Abstract
Purpose:
To evaluate the therapeutic effects of copper reduction on angiogenesis-related factors in patients with glioblastoma multiforme treated by gamma knife radiosurgery
Materials and Methods:
In the present block randomized, placebo-controlled trial, fifty eligible patients with a diagnosis of glioblastoma multiforme who were candidates for gamma knife radiosurgery were randomly assigned into two groups to receive daily either 1gr penicillamine and a low copper diet or placebo for three months. The intervention started on the same day as gamma knife radiosurgery. Serum interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), vascular endothelial growth factor (VEGF) and copper levels were measured at baseline and after the intervention. The serum copper level was used as the final index of compliance with the diet. In order to control probable side effects of intervention, laboratory tests were conducted at the beginning, middle and end of the study.
Results:
The patients had a mean age and Karnofsky Performance Scale of 43.7 years and 75 respectively. Mean serum copper levels were significantly reduced in intervention group. Mean survival time was 18.5 months in intervention group vs. 14.9 in placebo group. VEGF and IL-6 levels in the intervention group were also significantly reduced compared to the placebo group and TNF-α increased less.
Conclusions:
It seems that reducing the level of copper in the diet and dosing with penicillamine leads to decline of angiogenesis-related factors such as VEGF, IL-6 and TNF-α. Approaches targeting angiogenesis may improve survival and can be used as a future therapeutic strategy.
Keywords: Angiogenesis, copper, gamma knife, glioblastoma
Introduction
Glioma is the most common primary brain tumor in adults and glioblastoma multiforme (GBM) is the most aggressive of all human tumors which eventually have a very poor prognosis (Ni et al., 2014; Pashaki et al., 2014; Qin et al., 2015). In spite of multiple treatment strategies including surgery, external radiation therapy and chemotherapy (Qin et al., 2013), the average survival of these patients is about 6-11 months (Davis et al., 1998; Farah et al., 2016) and even less than 6 months in recurrent cases. GBM is more common in men than women (male to female ratio: 1.58 to 1) and the average age at the time of diagnosis is about sixty four (States, 2010). Glioblastoma is one of the most highly angiogenic solid tumors that its vasculature is both structurally and functionally abnormal (Weathers and de Groot, 2015). The improper vasculature enhances tumor hypoxia and impairs the delivery of cytotoxic chemotherapy (Weathers and de Groot, 2015). Copper is an essential trace mineral that its concentration is high in blood-rich organs such as liver, brain, heart and kidney, also known as an important mediator of angiogenesis (Hanahan and Folkman, 1996; Urso and Maffia, 2015). Copper reduction inhibits experimentally the growth and invasiveness of Glioma (Brem et al., 2005). Copper ions stimulate proliferation and migration of the endothelial cells through the induction of fibronectin synthesis (Hanahan and Folkman, 1996). Penicillamine is a chelating agent used to treat intracerebral copper overload in Wilson’s disease (Brem et al., 2005). A broad collection of cytokines displays modified expression in cancers, including glioblastoma multiforme (Albulescu et al., 2013). In the process of angiogenesis, several factors such as IL-6, TNF-α, VEGF, IL-8 and TGF are involved (Fukumura and Jain, 2007; Riaz et al., 2014). It is reported that a number of growth factors and cytokines have angiogenic activity; these include VEGF, bFGF (basic fibroblast growth factor), EGF (epidermal growth factor), TGF (transforming growth factor)-β, IL-6, IL-8, IL-1 and TNF-α (Mihara et al., 2012; Raluca et al., 2014). IL-6 has been implicated in the regulation of growth and differentiation in many cancers, and is associated with poor prognosis (Mihara et al., 2012) and its overproduction has been implicated in the pathogenesis of a variety of diseases, including numerous chronic inflammatory diseases and cancer (Mihara et al., 2012). In glioblastoma, IL-6 has been associated with tumor cell proliferation, invasion, angiogenesis, immune suppression, and poor prognosis (Anton and Glod, 2014). IL-6 enhances the expression of adhesion molecules such as VCAM (vascular cell adhesion molecule)-1 and ICAM-1 (intercellular adhesion molecule-1) in inflamed sites and endothelial cells (Mihara et al., 2012). IL-6 is a key tumor-promoting cytokine produced by both malignant and host cells in the tumor microenvironment (Gopinathan et al., 2015) and is an important regulator of cell survival, providing tumor cells with a mechanism to escape cell death induced by stress and cytotoxic drugs (Mihara et al., 2012). IL-6 level is lower in the central portion of tumors (Chang et al., 2013). These data suggest a role for IL-6 in promoting invasion and metastasis. Therefore IL-6 blockade is anticipated to constitute a new treatment strategy for inflammatory and autoimmune diseases, as well as for cancers.
