Abstract
Background
Hepatitis E virus (HEV) is endemic in India and causes epidemics and sporadic cases. However, the exact transmission route for sporadic hepatitis E remains unclear. This study investigated HEV in sporadic hepatitis cases and sewage samples, as sewage is the major source of contamination of water in developing countries.
Methods
Monthly sampling and testing for HEV in sewage samples from Vellore, India was carried out for 1 year (November 2009–October 2010) and plasma and/or fecal samples from sporadic hepatitis cases presenting to a hospital in Vellore during 2006–2010 were tested for HEV RNA. A total of 144 raw sewage samples and 94 samples from sporadic hepatitis cases were tested for HEV RNA using RT-PCR.
Results
The prevalence of HEV RNA in sewage and sporadic cases was 55.6% and 9.6%, respectively. HEV strains isolated from sewage showed 94–100% nucleotide sequence similarity with the HEV strains isolated from the sporadic hepatitis cases. HEV RNA in sewage was identified more often during the summer (81.2%) than the monsoon season (14.5%) (p < 0.001).
Conclusion
This study indicates that sewage may be a source of contamination for sporadic hepatitis and also underscores the need for preventive measures to protect drinking water from sewage contamination, particularly in the summer.
GenBank accession numbers
HEV strains isolated from this study were deposited in GenBank under accession numbers JF972766–JF972773, JN705651–JN705659 and JN705660–JN705662.
Keywords: Hepatitis E virus, Sporadic hepatitis E, Sewage, Transmission, RT PCR, India
Introduction
Hepatitis E virus (HEV) is transmitted enterically and causes acute, self-limiting hepatitis in persons with no risk factors. HEV is now classified under the genus Hepevirus and family Hepeviridae. Four phylogenetically distinct genotypes of HEV, belonging to a single serotype, have been identified and show specific geographic distribution.1 Genotypes 1 and 2 are implicated in outbreaks and sporadic cases of hepatitis E in Asia and Africa and are restricted to humans. Genotypes 3 and 4 infect animals other than humans and cause sporadic hepatitis E across Europe, North America, South America, Australia and eastern parts of Asia.1
Epidemic and sporadic hepatitis are the two epidemiological forms of hepatitis E seen worldwide. In developing countries where high standards of sanitation and hygiene are lacking, HEV is endemic. In areas with high endemicity, such as India, HEV infection is the most common cause of acute sporadic viral hepatitis.2,3 With the development of better assays for HEV, this pathogen has increasingly been identified as a major etiologic agent for sporadic acute hepatitis. This includes a growing number of reports of autochthonous, mainly foodborne, infections in developed countries where previously HEV was thought to be imported through travel to endemic countries.4 Sporadic hepatitis resembles epidemic hepatitis in age distribution, severity, duration of illness and greater severity in pregnant women in developing countries, whereas in developed countries such as the UK sporadic autochthonous HEV infection is epidemiologically very distinct, for reasons unexplained, and occurs more frequently in older men. Sporadic acute hepatitis E is usually self-limiting but leads to chronicity in immunosuppressed individuals. Specific risk factors for sporadic disease are incompletely characterized and acquisition is believed to be mainly through consumption of contaminated food or water. HEV is moderately resistant to heat inactivation and may cause frequent foodborne infections where animal reservoirs of HEV exist.5
In disease-endemic regions such as India, sewage acts as a possible reservoir of HEV, with HEV shed by symptomatic and asymptomatic individuals continuously contaminating sewage. The virus, though less stable in the environment than hepatitis A virus, is detectable and infective in sewage,6 and contamination of drinking water with HEV from sewage is common. Many large outbreaks of hepatitis E infection ascribed to sewage contamination of drinking water sources have occurred in India.7–9 By acting as a reservoir, sewage may maintain the endemicity of HEV in India and other resource-limited countries.
