Abstract
Bovine viral diarrhea virus (BVDV) is an economically important pathogen of the livestock industry worldwide. BVDV is classified into cytopathic (cp) and noncytopathic (ncp), depending on its effects on cultured cells. BVDV is known to alter the host’s immune response. Of this, major histocompatibility complex (MHC) class II molecules play a central role in the development and function of the immune system, and are comprised of two types, DR and DQ, in cattle. In this study, we investigated the expression of MHC class II on monocytes infected with ncp BVDV1 or ncp BVDV2. Using flow cytometry (P<0.01), mRNA level quantification (quantitative real time RT-PCR, P<0.01), and western blot (P<0.001), we found that the expressions of MHC class IIDQ was significantly decreased in ncp BVDV2-infected monocytes compared with that in ncp BVDV1-infected cells. Furthermore, interferon gamma (IFN) production was markedly decreased in ncp BVDV2-infected monocytes (P<0.001) compared to those with ncp BVDV1 infection. These findings suggest that ncp BVDV2 causes reduced expressions of MHC class II DQ and a decreased production of IFN, resulting in evasion of immune recognition and suppression of the antiviral defense mechanism of the innate immune response. Consequently, the results demonstrate that ncp BVDV1 and ncp BVDV2 interact differently with the host innate immune response. Thus, our data provide insight into the mechanism by which, unlike ncp BVDV1, ncp BVDV2 impairs antigen presentation, fails to control the viral infection, and causes more severe disease.
Key Words: Bovine viral diarrhea virus, Interferon gamma, Major histocompatibility complex, Non-cytopathic
Introduction
Bovine viral diarrhea virus (BVDV) is an important viral pathogen distributed worldwide, affecting the livestock industry and for which there is no approved and efficacious vaccine available. BVDV exists in two biotypes, cytopathic (cp) and noncytopathic (ncp), based on its ability to have pathogenic effects on cultured cells (Baker, 1995 ▶; Ridpath et al., 2006 ▶). The ncp BVDV generally causes a transient infection. In utero infection of cows during the first trimester of pregnancy with ncp BVDV can result in the birth of a calf that is persistently infected (PI). PI calves that are superinfected with a homologous strain of cp BVDV may develop lethal mucosal disease.
The two biotypes of the virus interact differently with the host innate immune response (Brackenbury et al., 2003 ▶; Peterhans et al., 2003 ▶; Chase, 2013 ▶). Whereas cp BVDV is known to strongly induce an innate immune response, ncp BVDV inhibits the innate immune response in the infected animal and causes immuno-suppression, which leads to secondary infection. This would result in the establishment and maintenance of a viable infection within the host, resulting in persistent infection. The differences between ncp BVDV and cp BVDV could be dependent on virus latency and the response they elicit in hosts, including cytokine production and activation of antigen processing and presentation pathways with T cells (Tortorella et al., 2000 ▶; Glew and Howard, 2001 ▶). Of these, CD4 T cells are a primary target of BVDV infection and are affected by the biotype of the virus influencing the signaling and the dynamics that determine whether it will be a Th1 or Th2 cell (Chase, 2013 ▶).
Major histocompatibility complex (MHC) class II molecules are expressed in so-called professional antigen presenting cells (APCs) and play a central role in the regulation of immune responses. MHC class II molecules presented on APCs are critical for antigen-specific immune recognition, which recognize exogenously processed peptide antigens. In cattle, MHC is also called bovine leukocyte antigen (BoLA) (Andersson et al., 1988 ▶) and two genes, DR and DQ, represent the main restriction elements (Lewin et al., 1999 ▶; Takeshima et al., 2007 ▶). MHC class II molecules in cattle have been evaluated as candidate markers for various diseases and immunological traits (Zanotti et al., 1996 ▶; Rupp et al., 2007 ▶). In addition, the level of cell surface expression of MHC class II molecules can influence the efficiency of antigen presentation and the subsequent immune response.
In this study, we investigated the expression of MHC class II molecules in monocytes infected with each BVDV to determine the differences in the effect on the host immune response between ncp BVDV1 and ncp BVDV2.