IL-6 is as potent as VEGF in inducing vessel sprouting and was also able to stimulate endothelial cell migration and proliferation (Gopinathan et al., 2015). VEGF plays a crucial role in IL-6-induced angiogenesis (Mihara et al., 2012). VEGF acts by different mechanisms such as induction of endothelial cells to form capillary-like structures and enzyme secretion for degradation of extracellular matrix and also increases the survival of tumoral cells by expressing anti-apoptotic proteins such as BcL-2 and its homologous A1 (Zhang et al., 2013; Kumar et al., 2016). TNF-α, a pro-inflammatory cytokine, is expressed in ischemic and injured tissues (Kwon et al., 2013). Effects of TNF-α on angiogenesis and vasculogenesis already have shown in experimental models (Kwon et al., 2013). TNF-α induces angiogenesis by NF-kappaB signaling pathway. Nuclear factor-kappaB (NF-kappaB) is a critical transcription factor that contributes to cancer development by regulating a number of genes involved in angiogenesis and tumorigenesis (Kim et al., 2010). TNF-α induces activity and expression of matrix metalloproteinase-9 (MMP-9), and cell-surface expression of intercellular adhesion molecule-1 (ICAM-1), which is associated with adhesion of leukocytes to endothelial cells (Yang et al., 2014). TNF-α leads to upregulation of VEGF and following upregulation of MMP-2 and MMP-9 production, and promote angiogenesis via pathways involving PI3K (phosphatidylinositol 3-kinase) and NF-κB (Shin et al., 2015). VEGF and TNF-α can induce angiogenesis. TNF-α affects on the expression of VEGF and these factors both result in angiogenesis (Chen et al., 2005). TNF-α stimulates blood perfusion and inhibits ischemic tissue damage in an IL-6- and IL-8-dependent mechanisms (Kwon et al., 2013).
Gamma knife radiosurgery utilizes highly accurate radiation techniques to allow dose escalation and delivery of ablative radiation doses to tumor by affecting vascular endothelium, while minimizing dose to the adjacent normal organs (Redmond and Mehta, 2015). Stereotactic radiosurgery (SRS) may be used as a considerable treatment option in the patients recurring after surgery, external radiotherapy and chemotherapy (Koca et al., 2014; Redmond and Mehta, 2015). Because glioblastoma shows the highest degree of angiogenesis among all human tumors (Brem et al., 2005), in this randomized clinical trial we have investigated whether reducing serum copper and consequently brain copper (by combination of low copper diet and penicillamine), associated with gamma knife radiosurgery could have a synergistic effect on survival rate and also inhibit angiogenic factors VEGF, IL-6 and TNF-α in GBM.
Materials and Methods
This clinical trial was designed to add a low-risk method to the treatment of patients suffering from recurrent glioblastoma which have not any specified and definite way to improve survival rate despite many efforts and researches. The study protocol approved by Tehran University of Medical Sciences Ethics Committee (Approval number: 9209161).
