Abstract
The presence of apoC-III on HDL impairs HDL’s inverse association with coronary heart disease (CHD). Little is known about modifiable factors explaining variation in HDL subspecies defined according to apoC-III. The aim was to investigate cross-sectional associations of anthropometry and lifestyle with HDL subspecies in 3,631 participants from the Diet, Cancer, and Health study originally selected for a case-cohort study (36% women; age 50–65 years) who were all free of CHD. Greater adiposity and less activity were associated with higher HDL containing apoC-III and lower HDL lacking apoC-III. Per each 15 cm higher waist circumference, the level of HDL containing apoC-III was 2.8% higher (95% CI: 0.4, 5.3; P = 0.024) and the level of HDL not containing apoC-III was 4.7% lower (95% CI: −6.0, −3.4; P = <0.0001). Associations for physical activity were most robust to multivariable modeling. Each 20 metabolic equivalent task hours per week reported higher physical activity was associated with 0.9% (95% CI: −1.7, −0.1; P = 0.031) lower HDL containing apoC-III and 0.5% higher (95% CI: 0.1, 1.0; P = 0.029) HDL lacking apoC-III. Lower alcohol consumption was associated with lower HDL lacking apoC-III (percent difference per 15 g/day: 1.58 (95% CI: 0.84, 2.32; P = <0.0001). Adiposity and sedentary lifestyle were associated with a less favorable HDL subspecies profile.
Keywords: high density lipoprotein, obesity, epidemiology
HDL carries diverse proteins differing in size and structure, which determine the functional properties and metabolism of HDL (1–3). The hepatosecretory apoC-III is carried on ∼8–15% of HDL particles, as estimated by cholesterol or apoA-I concentration (4). apoC-III functions as a key regulator of triglyceride metabolism (5, 6). Humans with genetically lower apoC-III levels have a more beneficial lipid profile and less subclinical atherosclerosis (7). Carriers of rare mutations in the gene encoding apoC-III have lower triglyceride, higher HDL cholesterol (HDL-C) levels (8), and up to 41% lower risks of cardiovascular diseases (8, 9). In a randomized controlled trial in participants with elevated triglyceride levels, the administration of an antisense inhibitor of apoC-III resulted in a decrease in apoC-III that was accompanied by a decrease in triglyceride levels and an increase in HDL-C (10). apoC-III has gained increasing attention, as the presence of apoC-III on HDL impairs HDL’s inverse association with coronary heart disease (CHD) (4, 11, 12). In our prior analyses in the Nurses’ Health Study and Health Professionals Follow-Up Study, HDL-C containing apoC-III was positively associated with the risk of CHD, whereas HDL-C that did not contain any apoC-III was inversely associated with the risk of CHD (4). Although these analyses suggest an important role of apoC-III-defined HDL subspecies in cardiovascular etiology, only sparse data point to potentially modifiable factors, including anthropometry and lifestyle factors, that relate to differences in plasma levels of HDL containing apoC-III and HDL not containing apoC-III. Identification of such modifiable factors that predict differential associations with HDL subspecies are of particular interest, as they may open new avenues for primordial and primary prevention.
Our previous study of US nurses and health professionals suggested that HDL subspecies with or without apoC-III might be differentially associated with anthropometrical measures and lifestyle factors (4). Compared with participants with normal weight, obese and overweight individuals had lower levels of HDL-C without apoC-III (4). A case-control study including 20 normal weight and 20 obese individuals matched for age, sex, and race/ethnicity also demonstrated higher HDL containing apoC-III levels in obese compared with normal weight individuals (13). Because body composition is not captured by anthropometrical measures like BMI, but might be more closely related to metabolic disturbances of importance to CHD, we aimed to systematically investigate associations of modifiable factors, including measures of waist and hip circumference, BMI, and bioelectrical impedance-derived fat mass, in addition to lifestyle factors with HDL with and without apoC-III in a population-based setting. We hypothesized that only HDL not containing apoC-III is inversely associated with anthropometrical measures of obesity and positively associated with favorable lifestyle factors.
MATERIALS AND METHODS
Study population and design
Between 1993 and 1997, 57,053 Danish-born residents living in Aarhus or Copenhagen aged 50–65 years with no record of cancer diagnosis in the Danish Cancer Registry were enrolled into the prospective Diet, Cancer, and Health study (14). All eligible persons were retrieved from the Civil Registration System established in 1986 in Denmark. Study participants were invited for a physical examination by trained laboratory technicians in two study clinics in Aarhus and Copenhagen. A detailed description of the study has been published previously (14). The study complied with the Helsinki declaration and was approved by the National Committee on Health Research Ethics and the Danish Data Protection Agency (KF 01-116/96). Informed consent was obtained from all study participants.
We performed a cross-sectional study in participants of the Diet, Cancer, and Health study that had apolipoprotein subspecies measured as part of a previous case-cohort study of incident CHD. The case-cohort study was conducted among participants who were free of CHD at baseline and included a subsample of (n = 1,824) individuals selected at random from all participants of the Diet, Cancer, and Health study at baseline, together with all participants who experienced a first confirmed CHD event during follow-up [baseline until May 2008 (n = 2,104)]. In line with the case-cohort design, 65 participants who later developed CHD were randomly selected into the subcohort as well. After exclusion of participants for whom HDL subspecies according to apoC-III were missing or who reported implausible energy intake (<800 and >4,200 kcal/day in men and <500 and >3,500 kcal/day in women), a total of 3,631 individuals were available for the present cross-sectional analysis in participants of the Diet, Cancer, and Health study that were all free of CHD.
