Abstract
The LDL receptor (LDLR) family has long been studied for its role in cholesterol transport and metabolism; however, the identification of ApoE4, an LDLR ligand, as a genetic risk factor for late-onset Alzheimer’s disease has focused attention on the role this receptor family plays in the CNS. Surprisingly, it was discovered that two LDLR family members, ApoE receptor 2 (Apoer2) and VLDL receptor (Vldlr), play key roles in brain development and adult synaptic plasticity, primarily by mediating Reelin signaling. This review focuses on Apoer2 and Vldlr signaling in the CNS and its role in human disease.
Keywords: apolipoproteins, Alzheimer’s disease, endocytosis, lipoprotein receptors, cell signaling, neurons, apolipoprotein E, very low density lipoprotein receptor, apolipoprotein E receptor 2
The LDL receptor (LDLR) family is an evolutionarily ancient family consisting of seven key members (1), several of which will be touched on by other reviews in this Journal of Lipid Research series. Here, we focus on ApoE receptor 2 (Apoer2) and VLDL receptor (Vldlr). To better understand these receptors, we begin with a close study of their structure. From there, we will expand to cover their function in brain development and synaptic plasticity, and we end on how disruptions in these pathways lead to human disease and potential avenues of therapeutic approach.
LDLR FAMILY STRUCTURE: FOCUS ON Apoer2 AND Vldlr
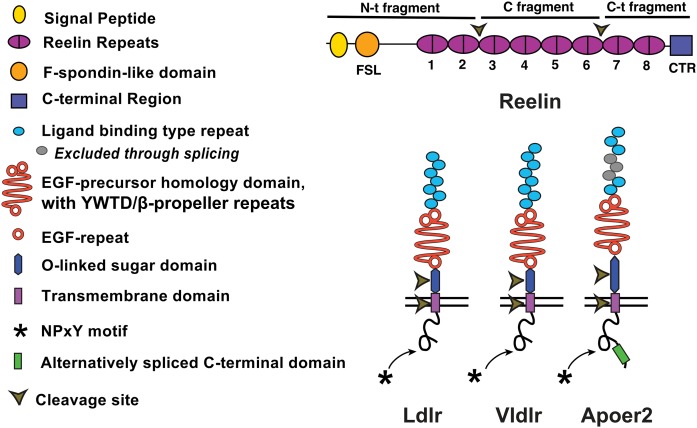
All LDLR family members are type I transmembrane receptors that share a highly conserved structure consisting of five structural domains (1). Figure 1 shows the structural similarities and differences between LDLR, the founding family member, and Vldlr and Apoer2, the focus of this review.
Fig. 1.
Structure of Reelin and the ApoE receptors, Ldlr, Vldlr, and Apoer2. Reelin comprises a signal peptide, an F-spondin-like domain, eight RRs that each contain a “A” and “B” parts separated by an EGF motif, and a C-terminal domain. Reelin is cleaved at two sites between RR2/RR3 and RR6/RR7. The ApoE receptors contain an extracellular ligand binding domain, EGF-precursor homology domain, an alternatively spliced OLS domain, a single transmembrane domain, and a short ICD containing one to three NPXY motifs. Apoer2 has an alternatively spliced 59 amino acid ICD.
At the extracellular N terminal, there is a ligand-binding domain with a variable number of cysteine-rich repeats (2). Vldlr has eight cysteine-rich repeats (3). Similarly, full-length Apoer2 mRNA encodes eight repeats; however, exon 5, which encodes repeats 4–6, is spliced out in most transcripts, leading to an expressed protein with five repeats (4, 5). Both receptors possess three extracellular epidermal growth factor (EGF) precursor homology domains, which are responsible for pH-dependent ligand release (6). They additionally have an alternatively spliced extracellular O-linked sugar (OLS) domain, which is larger in Apoer2 and whose processing and cleavage is described in more depth later in this review, and a single transmembrane domain (7, 8). Finally, at the C terminus, the LDLR family members have a small cytoplasmic domain containing one to three NPXY motifs, which is responsible for endocytic trafficking and signal transmission and is notable for a lack of tyrosine kinase activity (9). Apoer2 and Vldlr each have one NPXY motif. Apoer2 additionally has an alternatively spliced 59 amino acid proline-rich region in the C terminal, which is important for protein interaction and synaptic transmission (4, 5, 10). While Apoer2 is expressed by neurons throughout the brain, a full expression analysis of splicing variants in different neuronal subtypes and brain regions has not yet been completed. The mechanism by which this alternative splicing is controlled is currently unknown.
Overall, Apoer2 and Vldlr share approximately 50% sequence homology (11), with the regions of greatest difference being in the alternatively spliced OLS and intracellular domains (ICDs).
Reelin SIGNALING THROUGH Apoers IS REQUIRED FOR BRAIN DEVELOPMENT
The primary ligand in the CNS for Apoer2 and Vldlr is Reelin, a large secreted glycoprotein that is required for appropriate brain development (12, 13). In the prenatal brain, Reelin is secreted by Cajal-Retzius cells in the marginal zone of the cortex, where it sends signals to migrating neurons, leading to the characteristic “inside-out” pattern of the cortex, whereby the youngest neurons are in the most superficial layers and the oldest neurons are in the deepest layers (12, 14). Similarly, Reelin is expressed by cells in the external granule layer of the developing cerebellum, where it mediates Purkinje cell migration. Mice that are deficient in Reelin, known as reeler mice, have broad lamination defects due to failure of neuronal migration, including disrupted cortical layering and cerebellar hypoplasia (12). Reeler mice have a characteristic “reeling” ataxia, severe cognitive deficits, and early lethality (15).
The details of neuronal migration and its role for Reelin signaling are reviewed in (16). However, it is important to point out here that double knockout mice that are deficient in both Apoer2 and Vldlr recapitulate the reeler phenotype (17). Similarly, disabled-1 (Dab1) knockout (scrambler or yotari) mice (18–20) and Src and Fyn double knockout mice do so as well (21), pointing to a precise signaling cascade with these essential components that is responsible for transmitting the effects of Reelin to the neuron.