To understand the route of transmission of sporadic acute hepatitis E in Vellore, south India, HEV was detected and characterized in samples taken from patients with acute hepatitis presenting to a hospital and compared with the prevalence and strain diversity of HEV in sewage. This study also compared the frequency and seasonal pattern of HEV in sewage with another enteric pathogen, rotavirus.
Materials and methods
Collection of sewage samples
Sewage from Vellore, a city in the state of Tamil Nadu in south India, is collected and drained without treatment into the Palar riverbed, which is dry in most years. Drinking water for Vellore is sourced from bore wells in the Palar riverbed. Underground water is pumped and piped to filtration tanks from which it is distributed after filtration to overhead tanks in different parts of the city where it is chlorinated before distribution. For this study, the 12 major sewage outlets which discharge at one point into the Palar riverbed were identified and 144 samples of untreated sewage were collected between November 2009 and October 2010. Twelve sewage samples were collected each month in sterile plastic containers, transported to the Wellcome Trust Research Laboratory at the Christian Medical College (Vellore) and processed immediately.
Collection of samples from acute sporadic hepatitis E cases
Patients with acute viral hepatitis who were positive for IgM anti-HEV antibodies and who presented to the Christian Medical College and Hospital, a tertiary care center in Vellore, were enrolled in the study. In accordance with routine clinical investigations, a total of 94 plasma and/or fecal samples were collected from patients between 2006 and 2010 and stored at −70°C and tested. Details on the duration of symptoms for each patient were recorded. In addition, plasma and/or stool samples were collected from 37 patients with icteric hepatitis presenting to a native medicine practitioner in Vellore city between 2006 and 2007 and tested.
Concentration of sewage samples
Viral particles from raw sewage were concentrated by ultracentrifugation.10 Briefly, 30 mL of untreated sewage was centrifuged (110 000 g at 4°C for 1 h; Beckman Coulter, Optima L-100K, Brea, CA, USA) to pellet all viral particles, together with other suspended solids. The sediment was suspended in 8 mL of 0.25 N glycine buffer (pH 9.5) by vigorous shaking at room temperature for 30 min. The suspended solids were removed by centrifugation at 12 000 g at 4°C for 17 min. The virus in the suspension was pelleted by another round of ultracentrifugation (110 000 g at 4°C for 1 h). The final pellet was resuspended in 100 μL PBS (pH 6.8) and stored at −80°C.
Viral RNA extraction
Sewage concentrate (100 μL) was extracted with Trizol reagent (Invitrogen, Carlsbad, CA, USA) (1000 μL). Chloroform (200 μL) was added, mixed thoroughly and centrifuged at 12 000 g at 4°C for 15 min. The chloroform layer was separated and RNA precipitated by adding isopropanol (500 μL) and pelleted by centrifugation at 10 000 g at 4°C for 10 min. The RNA pellet was washed using 75% ethanol and air dried. The RNA pellet was dissolved in 40 μL of DEPC water containing RNase inhibitor (Invitrogen, Life Technologies, Paisley, UK) and stored at −80°C.
Detection of human HEV RNA
Total RNA was reverse-transcribed to cDNA using random hexamers (Pharmacia Biotech, Milton Keynes, UK) and 400 units of Moloney murine leukemia virus reverse transcriptase (Invitrogen, Life Technologies, Paisley, UK). Nested PCR was performed, with first-round primers, MJC ESP 5-CATGGTAAAGTGGGTCAGGGTAT-3 and MJC EAP 5-AGGGTGCCGGGCTCGCCGGA-3. Second-round primers MJC ISP 5-GTATTTCGGCCTGGAGTAAGAC-3 and MJC IAP 5-TCACCGGAGTGYTTCTTCCAGAA-3 amplified a 325 bp fragment which corresponds to the RNA dependent RNA polymerase (RdRp) gene.11 The amplified product was sequenced using ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Carlsbad, CA, USA). Phylogenetic analysis of nucleotide (nt) sequences was performed using BioEdit 7.0.5.3 (Ibis Biosciences, Carlsbad, CA, USA). Dendrograms were constructed using MEGA 4.0 (The Biodesign Institute, Tempe, AZ, USA) and genetic lineages were inferred by the neighbor-joining algorithm using 1000 pseudoreplicates. Genotypes were assigned based on >90% homology at the nt level with sequences from other published strains within a given genotype available at GenBank.