Materials and Methods
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the Kyungpook National University Animal Care and Use Committee.
Preparation of mononuclear cells
Blood from healthy Korean indigenous cattle was obtained by jugular venipuncture into heparinized vacutainer tubes (Becton Dickinson, New Jersey, USA). Briefly, bovine peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque density gradients (Amersham Pharmacia Biotech, Uppsala, Sweden), and contaminating erythrocytes were lysed with RBC lysis buffer (Sigma, St. Louis, MO, USA). PBMCs were suspended in RPMI 1640 medium with 10% FCS (Gibco Life Technology, Carlsbad, CA, USA), 100 U/ml penicillin, 100 g/ml streptomycin (Gibco), and 2 mM L-glutamine (Gibco). Cells were plated on 100-mm culture dishes and incubated for 3 h at 37°C. Non-adherent cells were discarded, and adherent cells were washed with sterile PBS, detached from plates, and pelleted by centrifugation for 10 min at 650 g. Monocytes were confirmed by flow cytometry using monoclonal antibodies to CD14 (Serotec, Raleigh, NC, USA). Monocyte purity was 95%, as determined microscopically after Diff-Quik staining (Thermo Fisher Scientific Inc., VA, USA), and viability was 98%, as assessed by trypan blue dye exclusion. Harvested monocytes were then counted and plated into 6-well plates for further experiments.
Virus infection
The ncp BVDV1 (11Q472b) and ncp BVDV2 (11F011) strains used in this study were isolated in the Republic of Korea (Seong et al., 2013 ▶). The viruses were grown in MDBK cells which were free of adventitious BVDV and maintained in minimum essential media (MEM) (Gibco) supplemented with 5% heat-inactivated horse serum (Gibco), 2 mM L-glutamine (Gibco), sodium pyruvate (Gibco), and MEM non-essential amino acids (Gibco). The virus preparation was obtained after 5 days in culture by one-freeze-thaw cycle and centrifuga-tion at 4000 g for 10 min to remove large cellular debris. The supernatant was used for infection. For the infection procedure, monocytes (5 106) were added to 6-well plates, infected with ncp BVDV1 and ncp BVDV2 at a MOI 0.02, and then incubated for 48 h. Infected cells were subsequently harvested using 0.05% trypsin-EDTA (Gibco), and suspended in medium for use in further experiments.
Flow cytometry
For flow cytometric analysis, mock-, ncp BVDV1-, or ncp BVDV2-infected monocytes were harvested, washed, and suspended in PBS containing 0.5% bovine serum albumin (BSA) (Sigma). Cells were incubated at 4C for 20 min with FITC-labeled IL-A21, which recognizes both bovine DQ and DR (Serotec, Raleigh, NC, USA), and with each single FITC-labeled DQ (CC158, Serotec) or FITC-labeled DR (CC108, Serotec). Stained cells were washed twice with PBS containing 0.5% BSA, fixed with 1% paraformaldehyde (Sigma), and examined by flow cytometry using a FACSCalibur (BD, San Diego, CA, USA). Data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR, USA), and gates were set based on isotype-matched control antibodies.
RNA extraction and quantitative real-time RT-PCR
Total RNA was extracted from mock-, ncp BVDV1-, or ncp BVDV2-infected monocytes using RNAiso Plus Reagent (Takara Bio, Otsu, Japan). RNA concentration was analyzed using a Nanodrop Lite Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) and 50 ng of RNA was used in each sample. Gene expression was quantified by real-time RT-PCR using One-Step SYBR PrimeScriptTM RT-PCR Kit (Takara). Reaction mixtures were incubated for 10 min at 42°C then 10 s at 95°C, followed by 40 cycles of 30 s at 95°C, 30 s at 60°C and 1 min at 72°C. The primers used to amplify DQ, DR, and GADPH were listed in Table 1. All reactions were performed in triplicate using an Applied Biosystems 7500 Fast Real-Time PCR System (Grand Island, NY, USA). Relative gene expression values were determined using the 2Ct method according to the User Bulletin Guide for 7500 Software (version 2.0.5) (Applied Biosystems).
