Abstract
By causing numerous changes in the cardiovascular system, ageing leads to a decreased threshold for clinical manifestation of heart disease. The aim of this study was to define the existence of cardiac structural and functional changes in healthy dogs of different age. Radiographic, electrocardiographic (ECG) and echocardiographic examinations of 20 clinically healthy German Shepherd dogs were performed in order to define the values of relevant parameters. Afterwards, the values of cardio examinations were compared between young and old dogs and statistically analyzed. The ECG recordings did not show the appearance of clinically significant arrhythmias, nor was their appearance significantly different between dogs of different age. Statistically significant differences in QRS duration (P<0.05) and R wave amplitude (P<0.05) existed between groups, but all values were within the range of ECG reference values for healthy dogs. Concerning structural changes, the left ventricle wall thickness at end-diastole (LVWd) and end-systole (LVWs), and the relative wall thickness (RWT) between young and old dogs differed significantly (P<0.001, P<0.05, P<0.05, respectively). These differences in heart structure were not accompanied by systolic dysfunction, estimated by a left ventricle shortening fraction. The diastolic wall stress index (WSID) was significantly decreased in old dogs (P<0.05). Young and old dogs showed similar electrical and systolic function. Old dogs had different cardiac structure compared to the young dogs, which could result in diastolic function change.
Key Words: Ageing, Cardiology, German Shepherd dog
Introduction
It was generally recognized that the frequency of cardiovascular diseases increases with ageing in dogs (Bonagura, 1981 ▶; Hamlin, 2005 ▶). Disorders of the cardiovascular system are one of the most commonly encountered disease entities in the ageing population (Saunders, 2012 ▶) and furthermore, are indicated as the second most common cause of death in dogs (Eichelberg and Seine, 1996 ▶). The mortality rate from heart disease, however, is dependent on breed, age and gender of dogs, and it was shown to be increased for Irish Wolfhound, Cavalier King Charles Spaniel and Great Dane (Egenvall et al., 2005 ▶) or defined like leading cause of death in Newfoundland, Maltese, Chihuahua, Doberman Pincher, Fox Terrier and Bernese (Fleming et al., 2011 ▶).
Ageing results in numerous changes of the cardio-vascular system that could be observed from morphological, functional, endocrinological, genetic and biochemical points of view. The precise relationship between the negative modifications of cardiovascular function with ageing and development of specific cardiovascular diseases is difficult to ascertain. In healthy humans, age-associated cardiac changes seem to have relevance to the steep increases in left ventricular (LV) hypertrophy, heart failure and arterial fibrillation (Lakatta and Levy, 2003 ▶; Redfield et al., 2005 ▶). Although the existence of cardiovascular functional modification with ageing was shown in anesthetized and unanesthetized dogs under experimental conditions (Miller et al., 1976 ▶; Templeton et al., 1976 ▶, 1979; Haidet, 1993 ▶; Haidet et al., 1996 ▶; Strasser et al., 1997 ▶), extensive clinical studies were not performed in dogs. Besides, the studies in dogs as experimental animals (Gan et al., 2013 ▶; Kim et al., 2013 ▶; Xu et al., 2013a ▶, b ▶) have been designed as researches in human cardiology. Knowing that the number and proportion of older dogs in the canine population is constantly increasing, the results of these studies could be very useful in canine cardiology.
Breed predisposition for certain acquired cardiac diseases imposes the need for studies in dogs of the same breed. German Shepherd dogs have low risk of death due to heart disease in comparison with other breeds (Egenvall et al., 2005 ▶), which makes them suitable for assessment of cardiac morphology and function in relation to ageing. Although data concerning values of electrocardiographic (ECG) (Rezakhani et al., 1990 ▶) and echocardiographic parameters (Kayar et al., 2006 ▶; Muzzi et al., 2006 ▶) in the healthy German Shepherd dog were published, these studies were not designed to analyze age-dependent cardiac changes. The study presented here was performed to define the existence of age-dependent changes of cardiac structural and functional characteris-tics in German Shepherd dogs, which could be registered with the conventional diagnostic procedures widely used in canine cardiology.
Materials and Methods
Animals
In this study, 20 clinically healthy German Shepherd dogs (16 males and 4 females) were examined. The dogs were divided into two groups on the basis of their age: young dogs from 1 to 3 years of age (n=10 dogs, 8 males and 2 females; average age 2 years) and old dogs from 8 to 13 years of age (n=10 dogs, 8 males and 2 females; average age 10.5 years). Knowing that different breeds age at different speeds, the groups of young and old dogs were defined in accordance with available data on the subject of ageing in German Shepherd dogs (Egenvall et al., 2005 ▶).