Exposure and covariate assessment
Participants completed self-administered questionnaires, including information on age, sex, educational attainment, menopausal status and hormone therapy use, medical history, and medication intake. Participants were asked whether they had ever been diagnosed with diabetes, hypertension, or hypercholesterolemia by a physician or if they took medication for these conditions. Missing information on medication intake was coded as nonusage. Information on CHD (nonfatal myocardial infarction and fatal CHD) during follow-up until 2008 (median follow-up length 10.8 years) was obtained as previously described (15). In brief, the unique personal identification number assigned to all Danish citizens in the Danish Civil Registration System allowed identification of hospital discharges in the Danish National Register of Patients and the Cause of Death Register. Registers provided information on myocardial infarction diagnosis [International Classification of Diseases (ICD), 8th revision codes 410–410.99; and ICD 10th revision codes I21.0–I21.9] and diagnosis of sudden cardiac death caused by myocardial infarction (ICD 8: 427.27 or ICD 10: I46.0–I46.9). Hospital medical records were retrieved, reviewed in accordance with current guidelines, and CHD events were found to be recorded with a high degree of validity in this register (16, 17).
A digital scale was used to measure the body weight to the nearest 100 g (Soehnle, Murrhardt, Germany). Body height and waist and hip circumference were measured to the nearest half centimeter by trained laboratory technicians with participants in standing position without shoes. Waist circumference was measured at the narrowest section between the lower rib and the iliac crest or halfway between the lower rib and iliac crest if no waist narrowing was determinable. The waist-to-hip ratio was calculated as waist circumference in centimeters divided by hip circumference in centimeters. BMI was calculated as weight in kilograms divided by height in meters squared. Normal weight was defined as a BMI <25 kg/m2, overweight was defined as BMI 25 to <30 kg/m2, and general obesity was defined as BMI ≥30 kg/m2. Body fat mass was quantified by validated bioelectrical impedance, which was conducted by trained laboratory technicians using a bioelectrical impedance 101-F device (Akern/RJL, Florence, Italy) with the participant in a supine position, as described elsewhere (18, 19). Percent fat mass was calculated by dividing total fat mass by total body weight.
Participants were asked to report the duration of time spent exercising, cycling, walking, and gardening in a typical week during the last year in summer and winter. The product of the metabolic equivalent task (MET) intensity level corresponding to each activity (6.0 for exercise and cycling, 3.0 for walking, and 4.0 for gardening) and the reported activity time per week was summed up for all activities for each participant to obtain the overall MET hours per week (20).
Individuals were categorized as never smokers, former smokers, and light (<15 g/day), moderate (15–25 g/day), or heavy (>25 g/day) current smokers based on self-reported information on smoking behavior. The number of pipes smoked per day was multiplied by 3 and the number of cigars was multiplied by 4.5, respectively, to obtain cigarette equivalents based on 1 g of tobacco per cigarette (15).
Dietary intake including alcohol consumption over the past year was assessed with a validated 192-item food-frequency questionnaire mailed to the study participants before the visit to the study clinic (21, 22). Information on serving size and frequency of consumption were used to determine the amount of a food item consumed in grams per day. A total of 12 predefined categories for frequency of intake ranged from “never or less than once per month” to “eight times or more per day.” Macro- and micronutrient intakes were obtained from the reported usual intake using the software program, FoodCalc. We calculated the modified Mediterranean diet score (23). Participants received one point if their consumption of presumed beneficial index components (vegetables, legumes, fruits and nuts, cereals, fish, lipid ratio) was at or above the sex-specific median. The lipid ratio was calculated as the ratio of the sum of monounsaturated and polyunsaturated lipids to saturated lipids. If consumption was below the sex-specific median for presumed detrimental index components (meat, dairy products), participants were assigned a value of one. Points were summed to obtain the total modified Mediterranean diet score, ranging from zero (indicating minimal adherence) to eight (indicating maximal adherence). Alcohol intake was initially a component of the modified Mediterranean diet score, but was considered as a separate lifestyle factor. The estimation of average alcohol content (grams per day) was based on reported alcoholic beverages (beer, wine, spirits, and mixed drinks).