Table 1.
Primers used in this study
| Specificity | Forward primer (5´3´) | Reverse primer (5´3´) |
|---|---|---|
| DR | TCCTCTCGCTCTCTATCCTCTGCTGT | AGGAAACCTTTCCATGCTGTGAAGAA |
| DQ | AGGATGGTCCTGAACAGAGCTCTGA | CTAGGGTGCAACTCACAAGGGA |
| GADPH | CCTGGAGAAACCTGCCAAGT | GCCAAATTCATTGTCGTACCA |
Western blot
Bovine monocytes were infected for 48 h with mock (control medium), ncp BVDV1, or ncp BVDV2. Cells were harvested, suspended in lysis buffer (Pierce, Rockford, IL, USA), and assayed for protein content (bicinchoninic acid protein assay; Pierce). Samples containing equal amounts of total protein (20 g per lane) were separated by 15% SDS-PAGE and transferred to nitrocellulose membranes, which were then blocked with 5% nonfat dry milk in TBS containing 0.1% Tween 20. After blocking, membranes were incubated with anti-bovine DQ (Abcam, Cambridge, UK), anti-sheep DR (Serotec), and anti-actin (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) antibodies. The nitrocellulose blots were then washed and incubated with horseradish peroxidase-conjugated secondary anti-mouse IgG (Santa Cruz Biotechnology). Bands were visualized on exposure film (Thermos Scientific Inc., Rockford, IL, USA) using an enhanced ECL (Bio-Rad, Hercules, CA, USA). To confirm equal protein loading in each experiment, blots were treated with RestoreTM PLUS Western Blot Stripping Buffer (Thermo Scientific) and then re-probed for actin.
Interferon gamma (IFN) assays
The concentration of IFN in the culture supernatants from the mock-, ncp BVDV1-, or ncp BVDV2-infected monocytes was determined using a bovine IFN ELISA kit (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. Briefly, the capture antibody was added to the 96-well plates and incubated overnight at room temperature (RT). The next day, the plates were washed, and blocking buffer (supplied with the kit) was added and incubated for 1 h at RT. The plates were washed again, and culture supernatant was added to the antibody capture wells (with standards added to wells in duplicate) and incubated for 2 h. After the plates were washed, the detection antibody was added and incubated for 2 h, and then horseradish peroxidase-labeled streptavidin was added to each well and incubated at RT for 30 min. Finally, the substrate solution was added to each well and allowed to incubate for 20 min, followed by the addition of stop solution. The optical density (OD) was measured at 450 nm on a microplate reader (Tecan, Switzerland).
Statistical analysis
Data were analyzed by one-way analysis of variance and Student’s t-test using GraphPad Prism version 5.0 software (GraphPad Software Inc., San Diego, CA, USA). All experiments were repeated at least four times, and data represent the mean ± standard errors (SE). P-values <0.05 were considered statistically significant.
Results
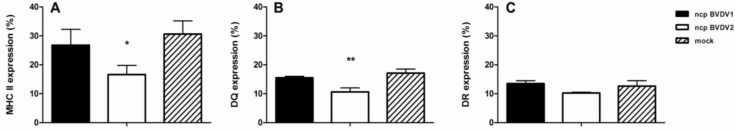
To investigate the cell surface’s expression of MHC class II molecules on monocytes after BVDV infection for 48 h, mock-, ncp BVDV1- or ncp BVDV2-infected monocytes were incubated with IL-A21, which recognizes an epitope common to both DR and DQ of MHC class II, and analyzed by flow cytometry. As shown in Fig. 1A, ncp BVDV1-infected monocytes (27.8 9.5%) displayed the expression of total MHC class II on their surface, which showed a similar expression to the mock-infected cells (30.6 7.9%). In contrast, the expression was significantly decreased in ncp BVDV2-infected cells (16.6 5.5%) compared to mock infection (P<0.05; Fig. 1A). We also examined the individual expression of DQ and DR by flow cytometry. Compared with ncp BVDV1- (15.5 0.7%) and mock-infected monocytes (17.1 1.9%), the expression of DQ was markedly decreased in ncp BVDV2-infected cells (10.6 1.9%, P<0.01; Fig. 1B). However, the expression of DR in ncp BVDV2-infected cells was decreased and its expression did not show a significant reduction compared with DQ expression (Fig. 1C).