Individual loss of the ApoE receptors has much milder effects on brain development. Single knockout mice for either Apoer2 or Vldlr each have reduced foliation of their cerebella and distinct cortical lamination defects, as well as splitting of the CA1 layer of the hippocampus; however, the cerebellar defects are more prominent in Vldlr knockouts, while the cortical and hippocampal defects are more pronounced in the Apoer2 knockouts, suggesting clear functional differences in how these two receptors transmit the Reelin signal to direct neuronal migration (17).
Reelin STRUCTURE AND CLEAVAGE
Full-length Reelin is a very large (>400 kDa) protein (12). The central domain of Reelin consists of eight Reelin repeats (RRs), each containing two subdomains separated by an EGF motif. At the N terminus, there is a signal peptide and an F-spondin domain, while the C terminus contains a small C-terminal region that is positively charged (Fig. 1).
Reelin possesses two primary cleavage sites between RR2 and RR3 and between RR6 and RR7, leading to N-terminal (N-t), central, and C-terminal fragments (22). The central fragment of Reelin is the binding site for Apoer2 and Vdlr and is sufficient for many aspects of Reelin signaling (23). However, the N-t fragment is required for homodimerization of Reelin (24, 25), while the C-terminal fragment is required for full Reelin signaling (26). Additionally, the N-t fragment has been reported to bind integrin α3β1, ephrine B (EphB) receptors, and cadherin-related neuronal receptors (CNRs) (27–29). More recently, a novel mutant was described in which just the C-terminal region was deleted. Interestingly, this region was shown to interact with Vldlr, mediating binding, but not Apoer2, and these mutants recapitulated the Vldlr null phenotype (30).
Several proteases have been identified that mediate cleavage at these sites, including tPA, meprin α and β, and matrix metalloproteinase-9, reviewed thoroughly in (31); however, it appears that these proteases have overlapping roles, as no single knockout mouse has altered levels of Reelin fragments. Overall, full-length Reelin appears to be the most potent form of Reelin, while Reelin fragments are more diffusible and carry the Reelin signal a greater distance (32, 33).
Reelin SIGNALING CASCADE
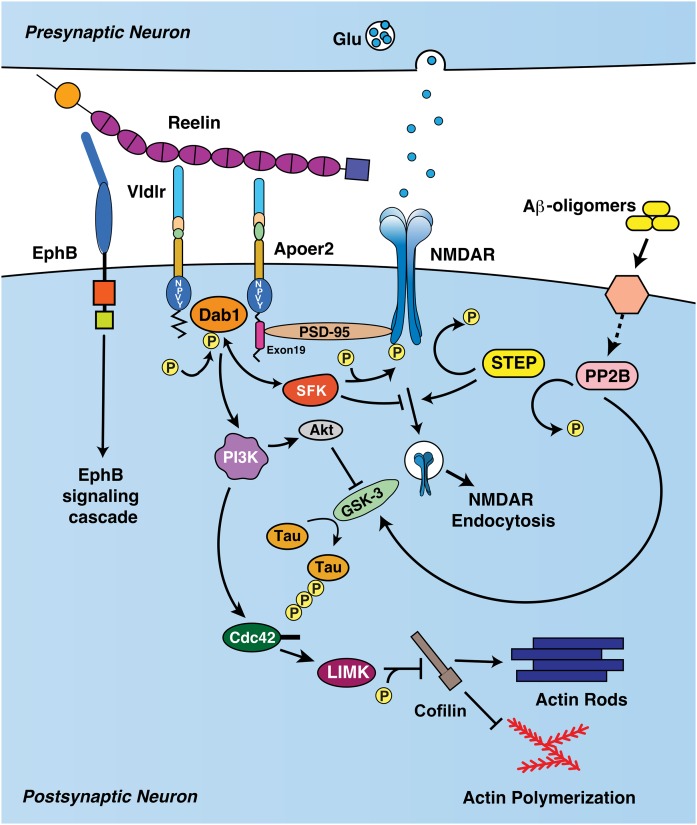
The Reelin signaling cascade is an evolutionarily conserved pathway that is important for numerous aspects of brain function, including laminar brain development, dendritic spine remodeling, and synaptic plasticity (Fig. 2). First, Reelin binding to Apoer2 and Vldlr induces clustering of the receptors (34). The NPXY domains of Apoer2 and Vldlr interact with the PTB domain of Dab1, and the receptor-adaptor cluster induces reciprocal phosphorylation of Dab1 and the Src family kinases (SFKs) (e.g., Fyn and Src) (13, 17, 35–39).
Fig. 2.
Reelin signaling cascade. The central fragment of Reelin binds to Vldlr and Apoer2, which clusters the two receptors and activates Dab1 signaling. Dab1 activates SFKs, which phosphorylate NMDARs, enhancing LTP and preventing NMDA endocytosis. NMDAR activation requires the presence of the alternatively spliced exon 19, which interacts with scaffolding protein PSD-95. Aβ has opposing effects on NMDAR phosphorylation and promotes NMDAR endocytosis, primarily through striatal-enriched protein tyrosine phosphatase (STEP) activation. Dab1 also activates PI3K, which leads to inhibition of GSK3β reduction of tau phosphorylation. PI3K additionally activates Cdc42, which leads to inhibitory phosphorylation of cofilin and promotes actin polymerization and dendritic spine growth. The N-t fragment of Reelin binds to EphB receptors and can induce EphB signaling (28). [Modified from (87).]
There are several effects of this activation. Dab1 activates phosphoinositol-3 kinase (PI3K), which in turn activates protein kinase B (Akt) (40). Akt phosphorylates glycogen synthase kinase 3β (GSK3β) at its inhibitory Ser-9 phosphorylation site. This reduces the phosphorylation of numerous targets of GSK3β, including tau, a microtubule associated protein whose hyperphosphorylation is a key initiator to development of the neurofibrillary tangles found in Alzheimer’s disease (AD) (41). Additionally, inhibition of GSK3β by Akt has been shown to inhibit long-term depression (42).
