Abstract
Background
We conducted a study of congenital Trypanosoma cruzi infection in Santa Cruz, Bolivia. Our objective was to apply new tools to identify weak points in current screening algorithms, and find ways to improve them.
Methods
Women presenting for delivery were screened by rapid and conventional serological tests. For infants of infected mothers, blood specimens obtained on days 0, 7, 21, 30, 90, 180, and 270 were concentrated and examined microscopically; serological tests were performed for the day 90, 180, and 270 specimens. Maternal and infant specimens, including umbilical tissue, were tested by polymerase chain reaction (PCR) targeting the kinetoplast minicircle and by quantitative PCR.
Results
Of 530 women, 154 (29%) were seropositive. Ten infants had congenital T. cruzi infection. Only 4 infants had positive results of microscopy evaluation in the first month, and none had positive cord blood microscopy results. PCR results were positive for 6 (67%) of 9 cord blood and 7 (87.5%) of 8 umbilical tissue specimens. PCR-positive women were more likely to transmit T. cruzi than were seropositive women with negative PCR results (P < .05). Parasite loads determined by quantitative PCR were higher for mothers of infected infants than for seropositive mothers of uninfected infants (P < .01). Despite intensive efforts, only 58% of at-risk infants had a month 9 specimen collected.
Conclusions
On the basis of the low sensitivity of microscopy in cord blood and high rate of loss to follow-up, we estimate that current screening programs miss one-half of all infected infants. Molecular techniques may improve early detection.
Since 1991, the estimated prevalence of Trypanosoma cruzi infection has decreased from 18 million to 8 million as a result of intensive vector control and blood bank screening [1, 2]. As other transmission routes have diminished, the proportion attributable to congenital infection has grown: an estimated 26% of new infections now occur through mother-to-child transmission [2]. Congenital transmission can occur from women who are themselves infected congenitally, perpetuating the disease in the absence of the vector [3]. Reported transmission rates from infected mothers vary from 1% to >10% [4–6]. Factors reported to increase risk include younger maternal age, human immunodeficiency virus infection, and (in an animal model) parasite strain [6–9].
Although an autochthonous enzootic cycle and competent vectors exist across the southern half of the country, the vast majority of human T. cruzi infections in the United States affect immigrants infected in their home countries [10–12]. One group of researchers estimates that 40,000 infected women of child-bearing age live in the United States and that 189 congenital T. cruzi infections occur annually [13]. This estimate indicates that congenital Chagas disease may be more common than 23 of 29 noninfectious disorders in the American College of Genetics recommended newborn screening panel [14].
Most congenital T. cruzi infections are asymptomatic or cause nonspecific signs, requiring laboratory screening for detection [15]. However, a small proportion causes severe morbidity, including low birthweight, hepatosplenomegaly, anemia, meningoencephalitis, and/or respiratory insufficiency, with high mortality [6]. Infants who survive the acute infection are presumed to carry the same 20%–30% lifetime risk of cardiac or gastrointestinal disease as other infected individuals [15]. Treatment during infancy is more effective and better tolerated than later treatment [16]. Although data are lacking, successful treatment is assumed to decrease or eliminate risk of later complications [8, 16]. Early diagnosis and treatment are, therefore, high priorities in control programs.
Bolivia has a congenital Chagas screening program in all departments where vector-borne T. cruzi transmission is endemic [17]. The program uses prenatal serological screening, followed by microscopic examination of concentrated cord blood from infants of seropositive mothers [17–19]. For infants who do not receive diagnoses at birth, conventional immunoglobulin G (IgG) serological testing is recommended after 6 months of age [17]. Similar programs exist in Argentina and Brazil [20, 21]. Because of logistical challenges, <20% of at-risk infants complete all steps of the algorithm [22].
Our objective was to use new tools to identify weak points in current congenital T. cruzi screening, and find ways to improve sensitivity and efficiency. We also investigated transmission dynamics, including quantification of parasitemia in mother and infant by real-time polymerase chain reaction (PCR). These data represent the most comprehensive recent study of a cohort of infants of T. cruzi–infected mothers, including systematic follow-up from birth to 9 months, and the use of conventional and novel diagnostic techniques over the full period.
METHODS
This study was conducted in the Hospital Universitario Japones, a public hospital in Santa Cruz, Bolivia. Although vectorborne T. cruzi transmission is absent, the infection prevalence is high, because the city acts as an economic magnet for migrants from rural areas with intense transmission [4, 23]. The protocol was approved by the ethics committees of the study hospital; Asociación Benéfica Proyectos en Informática, Salud, Medicina y Agricultura (Lima, Peru); Johns Hopkins University (Baltimore, MD; and the Centers for Disease Control and Prevention (Atlanta, GA). Trained study nurses explained the protocol to women presenting for delivery. After we obtained written informed consent, demographic data were solicited. A blood specimen was collected, centrifuged, and tested by 2 rapid diagnostic tests (RDTs), the indirect hemagglutination test (IHA) and Trypanosoma Detect (InBios International). The IHA utilized the Chagas Polychaco kit (Lemos Laboratories) with a positive cutoff value of 1:16 to provide high sensitivity. A subset of specimens was tested by Stat-Pak (Chembio Diagnostic Systems).