Fig. 1.
Analysis of total MHC class II (DR and DQ) expression was assessed by flow cytometry. Cells were stained with Abs, as described in the text. Total MHC class II expression was diminished in ncp BVDV1-infected monocytes (A). The expression of MHC class II DQ was significantly decreased on ncp BVDV2-infected monocytes (B). The expression of MHC class II DR was reduced in ncp BVDV2-infected cells (C). Results are representative of three independent experiments (* P<0.05, and ** P<0.01)
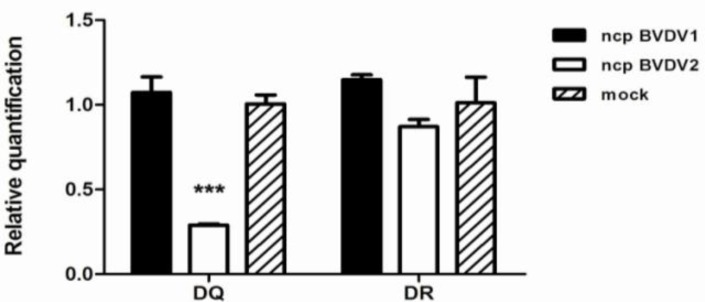
The flow cytometric analyses described above showed a reduced level of MHC class II DQ molecule on ncp BVDV2-infected monocytes. To confirm this observation, we examined the transcriptional levels of DR and DQ in mock-, ncp BVDV1- or ncp BVDV2-infected monocytes using quantitative real time RT-PCR. The average mRNA expression of DQ and DR was calculated relative to the expression of the GAPDH housekeeping gene. The expression of DQ mRNA in ncp BVDV2-infected monocytes was 3.72-fold lower than that in ncp BVDV1-infected cells (P<0.001; Fig. 2), while DR transcript levels in ncp BVDV2-infected monocytes were decreased 1.31-fold compared with ncp BVDV1-infected cells (Fig. 2). However, DQ and DR mRNA expression in ncp BVDV1-infected monocytes was increased compared to mock infection. We found a statistically significant reduction in DQ expression level in ncp BVDV2-infected monocytes.
Fig. 2.
The transcriptional levels in MHC class II DQ and DR mRNA expression were determined by quantitative real time RT-PCR analysis. Blood monocytes were infected with mock, ncp BVDV1 or ncp BVDV2. RNA was isolated after 48 h, and DR and DQ transcript levels were measured by RT-PCR. Two genes were assayed in triplicate for each sample. All values were normalized to the reference GAPDH gene and presented as the fold difference relative to GAPDH gene expression. Results are shown the mean SEM of 4 independent experiments (*** P<0.001
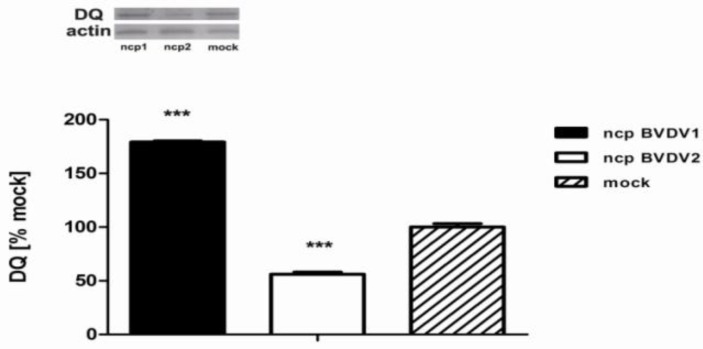
We then assessed the amount of DQ protein expression by western blot and independent blots were quantified using ImageJ software. A significant reduction in the levels of DQ protein was observed in ncp BVDV2- infected monocytes compared with ncp BVDV1-infected cells (Fig. 3), whereas the decrease of DR protein expression was not observed in ncp BVDV2-infected cells (data not shown). This correlated well with the quantification of DQ protein by western blot, which revealed an almost 56.1 3.4% decrease in ncp BVDV2-infected cells (P<0.001). The level of DQ protein expression was significantly increased in ncp BVDV1-infected monocytes (179.3 1.7%, P<0.001).