Dab1 signaling also impacts the development of dendritic spines. PI3K induces the phosphorylation of LIM kinase-1, which phosphorylates cofilin at an inhibitory site (43). Cofilin promotes the depolymerization of actin polymers, thus its inhibition by LIM kinase-1 leads to actin polymerization and spine growth. Reelin signaling also regulates dendritic growth via the Akt-dependent activation of mTor (44). As a result, mice that overexpress Reelin have higher spine density and complexity (45).
Interestingly, phosphorylation and activation of Dab1 leads to its ubiquitination and degradation, leading to self-limitation of the Reelin signal. As a result, reeler mice, as well as other mice with genetic interruptions to the Reelin cascade upstream of Dab1, have very high levels of Dab1 (38, 46).
Clearly, Dab1 signaling has numerous roles in the CNS. The effect of Dab1-induced SFK activation on synaptic function is described in the next section.
Reelin AS A SYNAPTIC MODULATOR
In the young postnatal brain, Cajal-Retzius cells slowly die off, and Reelin expression primarily switches to GABAergic inhibitory interneurons and a subset of excitatory pyramidal cells in the entorhinal cortex (47, 48). At this point, the Reelin signaling apparatus is repurposed as a modulator of synaptic plasticity. As described above, Reelin clustering of Apoer2 and Vldlr leads to the activation of SFKs via Dab1. SFKs, in turn, phosphorylate the N-methyl-D-aspartate receptor (NMDAR), which augments Ca2+ flux through the receptor when the receptor is open (49). As a result, application of Reelin to an acutely prepared hippocampal slice enhances long-term potentiation (LTP) (50). Reelin application does not enhance LTP in slices prepared from Apoer2 or Vldlr knockout mice (50). As an in vivo correlate of this enhancement, intraventricular injection of Reelin enhances performance of wild-type mice on the Morris water maze, a common behavioral test of learning (51).
In addition to its effect at the postsynaptic density, Reelin signaling affects presynaptic function. Apoer2 and Vldlr were recently demonstrated to be present in the presynaptic membrane, where Reelin signaling leads to a transient elevation of intracellular Ca2+, which increases the spontaneous release of vesicle-associated membrane protein 7 (VAMP-7) (52). Intriguingly, this effect is specific to VAMP-7-containing vesicles, and further research is required to determine the mechanism by which Reelin signaling affects this select vesicle pool.
Apoer2 ALTERNATIVE SPLICING
The ICD of Apoer2 has an alternatively spliced 59 amino acid domain that interacts with c-Jun N-terminal kinase (JNK) and postsynaptic density protein 95 (PSD-95), the latter of which acts as a scaffold to promote interaction with NMDAR. This interaction is required for the effect of Reelin on LTP, as hippocampal slices from mice that are constitutively lacking this region do not enhance LTP in the presence of Reelin (10). This is correlated in vivo by the finding that these mice have a deficit in fear learning (10).
The alternative splicing of exon 19 may play a role in human disease. Postmortem brain tissue from AD patients has fewer Apoer2 transcripts that include exon 19 compared with healthy controls. This dysregulation also occurs in the TgCRND8 mouse model of AD, and rescuing dysregulated splicing using an antisense oligonucleotide (ASO) improves synaptic function and cognitive performance (53).
Another alternatively spliced domain of Apoer2 is the OLS domain encoded by exon 16, which is important for receptor processing. Apoer2 is sequentially cleaved first by extracellular α-secretases [e.g., a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10)], leading to the release of the extracellular domain (ECD), followed by γ-secretase, which releases the ICD (7) (Fig. 1). This cleavage process leads to a negative feedback through two mechanisms. The ICD translocates to the nucleus, which alters the epigenetic signature and causes inhibition of Reelin transcription (54). At the same time, the ECD can act as a dominant-negative ligand to inhibit Reelin signaling (55). When the OLS is glycosylated or excluded by alternatively splicing, Apoer2 is protected from proteolytic processing (7, 56). This leads both to an abundance of Apoer2 and a reduction in these negative-feedback mechanisms (56).
Reelin RECEPTOR SUPERSTRUCTURE: ADDING EphB RECEPTORS AND AMYLOID PRECURSOR PROTEIN
The large size of Reelin suggests an ability to form a “superstructure,” bringing together multiple components of the synapse. While the central fragment of Reelin binds to Vldlr and Apoer2, the N-t fragment binds to EphB2 receptors (Fig. 2). Similar to its effect on the ApoE receptors, Reelin binding to EphB receptors induces their clustering and forward EphB signaling, independent of both the ApoE receptors and Dab1 (28). This leads to an interesting model whereby the N-t region and central region of Reelin bind to EphB receptors and the ApoE receptors, respectively, bringing together these two signaling cascades; however, this hypothesis has yet to be formally tested.
The role that the EphB/Reelin interaction plays in brain development has been difficult to tease apart. Though it had been previously reported that EphB1/2/3 triple knockout mice recapitulated the reeler phenotype (57), Pohlkamp et al. (58) failed to replicate this finding. Rather, it appears that EphB1/2 double knockout mice merely have a mild hippocampal positioning defect (28).
Another protein shown to interact with Apoer2 is the amyloid precursor protein (APP), which is sequentially cleaved to form amyloid-β (Aβ) (59). APP contains an NPXY domain in its ICD, similar to Apoer2 (60). As described above, Dab1 interacts with the NPXY motif, and Dab1 interaction with either APP or Apoer2 increases cell surface levels of the receptors and decreases levels of Aβ (61). Similarly, Fe65 interacts intracellularly with both APP and Apoer2 to form a trimeric complex (62). Surprisingly, APP and Apoer2 also directly interact, which slows the rate of APP internalization, increases its association with lipid rafts, and results in increased β-cleavage of APP and, thus, Aβ production (63). Additionally, APP and Apoer2 indirectly interact extracellularly through F-spondin, which enhances α-cleavage (i.e., nonamyloidogenic) of APP (64). Full-length F-spondin is required for this interaction, suggesting that it forms an extracellular bridge. Thus, there are several mechanisms by which APP and Apoer2 interact, which can modify Aβ production in opposite ways. The interactions of ApoE receptors and APP have been previously reviewed in depth by us and other groups.