Biochemical measurements
Blood samples were fasting for 6.3% of participants, nonfasting (≤6 h fasting) for 84.9%, and the fasting status was missing for 8.8% of participants. HDL-cholesterol (HDL-C) and triglycerides were measured by enzymatic assays (Thermo Scientific, Waltham, MA). apoA-I is the major apolipoprotein component of HDL and is strongly correlated with the number of HDLs, more-so than cholesterol concentrations. Nearly all apoA-I (>98%) is found in HDL. apoC-III-containing HDL is defined as apoA-I-containing lipoproteins that also contain apoC-III. Therefore, we quantified the concentration of apoC-III-containing HDL and apoC-III-deficient HDL by first fractionating plasma into the part containing apoC-III and the part deficient of apoC-III and then determining the concentration of apoA-I in each fraction. Each sample was thawed, filtered, and incubated overnight with anti-apoC-III resin, as previously described and validated (24, 25). Briefly, Econo-Pac columns (Bio-Rad Laboratories, Hercules, CA) were packed with anti-apoC-III resin (polyclonal goat anti-human apo C-III antibody bound to Sepharose 4B resin at 5 mg/ml; Academy Biomedical Co. Inc., Houston, TX). Samples and resin were incubated for 16 h at 4°C with mixing. The unbound fraction containing intact lipoproteins without apoC-III was eluted from the column by gravity followed by washes with PBS. The bound fraction, consisting of intact lipoproteins with apoC-III, was then eluted from the columns with 3 mol/l sodium thiocyanate in PBS and was immediately desalted by buffer exchange via centrifugal concentrators (Sigma-Aldrich, St. Louis, MO). All columns were tested to ensure efficiency of >95% before the start of laboratory analysis and periodically throughout the analysis by application of a quality control plasma sample to each column and measurement of apoC-III concentration of both the retained and unretained fractions. Following this fractionation of plasma, the two parts were assayed for apoA-I concentration by standard sandwich ELISA on 96-well plates coated with 0.005 mg/ml of anti-apoA-I polyclonal antibody (Academy Biomedical Co. Inc.) and blocked with casein blocking reagent (Thermo Scientific, catalog number 37528). Samples were diluted with PBS (10,000× for the apoC-III-containing fraction and 200,000× for apoC-III-deficient fraction) and were incubated for 1 h at 37°C along with a seven-point standard curve composed of calibrated plasma diluted with PBS ranging from 1.09 to 16.37 ng/ml of apoA-I, a diluent only blank, and two control pool samples to monitor plate performance. Following incubation, plates were emptied and washed three times with PBS wash buffer containing 0.1% Tween 20. Anti-apoA-I:HRP conjugate (Academy Biomedical Co. Inc.) was added at 0.005 mg/ml and incubated for 1 h at 37°C. Plates were emptied, washed as before, and o-phenylenediamine solution (Sigma-Aldrich) was added. After 1 h incubation at room temperature, absorbance at 450 nm was determined in each well and the on-plate standard curve was used to create a calibration curve to convert sample readings into concentrations. All samples and standard curve points were run in triplicate. Concentrations are average values of three repeated measurements and all intra-assay coefficients of variation were <15%.
Statistical analysis
We tested for the normal distribution of all continuous characteristics using the Kolmogorov-Smirnov test. We evaluated all variables by sex. We used robust linear regression models to investigate the association of anthropometry and lifestyle factors as exposure variables with HDL containing apoC-III or HDL not containing apoC-III as the outcome variable. All models were adjusted for potential confounders, including age, sex, future CHD case status, and the complementary HDL subtype. The model relating the Mediterranean diet score to the HDL with or without apoC-III was additionally adjusted for total energy intake. Log-transformation of the HDL subspecies according to apoC-III before the analysis allowed the calculation of the percent difference in the outcome variable associated with a one unit higher level of the exposure variable as follows: percent difference = [exp (coefficient) − 1] × 100.
In multivariable models, we additionally included waist circumference, physical activity, smoking status, and alcohol consumption simultaneously, as well as educational attainment, diabetes medication intake, diabetes, antihypertensive medication intake, and hypertension.
In sensitivity analyses, we explored whether differences in the association of anthropometry and lifestyle factors with the HDL subspecies according to apoC-III exist by age, sex, or future CHD case status. Models were adjusted for age, sex, future CHD case status, and the complementary HDL subtype. We compared the difference in HDL subspecies across sex-specific quartiles of three types of activities (sports, biking, walking, and gardening). We also examined the association of individual Mediterranean diet components with HDL containing apoC-III or HDL not containing apoC-III. All statistical tests were two-tailed and P values below 0.05 were considered statistically significant. Analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Participant characteristics
The median age of study participants from the Diet, Cancer, and Health study was 57 years (Table 1). Thirty-six percent of participants were women, of which 63% were postmenopausal. HDL containing apoC-III represents on average 9% of the total HDL in men, and 8% of the total HDL in women, respectively.
TABLE 1.