Fig. 3.
MHC class II DQ expression is decreased after ncp BVDV2 infection. Whole cell lysates were prepared from mock, ncp BVDV1- or ncp BVDV2-infected monocytes and then lysed, and the protein levels of MHC class II DQ were determined by western blot. Monocytes infected with ncp BVDV2 decreased expression of the DQ protein by 33%. Three independent blots were quantified using ImageJ software. Mock-infected monocytes were considered as 100% of DQ expression to compared band densities in different blots (*** P<0.001 versus ncp BVDV1). The actin in each sample was used to ensure equal protein loading
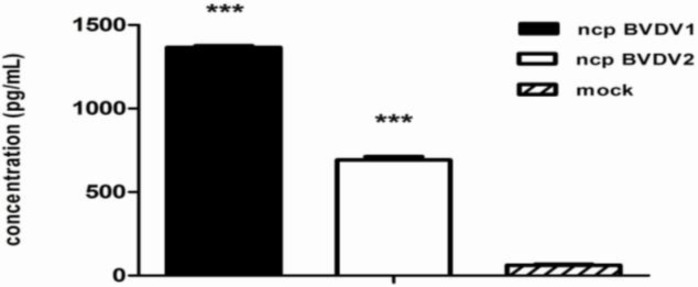
IFN is the most important antiviral cytokine of the adaptive defense system (Glew et al., 2003 ▶; Chase et al., 2004 ▶). IFN production in culture supernatants from ncp BVDV1-, ncp BVDV2- or mock-infected monocytes was measured using an ELISA. The result showed that IFN production was increased almost 2-fold in the super-natant from ncp BVDV1-infected monocytes (1366.2 13.6 pg) compared with ncp BVDV2-infected cells (692.1 27.9 pg). As shown in Fig. 4, the level of IFN in supernatants from ncp BVDV1-infected cells was approximately 22-fold higher (P<0.001) than that in supernatants from mock-infected cells (62.0 8.1 pg). However, the level of IFN in supernatants from ncp BVDV2-infected cells was approximately 11-fold higher (P<0.001) than that in supernatants from mock-infected cells.
Fig. 4.
Ncp BVDV2 infection decreases the secretion of IFN into culture supernatants. Blood monocytes were infected with mock, ncp BVDV1, or ncp BVDV2 for 48 h. Subsequently, the production of IFN by ncp BVDV2-infected monocytes was found to be lower than that by ncp BVDV1-infected cells. Data represent the mean SEM of duplicate measurements in each experiment (*** P<0.001). The figure shows a representative result from five independent experiments
Discussion
In the present study, we found that infection with ncp BVDV2 in monocytes caused a marked reduction of MHC class II DQ expression and an impairment of antigen presentation. This observation leads to evidence that ncp BVDV2, in comparison to ncp BVDV1, replicates more rapidly, disseminates more efficiently, and produces more severe clinical manifestations. Taken together, these results indicate that the down-regulation of MHC class II in ncp BVDV2-infected monocytes is due only to the reduction of DQ, but not DR. In full agreement with the findings presented above, antigen presentation was significantly impaired during ncp BVDV2 infection. Thus, our findings may provide clues to help understand the differences in pathogenesis between ncp BVDV1 and ncp BVDV2.