Reelin-INDEPENDENT ROLES OF Vldlr/Apoer2 SIGNALING IN THE CNS
The roles of the Vldlr/Apoer2 signaling cascade are not limited to Reelin as the ligand. Postnatal migration of neuronal precursors from the subventricular zone to the olfactory bulb is not dependent on Reelin, but does require Apoer2/Vldlr/Dab1 (65). One proposed ligand to mediate this effect is ApoJ (also known as clusterin), which can bind to Apoer2 and Vldlr and activate Dab1 (66). When clusterin is blocked in vitro, neuroblast chain formation and migration is blocked in the subventricular zone (66). Another proposed ligand is thrombospondin-1, which also binds Apoer2 and Vldlr, inducing phosphorylation of Dab1 and stabilization of neuronal precursor chains. Interestingly, thrombospondin-1 phosphorylation does not induce Dab1 degradation or Akt phosphorylation, and the reason for these differences in downstream signaling is currently unclear (67).
Additionally, Apoer2 is responsible for selenium uptake from selenoprotein P (Sepp1) at the blood-brain barrier, independent of Reelin (68). The importance of this trafficking role was highlighted by the finding that Apoer2-deficient mice are susceptible to neurodegeneration on a reduced selenium diet (69). It is likely that with further research, other noncanonical roles for Apoer2 and Vldlr signaling in the CNS will be found.
Reelin SIGNALING COMPONENTS IN HUMAN DISEASE
Given the importance of Reelin signaling in brain development, it is not surprising that mutations in the components of the Reelin signaling cascade often lead to neurodevelopmental disorders in humans. Homozygous loss of RELN leads to lissencephaly with cerebellar hypoplasia, a phenotype similar to that observed in reeler mice (70). Similarly, homozygous loss-of-function mutations in Vldlr cause disequilibrium syndrome, a nonprogressive cerebellar ataxia with mental retardation that is similar to, though less severe than, the phenotype observed in Reelin-deficient patients (71). Mutations in Apoer2 leading to neurodevelopmental disorders have so far not been identified. In contrast, individuals who are completely ApoE-deficient do not have neurodevelopmental changes; however, consistent with the role of ApoE in cholesterol metabolism, ApoE-deficient individuals do have high plasma cholesterol and triglycerides and early atherosclerosis (72).
Because Reelin signaling is required for normal brain development, it would be difficult for extensive structural Reelin defects to lead to a milder phenotype. However, numerous studies have identified RELN SNPs and de novo mutations in autism spectrum disorder (ASD), which have been recently reviewed in (73). It is important to note that these genetic changes are unlikely to be the sole cause of disease, but rather contributing factors to ASD development. Mutations in the other components of the Reelin signaling pathway (Vldlr, Apoer2, Dab1, etc.) have not been associated with ASD. Reelin has similarly been proposed to play a role in schizophrenia and depression, summarized in (74, 75).
Reelin RECEPTOR TRAFFICKING: A ROLE FOR ApoE4 IN GENERATING SYNAPTIC DYSFUNCTION
ApoE exists in the human populations in three common isoforms: ε2, ε3, and ε4 (ApoE2, -E3, and -E4, respectively). ApoE4, present in approximately 15% of the population, confers a dose-dependent increased risk of late-onset AD, with one allelic copy increasing risk 3-fold, and two copies increasing risk 12-fold (76). Conversely, ApoE2 is protective against AD development, while ApoE3, the most common allele, is considered to be risk neutral (77). ApoE3 and ApoE4 differ by one amino acid (Cys112 and Arg 112, respectively), which promotes a domain interaction between the N and C termini of ApoE4 and increases the cleavage of ApoE4 into fragments (78). Other review articles in this series will focus on the role of ApoE in Aβ clearance and neurodegeneration. Here, we focus on its effects on Reelin receptor signaling.
As described above, the ApoE receptors, Apoer2 and Vldlr, are important for both brain development and adult synaptic plasticity as mediators of Reelin signaling. Upon activation of the receptors by Reelin, they are endocytosed to the early endosome, then recycled back to the surface. The ApoE isoforms differentially affect this process, with ApoE4 severely impairing receptor recycling to the surface, and ApoE3 and ApoE2 having more moderate and mild effects respectively (79). The reduction of surface availability of Reelin receptors leads to a resistance to Reelin signaling whereby acute hippocampal slices pretreated with ApoE4 do not have enhanced LTP after Reelin application (79).
Reelin activation of downstream kinases and enhancement of LTP is important for protection against Aβ, which reduces LTP by enhancing NMDAR endocytosis via striatal-enriched protein tyrosine phosphatase (80). Thus, application of Reelin to acute hippocampal slices prevents Aβ-induced reduction of LTP, and this effect is abrogated in the presence of ApoE4 (79, 81). As an important in vivo correlate, adult Reelin conditional-knockout mice, which have normal brain development and normal cognition in the absence of Aβ, become severely cognitively impaired when crossed with an Aβ-overproducing mouse model (82).
Evidence for an effect of ApoE isoform on synaptic plasticity in humans comes from imaging studies done in children and young adults. Functional MRI in healthy, young ApoE4 carriers already demonstrates connectivity deficits that are similar to AD patients (83), and some changes have been demonstrated as early as infancy (84). It is important to note that these functional imaging changes are observed prior to the development of Aβ deposition and neuritic plaques, supporting the idea that ApoE affects synaptic function independent of amyloid accumulation.