Detection of group A rotavirus
In order to compare the frequency and seasonal pattern of HEV with another enteric pathogen, the frequency and seasonal pattern of rotavirus in sewage was also tested. Rotavirus was detected from cDNA obtained from the sewage concentrate using a single-round PCR for the amplification of a 379 bp region of the VP6 gene using VP6-F 5-GACGGVGCRACTACATGGT-3 and VP6-R 5-GTCCAATTCATNCCTGGTGG-3 primers.12
Statistical analysis
Comparison of HEV and rotavirus prevalence in different seasons was done using the two-tailed Fisher’s exact test in Epi Info 3.5.1 (CDC, Atlanta, GA, USA).
Results
Of the 144 sewage samples tested, 80 (55.6%) were positive for HEV and 111 (77.0%) for rotavirus. Of the 94 samples from sporadic hepatitis E cases tested using RT-PCR, 9 (9.6%) were positive for HEV RNA and of the 37 samples from icteric hepatitis cases, 3 (8.1%) were positive for HEV RNA.
PCR amplicons of HEV-positive samples were sequenced. The sequence of HEV strains isolated from sewage, hospital cases and community cases belonged to genotype 1. The HEV strains isolated from sewage samples collected in Vellore showed 94–100% nt sequence similarity with the HEV strains isolated from sporadic acute hepatitis E cases presenting at the hospital in Vellore. One of the HEV strains isolated from sewage showed 100% nt sequence similarity with one of the strains isolated from the sporadic hospital cases. HEV strains isolated from sewage in Vellore also showed 98% nt sequence similarity to HEV strains from Swedish nationals who were infected while travelling in India and 97% nt similarity with HEV strains implicated in a large outbreak in Nellore, south India (Figure 1).
Figure 1.
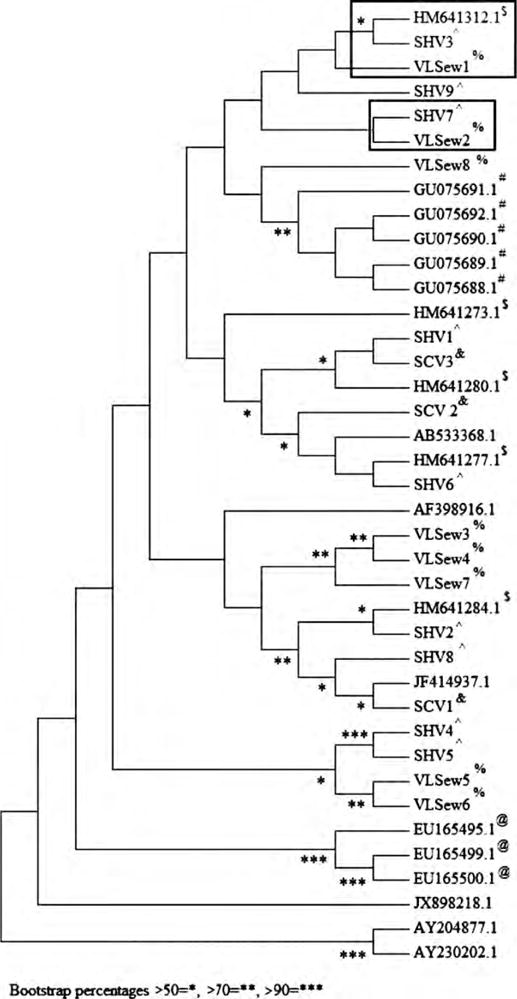
Phylogenetic dendrogram constructed by the neighbor-joining method based on the nucleotide sequence of the gene encoding the RNA dependent RNA polymerase from hepatitis E virus strains isolated from samples from sporadic cases and from sewage collected in Vellore, south India. Bootstrap values for 1000 pseudoreplicates are shown. Boxed sequences show the strains isolated from sewage in Vellore with high similarity to strains from sporadic cases in Vellore and Swedish nationals who were infected while travelling in India. Reference sequences from studies carried out in Sweden are shown with $ superscripts, sequences from Cuba with @ superscripts and sequences from the Vellore outbreak with # superscripts. GenBank reference sequences were also selected from other countries, Chad (AY204877) and Morocco (AY230202), and strains isolated from a Nepalese individual in Japan (AB533368), sewage from western India (AF398916), an outbreak in north India (JF414937) and an aymptomatic migrant in Italy (JX898218). Strains isolated from the current study have been deposited in GenBank with accession numbers: sewage samples: (%) VLSew1–VLSew8 (JF972766–JF972773); hospital samples: (ˆ) SHV1–SHV9 (JN705651–JN705659); and community samples: (&) SCV1–SCV3 (JN705660–JN705662).