Characteristics of participants of the Danish Diet, Cancer, and Health study
| Characteristics | Men (n = 2,314) | Women (n = 1,317) |
| Age (years) | 57.3 (53.3, 61.4) | 57.3 (53.2, 61.2) |
| Educational attainment (years), n = 3,628 | ||
| ≤7 | 945 (40.8) | 490 (37.2) |
| 8–10 | 864 (37.3) | 644 (48.9) |
| >10 | 503 (21.7) | 182 (13.8) |
| CHD during 10 years of follow-up | 1,437 (62.1) | 524 (39.8) |
| Diabetesa | 102 (4.4) | 30 (2.3) |
| Hypertension,a n = 3,129 | 446 (19.3) | 295 (22.4) |
| Hypercholesterolemia,a n = 2,114 | 266 (11.5) | 117 (8.9) |
| Hormone therapy use,b n = 834 | — | 179 (21.5) |
| Diabetes medication intake | 30 (1.3) | 11 (0.8) |
| Antihypertensive medication intake | 347 (15.0) | 229 (17.4) |
| Cholesterol lowering medication intake | 62 (2.7) | 27 (2.1) |
| HDL-C (mg/dl), n = 3,617 | 45.0 (34.8, 56.9) | 56.6 (45.1, 71.0) |
| Triglycerides (mmol/l) | 1.9 (1.3, 2.7) | 1.4 (1.0, 2.0) |
| Total HDLc (mg/dl) | 129 (105, 158) | 145 (119, 118) |
| HDL containing apoC-III (mg/dl) | 10.9 (7.4, 15.9) | 11.8 (8.2, 16.6) |
| HDL not containing apoC-III (mg/dl) | 117 (95, 143) | 133 (107, 163) |
| Proportion of HDL containing apoC-III (%) | 9 (6, 12) | 8 (6, 11) |
| Anthropometry | ||
| Waist circumference (cm), n = 3,630 | 96 (91, 103) | 81 (74, 90) |
| Waist-to-hip ratio, n = 3,629 | 0.96 (0.92, 1.00) | 0.81 (0.76, 0.86) |
| BMI (kg/m2) | 26.7 (24.6, 29.1) | 25.0 (22.7, 28.3) |
| BMI categories (kg/m2) | ||
| <25 | 696 (30.1) | 652 (49.5) |
| 25–<30 | 1,179 (51.0) | 447 (33.9) |
| ≥30 | 439 (19.0) | 218 (16.6) |
| Body composition, n = 3,619 | ||
| Fat mass (kg) | 22.4 (18.2, 27.7) | 23.8 (18.9, 29.6) |
| Fat percentage | 27.2 (23.8, 30.9) | 35.0 (30.6, 39.7) |
| Lifestyle factors | ||
| Physical activity (MET h/week), n = 3,620 | 57.5 (36.5, 86.5) | 62.8 (41.3, 92.5) |
| Time spent on sport activities (h/week) | 0 (0, 2) | 1 (0, 2) |
| Time spent cycling (h/week) | 1 (0, 3) | 1 (0, 3) |
| Time spent walking (h/week) | 3 (1, 5) | 3 (2, 5) |
| Time spent gardening (h/week) | 2 (1, 4) | 1 (0, 3) |
| Smoking status | ||
| Never smoker | 477 (20.6) | 474 (36.0) |
| Former smoker | 730 (31.6) | 262 (19.9) |
| Light current smoker (<15 g/day) | 265 (11.5) | 253 (19.2) |
| Moderate current smoker(15–25 g/day) | 500 (21.6) | 273 (20.7) |
| Heavy current smoker (>25 g/day) | 342 (14.8) | 55 (4.2) |
| Alcohol consumption (g/day) | 18.9 (8.4, 38.5) | 7.2 (2.2, 15.4) |
| Low Mediterranean diet score (0–2 points) | 879 (38.0) | 377 (28.6) |
| Medium Mediterranean diet score (3–4 points) | 968 (41.8) | 542 (41.2) |
| High Mediterranean diet score (5–8 points) | 467 (20.2) | 398 (30.2) |
| Mediterranean diet components | ||
| Vegetables (g/day) | 141 (90, 203) | 154 (100, 224) |
| Fruits (g/day) | 110 (45, 189) | 162 (88, 260) |
| Legumes (g/day) | 0.3 (0.0, 1.5) | 0.3 (0.0, 1.6) |
| Fish (g/day) | 41 (27, 59) | 34 (23, 51) |
| Meat (g/day) | 207 (167, 256) | 142 (110, 179) |
| Dairy (g/day) | 292 (146, 590) | 305 (156, 565) |
| Cereals (g/day) | 200 (151, 250) | 159 (121, 203) |
| Ratio of unsaturated to saturated fat | 1.3 (1.2, 1.5) | 1.3 (1.1, 1.5) |
Continous characteristics are shown as medians (25th to 75th percentiles); n = 3,631 if not stated otherwise.
Self-reported physician-diagnosis.
Postmenopausal women only.
The concentration of HDL was quantified based on apoA-I levels, the major apolipoprotein component of HDL.
Association of anthropometry and physical activity with HDL subspecies according to apoC-III
All anthropometrical measures and physical activity were differentially associated with HDL with and without apoC-III independent of age, sex, and CHD status during follow-up (Table 2). Greater adiposity and less physical activity were associated with higher HDL containing apoC-III and lower HDL not containing apoC-III. Per each 0.1 unit higher waist-to-hip ratio, the level of HDL containing apoC-III was 2.8% higher (95% CI: 0.3, 5.5) and the level of HDL not containing apoC-III was 3.9% lower (95% CI: –5.3, –2.6). Compared with normal weight individuals, HDL not containing apoC-III levels was lower in individuals who were overweight [percent difference: –3.2 (95% CI: –5.3, –1.1)] or obese [percent difference: –9.2 (95% CI: –11.7, –6.7)]. Similarly, HDL containing apoC-III was 7% higher in obese individuals compared with individuals with normal weight. We also saw differential associations for waist circumference and bioelectrical impedance measures of body composition, as well as physical activity, with the HDL subspecies.
TABLE 2.
Percent differences in the concentration of HDL with and without apoC-III by anthropometry and physical activity in participants of the Danish Diet, Cancer, and Health study
| Age- and Sex-Adjusted Percent Difference (95% CI)a | ||
| Exposures | HDL Containing apoC-III | HDL Not Containing apoC-III |
| Waist circumference (per 15 cm) | 2.80 (0.36, 5.30) | −4.70 (−5.95, −3.42) |
| Waist-to-hip ratio (per 0.1 units) | 2.83 (0.26, 5.46) | −3.93 (−5.28, −2.57) |
| BMI (per 5 kg/m2) | 2.13 (−0.03, 4.35) | −3.83 (−4.97, −2.69) |
| BMI categories (kg/m2) | ||
| 25 to <30 versus <25 | 0.72 (−3.11, 4.71) | −3.18 (−5.25, −1.06) |
| >30 versus <25 | 7.00 (1.80, 12.48) | −9.22 (−11.70, −6.67 |
| Body composition | ||
| Fat mass (per 5 kg) | 1.12 (0.07, 2.18) | −2.15 (−2.71, −1.58) |
| Fat percentage (per 5%) | 1.64 (0.15, 3.16) | −2.70 (−3.50, −1.90) |
| Physical activity (per 20 MET h/week) | −1.00 (−1.76, −0.23) | 0.64 (0.21, 1.09) |
n = 3,631 except for waist circumference (n = 3,630), waist-to-hip ratio (n = 3,629), body composition (n = 3,619), and physical activity (n = 3,620). Boldface indicates significant result.