A study nurse attended the delivery of each RDT-positive woman to collect cord blood specimens and specimens from the placenta and severed umbilical cord from the end closest to the infant (Figure 1). The cord and placenta were washed thoroughly in phosphate-buffered saline buffer to remove maternal blood prior to sampling. Tissue aliquots were placed in 95% ethanol and in formalin. The attending pediatrician recorded major neonatal examination findings. Cord blood was placed in 4–6 heparinized microhematocrit tubes, sealed, and processed within 24 h by centrifugation (12,000 rpm for 7 min [rotor diameter, 20 cm]) followed by microscopic examination. This technique, called the micromethod, is the standard method to diagnose congenital T. cruzi infection in the first months of life [17–19]. The formalin-fixed umbilical specimen was embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
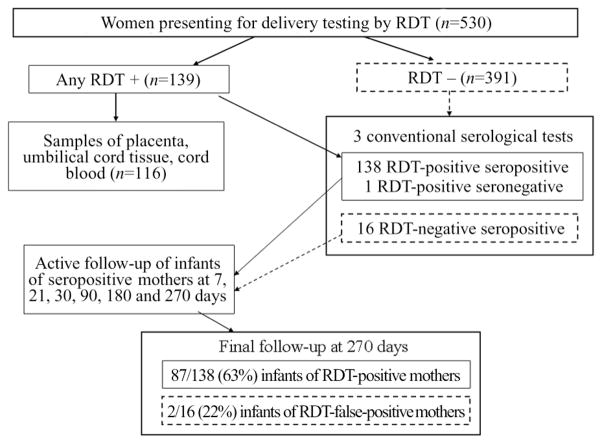
Figure 1.
Flow chart of the study design, with the number of mothers and infants completing each step of the follow-up plan. The rapid diagnostic tests (RDTs) used for maternal specimens were the InBios Trypanosoma Detect test (InBios International) and the indirect hemagglutination assay (IHA); a subset of specimens were tested by Stat Pak (Chembio Diagnostic Systems). One woman had a false-positive IHA result. Specimens from 16 confirmed seropositive women yielded false-negative RDT results; follow-up was significantly less complete for the infants of these women than for infants of women with true-positive RDT results.
All laboratory assays were run by technicians blinded to infection status of subjects. Maternal specimens were tested by an immunofluorescent antibody test (IFA) following standard methods [24], Chagatek enzyme-linked immunosorbent assay (ELISA; bioMérieux), and Chagatest Recombinante ELISA (Wiener Laboratories), as described in a previous publication [25]. Specimens that yielded positive results with ≥2 conventional tests were considered to be confirmed seropositive [26].
Infants of infected women had blood samples obtained at 7, 21, 30, 90, 180, and 270 days of age. At each follow-up visit, blood was examined by the micromethod, and additional blood was separated into clot and serum for subsequent testing. If infection was detected by parasite visualization at any age or by positive serological test results at 9 months, the infant was referred for treatment.
In maternal and infant serum specimens, we performed Western blots using trypomastigote excreted-secreted antigens in accordance with published methods [27, 28]. Blots with clear bands at the expected molecular weights were considered positive; blots with weak or ambiguous bands were repeated. The 150–160 kDa band on IgG TESA-blot corresponds to chronic infection, whereas ladder-like bands at 130–200 kDa on IgM TESA-blot demonstrate antibodies to Shed Acute Phase Antigens (SAPA), indicating acute or congenital infection [27, 29]. Bands below 95 kDa are considered to be nonspecific.
PCR was performed using 500-μL specimens of maternal and infant blood clot, and 25 mg specimens of placental and umbilical tissue. The use of clot was based on an analysis that demonstrated higher sensitivity for clot compared to buffy coat or whole blood mixed with guanidine [30]. DNA was extracted following a standard phenol-chloroform protocol [31]. Amplifications were performed using the 121/122 kinetoplast DNA primer set, as described elsewhere [30, 32].
Quantitative real-time PCR was performed on the basis of published methods [33]. The primer set Cruzi 1 (5′–ASTCGGCTGATCGTTTTCGA–3′) and Cruzi 2 (5′–AATTCCTCCAAGCAGCGGATA–3′) was used to amplify a 166–base pair DNA fragment. The probe Cruzi 3 (5′–CACACACTGGACACCAA–3′) was labeled with 5′FAM (6-carboxyfluorescein) and 3′MGB (minor groove binder). TaqMan Human RNase-P detection reagent (Applied Biosystems) was included as an internal control. Each reaction contained 10 μL of 1× Master Mix with uracil-N-glycosylase (Applied Biosystems), 0.4 μL of 0.1× Taqman RNase-P, 0.5 μmol/L of each primer, 0.2 μmol/L of Cruzi 3 probe, and 2 μL of extracted DNA in a final reaction volume of 20 μL. The reaction was performed in the Opticon 2 System under the following conditions: 50°C for 2 min, followed by 95°C for 10 min, during 45 cycles with 2 steps of amplification (95°C for 15 s and 1 min at 58°C) [33]. The threshold cycle was defined as the first cycle in which fluorescence was detected above baseline. A result was considered valid only when the internal control was efficiently amplified. We included 2 positive control specimens in each quantitative PCR run and checked their cycle number against their known cycle number from the standardization procedure, including a new standard curve, conducted for each batch of specimens. If the cycle number for the positive controls varied by more than 0.5 cycles, the run was repeated. A non-template negative control was included in each run. The threshold cycle was determined by the respective standard curve for the specimen batch and was always between 37 and 38 cycles. A clot specimen was inoculated with 1 × 106 T. cruzi Y strain trypomastigotes, extracted, and diluted successively to determine the minimum quantity detectable; the limit was found to be 1 parasite/mL.