All dogs were assessed by clinical examination including history of each dog, physical examination, complete blood count and serum chemistry, thoracic radiography, electrocardiography and echocardiography. All examinations were performed with manual restraint of the animals, without using sedation or anesthesia.
Methods
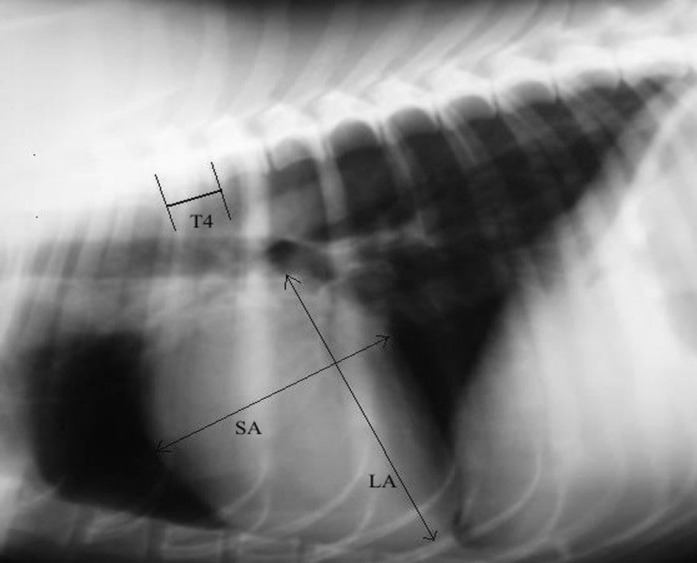
Radiographic examinations were performed from both laterolateral and dorsoventral radiographic views (Gierth HF 200, Japan). Radiographs of all dogs were analyzed subjectively by an experienced veterinary radiologist. Then, the vertebral heart size (VHS) was calculated from laterolateral thoracic radiographs of the dogs, according to modified Buchanan and Bücheler’s measurement (Buchanan and Bücheler, 1995 ▶; Spasojević Kosić et al., 2007 ▶). Briefly, the long axis (LA) and short axis (SA) of the heart were placed on the cardiac silhouette. By using calipers and ruler, the LA and SA were measured in mm, and then transformed into VHS unit (v) by dividing them with the length of the body and caudal disc of fourth thoracic vertebra (T4). Finally, the values of the LA and SA were summed up in order to define VHS (Fig. 1).
Fig. 1.
Laterolateral radiograph with marked LA and SA of the heart and VHS unit (body and disc of T4) according to the modified VHS measurement
Electrocardiographic examinations were done separately as resting electrocardiography in dogs’ right lateral recumbent position (Cardiopan, Philips and Schiller AT-1, Schiller AG, Switzerland) according to the Standards for Canine Electrocardiography (Hamlin and Patterson, 1977 ▶). The values of ECG parameters received from lead II were collected in a computer data base. The ECG recordings were analyzed according to the standard procedure (Edwards, 1987 ▶).
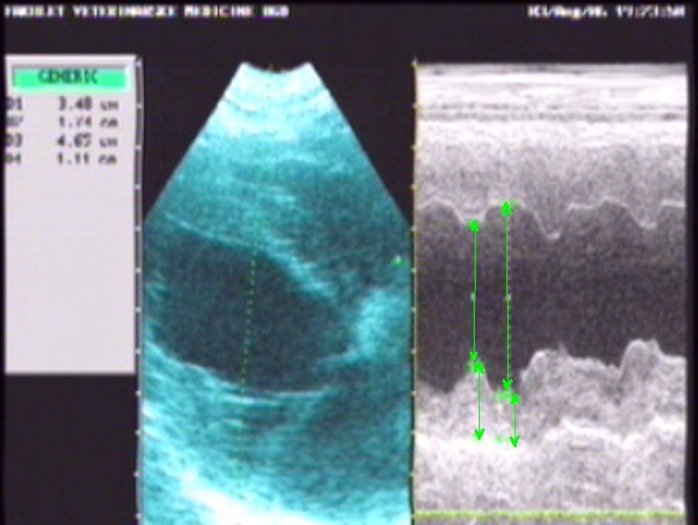
Echocardiographic examinations were performed in the standing position of dogs as two-dimensional (2D) (both longitudinal and transversal from right parasternal location, left caudal and left cranial parasternal location) and M-mode echocardiography, using a 5 MHz mechanical sector transducer (300 S Pandion Vet, Esaote Pie Medical, the Netherlands). Measurements were performed in accordance with recommendations of the American Society of Echocardiography (Sahn et al., 1978 ▶). LV parameters: left ventricular internal diameter in diastole (LVIDd), left ventricular internal diameter in systole (LVIDs), left ventricular wall thickness in diastole (LVWd), and left ventricular wall thickness in systole (LVWs) were measured from right parasternal location by using two-dimensional guided M-mode echocardiograms. The M-mode cursor line was placed at the LV chamber level, just below the tip of the mitral valve, with the beam directed perpendicular to the septum and LVW in order to avoid papillary muscles within the left ventricle (Fig. 2). The measurements were made from the leading edge of an echo (Kienle, 1998 ▶). For each parameter, the mean of three consecutive cardiac cycles was taken as the result of measurement. As indicator of LV geometric remodeling, the relative wall thickness (RWT) was calculated (Foppa et al., 2005 ▶). The relative wall thickness, the left ventricular fractional shortening (FS), the left ventricle free wall percent thickening (LVW%T), the systolic wall stress index (WSIS) and the diastolic wall stress index (WSID) were calculated using the following formulas (Minors and O’Grady, 1998 ▶; Foppa et al., 2005 ▶):
Fig. 2.