Patients and Treatment
The patients with recurrent GBM who were candidate for gamma knife radiosurgery, after obtaining written consent and according to the following inclusion criteria were randomly divided into two intervention and placebo groups: pathologically confirmed glioblastoma multiforme by open surgery or biopsy; tumor recurrence after operation, radiotherapy or chemotherapy; age≥ 18.0; Karnofsky performance score (KPS)≥ 60.0 based on Table 1 (Milstein et al., 1985); ability to provide written consent; proper hematological, renal and hepatic function. Before intervention, laboratory tests were performed and patients with following criteria were included: WBC≥ 4,000.0/mm3, Hb≥ 10.0 g/dl, platelets≥ 100,000.0/mm3, BUN≤ 40.0, creatinine≤ 1.5mg, AST and ALT≤ 4.0 folds normal, PT and PTT< 1.5 folds normal and serum copper 70.0-150.0µg/dl. The Karnofsky Performance Status Scale (KPS) is the most widely used method of quantifying the functional status of cancer (Mor et al., 1984). The KPS is an11-point rating scale which ranges from normal functioning (100.0) to dead (0.0). Exclusion criteria: Pregnancy; breast feeding; oral contraceptive use; sensitivity to penicillin; hepatic, renal or hematological insufficiency; concomitant use of Avastin (Bevacizumab) or any investigational agent. All two group of patients also received desired therapy consisting of gamma knife radiosurgery followed by adequate drugs such as anticonvulsant and corticosteroid. Using a randomized block design, patients were divided into intervention and placebo group. Weight, height, BMI and KPS of all patients were measured and also in a non-fasting state, 10.0 ml blood sample was taken and serum was separated and frozen in -70.0 ˚C to measure biomarkers such as IL-6, TNF-α and VEGF by ELISA (enzyme-linked immunosorbent assay) test. Duration of the intervention was three months and patients were followed for 12.0-24.0 months. Intervention began on the same day as the start of gamma knife radiosurgery, using an initial dose of 250 mg daily of penicillamine or placebo, with dose escalation every other day for one week to reach final dose of 1gr per day. For both intervention and placebo group, radiosurgery at a dose of 16 to 18 Gray (1,600 to 1,800 cGy) with 50% isodose in singlefractionwas performed. In intervention group, a low-copper diet was used simultaneously with penicillamine. Patients in the placebo group were asked to continue their usual diet. Pyridoxine, 40mg orally daily was given during the study to prevent the seizure secondary to vitamin B6 deficiency due topenicillamine consumption (Brem et al., 2005). In order to control probable side effects of intervention, laboratory tests including CBC, PT, PTT, BUN, Cr, AST, ALT and serum copper were conducted in the beginning, middle and end of the study.
Table 1.
Karnofsky Performance Score
| Performance | Score |
|---|---|
| Normal | 100 |
| Normal activity; minor signs/symptoms of disease | 90 |
| Subnormal activity; some signs/symptoms of disease | 80 |
| Self-care; unable to work and unable to continue normal | 70 |
| Requires occasional assistance | 60 |
| Requires considerable assistance and frequent medical care | 50 |
| Disabled; requires special care | 40 |
| Severely disabled hospitalized | 30 |
| Very sick hospitalized with active support treatment | 20 |
| Moribund | 10 |
| Dead | 0 |
Nutritional Intervention: Low-Copper Diet
Patients in the intervention group were asked to follow prescribed low copper diet for three months. A dietwith a limit of 0.5 mg of copper per day was developed. Foods high in copper (organ meat including liver; heart; brain, shellfish, bran bread, mushrooms and chocolate) were replaced with foods low in copper to provide adequate micronutrients and calories (Brem et al., 2005). The researcher called the patients every week; a diary of food intake of three days was used to confirm compliance with the diet at first and end of study. The serum copper level was used as the ultimate index of compliance with the diet.
Statistical Methods
Data were analyzed using SPSS version 23 software (SPSS Inc., Chicago, IL). The Kolmogorov–Smirnov test (KS test) was used to test normal distribution of data. Student’s t-test was adopted to compare two groups at the beginning and end of the study. For each group, we compared baseline with final measurement using paired t-test. Kaplan-Meier model was used to compare overall survival rates between the two groups. Failure rate was calculated as the numbers of deaths divided by total follow up time. All reported P values were two-sided.