A neonatologist (M.d.C.A.) treated infected infants in accordance with the Bolivian Chagas Disease Control Program guidelines [17]. The guidelines require positive micromethod results or positive results on 2 serological tests at ≥6 months; infants were only eligible to receive treatment by the program on basis of the results of these assays and not PCR results. However, for the research analysis, we considered an infant to have confirmed congenital infection if he or she met the program case definition or had reproducible detection of T. cruzi DNA in ≥2 specimens collected on separate occasions. The mother was interviewed to assure that the infant had not lived in an area with vectorborne transmission between birth and the date of the positive specimen.
Statistical analyses were performed in SAS software for Windows, version 9.0 (SAS Institute). Categorical variables were compared by the χ2 test or the Fisher exact test as appropriate, whereas continuous variables were analyzed using the Kruskal-Wallis test.
RESULTS
From November 2006 to June 2007, a total of 530 women had a prepartum blood specimen collected; 139 specimens tested positive by at least 1 RDT (Figure 1). Specimens from 154 women (29%) were confirmed to be seropositive; 151 had positive results by 3 conventional assays, whereas 3 had positive results by 2 of 3 assays. Sixteen RDT-negative specimens were confirmed to be seropositive. One IHA-positive, InBios-negative specimen yielded negative results by conventional serological testing and was classified as a false-positive IHA result. Umbilical tissue and cord blood specimens were collected for 116 births from confirmed seropositive mothers (Figure 1). One seropositive mother gave birth to twins. One seropositive mother had a stillborn infant, and 1 infant of a seronegative mother died at 2 months of age. Although many attempts were made to convince the 16 mothers with false-negative RDTs to bring their infants for testing, follow-up was much poorer than for infants of RDT-positive mothers (mean number of specimens, 0.25 vs 3.6 [P < .001]; percentage of infants who completed month 9 follow-up, 22% vs 63% [P < .001]).
Ten infants (6.5%) received a diagnosis of congenital T. cruzi infection (Table 1). All 10 infants had positive PCR results of specimens from the first month of life. The mean age at diagnosis by conventional techniques was 131 days, compared with 4 days for molecular diagnosis (P < .001). Only 4 infants received a diagnosis by micromethod in their first month, and all cord blood specimens had negative micromethod results. Two additional infants had parasites visualized in specimens at 103 and 189 days. By contrast, PCR results were positive in 6 for (67%) of 9 cord blood specimens, 7 (87.5%) of 8 umbilical tissue specimens, and 27 (75%) of 36 infant follow-up specimens collected prior to treatment. Among uninfected infants, all 109 cord blood, 107 umbilical tissue, and 137 of 138 follow-up specimens were PCR negative; one 7-day specimen from an infant who had been proven to be uninfected at 9 months was weakly positive by PCR (specificity, 99.6%). Placental specimens from 14 births were PCR positive, including specimens from 7 infected infants, 4 infants who were proven to be un-infected, and 3 infants who were lost to follow-up at 9 months but presumed to be uninfected on the basis of neonatal specimens. Two infected infants had negative placental PCR results. Parasite nests were visible in umbilical tissue specimens from 7 of 9 infected infants (Figure 2).
Table 1.
Comparison of Conventional and Detection Polymerase Chain Reaction (PCR) Targeting Trypanosoma cruzi Kinetoplast DNA in Specimens Obtained from 10 Infants Born in the Hospital Universitario Japones, Santa Cruz, Bolivia
| Conventional diagnosisa
|
PCR results in specimens from the first 90 days of life
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Infant | Means of diagnosis | Age at conventional diagnosis, days | Age at first PCR-positive specimen, days | Umbilical tissue | Cord blood | 7 days | 21 days | 30 days | 90 days |
| Infant 15 | Serological testing | 271 | 30 | − | − | ND | ND | + | + |
|
| |||||||||
| Infant 19 | Micromethodb | 33 | Birth | + | + | − | + | + | ND |
|
| |||||||||
| Infant 120 | Missed | Missed | Birth | + | + | + | ND | ND | ND |
|
| |||||||||
| Infant 179 | Micromethod | 6 | 6 | ND | ND | + | + | ND | ND |
|
| |||||||||
| Infant 203 | Micromethod | 20 | Birth | ND | + | + | + | ND | ND |
|
| |||||||||
| Infant 218 | Serological testing | 275 | Birth | + | + | + | − | + | + |
|
| |||||||||
| Infant 270 | Micromethod | 189 | Birth | + | + | + | + | + | + |
|
| |||||||||
| Infant 342 | Micromethod | 103 | Birth | + | − | − | − | − | + |
|
| |||||||||
| Infant 471 | Micromethod | 8 | Birth | + | + | + | + | ND | ND |
|
| |||||||||
| Infant 478 | Serological testing | 278 | Birth | + | − | − | − | − | + |
NOTE. The mean age at diagnosis by conventional techniques was 131 days, compared with 4 days for molecular diagnosis. ND, not done; +, positive; −, negative.
Conventional diagnosis defined by visualization of parasites in concentrated blood specimens at any age, or positive results of 2 conventional IgG serological tests at 270 days (9 months) of age.