Two-dimensional guided M-mode echocardiogram with LV parameters measurements
Statistical analysis
Statistical analyses were performed with a com-puterized statistical software package Statistica 12. Data are reported as the mean ± standard deviation (SD). Values of radiographic, ECG and echocardiographic parameters between dogs of different age were compared using Student’s t-test for independent samples. Rate of arrhythmias’ occurrence between groups was assessed by Fisher test of probability. Multiple regression analysis was used to assess the association between measured LV echocardiographic parameters (LVIDd, LVIDs, LVWd, LVWs) and calculated echocardiographic parameters (FS, LVW%T, WSIS, WSID, RWT). The correlation between measured and calculated LV echocardiographic parameters was evaluated by correlation coefficients. Statistical significance was defined as P<0.05.
Results
In this study, 20 German Shepherd dogs without clinical signs of cardiovascular or respiratory system diseases were clinically assessed. Dogs did not have pathological heart sounds or heart murmurs. Clinical and laboratory examinations revealed no evidence of relevant systemic disease existence. Average values of VHS in young and old German Shepherd dogs were 10.26 ± 0.34 v and 10.26 ± 0.61 v, respectively. There was no significant difference in VHS between dogs of different age.
Heart rhythm of all dogs was sinus in origin (Table 1). All registered arrhythmias in dogs were benign. Certain dogs had several arrhythmias (for example, sinus arrhythmia with sinus pause, wondering pacemaker with sinus arrest and AV block 1°). The appearance of AV block 1° in aged dogs was not statistically significant. The values of R wave amplitude and QRS complex duration were significantly different between dogs of different age (Table 2). However, all ECG parameter values were within the references range for healthy dogs (Edwards, 1987 ▶).
Table 1.
Heart rhythm in German Shepherd dogs of different age
| Heart rhythms | Young dogs n (% of all dogs) |
Old dogs n (% of all dogs) |
|---|---|---|
| Normal sinus rhythm | 1 (5%) | 1 (5%) |
| Sinus arrhythmia | 5 (25%) | 5 (25%) |
| Wandering pacemaker | 3 (15%) | 3 (15%) |
| Sinus bradycardia | 1 (5%) | 1 (5%) |
| Sinus block | 1 (5%) | - |
| Sinus pause | - | 1 (5%) |
| Sinus arrest | 1 (5%) | 3 (15%) |
| AV block 1° | - | 3 (15%) |
n: Number of dogs
Table 2.
ECG parameters in German Shepherd dogs of different age
| ECG parameters | Young dogs (mean±SD) |
Old dogs (mean±SD) |
|---|---|---|
| HR (beats/min) | 92.184 ± 13.577 | 87.458 ± 17.538 |
| P time (s) | 0.040 ± 0.000 | 0.044 ± 0.008 |
| P ampl. (mV) | 0.183 ± 0.073 | 0.229 ± 0.079 |
| PR time (s) | 0.116 ± 0.014 | 0.126 ± 0.021 |
| R ampl. (mV) | 2.228 ± 0.674 | 1.650 ± 0.501* |
| QRS time (s) | 0.060 ± 0.000 | 0.055 ± 0.007* |
| Tampl. (+) (mV) | +0.400 ± 0.000 | +0.450 ± 0.260 |
| Tampl. (-) (mV) | -0.427 ± 0.293 | -0.373 ± 0.083 |
| QT time (s) | 0.210 ± 0.019 | 0.206 ± 0.021 |
P<0.05
The echocardiographic parameters (Table 3), the values of the LVWd (P=0.000597) and LVWs (P=0.009259) in older dogs were significantly higher compared with values in young dogs. Also, the RWT was significantly different (P=0.007798) between the two groups. There was no significant difference for FS between dogs of different age, but WSID was significantly lower (P=0.007107) in older dogs compared to the young ones.