Results
Sixty three patients with recurrent glioblastoma assessed for eligibility for the study. Thirteen of them were excluded after the initial interview and laboratory tests, 9 patients not met inclusion criteria and 4 refused to follow participation (Figure 1). All of 50 selected patients had pathologically confirmed diagnosed glioblastoma multiforme (48 patients by open surgery and 2 patients with stereotactic biopsy). Patients were allocated to intervention and placebo group. The sample size was 21 patients in each group; with a 20% risk of loss, 25 persons allocated in intervention and 25.0 in placebo group. Unfortunately 6 patients (12%) died before the end of 3.0 months intervention period; four of which in the placebo and two patients were in the intervention group. Of the remaining 44 patients, 26 men and 18 women (59% and 41% respectively) followed the study. Patients’ characteristics at entry according to sex, age and KPS are listed in Table 2.
Figure 1.
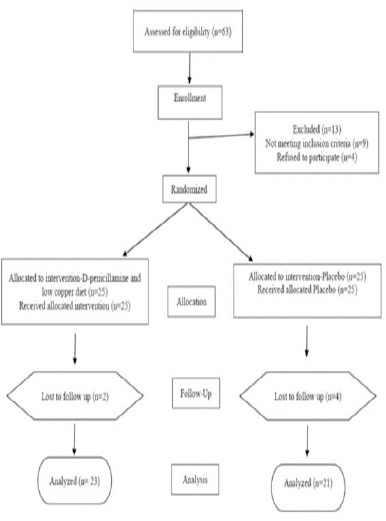
Flow Chart for the Study Subjects.
Table 2.
Patient Characteristics
| Characteristics* | Intervention group (n=23) | Placebo group (n=21) | p |
|---|---|---|---|
| Sex(male) | 14.0 (60.9%) | 12.0 (57.1%) | 0.8 |
| Age(years) | 44.8 ± 2.5 | 42.5 ± 2.5 | 0.51 |
| Karnofsky performance score (KPS) | 75.2 ± 2.4 | 74.8 ± 2.4 | 0.89 |
Data are presented as Mean ± Standard Error of Mean (SE)
The amount of copper used in the intervention group was reduced which was measured by food diary method. Reduction in serum copper was clinically tolerable throughout the study for the patients. In one patient, hemoglobin decreased below normal level that improved by reduction of penicillamine dose to 500 mg per day. One patient underwent debulking operation in the course of intervention. None of the patients developed skin, liver and bone complications. After 6 weeks (middle of the study), both intervention and placebo groups were examined clinically and paraclinically. Mean serum copper level was significantly decreased in intervention group compared to placebo group (86.8 ± 2.9 vs. 118.3 ± 3.4, P < 0.001) (Table 3). IL-6 and VEGF in intervention group were significantly decreased compared to placebo group (P< 0.001). For IL-6 mean ± SE, 1.4 ± 0.1 vs. 2.2 ± 0.2; (data not shown for VEGF). Level of TNF-α was not significantly increased in intervention group (Mean ± SE, 10.8 ± 0.9 vs. 12.5± 1.3; P=0.263) while it was increased significantly in placebo group (Mean ± SE, 12.6 ± 0.9 vs. 16.8 ± 1.5; P=0.018). (Table 3). Twenty four months after starting intervention, twenty of the 44 patients accrued to this clinical trial have died. Nine patients in intervention group and 11 in placebo group. Total follow up time in this study was 29.8 patient-years in intervention group and 22.6 in placebo group. The failure rate in intervention and placebo groups was 0.3 vs. 0.5 respectively. Overall survival time was 17.1 (95% CI, 15.6-21.4) months. Mean survival in intervention and placebo group were 18.5 and 14.9 months respectively. Overall survival in intervention group was improved however there was no significant (Figure 2).
Table 3.
Patients’ Serum Levels of Cytokines and Copper
| Variable | Intervention (Mean ± SE) | Placebo (Mean ± SE) | P | |
|---|---|---|---|---|
| IL-6 (pg/ml) | Before | 2.3 ± 0.3 | 2.3 ± 0.2 | 0.77 |
| After | 1.4 ± 0.1 | 2.2 ± 0.2 | < 0.001 | |
| TNF-α (pg/ml) | Before | 10.8 ± 0.9 | 12.6 ± 0.9 | 0.19 |
| After | 12.5 ± 1.3 | 16.8 ± 1.5 | 0.038 | |
| Copper(µg/dl) | Before | 116.2 ± 5.4 | 116.4 ± 3.5 | 0.98 |
| After | 86.8 ± 2.9 | 118.3 ± 3.4 | < 0.001 |
Figure 2.
