not show these correlations, as the hydrophilic β-MCA showed the toxicity at a level comparable to that of DCA (Table 2). These results suggest that the bactericidal activities of rodent FBAs may be determined not only by their hydrophobic surface, but also by the hydrophilic surface of the molecules. The hydrophilic nature of rodent FBAs, such as α-MCA (3α,6β,7α), β-MCA (3α,6β,7β), ω-MCA (3α,6α,7β), and HDCA, have been revealed in previous studies by reverse-phase LC analyses (16). Comparison of the orientation of the functional hydroxy groups and the hydrophilicities of the FBAs revealed that the 6α-hydroxy group renders the FBA molecules hydrophilic. HDCA (3α,6α) is more hydrophilic than either CDCA (3α,12α) or DCA (3α,7α) (k′ = 18.39 ± 0.08 vs. 29.37 ± 0.12 or 30.58 ± 0.11, respectively) (Table 1, Fig. 2) (16, 24). Also, the 7β-hydroxy group has been shown to be intrinsically hydrophilic based on a comparison between CDCA (3α,7α) and its 7β-hydroxy epimer, UDCA (3α,7β) (k′ = 29.37 ± 0.12 vs. 16.46 ± 0.10, respectively) (Table 1, Fig. 2) (16, 24). Our study showed that UCA (3α,7β,12α) is far more hydrophilic than its 7α-hydroxy epimer, CA (3α,7α,12α) (k′ = 3.82 ± 0.04 vs. 17.82 ± 0.08, respectively) (Table 1, Fig. 2). Model building (6α- and 7β-hydroxy groups) and X-ray diffraction analyses (6α-hydroxy groups) demonstrated that the orientations of the 6α- and 7β-hydroxy groups are equatorial to the plane of the steroid skeleton (24, 36). As the equatorial configuration in the hydroxy group at seventh carbon atom favors interaction with water when micelles are formed (37), the hydroxy group intrinsically renders the UCA and UDCA molecules more hydrophilic than their respective 7α-hydroxy epimers, CA and CDCA. The subtle variations in molecular structure strongly influence the hydrophilicity. Thus, by the same token, 6α-trihydroxy (λ-MCA), 7β-trihydroxy (β-MCA), and 6α,7β-trihydroxy (ω-MCA) rodent FBAs may also be highly hydrophilic (Table 1, Fig. 2). Taking these into consideration, one possible explanation for the relatively high bactericidal activities exhibited by rodent FBAs is that the highly hydrophilic structure of rodent FBA molecules may facilitate formation of the face-to-face dimer more tightly than that in the common FBAs due to the increased hydrophilicity of the α-faces of the bile acid molecules that face each other. Further study will be required to examine these speculations on the structure-function relationship in rodent FBAs.
On the other hand, we must also consider the hydrophobicity estimation method-dependent nature of FBAs. It was shown that the bile acid lipophilicities determined by the 1-octanol/water partition coefficient and reverse phase HPLC differ (25), and thus, the more hydrophilic surfaces of rodent FBA molecules may disturb their association with the hydrocarbon stationary phase of the UPLC column, which could have resulted in the faster retention time compared with those of the other FBAs.
Based on the determined toxic concentration range (Fig. 4), we evaluated the bactericidal activity of each FBA in the intestine. According to previously reported fecal bile acid concentrations in male DA/Slc rats (6 weeks old, n = 8) on a normal diet for 7 weeks (16), the major bactericidal FBA species detected and their concentrations (in micromoles per gram dry feces; roughly equal to millimoles) were DCA (0.58 ± 0.14), β-MCA (0.60 ± 0.17), HDCA (1.96 ± 0.63), and ω-MCA (2.79 ± 0.80). As these values seem to be within the ranges of bactericidal activity exhibited by each FBA, DCA, β-MCA, HDCA, and ω-MCA may exert strong selective pressure in the determination of gut microbiota composition in vivo.
We conducted a comprehensive study of the bactericidal activities of human and rodent FBAs. Among the common FBAs, the bactericidal activities of α-hydroxy FBAs were higher than those of their oxo-derivatives and β-epimers, and their hydrophobicities, as determined by UPLC/ESI-MS, correlated well with their bactericidal activities. In contrast, the bactericidal activities of rodent FBAs did not correlate with their hydrophobicities, and hydrophilic β-MCA exhibited fairly high bactericidal activity, similar to those of known toxic FBAs, such as DCA and CDCA. These results may add valuable information on the structure-function relationship of FBAs that interact with the bacterial cell membrane. Our results also suggest that the bacterial populations in the rodent intestine may be controlled in part by FBAs, particularly β-MCA, HDCA, ω-MCA, and DCA. These findings will help us gain a better understanding of the role that FBAs play in controlling the gut microbiota composition, especially under HFD administration in both humans and rodent models.
Acknowledgments
We thank the JCM RIKEN BioResource Center, participating in the National BioResource Project of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, for providing B. breve JCM 1192T and B. coccoides JCM 1395T.