Table 3.
Echocardiographic data for German Shepherd dogs of different age
| Echo parameters | Young dogs (mean±SD) |
Old dogs (mean±SD) |
|---|---|---|
| LVIDd (mm) | 43.88 ± 3.78 | 45.29 ± 3.34 |
| LVIDs (mm) | 31.23 ± 5.51 | 32.04 ± 3.55 |
| LVWd (mm) | 8.75 ± 0.71 | 10.99 ± 1.55** |
| LVWs (mm) | 10.52 ± 1.38 | 12.97 ± 2.27* |
| RWT | 0.40 ± 0.03 | 0.48 ± 0.09* |
| FS (%) | 28.94 ± 8.96 | 29.16 ± 6.77 |
| LVW%T (%) | 20.05 ± 10.78 | 18.17 ± 14.97 |
| WSIS | 3.03 ± 0.78 | 2.55 ± 0.62 |
| WSID | 5.03 ± 0.48 | 4.20 ± 0.72* |
P<0.05,
P<0.001
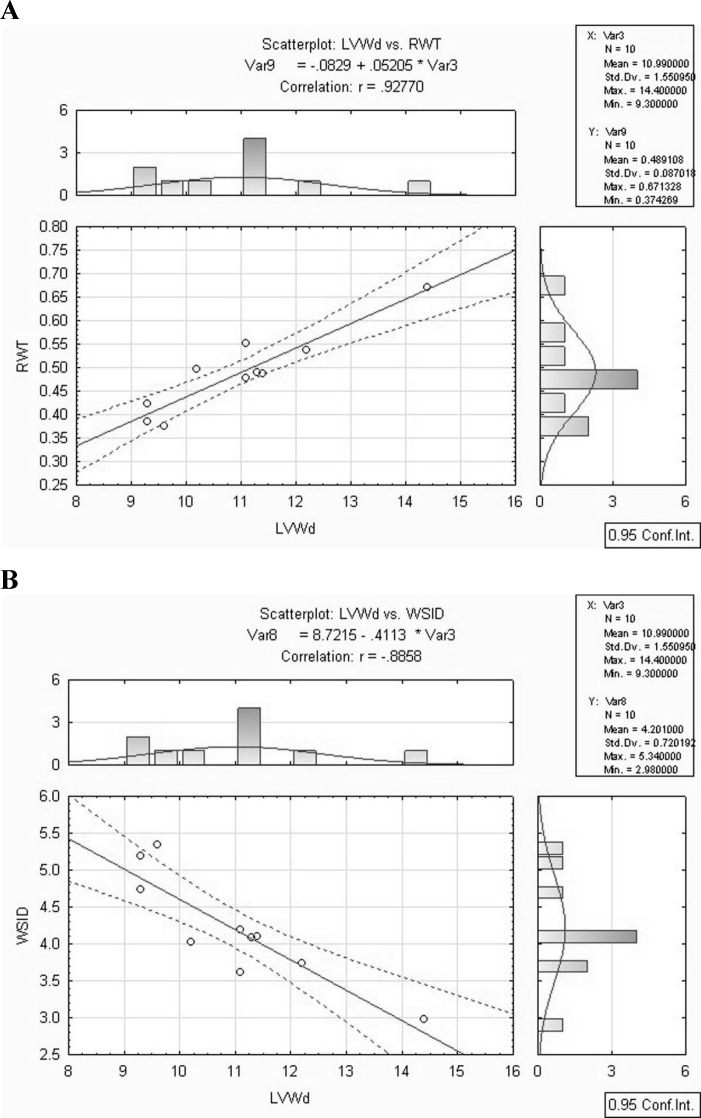
Results of the multivariable regression model by using echocardiographic parameters are shown in Table 4. Both echocardiographic parameters, RWT and WSID, were significantly correlated with LVWd (Figs. 3A-B).
Table 4.