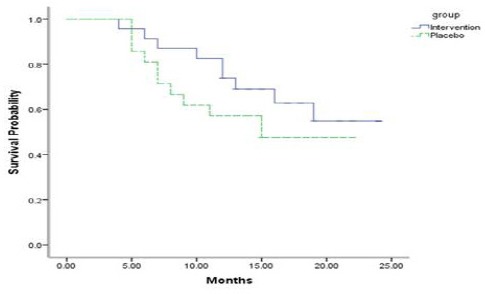
Overall Survival (Kaplan-Meier Curves) for Intervention Group (Solid Line) Compared to Placebo Group (Broken Line).
Discussion
Earlier, animal studies have shown that copper reduction can suppress malignant tumor growth and angiogenesis (Yoshida et al., 1995). We evaluated copper reduction by combining penicillamine with a nutritional method along with gamma knife radiosurgery, as a novel approach for treatment of recurrent glioblastoma at patients who have previously failed to respond to other treatments and have a survival rate less than 6 months. Only in one human study low copper diet and penicillamine was given to a number of patients with newly diagnosed GBM who had undergone conventional radiotherapy and the results were compared with a reference group (New Approaches to Brain Tumor Therapy (NABTT) CNS Consortium) that did not increase survival (Brem et al., 2005). In our study, patients suffering from recurrent GBM who were previously treated with open surgery and conventional radiotherapy (with or without chemotherapy) and now are candidate for gamma knife radiosurgery as one of the last palliative treatment modalities, were exposed to a safe, tolerable and feasible intervention. Nearly all patients cooperated with research team. In addition the placebo group was not deprived of desired treatment, the gamma knife radiosurgery. Up to this time, there have been few antiangiogenesis agents suitable for cancer therapy. Penicillamine was previously demonstrated to have antineoplastic (Wadhwa and Mumper, 2013) and antiangiogenic effects (Antoniades et al., 2013) in laboratory investigations, but it has been used rarely in clinical trials against human cancer such as malignant brain tumors (Brem et al., 2005) and had not encouraging results to increase survival rate. In this study, we used a nutritional intervention (reduction of copper as an essential micronutrient) accompanied by a precise radiation modality that discharges a high dose energy to the tumor and targets its vascular structures as the innovative aspect of the study. In gamma knife radiosurgery an accurate and high dose of gamma radiation (16-18Gy; BED [Biological Effective Dose] = 41.6-50.4Gy) is focused in the tumor center whereas area around the lesion receives a lower dose. Regarding to the invasive and highly malignant nature of glioblastoma, regrowth of malignant cells is probable and the margin of tumor where there is less gamma ray penetrated is one of the possible areas. So we tried to reduce copper, in order to minimize angiogenesis in the tumor margin where there is the most potential risk for tumor expansion. Also in this study the use of penicillamine and low copper diet was well tolerated by most patients and some complications were resolved by dose reduction or repletion of copper. We did not observe adverse reactions such as cerebral hemorrhage, thrombosis, cutaneous and hematological toxicity or neurological impairment. In this study, three important factors in angiogenesis, VEGF, IL-6 and TNF-α were assessed. Improper tumor vasculature enhances tumor hypoxia (Weathers and de Groot, 2015). Angiogenesis advances by both hypoxia-dependent and hypoxia-independent mechanisms (Carmeliet, 2005). Hypoxia activates hypoxia-inducible factor-1α (HIF-1α) that leads to activation of VEGF, one of the most important regulators of angiogenesis (Kaur et al., 2005). Hypoxia-independent mechanisms including Ras/mitogen activated protein kinase and phosphatidylinositol 3-kinase also upregulate VEGF. Mouse endothelial cell tube formation assay showed that anti-TNF-α monoclonal antibody could inhibit blood vessels formation directly and indirectly (Liu et al., 2015). Furthermore, the human-specific TNF-α antibody significantly inhibits in vivo angiogenesis, tumor growth and metastasis (Lai et al., 2016).