Footnotes
Abbreviations:
- CA
- cholic acid
- CDCA
- chenodeoxycholic acid
- cFDASE
- carboxyfluorescein diacetate succinimidyl ester
- cFSE
- carboxyfluorescein succinimidyl ester
- DCA
- deoxycholic acid
- FBA
- free bile acid
- GAM
- Gifu anaerobic medium
- HDCA
- hyodeoxycholic acid
- HFD
- high-fat diet
- JCM
- Japan Collection of Microorganisms
- LCA
- lithocholic acid
- MCA
- muricholic acid
- 3-oxo-12α
- 3-oxo-12α-hydroxy-5β-cholanoic acid
- UCA
- ursocholic acid
- UDCA
- ursodeoxycholic acid
- UPLC
- ultra-performance LC
This work was supported by the Institute for Fermentation, Osaka (A.Y.) and grants-in-aid for the Regional R&D Proposal-Based Program from the Northern Advancement Center for Science and Technology (M.W.). This work was also supported, in part, by the Regional Innovation Strategy Support Program of the Ministry of Education, Culture, Sports, Science and Technology through financial support to A.Y., S.F., and M.W.
REFERENCES
- 1.Begley M., Gahan C. G. M., and Hill C.. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29: 625–651. [DOI] [PubMed] [Google Scholar]
- 2.Ridlon J. M., Kang D. J., and Hylemon P. B.. 2006. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47: 241–259. [DOI] [PubMed] [Google Scholar]
- 3.Sacquet E. C., Raibaud P. M., Mejean C., Riottot M. J., Leprince C., and Leglise P. C.. 1979. Bacterial formation of ω-muricholic acid in rats. Appl. Environ. Microbiol. 37: 1127–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eyssen H. J., De Pauw G., and van Eldere J.. 1999. Formation of hyodeoxycholic acid from muricholic acid and hyocholic acid by an unidentified gram-positive rod termed HDCA-1 isolated from rat intestinal microflora. Appl. Environ. Microbiol. 65: 3158–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stacey M., and Webb M.. 1947. Studies on the antibacterial properties of the bile acids and some compounds derived from cholanic acid. Proc. R. Soc. Med. 134: 523–537. [DOI] [PubMed] [Google Scholar]
- 6.Floch M. H., Binder H. J., Filburn B., and Gershengoren W.. 1972. The effect of bile acids on intestinal microflora. Am. J. Clin. Nutr. 25: 1418–1426. [DOI] [PubMed] [Google Scholar]
- 7.Kurdi P., Kawanishi K., Mizutani K., and Yokota A.. 2006. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J. Bacteriol. 188: 1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devlin A. S., and Fischbach M. A.. 2015. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat. Chem. Biol. 11: 685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis M. B., Allen C. A., and Sorg J. A.. 2013. Muricholic acids inhibit Clostridium difficile spore germination and growth. PLoS One. 8: e73653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Islam K. B. M. S., Fukiya S., Hagio M., Fujii N., Ishizuka S., Ooka T., Ogura Y., Hayashi T., and Yokota A.. 2011. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 141: 1773–1781. [DOI] [PubMed] [Google Scholar]
- 11.Yokota A., Fukiya S., Islam K. B. M. S., Ooka T., Ogura Y., Hayashi T., Hagio M., and Ishizuka S.. 2012. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes. 3: 455–459. [DOI] [PubMed] [Google Scholar]
- 12.Kakiyama G., Pandak W. M., Gillevet P. M., Hylemon P. B., Heuman D. M., Daita K., Takei H., Muto A., Nittono H., Ridlon J. M., et al. 2013. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 58: 949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridlon J. M., Kang D. J., Hylemon P. B., and Bajaj J. S.. 2014. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 30: 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haslewood G. A. D. 1964. The biological significance of chemical differences in bile salts. Biol. Rev. Camb. Philos. Soc. 39: 537–574. [DOI] [PubMed] [Google Scholar]
- 15.Hagio M., Matsumoto M., Fukushima M., Hara H., and Ishizuka S.. 2009. Improved analysis of bile acids in tissues and intestinal contents of rats using LC/ESI-MS. J. Lipid Res. 50: 173–180. [DOI] [PubMed] [Google Scholar]
- 16.Hagio M., Matsumoto M., and Ishizuka S.. 2011. Bile acid analysis in various biological samples using ultra performance liquid chromatography/electrospray ionization-mass spectrometry (UPLC/ESI-MS). Methods Mol. Biol. 708: 119–129. [DOI] [PubMed] [Google Scholar]
- 17.Dinoto A., Marques T. M., Sakamoto K., Fukiya S., Watanabe J., Ito S., and Yokota A.. 2006. Population dynamics of Bifidobacterium species in human feces during raffinose administration monitored by fluorescence in situ hybridization-flow cytometry. Appl. Environ. Microbiol. 72: 7739–7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tap J., Mondot S., Levenez F., Pelletier E., Caron C., Furet J. P., Ugarte E., Muñoz-Tamayo R., Paslier D. L. E., Nalin R., et al. 2009. Towards the human intestinal microbiota phylogenetic core. Environ. Microbiol. 11: 2574–2584. [DOI] [PubMed] [Google Scholar]