We sequenced 10 sewage strains, randomly selecting one each from November 2009 to August 2010 (no HEV strains were detected in September 2010 and October 2010). Of the 10 sequences, 2 had poor readouts and so were not included in the report. Resources did not permit sequencing of all strains. The GenBank accession numbers assigned to the eight sequences of HEV strains isolated in sewage from Vellore are JF972766–JF972773, for the sporadic hospital strains from Vellore (SHV1–SHV9) are JN705651 – JN705659 and for the sporadic community strains from Vellore (SCV1–SCV3) are JN705660 –JN705662.
The prevalence of HEV and rotavirus in sewage during different seasons showed seasonal variation. The HEV prevalence during the monsoon season of July to October was low, while it was higher during the summer (March–June) (p < 0.001) and winter (November–February) (p < 0.001). The prevalence of rotavirus in sewage during winter (p < 0.001) and summer (p = 0.001) was higher than during the monsoon season. The detection of rotavirus was higher than that of HEV (p < 0.001).
The 94 sporadic acute hepatitis E patients presented to the hospital in Vellore at different times of the year. The number of patients presenting during the summer (p = 0.001) and winter (p = 0.023) was higher compared with the monsoon season (Table 1).
Table 1.
PCR detection of hepatitis E virus (HEV) and rotavirus in monthly sewage samples collected in Vellore, India, compared with the number of sporadic hepatitis E patients presenting to the Christian Medical College Hospital, Vellore, India
| Summer | Winter | Monsoon season | |
|---|---|---|---|
| HEV positivity in sewage (n = 48) | 39 (81.3) | 34 (70.8) | 7 (14.6) |
| Rotavirus positivity in sewage (n = 48) | 40 (83.3) | 47 (97.9) | 24 (50.0) |
| Sporadic hepatitis E patients (n = 94) | 41 (43.6) | 34 (36.2) | 19 (20.2) |
Data are number (%).
Discussion
HEV strains from sewage in Vellore were genotype 1, which is the predominant genotype in humans in India. This study did not detect any novel or animal genotypes,13 indicating that the sewage contamination is from infected humans, who may be symptomatic patients or asymptomatic carriers. Using phylogenetic analysis, the HEV strains from sewage in this study showed close nt similarity with strains causing sporadic hepatitis E in cases from Vellore. The HEV strains used in the phylogenetic analysis showed high sequence similarity to the study strains by BLAST search and represented strains isolated from sewage from western India, sporadic strains from individuals who were likely infected during their stay in India and strains implicated in outbreaks from south and north India.
Although the study samples were temporally separated by 3–4 years, the similarity of the nt sequences for the sewage samples and the human samples suggests the possibility of strains causing sporadic hepatitis E circulating in Vellore and also in other parts of India as indicated by the close nt similarity of strains isolated from Swedish nationals infected during their stay in India. As there are no studies to show the duration of HEV stability and its viability in sewage, the transmission of sporadic hepatitis in Vellore through sewage contamination of drinking water cannot be confirmed.