THERAPEUTIC APPROACHES
In the sections above, there are several points within the Reelin signaling cascade that could be targeted for therapeutics in AD and other diseases. The first point of intervention is at the level of Reelin itself. Overexpression of Reelin in a mouse model of AD rescues synaptic function and cognition (85). Similarly, intraventricular injection of Reelin improves memory acutely in wild-type mice (51). Though this has not been tested directly in AD mice, one could hypothesize that drugs that elevate levels of Reelin would be protective in AD. However, as described above, one of the mechanisms by which ApoE4 likely contributes to disease pathogenesis is by inducing a state of Reelin resistance through impaired endocytic recycling of receptors (79). Thus, in ApoE4 carriers, increasing Reelin levels may not be effective. Rather, therapeutics that restore endocytic recycling may be more effective.
At the level of the receptors themselves, one target may be the dysregulation of Apoer2 alternative splicing to promote the inclusion of exon 19. Using an ASO corrected this dysregulation in mice and rescued cognitive deficits (53). The first ASO treatment in clinical trials, nusinersen, developed for spinal muscular atrophy, a motor neurodegenerative disease that begins in infancy, was recently approved by the Food and Drug Administration for use in patients (86). This first clinical success indicates that ASOs are a viable method to treat neurodegenerative diseases.
CONCLUSIONS
The ApoE receptors, Apoer2 and Vldlr, and their primary ligand, Reelin, are vital for brain development and important for modulation of synaptic plasticity. Disruption of these pathways is implicated in various neurodevelopmental disorders, and ApoE4 appears to mediate some of its effects in AD pathogenesis by interfering with Reelin signaling in the adult brain. The Reelin-ApoE receptor signaling cascade presents an attractive therapeutic target for the treatment of AD and other diseases.
Footnotes
Abbreviations:
- Aβ
- amyloid-β AD, Alzheimer’s disease
- Akt
- protein kinase B
- Apoer2
- ApoE receptor 2
- APP
- amyloid precursor protein
- ASD
- autism spectrum disorder
- ASO
- antisense oligonucleotide
- Dab1
- disabled-1
- EGF
- epidermal growth factor
- EphB
- ephrin B
- GSK3β
- glycogen synthase kinase 3β ICD, intracellular domain
- LDLR
- LDL receptor
- LTP
- long-term potentiation
- NMDAR
- N-methyl-D-aspartate receptor
- N-t
- N-terminal
- OLS
- O-linked sugar
- PI3K
- phosphoinosital-3 kinase
- PSD-95
- postsynaptic density protein 95
- RR
- Reelin repeat
- SFK
- Src family kinase
- Vldlr
- VLDL receptor
This work was supported by National Institutes of Health Grants F30-AG047799 (to C.L-D.) and HL063762, NS093382, and AG053391 (to J.H.); the Consortium for Frontotemporal Dementia Research; and the BrightFocus Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Herz J. 2001. The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron. 29: 571–581. [DOI] [PubMed] [Google Scholar]
- 2.Davis C. G., Goldstein J. L., Sudhof T. C., Anderson R. G., Russell D. W., and Brown M. S.. 1987. Acid-dependent ligand dissociation and recycling of LDL receptor mediated by growth factor homology region. Nature. 326: 760–765. [DOI] [PubMed] [Google Scholar]
- 3.Oka K., Ishimura-Oka K., Chu M. J., Sullivan M., Krushkal J., Li W. H., and Chan L.. 1994. Mouse very-low-density-lipoprotein receptor (VLDLR) cDNA cloning, tissue-specific expression and evolutionary relationship with the low-density-lipoprotein receptor. Eur. J. Biochem. 224: 975–982. [DOI] [PubMed] [Google Scholar]
- 4.Clatworthy A. E., Stockinger W., Christie R. H., Schneider W. J., Nimpf J., Hyman B. T., and Rebeck G. W.. 1999. Expression and alternate splicing of apolipoprotein E receptor 2 in brain. Neuroscience. 90: 903–911. [DOI] [PubMed] [Google Scholar]
- 5.Brandes C., Kahr L., Stockinger W., Hiesberger T., Schneider W. J., and Nimpf J.. 2001. Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding reelin but not alpha 2-macroglobulin. J. Biol. Chem. 276: 22160–22169. [DOI] [PubMed] [Google Scholar]
- 6.Esser V., and Russell D. W.. 1988. Transport-deficient mutations in the low density lipoprotein receptor. Alterations in the cysteine-rich and cysteine-poor regions of the protein block intracellular transport. J. Biol. Chem. 263: 13276–13281. [PubMed] [Google Scholar]
- 7.May P., Bock H. H., Nimpf J., and Herz J.. 2003. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J. Biol. Chem. 278: 37386–37392. [DOI] [PubMed] [Google Scholar]
- 8.Sakai J., Hoshino A., Takahashi S., Miura Y., Ishii H., Suzuki H., Kawarabayasi Y., and Yamamoto T.. 1994. Structure, chromosome location, and expression of the human very low density lipoprotein receptor gene. J. Biol. Chem. 269: 2173–2182. [PubMed] [Google Scholar]
- 9.Gotthardt M., Trommsdorff M., Nevitt M. F., Shelton J., Richardson J. A., Stockinger W., Nimpf J., and Herz J.. 2000. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J. Biol. Chem. 275: 25616–25624. [DOI] [PubMed] [Google Scholar]
- 10.Beffert U., Weeber E. J., Durudas A., Qiu S., Masiulis I., Sweatt J. D., Li W. P., Adelmann G., Frotscher M., Hammer R. E., et al. . 2005. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 47: 567–579. [DOI] [PubMed] [Google Scholar]
- 11.Kim D. H., Iijima H., Goto K., Sakai J., Ishii H., Kim H. J., Suzuki H., Kondo H., Saeki S., and Yamamoto T.. 1996. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J. Biol. Chem. 271: 8373–8380. [DOI] [PubMed] [Google Scholar]
- 12.D’Arcangelo G., Miao G. G., Chen S. C., Soares H. D., Morgan J. I., and Curran T.. 1995. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 374: 719–723. [DOI] [PubMed] [Google Scholar]
- 13.D’Arcangelo G., Homayouni R., Keshvara L., Rice D. S., Sheldon M., and Curran T.. 1999. Reelin is a ligand for lipoprotein receptors. Neuron. 24: 471–479. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa M., Miyata T., Nakajima K., Yagyu K., Seike M., Ikenaka K., Yamamoto H., and Mikoshiba K.. 1995. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 14: 899–912. [DOI] [PubMed] [Google Scholar]