The micromethod is the standard assay for early diagnosis of congenital infection. The assay consists of concentration by centrifugation of 4–6 microhematocrit tubes of heparinized blood, followed by microscopic examination of the buffy coat layer. See text for further description.
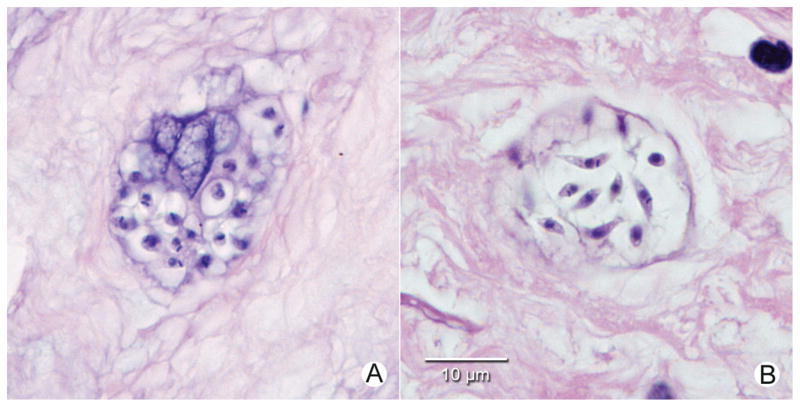
Figure 2.
A, Nest of Trypanosoma cruzi amastigotes in hematoxylin-and-eosin–stained section of proximal umbilical cord tissue from infant 203. B, Nest of T. cruzi showing forms intermediate between amastigotes and trypomastigotes in hematoxylin-and-eosin–stained section of proximal umbilical cord tissue from infant 120. (Original magnification, ×1000). No inflammatory response was seen in infected umbilical cord tissue specimens.
Quantitative PCR demonstrated higher parasite loads in umbilical tissue than cord blood specimens (median, 5752 vs 21 copies/mL; P < .05). Parasite loads increased after birth, peaked at days 30–90, and then decreased as infections entered the chronic phase (Table 2). Only 10 (42%) of 24 infant specimens positive by kinetoplast DNA PCR yielded positive results of microscopy evaluation. Although micromethod-positive specimens had significantly higher parasite loads than did micro-method-negative specimens (median, 9395 vs 78 copies/mL; P < .01), some specimens with >60,000 copies/mL yielded negative micromethod results, whereas others with <200 copies/mL were read as positive (Table 2).
Table 2.
Course of Parasitemia in Infected Infants Based on Detection Polymerase Chain Reaction (PCR) Targeting Trypanosoma cruzi Kinetoplast DNA (kDNA) and Quantitative PCR (qPCR) and Comparison with Micromethod (ie, Microscopy of Concentrated Heparinized Blood in Microhematocrit Tubes)
| Infant, technique | Cord blood specimen | Sample time | Age at treatment, days | |||||
|---|---|---|---|---|---|---|---|---|
| 7 days | 21 days | 30 days | 90 days | 180 days | 270 days | |||
| Infant 19 | ||||||||
| kDNA | − | ND | ND | + | + | + | + | 275 |
| qPCR, copies/mL | ND | ND | ND | <1a | 48,883 | 3127 | 8 | … |
| Micromethod | − | ND | ND | − | − | − | − | … |
| Infant 19 | ||||||||
| kDNA | + | − | + | + | ND | ND | − | 48 |
| qPCR, copies/mL | <1 | <1 | 825 | 10,539 | ND | ND | <1 | … |
| Micromethod | − | − | − | + | ND | ND | − | … |
| Infant 120 | ||||||||
| kDNA | + | + | ND | ND | ND | ND | ND | Not treatedb |
| qPCR, copies/mL | 42 | 44 | ND | ND | ND | ND | ND | … |
| Micromethod | − | − | ND | ND | ND | ND | ND | … |
| Infant 179 | ||||||||
| kDNA | ND | + | + | ND | ND | + | + | 27c |
| qPCR, copies/mL | ND | 1470 | 8251 | ND | ND | 5188 | 29 | … |
| Micromethod | ND | + | + | ND | ND | + | − | … |
| Infant 203 | ||||||||
| kDNA | + | + | + | ND | ND | − | − | 51 |
| qPCR, copies/mL | 9770 | 45,320 | 207,943 | ND | ND | <1 | <1 | … |
| Micromethod | − | + | + | ND | ND | − | − | … |
| Infant 218 | ||||||||
| kDNA | + | + | − | + | + | + | + | Not treatedd |
| qPCR, copies/mL | 1 | <1 | <1 | 3 | 61,146 | 4377 | 70 | … |
| Micromethod | − | − | − | − | − | − | − | … |
| Infant 270 | ||||||||
| kDNA | + | + | + | + | + | + | − | 230 |
| qPCR, copies/mL | 43 | 10 | 86 | 599 | 5412 | 875 | <1 | … |
| Micromethod | − | − | − | − | − | + | − | … |
| Infant 342 | ||||||||
| kDNA | − | − | − | − | + | ND | − | 122 |
| qPCR, copies/mL | <1 | <1 | <1 | <1 | 19,504 | ND | <1 | … |
| Micromethod | − | − | − | − | + | ND | − | … |
| Infant 471 | ||||||||
| kDNA | + | + | +e | ND | ND | − | − | 15 |
| qPCR, copies/mL | 174 | 12,274 | 12,508 | ND | ND | <1 | <1 | … |
| Micromethod | − | + | − | ND | ND | − | − | … |
| Infant 478 | ||||||||
| kDNA | − | − | − | − | + | + | + | 314 |
| qPCR, copies/mL | <1 | <1 | <1 | <1 | 8 | 1032 | 127 | … |
| Micromethod | − | − | − | − | − | − | + | … |
NOTE. ND, not done; +, positive; −, negative.
qPCR result less than the threshold of detection of 1 parasite copy/mL.