In addition to VEGF, IL-6 and TNF-α, glioblastoma usually expresses other proangiogenic factors such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and interleukin-8 (Schmidt et al., 1999; Brat et al., 2005; Reiss et al., 2005; Wang et al., 2008) so blocking these elements or their receptors can inhibit the expression of their signals (Yu et al., 2013). Copper reduction impairs function of the angiogenic factors such as VEGF and its receptors, VEGFR-1 and VEGFR-2 which are the main and primary activators of angiogenesis (Hanahan and Folkman, 1996; Fukumura and Jain, 2007; Xu et al., 2015) and inactivates cytokines such as IL-8, IL-6, TNFα and PG. It has already reported that low copper diet and penicillamine can inhibit glioma cell growth in animal model (Yoshida et al., 1995). Also reduced copper inhibits the angiogenic response induced by the human brain tumor cells (Nasulewicz et al., 2004). Since angiogenesis is a critical factor in the growth of some tumors such as glioblastoma and copper is a known regulator of angiogenesis (Brem et al., 2005), this study describes serum copper reduction by nutritional and pharmacological intervention may inhibit glioblastoma growth. Our study suggests that reduction of IL-6, TNF-α and VEGF along with copper depletion probably decreases tumor angiogenesis, expansion and invasiveness. Also the study demonstrates practical achievement to serum copper reduction as a safe and inexpensive procedure for one of the most malignant human tumors. The horizon of anti-angiogenesis research demonstrates opportunity in regard to brain cancer. Angiogenesis inhibitors seem likely to become a leading part of therapeutic strategies aimed at invasive tumors such as GBM. However further work is nedded to understand other complex mechanisms involved in angiogenesis.
According to our observations, it seems that reducing the level of copper by diet and penicillamine that was accompanied by a decrease in angiogenesis-related factors such as VEGF, IL-6 and TNF-α, may lead to decline in angiogenesis as a main phenomenon of tumor spread. To better understand the possibilities of antiangiogenic tumor therapy and to assess possible side effects further investigations will be needed.
Acknowledgments
This study was supported by Tehran University of Medical Sciences grant (ID: 23607), and carried out at School of Nutritional Sciences and Dietetics.
Conflict of interest
The authors declare that there is no conflict of interest
References
- Albulescu R, Codrici E, Popescu ID, et al. Cytokine patterns in brain tumour progression. Mediators Inflamm. 2013;2013:979748. doi: 10.1155/2013/979748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton K, Glod J. An Orchestrated Response To Tumor Signals By Macrophages and Mesenchymal Stem Cells Potentiates Interleukin-6 Secretion In Glioblastoma. Cell death in therapy. 2014:1. [Google Scholar]
- Antoniades V, Sioga A, Dietrich EM, et al. Is copper chelation an effective anti-angiogenic strategy for cancer treatment? Med Hypotheses. 2013;81:1159–63. doi: 10.1016/j.mehy.2013.09.035. [DOI] [PubMed] [Google Scholar]
- Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–33. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Grossman SA, Carson KA, et al. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro Oncol. 2005;7:246–53. doi: 10.1215/S1152851704000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Chang Q, Bournazou E, Sansone P, et al. The IL-6/JAK/stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15:848–IN45. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, Chen Y, Cui GH. Effects of TNF-alpha and curcumin on the expression of VEGF in Raji and U937 cells and on angiogenesis in ECV304 cells. Chin Med J (Engl) 2005;118:2052–7. [PubMed] [Google Scholar]