The echocardiographic parameters examined for association: multiple regression results. The values are expressed as coefficient b (SE
| Dependent variables | Age | Independent variables |
|||
|---|---|---|---|---|---|
| LVIDd | LVIDs | LVWd | LVWs | ||
| FS | Young | 0.68 (0.04)** | -1.34 (0.03)** | NS | NS |
| Old | 0.71 (0.03)** | -1.07 (0.03)** | NS | NS | |
| LVW%T | Young | NS | NS | -0.87 (0.04)** | 1.47 (0.03)** |
| Old | NS | NS | -1.02 (0.04)** | 1.36 (0.03)** | |
| WSIS | Young | NS | 0.70 (0.07)** | NS | -0.50 (0.07)* |
| Old | NS | 0.58 (0.05)** | NS | -0.71 (0.06)** | |
| WSID | Young | 0.95 (0.02)** | NS | -0.88 (0.03)** | NS |
| Old | 0.46 (0.06)** | NS | -0.65 (0.08)** | NS | |
| RWT | Young | -0.93 (0.05)** | NS | 0.86 (0.05)** | NS |
| Old | -0.38 (0.02)** | NS | 0.81 (0.03)** | NS | |
P<0.05,
P<0.001. NS: Non-significant
Fig. 3.
Correlations between LVWd and RWT (A), and LVWd and WSID (B) in old German Shepherd dogs. The graphics illustrate the linear regression line with a 95% confidence interval
Discussion
In this study, cardiac structural and functional differences were found between young and old German Shepherd dogs. An increase in LVW, and LV hyper-trophy was registered with aging. A significant systolic dysfunction was not registered with aging and there was no correlation between LVW and FS in healthy German Shepherd dogs. Ageing leads to statistically significant decrease of electrical and diastolic function in German Shepherd dogs.
Earlier studies have shown changes of cardiovascular function with aging, such as decreased blood flow, blood velocity, arterial compliance and distensibility (Miller et al., 1976 ▶; Haidet, 1993 ▶; Haidet et al., 1996 ▶), as well as increased systemic vascular resistance and decreased cardiac output (Haidet et al., 1996 ▶). Also, decreased contractile function, increase in LV systolic and diastolic stiffness and prolonged duration of contraction were demonstrated in senescent heart (Templeton et al., 1976 ▶, 1979). According to the results of our study, aging leads to changes of cardiac electrical characteristics in German Shepherd dogs. Both parameters of ventricles depolari-zation, QRS duration and R wave amplitude were significantly different between dogs of different age. However, values of QRS duration and R wave amplitude of both groups were within the reference range. Significant difference in R wave amplitude could be explained by the existence of low R wave amplitudes (R <1.5 mV) in 3 older dogs. Among the geriatric dogs, three dogs had first degree AV block (AV block 1°). Generally speaking, this type of impulse conduction disturbance increases with age in dogs. Although clinically important, the appearance of first degree AV block did not show statistically significant differences between the two groups. Our results are slightly different from the previously published ECG values of German Shepherd dogs (Rezakhani et al., 1990 ▶), and higher values of P wave amplitude, PR duration and R wave amplitude were measured in our study.
The recognition of interbreed variations in cardiac dimensions has already led to the development of breed-specific ranges for echocardiography (Morrison et al., 1992 ▶; Kayar et al., 2006 ▶) and researches have suggested their importance in radiographic measurement as well (Lamb et al., 2000 ▶, 2001). The values of VHS and LV echocardiographic parameters in our study were similar to previously published values for German Shepherd dogs (Lamb et al., 2001 ▶; Kayar et al., 2006 ▶; Muzzi et al., 2006 ▶). There was no significant difference in VHS between young and old dogs, and average value of 10.26 v for VHS in German Shepherd dogs in our study is in the reference range for this breed (Lamb et al., 2001 ▶). In the previous studies in German Shepherd dogs, measurements of LVWd and LVWs were 0.88 cm and 13.0 cm (Muzzi et al., 2006 ▶), and significant correlation with body weight and gender was observed with no age correlation (Kayar et al., 2006 ▶; Muzzi et al., 2006 ▶). However, in our study, the echocardiographic results showed the existence of significant differences in LVWs (P=0.009259) and LVWd (P=0.000597) values between dogs of different age. A possible explanation could be the different age spans which were included in these studies. The study of Kayar et al. (2006) ▶ was performed in German Shepherd dogs from 12 months to 8 years of age, whilst in the study of Muzzi et al. (2006) ▶, German Shepherd dogs ranging in age from 1 to 5 years were examined. In our study, comparison between young dogs (1-3 years of age) and aged dogs (8-13 years of age) was performed. The study group of dogs in our study, although smaller, included dogs on both ends of the scale of age span.