- 15.Falconer D. S. 1951. Two new mutants, ‘trembler’ and ‘reeler’, with neurological actions in the house mouse (Mus musculus L.). J. Genet. 50: 192–201. [DOI] [PubMed] [Google Scholar]
- 16.Zhao S., and Frotscher M.. 2010. Go or stop? Divergent roles of Reelin in radial neuronal migration. Neuroscientist. 16: 421–434. [DOI] [PubMed] [Google Scholar]
- 17.Trommsdorff M., Gotthardt M., Hiesberger T., Shelton J., Stockinger W., Nimpf J., Hammer R. E., Richardson J. A., and Herz J.. 1999. Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 97: 689–701. [DOI] [PubMed] [Google Scholar]
- 18.Sheldon M., Rice D. S., D’Arcangelo G., Yoneshima H., Nakajima K., Mikoshiba K., Howell B. W., Cooper J. A., Goldowitz D., and Curran T.. 1997. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature. 389: 730–733. [DOI] [PubMed] [Google Scholar]
- 19.Sweet H. O., Bronson R. T., Johnson K. R., Cook S. A., and Davisson M. T.. 1996. Scrambler, a new neurological mutation of the mouse with abnormalities of neuronal migration. Mamm. Genome. 7: 798–802. [DOI] [PubMed] [Google Scholar]
- 20.Howell B. W., Hawkes R., Soriano P., and Cooper J. A.. 1997. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 389: 733–737. [DOI] [PubMed] [Google Scholar]
- 21.Kuo G., Arnaud L., Kronstad-O’Brien P., and Cooper J. A.. 2005. Absence of Fyn and Src causes a reeler-like phenotype. J. Neurosci. 25: 8578–8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Arcangelo G., Nakajima K., Miyata T., Ogawa M., Mikoshiba K., and Curran T.. 1997. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J. Neurosci. 17: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jossin Y., Ignatova N., Hiesberger T., Herz J., Lambert de Rouvroit C., and Goffinet A. M.. 2004. The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J. Neurosci. 24: 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Utsunomiya-Tate N., Kubo K., Tate S., Kainosho M., Katayama E., Nakajima K., and Mikoshiba K.. 2000. Reelin molecules assemble together to form a large protein complex, which is inhibited by the function-blocking CR-50 antibody. Proc. Natl. Acad. Sci. USA. 97: 9729–9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubo K., Mikoshiba K., and Nakajima K.. 2002. Secreted Reelin molecules form homodimers. Neurosci. Res. 43: 381–388. [DOI] [PubMed] [Google Scholar]
- 26.Kohno T., Honda T., Kubo K., Nakano Y., Tsuchiya A., Murakami T., Banno H., Nakajima K., and Hattori M.. 2015. Importance of Reelin C-terminal region in the development and maintenance of the postnatal cerebral cortex and its regulation by specific proteolysis. J. Neurosci. 35: 4776–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dulabon L., Olson E. C., Taglienti M. G., Eisenhuth S., McGrath B., Walsh C. A., Kreidberg J. A., and Anton E. S.. 2000. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron. 27: 33–44. [DOI] [PubMed] [Google Scholar]
- 28.Bouché E., Romero-Ortega M. I., Henkemeyer M., Catchpole T., Leemhuis J., Frotscher M., May P., Herz J., and Bock H. H.. 2013. Reelin induces EphB activation. Cell Res. 23: 473–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senzaki K., Ogawa M., and Yagi T.. 1999. Proteins of the CNR family are multiple receptors for Reelin. Cell. 99: 635–647. [DOI] [PubMed] [Google Scholar]
- 30.Ha S., Tripathi P. P., Mihalas A. B., Hevner R. F., and Beier D. R.. 2017. C-terminal region truncation of RELN disrupts an interaction with VLDLR causing abnormal development of the cerebral cortex and hippocampus. J. Neurosci. 37: 960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lussier A. L., Weeber E. J., and Rebeck G. W.. 2016. Reelin proteolysis affects signaling related to normal synapse function and neurodegeneration. Front. Cell. Neurosci. 10: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koie M., Okumura K., Hisanaga A., Kamei T., Sasaki K., Deng M., Baba A., Kohno T., and Hattori M.. 2014. Cleavage within Reelin repeat 3 regulates the duration and range of the signaling activity of Reelin protein. J. Biol. Chem. 289: 12922–12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jossin Y., Gui L., and Goffinet A. M.. 2007. Processing of Reelin by embryonic neurons is important for function in tissue but not in dissociated cultured neurons. J. Neurosci. 27: 4243–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strasser V., Fasching D., Hauser C., Mayer H., Bock H. H., Hiesberger T., Herz J., Weeber E. J., Sweatt J. D., Pramatarova A., et al. . 2004. Receptor clustering is involved in Reelin signaling. Mol. Cell. Biol. 24: 1378–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howell B. W., Lanier L. M., Frank R., Gertler F. B., and Cooper J. A.. 1999. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol. Cell. Biol. 19: 5179–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howell B. W., Herrick T. M., and Cooper J. A.. 1999. Reelin-induced tyrosine [corrected] phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 13: 643–648. [Erratum. 1999. Genes Dev. 13: 1642.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bock H. H., and Herz J.. 2003. Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 13: 18–26. [DOI] [PubMed] [Google Scholar]
- 38.Arnaud L., Ballif B. A., and Cooper J. A.. 2003. Regulation of protein tyrosine kinase signaling by substrate degradation during brain development. Mol. Cell. Biol. 23: 9293–9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiesberger T., Trommsdorff M., Howell B. W., Goffinet A., Mumby M. C., Cooper J. A., and Herz J.. 1999. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 24: 481–489. [DOI] [PubMed] [Google Scholar]
- 40.Bock H. H., Jossin Y., Liu P., Forster E., May P., Goffinet A. M., and Herz J.. 2003. Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J. Biol. Chem. 278: 38772–38779. [DOI] [PubMed] [Google Scholar]