Family refused follow-up.
Infant diagnosed with treatment failure at 270 days on the basis of increasing positive serological test values.
Family moved to an area without access to treatment before serological results were available.
Specimen collected at 15 days.
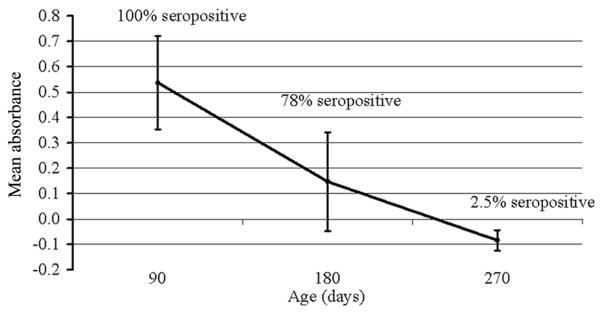
The IgG TESA-blot results was positive for 151 (98.7%) of 154 seropositive women and negative for 371 (98.1%) of 376 seronegative women. Among 101 infants of seropositive women, 90 (89%) had positive IgG TESA-blot results, with bands at 150–160 kDa in cord blood, reflecting passively transferred maternal antibody. Among 9 infected infants with cord blood IgM TESA-blot data, 5 demonstrated SAPA bands, whereas 4 tested negative (sensitivity, 56%). One infected infant had negative IgM TESA-blot results at birth but positive IgM TESA-blot results, with SAPA bands, at 90 days. Among uninfected infants, 92 cord blood specimens and 207 follow-up specimens were tested; none demonstrated SAPA bands (specificity, 100%). The prevalence of positive Chagatek ELISA results in specimens from uninfected infants of seropositive mothers decreased from 100% at 3 months to 78% at 6 months and 2.5% at 9 months of age (Figure 3).
Figure 3.
Mean absorbance (optical density minus cutoff) for the whole epimastigote lysate enzyme-linked immunosorbent assay in specimens from uninfected infants of seropositive mothers at 3, 6, and 9 months of age. Error bars represent 1 standard deviation above and below the mean. The curve demonstrates the elimination of passively transferred maternal immunoglobulin G (IgG) antibodies over the first 9 months of life and confirms that conventional IgG serology should not be applied for infant diagnosis until at least 9 months of age.
Infected infants did not differ from uninfected infants of seropositive mothers with regard to birth weight (mean, 3238 vs 3303 g; P = .54) or Apgar score (mean, 8.0 vs 7.7 at 1 min [P = .68] and 9.1 vs 9.0 at 5 min [P = .48]); no abnormalities were found on their newborn examinations. Three of 7 infants with available data had splenomegaly noted on pretreatment examination at 51, 230, and 314 days of age. Of 6 infected infants with posttreatment specimens available, 4 had negative PCR and serological test results. One infant, treated at 230 days, had low positive ELISA (absorbance, 0.157) and negative PCR results at 350 days. One infant, treated at 27 days, had PCR-positive specimens and increasing positive serological test values at 176 and 294 days; these were thought to reflect treatment failure. Two infants were not treated, one because the family refused to return for follow-up, and the other because mother and infant moved before month 9 serological test results were available and, when contacted, were living in a nonendemic department where antitrypanosomal treatment was not available.
Ninety-seven (63%) of 154 seropositive women had positive peripheral blood PCR results. Only 12.5% of women with false-negative RDT results had positive PCR results, compared with 68.8% of those with positive RDT results (P < .001). Mothers with PCR-positive specimens were significantly more likely to transmit T. cruzi to their infants (assuming all infants without follow-up were uninfected, 10 of 97 vs 0 of 57 [P = .014, by 2-sided Fisher exact test]; including only infants with final follow-up data, 10 of 57 vs 0 of 33 [P = .012]). Mothers of infected infants also had higher parasite loads than did seropositive mothers of uninfected infants (mean, 85.6 copies [32.8 cycles] vs 20.9 copies [38.9 cycles]; P < .01), but there were no difference in age (P = .64) or parity (P = .41).
DISCUSSION
Our study indicates that current programs miss many T. cruzi–infected infants. Cord blood microscopy has a reported sensitivity of ≤60%; an additional neonatal specimen is often recommended, usually at 30 days [4]. However, programs seldom include additional specimens, because women have difficulty returning for follow-up; some programs seek additional specimens only from symptomatic infants [17, 22]. On the basis of this practice, none of the infected infants in our study would have received diagnoses during the neonatal period. Even with intensive efforts by our full-time study nurses, only 58% of infants had the 9-month specimen collected. On the basis of our data, routine programs may miss one-half of the estimated 14,000 annual congenital T. cruzi infections [2]. Investigators in Argentina have raised similar concerns [21, 22, 34].