Although in the absence of an epidemic it is not often possible to define the route of transmission in sporadic cases, the 100% nt sequence similarity of one of the HEV strains from sewage and one of the strains from hospital cases suggests that contamination of drinking water with sewage could be a further mode of transmission. In addition, environmental surveillance through monitoring of sewage could aid understanding of the epidemiology of sporadic and epidemic hepatitis E in a given geographic area.14
This study reports HEV RNA prevalence of 55.6% in sewage from Vellore, which is higher than reports in sewage from other parts of India and elsewhere, which range from 11% to 41%.10,15,16 HEV prevalence in sewage was highest in the summer and lowest during the monsoon season. This variation may be due to concentration of viruses in sewage due to evaporation in summer months and dilution of viruses in sewage due to flooding during the monsoon season. A similar seasonal pattern was observed in a previous study in Lucknow in north India.10 The high prevalence of HEV in sewage during the summer corresponds to increased occurrence of HEV during this season in India. This is strongly supported by data from this study that shows the number of cases of sporadic hepatitis E presenting to the hospital was significantly higher during summer than the other seasons of the year.
This study also reported a higher prevalence of rotavirus than HEV RNA in sewage. This could reflect the greater circulation of rotavirus in the population. The high prevalence of rotavirus in sewage during winter correlates with the higher incidence of rotavirus infections during this season, although seasonality is less marked in tropical countries.17
In developed countries, HEV transmission to humans through consumption of water contaminated by sewage has been reported.18 Consumption of shellfish harvested from water contaminated by sewage has also been indirectly implicated in sporadic hepatitis E.19 HEV of human and animal origin in sewage can be a source of human infection; therefore, environmental surveillance of HEV in developed countries cannot be overlooked.
Limitations
This study shows the similarity in sequences isolated from sewage samples with the sequences isolated from human samples from the same region; however, these HEV strains were isolated at different time points. The exact duration of HEV stability and viability in sewage is not known and therefore water contaminated by sewage cannot be conclusively established as the only mode of transmission for sporadic hepatitis E in Vellore.
Conclusion
This study shows a high frequency of HEV RNA in sewage throughout the year and particularly a very high frequency during summer. Improved engineering of sewage canals and careful discharge of sewage along with regular maintenance and repair of drinking water pipelines can prevent HEV and other enteric infections. Though it is evident from this study that summer remains the most vulnerable period in the year, water purification and treatment measures cannot be over-looked during the rest of the year. Therefore, extra precautions such as boiling drinking water, may be warranted throughout the year for effective control and prevention of HEV infection.
Acknowledgments
Funding: This work was supported by the US National Institutes of Health through the Fogarty International Clinical Research Scholars and Fellows Program [grant no. R24 TW007988].
Footnotes
Authors’ contributions: GK, JR and CEE conceived the study; GK, DPR and RV designed the study protocol; UGZ, JR and CEE carried out the clinical assessment; RV carried out virus concentration, RT-PCR, phylogenetic analysis and interpretation of the data and drafted the manuscript; UGZ interpreted the data; GK, UGZ, JR, CEE and DPR revised the manuscript for intellectual content. All authors read and approved the final manuscript. GK is guarantor of the paper.
Competing interests: None declared.
Ethical approval: The study qualified for a waiver of informed consent from the Christian Medical College and Hospital Institutional Review Board, Vellore, India.