Adjusted for age (continuous), sex (male, female), future CHD case status (whether participant developed CHD during 10 year follow-up), and HDL with or without apoC-III concentration, respectively.
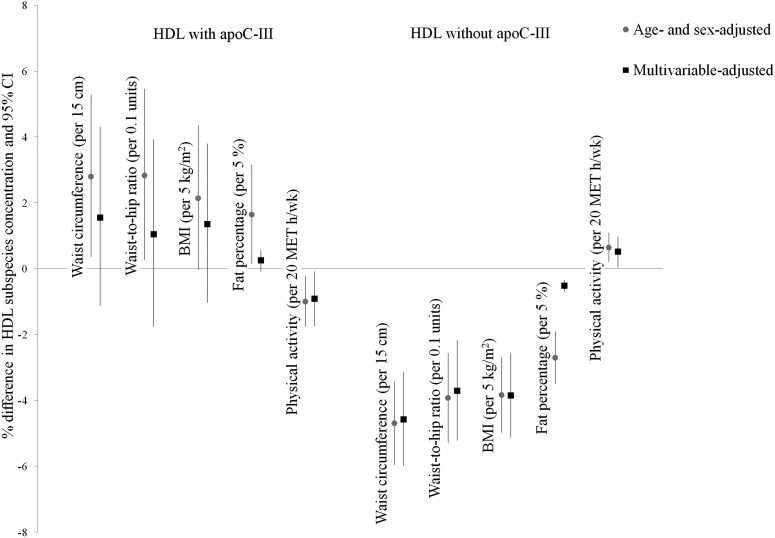
These differential associations persisted in multivariable models, but were attenuated (Fig. 1; supplemental Table S1). The attenuation and loss of statistical significance of anthropometric measures with HDL containing apoC-III was mainly attributable to adjustment for physical activity, which remained associated with HDL containing apoC-III. HDL containing apoC-III levels were 0.9% lower (95% CI: –1.7, –0.1) per each 20 MET hours per week higher physical activity. For HDL not containing apoC-III, associations with anthropometrical measures and physical activity were similar in the multivariable-adjusted model compared with the age- and sex-adjusted model.
Fig. 1.
Percent differences (95% CI) in concentrations of HDL with and without apoC-III in the Danish Diet, Cancer, and Health study (n = 3,631) adjusted for age (continuous), sex (male, female), future CHD case status (whether participant developed CHD during 10 years of follow-up), and the HDL with or without apoC-III concentration, respectively (age- and sex-adjusted model). The multivariable model is additionally adjusted for educational attainment (≤7 years, 8–10 years, >10 years), diabetes medication intake (yes, no), diabetes (yes, no), antihypertensive medication intake (yes vs. no), hypertension (yes, no), waist circumference (continuous; physical activity only), physical activity (continuous), smoking status [never smoker, former smoker, light current smoker (<15 g/day), moderate current smoker (15–25 g/day), heavy current smoker (≥25 g/day)], and alcohol consumption (continuous).
We also addressed whether the association of physical activity with HDL subspecies differs by type of activity (supplemental Table S2). We found no difference in HDL subspecies by time spent on sport activities, biking, or gardening. Compared with participants spending the least amount of time walking (<1.5 h/week in men; <2 h/week in women), participants with the most time spent walking (≥5.5 h/week in men and women) had statistically significant lower levels of HDL containing apoC-III levels and higher levels of HDL lacking apoC-III.
Association of lifestyle factors and medication intake with HDL subspecies according to apoC-III
Participants’ smoking status and level of adherence to the Mediterranean diet pattern were not statistically significantly related to the concentration of HDL with and without apoC-III (Table 3). The individual Mediterranean diet pattern components were not associated with the concentration of HDL with and without apoC-III, except for legumes and the ratio of unsaturated to saturated fat (supplemental Table S3). No association was observed between alcohol intake and HDL containing apoC-III. HDL not containing apoC-III levels were 1.6% higher (95% CI: 0.8, 2.3) per 15 g/day higher alcohol consumption. HDL with or without apoC-III did not differ by hormone therapy use (Table 3).
TABLE 3.
Percent difference in the concentration of HDL with and without apoC-III by lifestyle factors and medication intake in participants of the Danish Diet Cancer and Health study
| Age- and Sex-Adjusted Percent Difference (95% CI)a | Multivariable-Adjusted Percent Difference (95% CI)b | |||
| Exposures | HDL Containing apoC-III | HDL Not Containing apoC-III | HDL Containing apoC-III | HDL Not Containing apoC-III |
| Smoking status | ||||
| Former versus never | −0.78 (−5.40, 4.07) | 0.83 (−1.82, 3.56) | −2.06 (−6.89, 3.02) | 2.08 (−0.77, 5.00) |
| Light (<15 g/day) versus never | −1.67 (−7.09, 4.08) | −0.05 (−3.17, 3.18) | −1.37 (−6.89, 4.77) | 0.19 (−3.13, 3.62) |
| Moderate (15–25 g/day) versus never | −1.67 (−7.09, 4.08) | −2.06 (−4.80, 0.76) | −0.32 (−5.67, 5.34) | −2.36 (−5.32, 0.69) |
| Heavy (≥25 g/day) versus never | −2.78 (−8.77, 3.59) | −0.95 (−4.40, 2.64) | −4.69 (−11.11, 2.20) | −0.83 (−4.62, 3.12) |
| Alcohol consumption (per 15 g/day) | 0.43 (−0.76, 1.63) | 1.42 (0.75, 2.10) | 0.58 (−0.72, 1.90) | 1.58 (0.84, 2.32) |
| Mediterranean diet scorec | ||||
| Medium (3–4) versus low (0–2) | −1.28 (−5.14, 2.75) | −0.45 (−2.65, 1.80) | −1.15 (−5.36, 3.25) | −1.09 (−3.47, 1.34) |
| High (5–8) versus low (0–2) | 0.07 (−4.54, 4.91) | −0.89 (−3.48, 1.77) | −0.17 (−5.24, 5.17) | −1.93 (−4.74, 0.96) |
| Medication intake | ||||
| Hormone therapy use | −1.96 (−9.69, 6.43) | −2.67 (−7.25, 2.13) | 0.17 (−8.39, 9.54) | −3.43 (−8.24, 1.63) |
n = 3,631 except for hormone therapy use (n = 831; postmenopausal women only). Boldface indicates significant result.