- 19.Turnbaugh P. J., Bäckhed F., Fulton L., and Gordon J. I.. 2008. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 3: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kibe R., Sakamoto M., Hayashi H., Yokota H., and Benno Y.. 2004. Maturation of the murine cecal microbiota as revealed by terminal restriction fragment length polymorphism and 16S rRNA gene clone libraries. FEMS Microbiol. Lett. 235: 139–146. [DOI] [PubMed] [Google Scholar]
- 21.Matsuki T., Watanabe K., Fujimoto J., Takada T., and Tanaka R.. 2004. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microbiol. 70: 7220–7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tannock G. W. 1977. Characteristics of Bacteroides isolates from the cecum of conventional mice. Appl. Environ. Microbiol. 33: 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin J., Li R., Raes J., Arumugam M., Burgdorf K. S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 464: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong M. J., and Carey M. C.. 1982. The hydrophobic-hydrophilic balance of bile salts. Inverse correlation between reverse-phase high performance liquid chromatographic mobilities and micellar cholesterol-solubilizing capacities. J. Lipid Res. 23: 70–80. [PubMed] [Google Scholar]
- 25.Roda A., Minutello A., Angellotti M. A., and Fini A.. 1990. Bile acid structure-activity relationship: evaluation of bile acid lipophilicity using 1-octanol/water partition coefficient and reverse phase HPLC. J. Lipid Res. 31: 1433–1443. [PubMed] [Google Scholar]
- 26.Dinoto A., Suksomcheep A., Ishizuka S., Kimura H., Hanada S., Kamagata Y., Asano K., Tomita F., and Yokota A.. 2006. Modulation of rat cecal microbiota by administration of raffinose and encapsulated Bifidobacterium breve. Appl. Environ. Microbiol. 72: 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruas-Madiedo P., Gueimonde M., Arigoni F., de los Reyes-Gavilán C. G., and Margolles A.. 2009. Bile affects the synthesis of exopolysaccharides by Bifidobacterium animalis. Appl. Environ. Microbiol. 75: 1204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokota A., Veenstra M., Kurdi P., van Veen H. W., and Konings W. N.. 2000. Cholate resistance in Lactococcus lactis is mediated by an ATP-dependent multispecific organic anion transporter. J. Bacteriol. 182: 5196–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gueimonde M., Garrigues C., van Sinderen D., de los Reyes-Gavilán C. G., and Margolles A.. 2009. Bile-inducible efflux transporter from Bifidobacterium longum NCC2705, conferring bile resistance. Appl. Environ. Microbiol. 75: 3153–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz L., Sánchez B., Ruas-Madiedo P., de los Reyes-Gavilán C. G., and Margolles A.. 2007. Cell envelope changes in Bifidobacterium animalis ssp. lactis as a response to bile. FEMS Microbiol. Lett. 274: 316–322. [DOI] [PubMed] [Google Scholar]
- 31.Margolles A., and Yokota A.. 2011. Bile acid stress in lactic acid bacteria and bifidobacteria. In Lactic Acid Bacteria and Bifidobacteria: Current Progress in Advanced Research. Sonomoto K. and Yokota A., editors. Caister Academic Press, Norfolk, UK: 111–142. [Google Scholar]
- 32.Kamp F., and Hamilton J. A.. 1993. Movement of fatty acids, fatty acid analogues, and bile acids across phospholipid bilayers. Biochemistry. 32: 11074–11086. [DOI] [PubMed] [Google Scholar]
- 33.Kurdi P., van Veen H. W., Tanaka H., Mierau I., Konings W. N., Tannock G. W., Tomita F., and Yokota A.. 2000. Cholic acid is accumulated spontaneously, driven by membrane ΔpH, in many lactobacilli. J. Bacteriol. 182: 6525–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurdi P., Tanaka H., van Veen H. W., Asano K., Tomita F., and Yokota A.. 2003. Cholic acid accumulation and its diminution by short-chain fatty acids in bifidobacteria. Microbiology. 149: 2031–2037. [DOI] [PubMed] [Google Scholar]
- 35.Hofmann A. F. 1994. Bile acids. In The Liver: Biology and Pathobiology. 3rd edition Arias I. M., Boyer J. L., Fausto N., et al., editors. Raven Press, New York: 677–718. [Google Scholar]
- 36.Hall S. R., Maslen E. N., and Cooper A.. 1974. The crystal and molecular structure of 3α,6α-dihydroxy-5β-cholan-24-oic acid, C24O4H40. Acta Crystallogr. B. 30: 1441–1447. [Google Scholar]
- 37.Mazer N. A., Carey M. C., Kwasnick R. F., and Benedek G. B.. 1979. Quasielastic light scattering studies of aqueous biliary lipid systems. Size, shape, and thermodynamics of bile salt micelles. Biochemistry. 18: 3064–3075. [DOI] [PubMed] [Google Scholar]