References
- 1.Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16:5–36. doi: 10.1002/rmv.482. [DOI] [PubMed] [Google Scholar]
- 2.Chadha MS, Walimbe AM, Chobe LP, et al. Comparison of etiology of sporadic acute and fulminant viral hepatitis in hospitalized patients in Pune, India during 1978–81 and 1994–97. Indian J Gastroenterol. 2003;22:11–5. [PubMed] [Google Scholar]
- 3.Kumar S, Ratho RK, Chawla YK, et al. The incidence of sporadic viral hepatitis in North India: a preliminary study. Hepatobiliary Pancreat Dis Int. 2007;6:596–9. [PubMed] [Google Scholar]
- 4.Teo CG. The two clinico-epidemiological forms of hepatitis E. J Viral Hepat. 2007;14:295–7. doi: 10.1111/j.1365-2893.2007.00857.x. [DOI] [PubMed] [Google Scholar]
- 5.Emerson SU, Arankalle VA, Purcell RH. Thermal stability of hepatitis E virus. J Infect Dis. 2005;192:930–3. doi: 10.1086/432488. [DOI] [PubMed] [Google Scholar]
- 6.Pina S, Jofre J, Emerson SU, et al. Characterization of a strain of infectious hepatitis E virus isolated from sewage in an area where hepatitis E is not endemic. Appl Environ Microbiol. 1998;64:4485–8. doi: 10.1128/aem.64.11.4485-4488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khuroo MS. Study of an epidemic of non-A, non-B hepatitis: possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med. 1980;68:818–23. doi: 10.1016/0002-9343(80)90200-4. [DOI] [PubMed] [Google Scholar]
- 8.Naik SR, Aggarwal R, Salunke PN, et al. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull World Health Organ. 1992;70:597–604. [PMC free article] [PubMed] [Google Scholar]
- 9.Vivek R, Nihal L, Illiayaraja J, et al. Investigation of an epidemic of hepatitis E in Nellore in south India. Trop Med Int Health. 2010;15:1333–9. doi: 10.1111/j.1365-3156.2010.02624.x. [DOI] [PubMed] [Google Scholar]
- 10.Ippagunta SK, Naik S, Sharma B, et al. Presence of hepatitis E virus in sewage in Northern India: frequency and seasonal pattern. J Med Virol. 2007;79:1827–31. doi: 10.1002/jmv.21017. [DOI] [PubMed] [Google Scholar]
- 11.Zhai L, Dai X, Meng J. Hepatitis E virus genotyping based on full-length genome and partial genomic regions. Virus Res. 2006;120:57–69. doi: 10.1016/j.virusres.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Iturriza Gomara M, Wong C, Blome S, et al. Molecular characterization of VP6 genes of human rotavirus isolates: correlation of genogroups with subgroups and evidence of independent segregation. J Virol. 2002;76:6596–601. doi: 10.1128/JVI.76.13.6596-6601.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivek R, Kang G. Hepatitis E virus infections in Swine and Swine handlers in Vellore, southern India. Am J Trop Med Hyg. 2011;84:647–9. doi: 10.4269/ajtmh.2011.10-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazam RK, Singla R, Kishore J, et al. Surveillance of hepatitis E virus in sewage and drinking water in a resettlement colony of Delhi: what has been the experience? Arch Virol. 2010;155:1227–33. doi: 10.1007/s00705-010-0707-z. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad T, Waheed Y, Tahir S, et al. Frequency of HEV contamination in sewerage waters in Pakistan. J Infect Dev Ctries. 2010;4:842–5. doi: 10.3855/jidc.612. [DOI] [PubMed] [Google Scholar]
- 16.Vaidya SR, Chitambar SD, Arankalle VA. Polymerase chain reaction-based prevalence of hepatitis A, hepatitis E and TT viruses in sewage from an endemic area. J Hepatol. 2002;37:131–6. doi: 10.1016/s0168-8278(02)00106-x. [DOI] [PubMed] [Google Scholar]
- 17.Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38:1487–96. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wichmann O, Schimanski S, Koch J, et al. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J Infect Dis. 2008;198:1732–41. doi: 10.1086/593211. [DOI] [PubMed] [Google Scholar]
- 19.Koizumi Y, Isoda N, Sato Y, et al. Infection of a Japanese patient by genotype 4 hepatitis E virus while traveling in Vietnam. J Clin Microbiol. 2004;42:3883–5. doi: 10.1128/JCM.42.8.3883-3885.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