In addition, in our study the RWT was significantly higher in old dogs. Parietal thickness and its relation to LV chamber size have been recognized as measure of hypertrophy for more than 30 years (Sjogren, 1971 ▶). A RWT provides information regarding LV geometry independent of other calculations (Li et al., 2001 ▶), precluding the requirement of most corrections. In the old dogs in our study, significant correlation was shown between LVIDd, LVWd and RWT. In humans, the reference cut point value for increased RWT derived from upper limits of normal samples is 0.44 (Ganau et al., 1992 ▶) or 0.45 (Savage et al., 1987 ▶). There are no data concerning reference values for the RWT in canine cardiology. In healthy Beagle dogs, the LV geometry estimated by the sphericity index and the RWT was not significantly different between young and old ones (Suzuki et al., 2013 ▶). There was no significant difference in FS values between young and aged dogs in our study. These findings may suggest that there was no significant change in cardiac pump function in systole with ageing. According to our study no significant correlation was found between systolic cardiac function and LVWd and LVWs both in young and old dogs. Since increased LVW dimension leads to increased stiffness of the left ventricle and contributes to diastolic dysfunction, it could be assumed that diastolic function changes with aging. In humans, combined ventricular-vascular stiffening may contribute to the increased prevalence of heart failure with preserved ejection fraction in elderly person, particularly women (Redfield et al., 2005 ▶). This is in accordance with findings of different myocardial diastolic functions between young and old Beagle dogs documented by a more sensitive echocardiographic technique (Suzuki et al., 2013 ▶). In our study, the WSID, as an echocardiographic parameter of diastolic function, was significantly lower in old dogs. Since WSID represents a measure of LV preload (Aurigemma and Gaasch, 2007 ▶), it could be presumed that preload is reduced in old dogs.
Because of the simplicity of their calculation, both RWT and WSID should be assessed more frequently in dogs. They can be useful as screening methods for LV hypertrophy and diastolic dysfunction, as it was shown in our study. Further studies are needed in order to prove this assumption. In spite of the fact that systemic hypertension in dogs is usually secondary to another disease, further studies with blood pressure measurement should additionally explain the structural cardiac changes in old dogs. The small number of dogs and the use of a specific breed (German Shepherd) in our study may have an influence on the extrapolation of these findings to other breeds.
In conclusion, conventional methods of cardiac examination, such as electrocardiography and echo-cardiography register structural and functional changes in aged German Shepherd dogs. Cardiac electrical activity changes were benign with no significant difference in the appearance of arrhythmias between dogs of different age. Systolic cardiac function was similar in young and old dogs. Significant differences in the values of LVW in systole and diastole, RWT and WSID were found between dogs of different age, highlighting that age should be taken into account in the assessment of LV hypertrophy and diastolic function in German Shepherd dogs.
Acknowledgements
The authors would like to thank Dr V. Magaš, Dr Lj. Popadić, and Dr A. Spasović for their technical assistance.
References
- Aurigemma GP, Gaasch WH. Quantitative evaluation of left ventricular structure, wall stress and systolic function. InOtto, CM (Ed.), The practice of clinical echocardiography. 3rd Edn. Philadelphia: Saunders; 2007. pp. 187–212. [Google Scholar]
- Bonagura, JD. Cardiopulmonary disorders in the geriatric dog. Vet. Clin. North. Am. (Small Anim. Pract.) 1981;11:705–726. doi: 10.1016/s0195-5616(81)50082-9. [DOI] [PubMed] [Google Scholar]
- Buchanan, JW, Bücheler, J. Vertebral scale system to measure canine heart size in radiographs. JAVMA. 1995;206:194–199. [PubMed] [Google Scholar]