Adjusted for age (continuous), sex (male, female), future CHD case status (whether participant developed CHD during 10 year follow-up), and HDL with or without apoC-III concentration, respectively.
Adjusted for age (continuous), sex (male, female), future CHD case status (developed CHD during 10 year follow-up), waist circumference (centimeters), physical activity (continuous), smoking status [never smoker, former smoker, light current smoker (<15 g/day), moderate current smoker (15–25 g/day), heavy current smoker (≥25 g/day)], alcohol consumption (continuous), HDL with or without apoC-III concentration, educational attainment (≤7 years, 8–10 years, >10 years), diabetes medication intake (yes, no), diabetes (yes, no), antihypertensive medication intake (yes vs. no), and hypertension (yes, no).
Additionally adjusted for total caloric intake (continuous).
Investigation of potential modifiers
In sensitivity analyses, we explored whether differences in the association of continuous anthropometric measures and lifestyle factors with HDL with and without apoC-III exist by age, sex, and future CHD case status (whether the participant developed CHD during the 10 years of follow-up). We observed no evidence that associations differed by age, or if participants developed CHD during follow-up (P for interaction > 0.05). We observed a tendency for effect modification of the association between smoking status and HDL not containing apoC-III by sex (P for interaction = 0.005). Whereas no difference in HDL with or without apoC-III by smoking status was observed in the overall study population, women currently moderately smoking had lower HDL not containing apoC-III levels compared with women that had never smoked [percent difference: 1.58% (95% CI: 0.84, 2.32)]. No difference in HDL not containing apoC-III by smoking status was observed for men (supplemental Table S4).
DISCUSSION
In this large study of 3,631 men and women from the Diet, Cancer, and Health study, we found strong differential associations of anthropometrical measures and physical activity with HDL subspecies. Physical activity was the key factor of these associations. HDL not containing apoC-III was also higher with higher alcohol consumption. Adherence to the Mediterranean dietary pattern and smoking were unrelated to the HDL subspecies in this study.
So far, little is known about associations of modifiable factors with HDL with and without apoC-III. Results from the Nurses’ and Health Professionals Follow-Up studies suggested that HDL subspecies with or without apoC-III might be differentially associated with anthropometry and lifestyle factors (4). In the present study, we also found that HDL not containing apoC-III was lower in individuals who were obese or overweight and that HDL containing apoC-III levels was higher in obese compared with normal weight individuals. However, we found that HDL subspecies were more strongly related to measures of abdominal fat, such as waist circumference, than with BMI, a measure of more general obesity. These findings may suggest that visceral fat accumulation might be more strongly related than overweight per se to higher HDL containing apoC-III levels. However, the associations of HDL containing apoC-III and waist circumference, waist-to-hip ratio, and bioelectrical impedance fat mass were attenuated by adjustment for physical activity. Physical activity remained differentially associated with both HDL subspecies according to apoC-III, and although the magnitude of association was smaller for physical activity, the statistical significance remained in the fully adjusted models. We found that only the time spent walking was differentially associated with the HDL subspecies, but the time spent on sport activities, cycling, and gardening was substantially lower compared with the time spent walking. In the Nurses’ and Health Professionals studies, HDL subtype concentrations did not differ by level of physical activity. As physical activity is a strong determinant of body weight and abdominal obesity (26), it can be difficult to disentangle independent associations of physical activity and anthropometry with cardiometabolic risk. It is possible that differences in how these exposures were assessed in the different studies (weight and height were self-reported in the Nurses’ and Health Professionals studies and types of physical activity are very different in Denmark vs. USA) may capture slightly different aspects and explain such slight differences.
In this analysis, we did not replicate the previous observation of higher concentrations of HDL containing apoC-III in smokers compared with nonsmokers (4). Alcohol, a powerful predictor of total HDL-C concentration (27), was positively correlated with both HDL subspecies in both studies (4), but associations were only statistically significant for HDL not containing apoC-III in the present analysis. Surprisingly, adherence to the Mediterranean dietary pattern, investigated for the first time in relation to the apolipoprotein subspecies, was not related to either of the HDL subspecies in the present study. Dietary pattern analysis can dilute or obscure an association if only some pattern components are associated with the outcome of interest. In our study, individual components of the Mediterranean diet pattern were not associated with the concentration of HDL containing or lacking apoC-III, except for legumes and the ratio of unsaturated to saturated fat. However, the observed associations were very weak and the intake of legumes was very low in our study population. Recent evidence from the PREDIMED study (Prevención con Dieta Mediterránea) revealed that two types of Mediterranean diet interventions did not statistically significantly alter HDL-C or apoA-I levels (28). In observational epidemiological studies, the Mediterranean diet is directly related to HDL-C levels (29, 30), but whether diet is differentially related to HDL subspecies should be evaluated in further studies and prospective settings.