- 41.Beffert U., Morfini G., Bock H. H., Reyna H., Brady S. T., and Herz J.. 2002. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3beta. J. Biol. Chem. 277: 49958–49964. [DOI] [PubMed] [Google Scholar]
- 42.Peineau S., Taghibiglou C., Bradley C., Wong T. P., Liu L., Lu J., Lo E., Wu D., Saule E., Bouschet T., et al. . 2007. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 53: 703–717. [DOI] [PubMed] [Google Scholar]
- 43.Chai X., Forster E., Zhao S., Bock H. H., and Frotscher M.. 2009. Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3. J. Neurosci. 29: 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jossin Y., and Goffinet A. M.. 2007. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol. Cell. Biol. 27: 7113–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bosch C., Masachs N., Exposito-Alonso D., Martinez A., Teixeira C. M., Fernaud I., Pujadas L., Ulloa F., Comella J. X., DeFelipe J., et al. . 2016. Reelin regulates the maturation of dendritic spines, synaptogenesis and glial ensheathment of newborn granule cells. Cereb. Cortex. 26: 4282–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bock H. H., Jossin Y., May P., Bergner O., and Herz J.. 2004. Apolipoprotein E receptors are required for reelin-induced proteasomal degradation of the neuronal adaptor protein disabled-1. J. Biol. Chem. 279: 33471–33479. [DOI] [PubMed] [Google Scholar]
- 47.Alcántara S., Ruiz M., D’Arcangelo G., Ezan F., de Lecea L., Curran T., Sotelo C., and Soriano E.. 1998. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J. Neurosci. 18: 7779–7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Botella-López A., Burgaya F., Gavin R., Garcia-Ayllón M. S., Gómez-Tortosa E., Peña-Casanova J., Ureña J. M., Del Rio J. A., Blesa R., Soriano E., et al. . 2006. Reelin expression and glycosylation patterns are altered in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 103: 5573–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y., Beffert U., Ertunc M., Tang T. S., Kavalali E. T., Bezprozvanny I., and Herz J.. 2005. Reelin modulates NMDA receptor activity in cortical neurons. J. Neurosci. 25: 8209–8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weeber E. J., Beffert U., Jones C., Christian J. M., Forster E., Sweatt J. D., and Herz J.. 2002. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 277: 39944–39952. [DOI] [PubMed] [Google Scholar]
- 51.Rogers J. T., Rusiana I., Trotter J., Zhao L., Donaldson E., Pak D. T., Babus L. W., Peters M., Banko J. L., Chavis P., et al. . 2011. Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn. Mem. 18: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bal M., Leitz J., Reese A. L., Ramirez D. M., Durakoglugil M., Herz J., Monteggia L. M., and Kavalali E. T.. 2013. Reelin mobilizes a VAMP7-dependent synaptic vesicle pool and selectively augments spontaneous neurotransmission. Neuron. 80: 934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hinrich A. J., Jodelka F. M., Chang J. L., Brutman D., Bruno A. M., Briggs C. A., James B. D., Stutzmann G. E., Bennett D. A., Miller S. A., et al. . 2016. Therapeutic correction of ApoER2 splicing in Alzheimer’s disease mice using antisense oligonucleotides. EMBO Mol. Med. 8: 328–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Telese F., Ma Q., Perez P. M., Notani D., Oh S., Li W., Comoletti D., Ohgi K. A., Taylor H., and Rosenfeld M. G.. 2015. LRP8-Reelin-regulated neuronal enhancer signature underlying learning and memory formation. Neuron. 86: 696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koch S., Strasser V., Hauser C., Fasching D., Brandes C., Bajari T. M., Schneider W. J., and Nimpf J.. 2002. A secreted soluble form of ApoE receptor 2 acts as a dominant-negative receptor and inhibits Reelin signaling. EMBO J. 21: 5996–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wasser C. R., Masiulis I., Durakoglugil M. S., Lane-Donovan C., Xian X., Beffert U., Agarwala A., Hammer R. E., and Herz J.. 2014. Differential splicing and glycosylation of Apoer2 alters synaptic plasticity and fear learning. Sci. Signal. 7: ra113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sentürk A., Pfennig S., Weiss A., Burk K., and Acker-Palmer A.. 2011. Ephrin Bs are essential components of the Reelin pathway to regulate neuronal migration. Nature. 472: 356–360. [DOI] [PubMed] [Google Scholar]
- 58.Pohlkamp T., Xiao L., Sultana R., Bepari A., Bock H. H., Henkemeyer M., and Herz J.. 2016. Ephrin Bs and canonical Reelin signalling. Nature. 539: E4–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Brien R. J., and Wong P. C.. 2011. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 34: 185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fiore F., Zambrano N., Minopoli G., Donini V., Duilio A., and Russo T.. 1995. The regions of the Fe65 protein homologous to the phosphotyrosine interaction/phosphotyrosine binding domain of Shc bind the intracellular domain of the Alzheimer’s amyloid precursor protein. J. Biol. Chem. 270: 30853–30856. [DOI] [PubMed] [Google Scholar]
- 61.Hoe H. S., Tran T. S., Matsuoka Y., Howell B. W., and Rebeck G. W.. 2006. DAB1 and Reelin effects on amyloid precursor protein and ApoE receptor 2 trafficking and processing. J. Biol. Chem. 281: 35176–35185. [DOI] [PubMed] [Google Scholar]
- 62.Hoe H. S., Magill L. A., Guenette S., Fu Z., Vicini S., and Rebeck G. W.. 2006. FE65 interaction with the ApoE receptor ApoEr2. J. Biol. Chem. 281: 24521–24530. [DOI] [PubMed] [Google Scholar]