Our data confirm that the use of molecular methods substantially increases early detection [35–37]. We found that PCR was even more sensitive in umbilical tissue than cord blood, a finding explained by higher copy numbers reflecting active parasite replication in neonatal tissues. Collection of an umbilical tissue specimen after the cord is severed from the neonate is noninvasive and requires minimal additional logistics beyond those necessary for cord blood collection, which is currently recommended as standard of care during all deliveries of T. cruzi–infected women. In common with other observers, we found positive placental PCR from births of uninfected as well as infected infants; this finding was not unexpected and does not offer useful diagnostic avenues [4, 38]. Decades ago, investigators observed that parasite loads increase in infected infants after birth [38], and we found that peak parasite loads occurred at 30–90 days. In several infants who had not been treated until their positive 9-month serological test results, the subsequent decrease in parasite load reflected the transition from acute to chronic T. cruzi infection.
Our data suggest that current micromethod sensitivity is disturbingly low, even in a highly experienced research laboratory following standard quality control procedures, perhaps in part due to relatively low parasite loads at birth. Although it is possible that discordance between quantitative PCR results and micromethod detection was due in part to heterogeneity in the recovery of DNA from small volume neonatal specimens, the internal consistency of the quantitative PCR results suggests that most of the discordance was related to low micromethod sensitivity. Of note, specimens were transported from hospital to laboratory daily, and the delay between collection and reading varied from a few hours to 18 h (in births that occurred in the evening); microscopic sensitivity may have been lower if trypomastigotes were no longer motile. Other investigators have noted decreased morbidity associated with congenital infection over time and have suggested that parasite levels in infected infants may also be decreasing, leading to lower sensitivity [6, 39]. Nevertheless, we found that higher parasite loads in the mother were predictive of vertical transmission risk, as documented in other publications [40, 41]. Although the need for specialized equipment and expertise limits the use of PCR in routine settings, there is an increasing effort to incorporate these technologies into congenital Chagas disease screening programs [42].
Recently introduced point-of-care rapid diagnostic tests represent a major advance in prenatal screening. However, the Stat-Pak and InBios tests had sensitivities of 87.5% and 90.7% in this population [25], and women with false-negative results seldom brought back their babies, despite repeated counseling. These women had lower parasite loads and may be less likely to transmit to infants, although loss to follow-up precludes a definitive conclusion. Commercial RDT performance also varies geographically, with lower sensitivity reported in Peru and Mexico than in Bolivia, Brazil, or Honduras [25, 43]. Because RDTs are now used as the sole screen—not just for pregnant women, but also for children in communities where T. cruzi infection is endemic—technical changes to increase RDT sensitivity to 98%–99% would be well worth the trade-off of lowered specificity. Confirmatory testing is required in all current algorithms and would identify false-positive RDT results.
Congenital T. cruzi screening continues to pose a challenge, even as this route of transmission is taking on proportionately greater importance. The next step may be adaptation of molecular methods to more field-friendly formats, and their validation in settings with the greatest need [44, 45]. Our data suggest an alternate strategy of screening of mother and then infant with an improved RDT during routine immunization visits at 1 year of age. This strategy may also be the most feasible for at-risk populations in the United States and other low prevalence areas. However, systematic studies are necessary to document acceptable RDT sensitivity and to ensure that long-term prognosis for infants treated at 1 year equals that for infants treated earlier in life.
Acknowledgments
We are grateful to Henry Bishop and Michael Arrowood for reviewing the umbilical tissue slides and for providing slide images.
Financial support. National Institutes of Health (NIH; 1R21 AI072093–01, NIH Fogarty Scholars Program R24TW007988, NIH Training Grant in Infectious and Tropical Diseases #5 T35 AI065385, and NIH Global Research Training Grant D43 TW006581) and a private Swiss foundation.
Potential conflicts of interest. All authors: no conflicts.
THE CHAGAS DISEASE WORKING GROUP
Members of the Chagas Disease Working Group in Peru and Bolivia include Viviana Pinedo-Cancino, Lilia Cabrera, Mike Levy, Sara Quispe, Edith Hinojosa, Nur Selum, Walter Selum, Juan Justiniano, Elisabeth de LaFuente, Milagros Bastos, Teresa Rojas, Nina Castro, Kathy Lancaster, Margaret Lin, Corrine Walker, Romeo Pomari, Eduardo Valencia, Sean Fitzwater, Susy Espetia Anco, and Elizabeth Chavez.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. a Members of the Working Group are listed at the end of the text.