- Davis FG, Freels S, Grutsch J, et al. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973-1991. J Neurosurg. 1998;88:1–10. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- Farah P, Blanda R, Kromer C, et al. Conditional survival after diagnosis with malignant brain and central nervous system tumor in the United States, 1995-2012. J Neurooncol. 2016;128:419–29. doi: 10.1007/s11060-016-2127-8. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinathan G, Milagre C, Pearce OM, et al. Interleukin-6 Stimulates Defective Angiogenesis. Cancer Res. 2015;75:3098–107. doi: 10.1158/0008-5472.CAN-15-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Kaur B, Khwaja FW, Severson EA, et al. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol. 2005;7:134–53. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NH, Jung HJ, Shibasaki F, et al. NBBA, a synthetic small molecule, inhibits TNF-alpha-induced angiogenesis by suppressing the NF-kappaB signaling pathway. Biochem Biophys Res Commun. 2010;391:1500–5. doi: 10.1016/j.bbrc.2009.12.101. [DOI] [PubMed] [Google Scholar]
- Koca T, Basaran H, Sezen D, et al. Comparison of linear accelerator and helical tomotherapy plans for glioblastoma multiforme patients. Asian Pac J Cancer Prev. 2014;15:7811–6. doi: 10.7314/apjcp.2014.15.18.7811. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sunita P, Jha S, et al. Daphnetin inhibits TNF-alpha and VEGF-induced angiogenesis through inhibition of the IKKs/IkappaBalpha/NF-kappaB, Src/FAK/ERK1/2 and Akt Signaling Pathways. Clin Exp Pharmacol Physiol. 2016;14:1440–681. doi: 10.1111/1440-1681.12608. [DOI] [PubMed] [Google Scholar]
- Kwon YW, Heo SC, Jeong GO, et al. Tumor necrosis factor-alpha-activated mesenchymal stem cells promote endothelial progenitor cell homing and angiogenesis. Biochim Biophys Acta. 2013;1832:2136–44. doi: 10.1016/j.bbadis.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Lai KC, Liu CJ, Lin TJ, et al. Blocking TNF-alpha inhibits angiogenesis and growth of IFIT2-depleted metastatic oral squamous cell carcinoma cells. Cancer Lett. 2016;370:207–15. doi: 10.1016/j.canlet.2015.10.016. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang G, Zhang J, et al. Anti-TNF-alpha monoclonal antibody reverses psoriasis through dual inhibition of inflammation and angiogenesis. Int Immunopharmacol. 2015;28:731–43. doi: 10.1016/j.intimp.2015.07.036. [DOI] [PubMed] [Google Scholar]
- Mihara M, Hashizume M, Yoshida H, et al. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122:143–59. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- Milstein JM, Cohen ME, Sinks LF. The influence and reliability of neurologic assessment and Karnofsky performance score on prognosis. Cancer. 1985;56:1834–6. doi: 10.1002/1097-0142(19851001)56:7+<1834::aid-cncr2820561323>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Mor V, Laliberte L, Morris JN, et al. The Karnofsky performance status scale: an examination of its reliability and validity in a research setting. Cancer. 1984;53:2002–7. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Nasulewicz A, Mazur A, Opolski A. Role of copper in tumour angiogenesis-clinical implications. J Trace Elem Med Biol. 2004;18:1–8. doi: 10.1016/j.jtemb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Ni W, Luo L, Ping Z, et al. Prognostic value of ALDH1A3 promoter methylation in gliob;astoma: a single center experience in Western China. Asian Pac J Cancer Prev. 2014;16:591–4. doi: 10.7314/apjcp.2015.16.2.591. [DOI] [PubMed] [Google Scholar]
- Pashaki AS, Hamed EA, Mohamadian K, et al. Efficacy of high dose radiotherapy in post-operative treatment of glioblastoma multiform--a single institution report. Asian Pac J Cancer Prev. 2014;15:2793–6. doi: 10.7314/apjcp.2014.15.6.2793. [DOI] [PubMed] [Google Scholar]