- 63.Fuentealba R. A., Barria M. I., Lee J., Cam J., Araya C., Escudero C. A., Inestrosa N. C., Bronfman F. C., Bu G., and Marzolo M. P.. 2007. ApoER2 expression increases Abeta production while decreasing amyloid precursor protein (APP) endocytosis: possible role in the partitioning of APP into lipid rafts and in the regulation of gamma-secretase activity. Mol. Neurodegener. 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoe H. S., Wessner D., Beffert U., Becker A. G., Matsuoka Y., and Rebeck G. W.. 2005. F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol. Cell. Biol. 25: 9259–9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andrade N., Komnenovic V., Blake S. M., Jossin Y., Howell B., Goffinet A., Schneider W. J., and Nimpf J.. 2007. ApoER2/VLDL receptor and Dab1 in the rostral migratory stream function in postnatal neuronal migration independently of Reelin. Proc. Natl. Acad. Sci. USA. 104: 8508–8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leeb C., Eresheim C., and Nimpf J.. 2014. Clusterin is a ligand for apolipoprotein E receptor 2 (ApoER2) and very low density lipoprotein receptor (VLDLR) and signals via the Reelin-signaling pathway. J. Biol. Chem. 289: 4161–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blake S. M., Strasser V., Andrade N., Duit S., Hofbauer R., Schneider W. J., and Nimpf J.. 2008. Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration. EMBO J. 27: 3069–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olson G. E., Winfrey V. P., Nagdas S. K., Hill K. E., and Burk R. F.. 2007. Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J. Biol. Chem. 282: 12290–12297. [DOI] [PubMed] [Google Scholar]
- 69.Burk R. F., Hill K. E., Motley A. K., Winfrey V. P., Kurokawa S., Mitchell S. L., and Zhang W.. 2014. Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J. 28: 3579–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hong S. E., Shugart Y. Y., Huang D. T., Shahwan S. A., Grant P. E., Hourihane J. O., Martin N. D., and Walsh C. A.. 2000. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat. Genet. 26: 93–96. [DOI] [PubMed] [Google Scholar]
- 71.Boycott K. M., Flavelle S., Bureau A., Glass H. C., Fujiwara T. M., Wirrell E., Davey K., Chudley A. E., Scott J. N., McLeod D. R., et al. . 2005. Homozygous deletion of the very low density lipoprotein receptor gene causes autosomal recessive cerebellar hypoplasia with cerebral gyral simplification. Am. J. Hum. Genet. 77: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mak A. C., Pullinger C. R., Tang L. F., Wong J. S., Deo R. C., Schwarz J. M., Gugliucci A., Movsesyan I., Ishida B. Y., Chu C., et al. . 2014. Effects of the absence of apolipoprotein e on lipoproteins, neurocognitive function, and retinal function. JAMA Neurol. 71: 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lammert D. B., and Howell B. W.. 2016. RELN mutations in autism spectrum disorder. Front. Cell. Neurosci. 10: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caruncho H. J., Brymer K., Romay-Tallon R., Mitchell M. A., Rivera-Baltanas T., Botterill J., Olivares J. M., and Kalynchuk L. E.. 2016. Reelin-related disturbances in depression: implications for translational studies. Front. Cell. Neurosci. 10: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guidotti A., Grayson D. R., and Caruncho H. J.. 2016. Epigenetic RELN dysfunction in schizophrenia and related neuropsychiatric disorders. Front. Cell. Neurosci. 10: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., and Pericak-Vance M. A.. 1993. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 261: 921–923. [DOI] [PubMed] [Google Scholar]
- 77.Corder E. H., Saunders A. M., Risch N. J., Strittmatter W. J., Schmechel D. E., Gaskell P. C. Jr., Rimmler J. B., Locke P. A., Conneally P. M., Schmader K. E., et al. . 1994. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat. Genet. 7: 180–184. [DOI] [PubMed] [Google Scholar]
- 78.Mahley R. W., Weisgraber K. H., and Huang Y.. 2006. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 103: 5644–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y., Durakoglugil M. S., Xian X., and Herz J.. 2010. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc. Natl. Acad. Sci. USA. 107: 12011–12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Snyder E. M., Nong Y., Almeida C. G., Paul S., Moran T., Choi E. Y., Nairn A. C., Salter M. W., Lombroso P. J., Gouras G. K., et al. . 2005. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 8: 1051–1058. [DOI] [PubMed] [Google Scholar]
- 81.Durakoglugil M. S., Chen Y., White C. L., Kavalali E. T., and Herz J.. 2009. Reelin signaling antagonizes beta-amyloid at the synapse. Proc. Natl. Acad. Sci. USA. 106: 15938–15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lane-Donovan C., Philips G. T., Wasser C. R., Durakoglugil M. S., Masiulis I., Upadhaya A., Pohlkamp T., Coskun C., Kotti T., Steller L., et al. . 2015. Reelin protects against amyloid beta toxicity in vivo. Sci. Signal. 8: ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sheline Y. I., Morris J. C., Snyder A. Z., Price J. L., Yan Z., D’Angelo G., Liu C., Dixit S., Benzinger T., Fagan A., et al. . 2010. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J. Neurosci. 30: 17035–17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dean D. C. III, Jerskey B. A., Chen K., Protas H., Thiyyagura P., Roontiva A., O’Muircheartaigh J., Dirks H., Waskiewicz N., Lehman K., et al. . 2014. Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurol. 71: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pujadas L., Rossi D., Andres R., Teixeira C. M., Serra-Vidal B., Parcerisas A., Maldonado R., Giralt E., Carulla N., and Soriano E.. 2014. Reelin delays amyloid-beta fibril formation and rescues cognitive deficits in a model of Alzheimer’s disease. Nat. Commun. 5: 3443. [DOI] [PubMed] [Google Scholar]
- 86.Finkel R. S., Chiriboga C. A., Vajsar J., Day J. W., Montes J., De Vivo D. C., Yamashita M., Rigo F., Hung G., Schneider E., et al. . 2016. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 388: 3017–3026. [DOI] [PubMed] [Google Scholar]
- 87.Lane-Donovan C., and Herz J.. 2017. ApoE, ApoE receptors, and the synapse in Alzheimer’s disease. Trends Endocrinol. Metab. 28: 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]