Monocytes/macrophages perform several important functions, including phagocytosis, the clearance of invading pathogens, and the secretion of cytokines/ chemokines that modulate the innate and adaptive immune response. In addition, these cells process microbial antigens and display antigenic peptides in the context of MHC molecules for their recognition by specific T cells (Kumar et al., 2011 ▶; La Gruta and Turner, 2014 ▶). Even though BVDV infection has already been reported to modulate MHC class II expression in monocytes (Archambault et al., 2000 ▶; Chase et al., 2004 ▶; Lee et al., 2009 ▶), our results demonstrate that this effect is specific only to MHC class II DQ (Fig. 2). Indeed, consistent, more specific down-regulation of DQ compared to DR, was observed in ncp BVDV2-infected monocytes, whereas no concomitant effect on DQ expression could be detected in ncp BVDV1-infected cells. We suspect that certain MHC class II molecules may be closely linked to ncp BVDV2 infection, and these molecules seem likely to participate in virus-host interaction. Thus, pathogens that can induce a down-regulation of the MHC class II may prevent the recognition of infected cells by specific T lymphocytes, and evade some adaptive immune responses (Kumar et al., 2011 ▶; La Gruta and Turner, 2014 ▶). Our findings suggest that ncp BVDV2-induced down-regulation of DQ expression seems to interfere with efficient antigen presentation by monocytes; it would be beneficial for the virus in its attempt to interfere with the host immune response. This provides evidence of how ncp BVDV2 may facilitate its own spread in the host and prevent its removal by the immune system, thus leading to life-long viremia, and severe immunosuppression in cattle.
IFN is known to be a key element that regulates the innate and adaptive immune response (Peterhans et al., 2003 ▶) and is the most important adaptive defense antiviral cytokine (Chase et al., 2004 ▶). IFN is also required for adequate presentation and stimulation of T helper cells (Chase et al., 2004 ▶). According to our data, IFN production was significantly decreased in culture supernatants from ncp BVDV2-infected monocytes. This may be associated with down-regulation of MHC class II expression, indicating the decreased ability to present antigens to T helper cells and eventually control the clearance of the ncp BVDV2 infection. The result demonstrated that there were significant differences in the immune response according to genotypes (ncp BVDV1 or ncp BVDV2). Consequently, our data support the evidence that these interactions with the immune system may be genotype specific (Seong et al., 2016 ▶). In this study, the results suggest that unlike ncp BVDV1, ncp BVDV2 may be accelerating the replication due to the limited capability to control virus replication and spread of the virus in the lymphatic system, impairing the immunity of the infected animals. As the ncp BVDV2 infection progresses in hosts, the viral load would cause the down-regulation of MHC class II expression and deficits in antigen presentation.
In summary, these results suggest that the expression of MHC class II DQ is significantly diminished in ncp BVDV2-infected monocytes. The reduction of MHC class II DQ may weaken host protective immunity against ncp BVDV2 infection. Evidently, the conse-quential defect in antigen presentation inhibits the appropriate switch to an adaptive immune response and prevents virus clearance, resulting in enhanced viral spread and impaired antiviral defense mechanisms in the host. Thus, these results provide understanding of the mechanism by which ncp BVDV2 alters the host immune response and evades immune recognition, leading to defective virus control and more severe clinical signs.
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (No. 2015R1C1A2A01053080).
References
- Andersson, L, Lundén, A, Sigurdardottir, S, Davies, CJ, Rask, L. Linkage relationships in the bovine MHC region High recombination frequency between class II subregions. Immunogenetics. 1988;27:273–280. doi: 10.1007/BF00376122. [DOI] [PubMed] [Google Scholar]
- Archambault, D, Beliveau, C, Couture, Y, Carman, S. Clinical response and immunomodulation follow-ing experimental challenge of calves with type 2 noncyto-pathogenic bovine viral diarrhea virus. Vet. Res. 2000;31:215–227. doi: 10.1051/vetres:2000117. [DOI] [PubMed] [Google Scholar]