- Qin JJ, Wang JM, Du J, et al. Radixin knockdown by RNA interference suppresses human glioblastoma cell growth in vitro and in vivo. Asian Pac J Cancer Prev. 2013;15:9805–12. doi: 10.7314/apjcp.2014.15.22.9805. [DOI] [PubMed] [Google Scholar]
- Qin JJ, Liu ZX, Wang JM, et al. Prognostic factors influencing clinical outcomes of malignant glioblastoma multiforme: clinical, immunophenotypic, and fluorescence in situ hybridization findings for 1p19q in 816 chinese cases. Asian Pac J Cancer Prev. 2015;16:971–7. doi: 10.7314/apjcp.2015.16.3.971. [DOI] [PubMed] [Google Scholar]
- Raluca BA, Cimpean AM, Cioca A, et al. Endothelial Cell Proliferation and Vascular Endothelial Growth Factor Expression in Primary Colorectal Cancer and Corresponding Liver Metastases. Asian Pac J Cancer Prev. 2014;16:4549–53. doi: 10.7314/apjcp.2015.16.11.4549. [DOI] [PubMed] [Google Scholar]
- Redmond KJ, Mehta M. Stereotactic Radiosurgery for Glioblastoma. Cureus. 2015;7:413. doi: 10.7759/cureus.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss Y, Machein MR, Plate KH. The role of angiopoietins during angiogenesis in gliomas. Brain Pathol. 2005;15:311–7. doi: 10.1111/j.1750-3639.2005.tb00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz SK, Iqbal Y, Malik M. Diagnostic and therapeutic implications of the vascular endothelial growth factor family in cancer. Asian Pac J Cancer Prev. 2014;16:1677–82. doi: 10.7314/apjcp.2015.16.5.1677. [DOI] [PubMed] [Google Scholar]
- Schmidt NO, Westphal M, Hagel C, et al. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer. 1999;84:8–10. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Shin MR, Kang SK, Kim YS, et al. TNF-alpha and LPS activate angiogenesis via VEGF and SIRT1 signalling in human dental pulp cells. Int Endod J. 2015;48:705–16. doi: 10.1111/iej.12396. [DOI] [PubMed] [Google Scholar]
- Urso E, Maffia M. Behind the Link between Copper and Angiogenesis: established mechanisms and an overview on the role of vascular copper transport systems. J Vasc Res. 2015;52:172–96. doi: 10.1159/000438485. [DOI] [PubMed] [Google Scholar]
- Wadhwa S, Mumper RJ. D-penicillamine and other low molecular weight thiols: review of anticancer effects and related mechanisms. Cancer Lett. 2013;337:8–21. doi: 10.1016/j.canlet.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Wang L-F, Fokas E, Juricko J, et al. Increased expression of EphA7 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. BMC Cancer. 2008;8:1. doi: 10.1186/1471-2407-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers SP, de Groot J. VEGF manipulation in glioblastoma. J Clin Oncol. 2015;29:719. [PubMed] [Google Scholar]
- Xu H, Zhu J, Gu L, et al. VEGFR2 Expression in Head and Neck Squamous Cell Carcinoma Cancer Cells Mediates Proliferation and Invasion. Asian Pac J Cancer Prev. 2015;17:2217–21. doi: 10.7314/apjcp.2016.17.4.2217. [DOI] [PubMed] [Google Scholar]
- Yang HL, Chang HC, Lin SW, et al. Antrodia salmonea inhibits TNF-alpha-induced angiogenesis and atherogenesis in human endothelial cells through the down-regulation of NF-kappaB and up-regulation of Nrf2 signaling pathways. J Ethnopharmacol. 2014;151:394–406. doi: 10.1016/j.jep.2013.10.052. [DOI] [PubMed] [Google Scholar]
- Yoshida D, Ikeda Y, Nakazawa S. Suppression of tumor growth in experimental 9L gliosarcoma model by copper depletion. Neurol Med Chir. 1995;35:133–5. doi: 10.2176/nmc.35.133. [DOI] [PubMed] [Google Scholar]
- Yu J, Cao X-F, Zheng Y, et al. Anti-VEGF Therapy with Bevacizumab--limited cardiovascular toxicity. Asian Pac J Cancer Prev. 2013;15:10769–72. doi: 10.7314/apjcp.2014.15.24.10769. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yu LK, Lu GJ, et al. Prognostic values of VEGF and endostatin with malignant pleural effusions in patients with lung cancer. Asian Pac J Cancer Prev. 2013;15:8435–40. doi: 10.7314/apjcp.2014.15.19.8435. [DOI] [PubMed] [Google Scholar]