- Edwards NJ. Interpreting the electrocardiogram. In: Pedersen, D (Ed.), Bolton’s handbook of canine and feline electrocardiography. 2nd Edn. Philadelphia: W. B. Saunders; 1987. pp. 32–60. [Google Scholar]
- Egenvall, A, Bonnett, BN, Hedhammar, A, Olson, P. Mortality in over 350000 insured Swedish dogs from 1995-2000: I breed-specific age and survival patterns and relative risk for cause of death. Acta Vet. Scand. 2005;46:121–136. doi: 10.1186/1751-0147-46-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelberg, H, Seine, R. Life expectancy and cause of death in dogs I: The situation in mixed breeds and various dog breeds. Berl. Munch. Tierarztl. 1996;109:292–303. [PubMed] [Google Scholar]
- Fleming, JM, Creevy, KE, Promislow, DEL. Mortality in North American dogs from 1984 to 2004: an investigation into age-, size-, and breed-related causes of death. J. Vet. Intern. Med. 2011;25:187–198. doi: 10.1111/j.1939-1676.2011.0695.x. [DOI] [PubMed] [Google Scholar]
- Foppa, M, Duncan, BB, Rohde, LEP. Echocardiography-based left ventricular mass estimation How should we define hypertrophy. Cardiovasc. Ultrasound. . 2005 doi: 10.1186/1476-7120-3-17. doi: 10.1186/1476-7120-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, TY, Qiao, W, Xu, GJ, Zhen, XH, Tang, BP, Song, JG, Li, YD, Zhang, Y, Li, FP, Mao, T, Jiang, T. Aging-associated changes in L-type calcium channels in the left atria of dogs. Exp. Ther. Med. 2013;6:919–924. doi: 10.3892/etm.2013.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganau, A, Devereux, RB, Roman, MJ, DeSimone, G, Pickering, TG, Saba, PS, Vargin, P, Simongini, I, Laragh, JH. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J. Am. Coll. Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- Haidet, GC. Effects of age on beta-adrenergic-mediated reflex responses to induced muscular contraction in beagles. Mech. Ageing Dev. 1993;68:89–104. doi: 10.1016/0047-6374(93)90142-e. [DOI] [PubMed] [Google Scholar]
- Haidet, GC, Wennberg, PW, Finkelstein, SM, Morgan, DJ. Effects of aging per se on arterial stiffness: systemic and regional compliance in beagles. Am. Heart J. 1996;132:319–327. doi: 10.1016/s0002-8703(96)90428-7. [DOI] [PubMed] [Google Scholar]
- Hamlin, RL. Geriatric heart diseases in dogs. Vet. Clin. North Am. (Small Anim. Pract.) 2005;5:597–617. doi: 10.1016/j.cvsm.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hamlin, RL, Patterson, DF. Standards for canine electrocardiography. The Academy of Veterinary Cardio-logy Committee Report 1977. The Academy of Veterinary Cardiology. 1977.
- Kayar, A, Gonul, R, Or, ME, Uzsal, A. 2006) M-mode echocardiographic parameters and indices in the normal German Shepherd dog. Vet. Radiol. Ultrasound. 47:482–486. doi: 10.1111/j.1740-8261.2006.00166.x. [DOI] [PubMed] [Google Scholar]
- Kienle, RD. Echocardiography. In: Kittleson, MD, Kienle, RD, editors. Small animal cardiovascular medicine. 1st Edn. St. Louis: Mosby; 1998. pp. 95–117. [Google Scholar]
- Kim, SH, Lee, KH, Won, HY, Park, S, Chung, JH, Jang, Y, Ha, JW. Quantitative assessment of aortic elasticity with aging using velocity-vector imaging and its histologic correlation. Arterioscler. Thromb. Vasc. Biol. 2013;33:1306–1312. doi: 10.1161/ATVBAHA.113.301312. [DOI] [PubMed] [Google Scholar]
- Lakatta, EG, Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part II: The aging heart in health: link to heart disease. Circulation. 2003;107:346–352. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- Lamb, CR, Tyler, M, Boswood, A, Skelly, BJ, Cain, M. Assesment of the value of the vertebral heart scale in the radiographic diagnosis of cardiac disease in dogs. Vet. Rec. 2000;146:687–690. doi: 10.1136/vr.146.24.687. [DOI] [PubMed] [Google Scholar]
- Lamb, CR, Wikeley, H, Boswood, A, Pfeiffer, DU. Use of breed specific ranges for the vertebral heart scale as an aid to the radiographic diagnosis in dogs. Vet. Rec. 2001;148:707–711. doi: 10.1136/vr.148.23.707. [DOI] [PubMed] [Google Scholar]
- Li, L, Shigematsu, Y, Hamada, M, Hiwada, K. Relative wall thickness is an independent predictor of left ventricular systolic and diastolic dysfunctions in essential hypertension. Hypertens. Res. 2001;24:493–499. doi: 10.1291/hypres.24.493. [DOI] [PubMed] [Google Scholar]