- Baker, JC. The clinical manifestations of bovine viral diarrhea infection. Vet. Clin. North Am. Food Anim. Pract. 1995;11:425–445. doi: 10.1016/s0749-0720(15)30460-6. [DOI] [PubMed] [Google Scholar]
- Brackenbury, LS, Carr, BV, Charleston, B. Aspects of the innate and adaptive immune responses to acute infections with BVDV. Vet. Microbiol. 2003;96:337–344. doi: 10.1016/j.vetmic.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Chase, CC. The impact of BVDV infection on adaptive immunity. Biologicals. 2013;41:52–60. doi: 10.1016/j.biologicals.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Chase, CC, Elmowalid, G, Yousif, AA. The immune response to bovine viral diarrhea virus: a constant-ly changing picture. Vet. Clin. North Am. Food Anim. Pract. 2004;20:95–114. doi: 10.1016/j.cvfa.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Glew, EJ, Carr, BV, Brackenbury, LS, Hope, JC, Charleston, B andHoward, CJ. Differential effects of bovine viral diarrhoea virus on monocytes and dendritic cells. J. Gen. Virol. 2003;84:1771–1780. doi: 10.1099/vir.0.18964-0. [DOI] [PubMed] [Google Scholar]
- Glew, EJ, Howard, CJ. Antigen-presenting cells from calves persistently infected with bovine viral diarrhoea virus, a member of the Flaviviridae, are not compromised in their ability to present viral antigen. J. Gen. Virol. 2001;82:1677–1685. doi: 10.1099/0022-1317-82-7-1677. [DOI] [PubMed] [Google Scholar]
- Kumar, H, Kawai, T, Akira, S. Pathogen recogni-tion by the innate immune system. Int. Rev. Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- La Gruta, NL, Turner, SJ. T cell mediated immunity to influenza: mechanisms of viral control. Trends Immunol. 2014;35:396–402. doi: 10.1016/j.it.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Lee, SR, Nanduri, B, Pharr, GT, Stokes, JV andPinchuk, LM. Bovine viral diarrhea virus infection affects the expression of proteins related to professional antigen presentation in bovine monocytes. Biochim. Biophys. Acta. 2009;1794:14–22. doi: 10.1016/j.bbapap.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Lewin, HA, Russell, GC, Glass, EJ. Comparative organization and function of the major histocompatibility complex of domesticated cattle. Immunol. Rev. 1999;167:145–158. doi: 10.1111/j.1600-065x.1999.tb01388.x. [DOI] [PubMed] [Google Scholar]
- Peterhans, E, Jungi, TW, Schweizer, M. BVDV and innate immunity. Biologicals. 2003;31:107–112. doi: 10.1016/s1045-1056(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Ridpath, JF, Bendfeldt, S, Neill, JD, Liebler-Tenorio, E. Lymphocytopathogenic activity in vitro correlates with high virulence in vivo for BVDV type 2 strains: criteria for a third biotype of BVDV. Virus Res. 2006;118:62–69. doi: 10.1016/j.virusres.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Rupp, R, Hernandez, A, Mallard, BA. Associa-tion of bovine leukocyte antigen (BoLA) DRB3.2 with immune response, mastitis, and production and type traits in Canadian Holsteins. J. Dairy Sci. 2007;90:1029–1038. doi: 10.3168/jds.S0022-0302(07)71589-8. [DOI] [PubMed] [Google Scholar]
- Seong, G, Lee, JS, Lee, KH, Shin, SU, Yoon, JY, Choi, KS. Noncytopathic bovine viral diarrhea virus 2 impairs virus control in a mouse model. Arch. Virol. 2016;161:395–403. doi: 10.1007/s00705-015-2665-y. [DOI] [PubMed] [Google Scholar]
- Seong, G, Oem, JK, Choi, KS. Pathogenetic differences after experimental infection of calves with Korean non-cytopathic BVDV-1 and BVDV-2 isolates. Vet. Immunol. Immunopathol. 2013;156:147–152. doi: 10.1016/j.vetimm.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Takeshima, S, Miki, A, Kado, M, Aida, Y. Establishment of a sequence-based typing system for BoLA-DQA1 exon 2. Tissue Antigens. 2007;69:1891–1899. doi: 10.1111/j.1399-0039.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- Tortorella, D, Gewurz, BE, Furman, MH, Schust, DJ, Ploegh, HL. Viral subversion of the immune system. Annu. Rev. Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- Zanotti, M, Poli, G, Ponti, W, Polli, M, Rocchi, M, Bolzani, E, Longeri, M, Russo, S, Lewin, HA, van Eijk, MJ. Association of BoLA class II haplotypes with subclinical progression of bovine leukaemia virus infection in Holstein-Friesian cattle. Anim. Genet. 1996;27:337–341. [PubMed] [Google Scholar]