References
- 1.Dias JC, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz. 2002;97:603–12. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- 2.Organización Panamericana de la Salud. Estimación cuantitativa de la enfermedad de Chagas en las Americas. Montevideo, Uruguay: Organización Panamericana de la Salud; 2006. [Google Scholar]
- 3.Schenone H, Gaggero M, Sapunar J, Contreras MC, Rojas A. Congenital Chagas disease of second generation in Santiago, Chile: report of two cases. Rev Inst Med Trop Sao Paulo. 2001;43:231–2. doi: 10.1590/s0036-46652001000400011. [DOI] [PubMed] [Google Scholar]
- 4.Azogue E, Darras C. Prospective study of Chagas disease in newborn children with placental infection caused by Trypanosoma cruzi (Santa Cruz-Bolivia) Rev Soc Bras Med Trop. 1991;24:105–9. doi: 10.1590/s0037-86821991000200007. [DOI] [PubMed] [Google Scholar]
- 5.Basombrio MA, Nasser J, Segura MA, et al. The transmission de Chagas disease in Salta and the detection of congenital cases. Medicina (B Aires) 1999;59(Suppl 2):143–6. [PubMed] [Google Scholar]
- 6.Torrico F, Alonso-Vega C, Suarez E, et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70:201–9. [PubMed] [Google Scholar]
- 7.Bittencourt AL. Possible risk factors for vertical transmission of Chagas’ disease. Rev Inst Med Trop Sao Paulo. 1992;34:403–8. doi: 10.1590/s0036-46651992000500006. [DOI] [PubMed] [Google Scholar]
- 8.Freilij H, Altcheh J. Congenital Chagas’ disease: diagnostic and clinical aspects. Clin Infect Dis. 1995;21:551–5. doi: 10.1093/clinids/21.3.551. [DOI] [PubMed] [Google Scholar]
- 9.Andrade SG. The influence of the strain of Trypanosoma cruzi in placental infections in mice. Trans R Soc Trop Med Hyg. 1982;76:123–8. doi: 10.1016/0035-9203(82)90036-0. [DOI] [PubMed] [Google Scholar]
- 10.Bern C, Montgomery SP, Katz L, Caglioti S, Stramer SL. Chagas disease and the US blood supply. Curr Opin Infect Dis. 2008;21:476–82. doi: 10.1097/QCO.0b013e32830ef5b6. [DOI] [PubMed] [Google Scholar]
- 11.Navin TR, Roberto RR, Juranek DD, et al. Human and sylvatic Trypanosoma cruzi infection in California. Am J Public Health. 1985;75:366–9. doi: 10.2105/ajph.75.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yabsley MJ, Noblet GP. Seroprevalence of Trypanosoma cruzi in raccoons from South Carolina and Georgia. J Wildl Dis. 2002;38:75–83. doi: 10.7589/0090-3558-38.1.75. [DOI] [PubMed] [Google Scholar]
- 13.Buekens P, Almendares O, Carlier Y, et al. Mother-to-child transmission of Chagas’ disease in North America: why don’t we do more? Matern Child Health J. 2008;12:283–6. doi: 10.1007/s10995-007-0246-8. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Impact of expanded newborn screening--United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:1012–5. [PubMed] [Google Scholar]
- 15.Maguire JH. Trypanosoma. In: Gorbach S, Bartlett J, Blacklow N, editors. Infectious diseases. 2. Philadelphia: Lippincott, Williams & Wilkins; 2004. pp. 2327–34. [Google Scholar]
- 16.Schijman AG. Congenital Chagas disease. In: Mushahwar IK, editor. Congenital and other related infectious diseases of the newborn. Vol. 13. Amsterdam, Netherlands: Elsevier; 2006. pp. 223–59. [Google Scholar]
- 17.Programa Nacional de Control de Chagas. Chagas Congénito: Estrategias de Diagnóstico y Control. 2. Cochabamba, Bolivia: Digital Dreams; 2007. pp. 1–89. [Google Scholar]
- 18.La Fuente C, Urjel R, Darras C, Saucedo E. Use of microhematocrit tubes for the rapid diagnosis of Chagas disease and malaria. Ann Soc Belg Med Trop. 1985;65(Suppl 1):95–9. [PubMed] [Google Scholar]
- 19.Freilij H, Muller L, Gonzalez Cappa SM. Direct micromethod for diagnosis of acute and congenital Chagas’ disease. J Clin Microbiol. 1983;18:327–30. doi: 10.1128/jcm.18.2.327-330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luquetti AO, Dias JC, Prata A. Diagnosis and treatment of congenital infection caused by Trypanosoma cruzi in Brazil [in Spanish] Rev Soc Bras Med Trop. 2005;38(Suppl 2):27–8. [PubMed] [Google Scholar]
- 21.Sosa-Estani S. Congenital transmission of Trypanosoma cruzi infection in Argentina [in Spanish] Rev Soc Bras Med Trop. 2005;38(Suppl 2):29–32. [PubMed] [Google Scholar]
- 22.Blanco SB, Segura EL, Cura EN, et al. Congenital transmission of Trypanosoma cruzi: an operational outline for detecting and treating infected infants in north-western Argentina. Trop Med Int Health. 2000;5:293–301. doi: 10.1046/j.1365-3156.2000.00548.x. [DOI] [PubMed] [Google Scholar]
- 23.Carrasco R, Miguez H, Camacho C, et al. Prevalence of Trypanosoma cruzi infection in blood banks of seven departments of Bolivia. Mem Inst Oswaldo Cruz. 1990;85:69–73. doi: 10.1590/s0074-02761990000100011. [DOI] [PubMed] [Google Scholar]
- 24.Camargo ME, Rebonato C. Cross-reactivity in fluorescence tests for Trypanosoma and Leishmania antibodies. A simple inhibition procedure to ensure specific results. Am J Trop Med Hyg. 1969;18:500–5. doi: 10.4269/ajtmh.1969.18.500. [DOI] [PubMed] [Google Scholar]