- Miller, CW, Nealeigh, RC, Crowder, ME. Evaluation of the cardiovascular changes associated with aging in a colony of dogs. Biomed. Sci. Instrum. 1976;12:107–110. [PubMed] [Google Scholar]
- Minors, SL, O’Grady, MR. Resting and dobu-tamine stress echocardiography factors associated with the development of occult dilated cardiomyopathy in healthy Doberman Pincher dogs. J. Vet. Intern. Med. 1998;12:369–380. doi: 10.1111/j.1939-1676.1998.tb02137.x. [DOI] [PubMed] [Google Scholar]
- Morrison, SA, Moise, NS, Scarlett, J, Mohammed, H, Zeager, AE. Effect of breed and bodyweight on ehocardiographic values in four breeds of dogs of differing somatotype. J. Vet. Intern. Med. 1992;6:220–224. doi: 10.1111/j.1939-1676.1992.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Muzzi, RAL, Muzzi, LAL, deAraujo, RB, Cherem, M. Echocardiographic indices in normal German Shepherd dogs. J. Vet. Sci. 2006;7:193–198. doi: 10.4142/jvs.2006.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfield, MM, Jacobsen, SJ, Borlaug, BA, Rodeheffer, RJ, Kass, DA. Age- and gender-related ventricular-vascular stiffening. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- Rezakhani, A, Atwell, RB, Webster, J. Electro-cardiographic value of German Shepherd dogs. Aust. Vet. J. 1990;67:307–309. doi: 10.1111/j.1751-0813.1990.tb07806.x. [DOI] [PubMed] [Google Scholar]
- Sahn, DJ, DeMaria, A, Kisslo, J, Weyman, A. Recommendations regarding quantitation in M-mode echo-cardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- Saunders, AB. The diagnosis and management of age-related veterinary cardiovascular disease. Vet. Clin. North Am. (Small Anim. Pract.) 2012;42:655–658. doi: 10.1016/j.cvsm.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Savage, DD, Garrison, RJ, Kannel, WB, Levy, D, Anderson, JJ, Stones, JIII, Feinleib, M, Castell, WP. The spectrum of left ventricular hypertrophy in a general population sample: the Framingham study. Circulation. 1987;75:125–133. [PubMed] [Google Scholar]
- Sjogren, AL. Left ventricular wall thickness determined by ultrasound in 100 subjects without heart disease. Chest. 1971;60:341–346. doi: 10.1378/chest.60.4.341. [DOI] [PubMed] [Google Scholar]
- Spasojević Kosić, Lj, Trailović, D, Krstić, N. Comparison of three methods of measurement vertebral heart size in German Shepherd dogs. Acta Vet. 2007;57:133–141. [Google Scholar]
- Strasser, A, Simunek, M, Seiser, M, Hofecker, NG. Age-dependent changes in cardiovascular and metabolic responses to exercise in beagle dogs. Zentrabl. Veterinarmed. 1997;44:449–460. doi: 10.1111/j.1439-0442.1997.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Suzuki, R, Matsumoto, H, Testima, T, Koyama, H. Effect of age on myocardial functional assessed by two-dimensional speckle-tracking echocardiography in healthy beagle dogs. J. Vet. Cardiol. 2013;15:243–252. doi: 10.1016/j.jvc.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Templeton, GH, Willerson, JT, Platt, MR, Wiesfeldt, M. Contraction duration and diastolic stiffness in aged canine left ventricle. Recent Adv. Stud. Card. Struct. Metabol. 1976;11:169–173. [PubMed] [Google Scholar]
- Templeton, GH, Willerson, JT, Platt, MR, Wiesfeldt, ML. Influence of aging on left ventricular hemo-dynamics and stiffness in beagles. Circ. Res. 1979;44:189–194. doi: 10.1161/01.res.44.2.189. [DOI] [PubMed] [Google Scholar]
- Xu, GJ, Gan, TY, Tang, BP, Chen, ZH, Mahemuti, A, Jiang, T, Song, JG, Guo, X, Li, YD, Zhen, XH, Zhang, Y, Li, JX. Alterations in the expression of atrial calpains in electrical and structural remodeling during aging and atrial fibrillation. Mol. Med. Rep. 2013a;8:1343–1352. doi: 10.3892/mmr.2013.1684. [DOI] [PubMed] [Google Scholar]
- Xu, GJ, Gan, TY, Tang, BP, Chen, ZH, Mahemuti, A, Zhen, XH, Jiang, T, Song, JG, Guo, X, Li, YD, Miao, HJ, Zhang, Y, Li, J. Changes in microRNAs expression are involved in age-related atrial structural remodeling and atrial fibrillation. Chin. Med. J. 2013b;126:1458–1463. [PubMed] [Google Scholar]