As yet, little is known about the relation of medication intake and HDL subspecies. Whereas hormone therapy use was associated with higher HDL not containing apoC-III levels in the Nurses’ Health Study, in the present analysis, hormone therapy use was unrelated to the HDL with and without apoC-III in postmenopausal women. This discrepancy might be attributable to the lower prevalence of hormone therapy use in our sample compared with the Nurses’ Health Study (4). Furthermore, hormone therapy prescription patterns have been shown to vary in Europe compared with the USA and it may be that different forms of hormone therapy could be differentially associated with measures of HDL subspecies (31, 32).
The strengths of this study include the unique opportunity to study body composition as assessed with bioelectrical impedance and dietary quality as assessed by the Mediterranean diet index in relation to HDL subspecies according to apoC-III for the first time. Furthermore, so far, the Diet, Cancer, and Health study is the only European cohort with HDL subspecies measurements and allows for direct comparison of those obtained from US-based cohorts to a Danish population sample. We performed many statistical tests and further studies are warranted to investigate the association of anthropometry and lifestyle factors with HDL subspecies in different populations with different racial and ethnic compositions in prospective settings. We tried to minimize measurement error in the HDL subspecies assessment by performing each measurement in triplicate, but measurement error might have contributed to the null results. Mechanisms by which lifestyle factors might differentially affect HDL subspecies might be exploited in future investigations. Besides apoC-III, several other apolipoproteins exist that might be important for the functionality of HDL (33), which should be explored in further investigations in relation to clinical characteristics and modifiable lifestyle factors.
CONCLUSIONS
In conclusion, we found that anthropometrical measures and physical activity were differentially associated with HDL subspecies and the association of physical activity with HDL subspecies was the most robust in multivariable modeling. It should be explored in intervention studies whether an increase in physical activity differentially affects HDL subspecies via lowering abdominal obesity or by another mechanism. Findings of this study highlight that, beyond overall HDL level, the protein composition of HDL might be relevant for the evaluation of primordial and primary prevention.
Supplementary Material
Acknowledgments
The authors thank all participants of the Diet, Cancer, and Health study for their invaluable contribution to the study.
Footnotes
Abbreviations:
- CHD
- coronary heart disease
- HDL-C
- HDL cholesterol
- ICD
- International Classification of Diseases
- MET
- metabolic equivalent task
This work was supported by a Postdoctoral Research Fellowship from the German Research Foundation (KO 5187/1-1) to M.K. HDL apoC-III measurements were funded by a Young Elite Research Award from the Danish Council of Independent Research, Ministry of Higher Education and Science. The Diet, Cancer, and Health Study is supported by the Danish Research Council and the Danish Cancer Society.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Davidson W. S., Silva R. A., Chantepie S., Lagor W. R., Chapman M. J., and Kontush A.. 2009. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 29: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaisar T., Pennathur S., Green P. S., Gharib S. A., Hoofnagle A. N., Cheung M. C., Byun J., Vuletic S., Kassim S., Singh P., et al. 2007. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J. Clin. Invest. 117: 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Movva R., and Rader D. J.. 2008. Laboratory assessment of HDL heterogeneity and function. Clin. Chem. 54: 788–800. [DOI] [PubMed] [Google Scholar]
- 4.Jensen M. K., Rimm E. B., Furtado J. D., and Sacks F. M.. 2012. Apolipoprotein C-III as a potential modulator of the association between HDL-cholesterol and incident coronary heart disease. J. Am. Heart Assoc. 1: jah3-e000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendivil C. O., Rimm E. B., Furtado J., Chiuve S. E., and Sacks F. M.. 2011. Low-density lipoproteins containing apolipoprotein C-III and the risk of coronary heart disease. Circulation. 124: 2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ooi E. M., Barrett P. H., Chan D. C., and Watts G. F.. 2008. Apolipoprotein C-III: understanding an emerging cardiovascular risk factor. Clin. Sci. (Lond.). 114: 611–624. [DOI] [PubMed] [Google Scholar]
- 7.Pollin T. I., Damcott C. M., Shen H., Ott S. H., Shelton J., Horenstein R. B., Post W., McLenithan J. C., Bielak L. F., Peyser P. A., et al. 2008. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 322: 1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crosby J., Peloso G. M., Auer P. L., Crosslin D. R., Stitziel N. O., Lange L. A., Lu Y., Tang Z. Z., Zhang H., Hindy G., et al. 2014. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 371: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jørgensen A. B., Frikke-Schmidt R., Nordestgaard B. G., and Tybjaerg-Hansen A.. 2014. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 371: 32–41. [DOI] [PubMed] [Google Scholar]
- 10.Gaudet D., Alexander V. J., Baker B. F., Brisson D., Tremblay K., Singleton W., Geary R. S., Hughes S. G., Viney N. J., Graham M. J., et al. 2015. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N. Engl. J. Med. 373: 438–447. [DOI] [PubMed] [Google Scholar]