- 25.Verani J, Seitz A, Gilman R, et al. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am J Trop Med Hyg. 2009;80:410–5. [PubMed] [Google Scholar]
- 26.World Health Organization Expert Committee. Control of Chagas disease. Brasilia, Brazil: World Health Organization; 2002. pp. 1–109. [PubMed] [Google Scholar]
- 27.Umezawa ES, Nascimento MS, Kesper N, Jr, et al. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas’ disease. J Clin Microbiol. 1996;34:2143–7. doi: 10.1128/jcm.34.9.2143-2147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umezawa ES, Nascimento MS, Stolf AM. Enzyme-linked immunosorbent assay with Trypanosoma cruzi excreted-secreted antigens (TESA-ELISA) for serodiagnosis of acute and chronic Chagas’ disease. Diagn Microbiol Infect Dis. 2001;39:169–76. doi: 10.1016/s0732-8893(01)00216-4. [DOI] [PubMed] [Google Scholar]
- 29.Breniere SF, Yaksic N, Telleria J, et al. Immune response to Trypanosoma cruzi shed acute phase antigen in children from an endemic area for Chagas’ disease in Bolivia. Mem Inst Oswaldo Cruz. 1997;92:503–7. doi: 10.1590/s0074-02761997000400011. [DOI] [PubMed] [Google Scholar]
- 30.Fitzwater S, Calderon M, Lafuente C, et al. Polymerase chain reaction for chronic Trypanosoma cruzi infection yields higher sensitivity in blood clot than buffy coat or whole blood specimens. Am J Trop Med Hyg. 2008;79:768–70. [PubMed] [Google Scholar]
- 31.Wincker P, Britto C, Pereira JB, Cardoso MA, Oelemann W, Morel CM. Use of a simplified polymerase chain reaction procedure to detect Trypanosoma cruzi in blood samples from chronic chagasic patients in a rural endemic area. Am J Trop Med Hyg. 1994;51:771–7. doi: 10.4269/ajtmh.1994.51.771. [DOI] [PubMed] [Google Scholar]
- 32.Sturm NR, Degrave W, Morel C, Simpson L. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas’ disease. Mol Biochem Parasitol. 1989;33:205–14. doi: 10.1016/0166-6851(89)90082-0. [DOI] [PubMed] [Google Scholar]
- 33.Piron M, Fisa R, Casamitjana N, et al. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop. 2007;103:195–200. doi: 10.1016/j.actatropica.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Gurtler RE, Segura EL, Cohen JE. Congenital transmission of Trypanosoma cruzi infection in Argentina. Emerg Infect Dis. 2003;9:29–32. doi: 10.3201/eid0901.020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schijman AG, Altcheh J, Burgos JM, et al. Aetiological treatment of congenital Chagas’ disease diagnosed and monitored by the polymerase chain reaction. J Antimicrob Chemother. 2003;52:441–9. doi: 10.1093/jac/dkg338. [DOI] [PubMed] [Google Scholar]
- 36.Diez CN, Manattini S, Zanuttini JC, Bottasso O, Marcipar I. The value of molecular studies for the diagnosis of congenital chagas disease in northeastern Argentina. Am J Trop Med Hyg. 2008;78:624–7. [PubMed] [Google Scholar]
- 37.Mora MC, Sanchez Negrette O, Marco D, et al. Early diagnosis of congenital Trypanosoma cruzi infection using PCR, hemoculture, and capillary concentration, as compared with delayed serology. J Parasitol. 2005;91:1468–73. doi: 10.1645/GE-549R.1. [DOI] [PubMed] [Google Scholar]
- 38.Bittencourt AL. Congenital Chagas disease. Am J Dis Child. 1976;130:97–103. doi: 10.1001/archpedi.1976.02120020099020. [DOI] [PubMed] [Google Scholar]
- 39.Torrico F, Vega CA, Suarez E, et al. Are maternal re-infections with Trypanosoma cruzi associated with higher morbidity and mortality of congenital Chagas disease? Trop Med Int Health. 2006;11:628–35. doi: 10.1111/j.1365-3156.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- 40.Burgos JM, Altcheh J, Bisio M, et al. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int J Parasitol. 2007;37:1319–27. doi: 10.1016/j.ijpara.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Hermann E, Truyens C, Alonso-Vega C, et al. Congenital transmission of Trypanosoma cruzi is associated with maternal enhanced parasitemia and decreased production of interferon- gamma in response to parasite antigens. J Infect Dis. 2004;189:1274–81. doi: 10.1086/382511. [DOI] [PubMed] [Google Scholar]
- 42.Russomando G, Almiron M, Candia N, Franco L, Sanchez Z, de Guillen I. Implementation and evaluation of a locally sustainable system of prenatal diagnosis to detect cases of congenital Chagas disease in endemic areas of Paraguay [in Spanish] Rev Soc Bras Med Trop. 2005;38(Suppl 2):49–54. [PubMed] [Google Scholar]
- 43.Sosa-Estani S, Gamboa-Leon MR, Del Cid-Lemus J, et al. Use of a rapid test on umbilical cord blood to screen for Trypanosoma cruzi infection in pregnant women in Argentina, Bolivia, Honduras, and Mexico. Am J Trop Med Hyg. 2008;79:755–9. [PubMed] [Google Scholar]
- 44.Adams ER, Hamilton PB. New molecular tools for the identification of trypanosome species. Future Microbiol. 2008;3:167–76. doi: 10.2217/17460913.3.2.167. [DOI] [PubMed] [Google Scholar]
- 45.Deborggraeve S, Claes F, Laurent T, et al. Molecular dipstick test for diagnosis of sleeping sickness. J Clin Microbiol. 2006;44:2884–9. doi: 10.1128/JCM.02594-05. [DOI] [PMC free article] [PubMed] [Google Scholar]