- 11.Di Angelantonio E., Sarwar N., Perry P., Kaptoge S., Ray K. K., Thompson A., Wood A. M., Lewington S., Sattar N., Packard C. J., et al. 2009. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 302: 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., and Dawber T. R.. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62: 707–714. [DOI] [PubMed] [Google Scholar]
- 13.Talayero B., Wang L., Furtado J., Carey V. J., Bray G. A., and Sacks F. M.. 2014. Obesity favors apolipoprotein E- and C-III-containing high density lipoprotein subfractions associated with risk of heart disease. J. Lipid Res. 55: 2167–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tjønneland A., Olsen A., Boll K., Stripp C., Christensen J., Engholm G., and Overvad K.. 2007. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand. J. Public Health. 35: 432–441. [DOI] [PubMed] [Google Scholar]
- 15.Jensen M. K., Chiuve S. E., Rimm E. B., Dethlefsen C., Tjonneland A., Joensen A. M., and Overvad K.. 2008. Obesity, behavioral lifestyle factors, and risk of acute coronary events. Circulation. 117: 3062–3069. [DOI] [PubMed] [Google Scholar]
- 16.Joensen A. M., Jensen M. K., Overvad K., Dethlefsen C., Schmidt E., Rasmussen L., Tjonneland A., and Johnsen S.. 2009. Predictive values of acute coronary syndrome discharge diagnoses differed in the Danish National Patient Registry. J. Clin. Epidemiol. 62: 188–194. [DOI] [PubMed] [Google Scholar]
- 17.Luepker R. V., Apple F. S., Christenson R. H., Crow R. S., Fortmann S. P., Goff D., Goldberg R. J., Hand M. M., Jaffe A. S., Julian D. G., et al. 2003. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 108: 2543–2549. [DOI] [PubMed] [Google Scholar]
- 18.Frost L., Benjamin E. J., Fenger-Gron M., Pedersen A., Tjonneland A., and Overvad K.. 2014. Body fat, body fat distribution, lean body mass and atrial fibrillation and flutter. A Danish cohort study. Obesity (Silver Spring). 22: 1546–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heitmann B. L. 1990. Prediction of body water and fat in adult Danes from measurement of electrical impedance. A validation study. Int. J. Obes. 14: 789–802. [PubMed] [Google Scholar]
- 20.Johnson B. L., Trentham-Dietz A., Koltyn K. F., and Colbert L. H.. 2009. Physical activity and function in older, long-term colorectal cancer survivors. Cancer Causes Control. 20: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Overvad K., Tjonneland A., Haraldsdottir J., Ewertz M., and Jensen O. M.. 1991. Development of a semiquantitative food frequency questionnaire to assess food, energy and nutrient intake in Denmark. Int. J. Epidemiol. 20: 900–905. [DOI] [PubMed] [Google Scholar]
- 22.Tjønneland A., Overvad K., Haraldsdottir J., Bang S., Ewertz M., and Jensen O. M.. 1991. Validation of a semiquantitative food frequency questionnaire developed in Denmark. Int. J. Epidemiol. 20: 906–912. [DOI] [PubMed] [Google Scholar]
- 23.Trichopoulou A., Bamia C., Norat T., Overvad K., Schmidt E. B., Tjonneland A., Halkjaer J., Clavel-Chapelon F., Vercambre M. N., Boutron-Ruault M. C., et al. 2007. Modified Mediterranean diet and survival after myocardial infarction: the EPIC-Elderly study. Eur. J. Epidemiol. 22: 871–881. [DOI] [PubMed] [Google Scholar]
- 24.Campos H., Perlov D., Khoo C., and Sacks F. M.. 2001. Distinct patterns of lipoproteins with apoB defined by presence of apoE or apoC-III in hypercholesterolemia and hypertriglyceridemia. J. Lipid Res. 42: 1239–1249. [PubMed] [Google Scholar]
- 25.Khoo C., Campos H., Judge H., and Sacks F. M.. 1999. Effects of estrogenic oral contraceptives on the lipoprotein B particle system defined by apolipoproteins E and C-III content. J. Lipid Res. 40: 202–212. [PubMed] [Google Scholar]
- 26.Ekelund U., Besson H., Luan J., May A. M., Sharp S. J., Brage S., Travier N., Agudo A., Slimani N., Rinaldi S., et al. 2011. Physical activity and gain in abdominal adiposity and body weight: prospective cohort study in 288,498 men and women. Am. J. Clin. Nutr. 93: 826–835. [DOI] [PubMed] [Google Scholar]
- 27.De Oliveira E Silva E. R., Foster D., McGee Harper M., Seidman C. E., Smith J. D., Breslow J. L., and Brinton E. A.. 2000. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation. 102: 2347–2352. [DOI] [PubMed] [Google Scholar]
- 28.Hernáez Á., Castaner O., Elosua R., Pinto X., Estruch R., Salas-Salvado J., Corella D., Aros F., Serra-Majem L., Fiol M., et al. 2017. Mediterranean diet improves high-density lipoprotein function in high-cardiovascular-risk individuals: a randomized controlled trial. Circulation. 135: 633–643. [DOI] [PubMed] [Google Scholar]
- 29.Rumawas M. E., Meigs J. B., Dwyer J. T., McKeown N. M., and Jacques P. F.. 2009. Mediterranean-style dietary pattern, reduced risk of metabolic syndrome traits, and incidence in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 90: 1608–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steffen L. M., Van Horn L., Daviglus M. L., Zhou X., Reis J. P., Loria C. M., Jacobs D. R., and Duffey K. J.. 2014. A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Br. J. Nutr. 112: 1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jolleys J. V., and Olesen F.. 1996. A comparative study of prescribing of hormone replacement therapy in USA and Europe. Maturitas. 23: 47–53. [DOI] [PubMed] [Google Scholar]
- 32.Sonigo C., Dray G., and Chabbert-Buffet N.. 2012. Hormone replacement therapy: practical aspects [article in French]. J. Gynecol. Obstet. Biol. Reprod. (Paris). 41: F3–F12. [DOI] [PubMed] [Google Scholar]
- 33.Vaisar T., Mayer P., Nilsson E., Zhao X. Q., Knopp R., and Prazen B. J.. 2010. HDL in humans with cardiovascular disease exhibits a proteomic signature. Clin. Chim. Acta. 411: 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.