Abstract
Purpose:
To evaluate palliative care for patients with gynecologic cancer in Japan.
Materials and Method:
A questionnaire asking facility characteristics, systems to coordinate palliative care, current status of end-of-life care, provision of symptom relief, palliative radiation therapy and chemotherapy, and cases of death from gynecological cancer, was mailed to facilities treating gynecologic cancer.
Results:
A total of 115 facilities (29.3% of the total) responded to the questionnaire. Of these, 33.0 (29.0%) had a palliative care ward. End-of-life care was managed by obstetricians and gynecologists in 72.0% of the facilities. The site where end-of-life care was provided was most often a ward in the department where the respondent worked. The waiting period for transfer to a hospice was 2 weeks or more in 52% of facilities. Before the start of primary treatment, pain control was managed by obstetrians and gynecologists in 98.0% of facilities. Palliative radiation therapy or chemotherapy was administered at 93.9% and 92.0% of facilities, respectively. Of the 115 facilities, 34.0 (29.6%) reported cases of death from gynecological cancer. There were 1,134 cases of death. The median time between the last cycle of chemotherapy and death was 85 days for all gynecological cancers. The proportion of patients receiving chemotherapy in the last 30 and 14 days of life were 17.4% and 7.1%, respectively.
Conclusions:
This large-scale survey showed characteristics of palliative care given to patients with gynecologic cancer in Japan. Assessment of death cases showed that the median time between the last cycle of chemotherapy and death was relatively short.
Keywords: Palliative care, gynecologic cancer, palliative chemotherapy, anonymous questionnaire, death cases
Introduction
The majority of patients with advanced gynecologic cancer experience recurrence and die despite aggressive treatment. Since it is difficult to cure recurrence in patients with solid cancer, the main purpose of the treatment for recurrence is to relief symptoms associated with cancer, to maintain or improve the quality of life (QOL), and if possible, to prolong overall survival. Continuing aggressive treatment for incurable patients with advanced cancer until the phase very close to death is associated with decreased QOL and does not prolog survival, suggesting that physicians should encourage patients to enter the hospice for better palliative care (Saito et al.,2011). In contrast, despite the poor outcome, the majority of patients with recurrent ovarian cancer indicated a desire for continuing aggressive treatment, such as chemotherapy, to prolong survival rather than maintaining QOL (Donovan et al.,2002). The optimal timing to discontinue aggressive treatment for incurable patients with recurrent/advanced cancer is still unknown. Consequently, it is necessary to clarify the optimal timing of discontinuing aggressive treatment for incurable patients by conducting a multicenter cooperative study. However, most of the reported studies on palliative care for patients with cancer are case reports or retrospective studies at a single center.
The Japan Society of Gynecologic Palliative Medicine (JSGPM) was organized in November 2013 to unveil current status of palliative care for women with gynecologic cancer and to provide better end-of-life palliative care. JSGPM conducted the current survey to assess how palliative care are given at facilities treating gynecologic cancer, and to identify problems and issues of palliative care for patients with gynecologic cancer. In addition, we retrospectively examined the cases of death from gynecological cancer to determine the interval between the final cycle of chemotherapy and death. This is the first multicenter cooperative survey to ascertain the current state of palliative care for patients with gynecologic cancer in Japan.
Materials and Methods
Of 393 facilities that were sent the questionnaire, 115 facilities treating gynecologic cancer responded to the current study by mail. This study was approved by the Institutional Review Board of Hirosaki University Graduate School of Medicine (Reference number: 2013-202). The questionnaire asked each facility about 1) patient characteristics, 2) its system for provision of palliative care, 3) its system for coordination of palliative care, 4) the current state of end-of-life care, 5) the current state of provision of symptom relief, 6) palliative radiation therapy, and 7) palliative chemotherapy. Facilities that responded to this study were also asked to provide information about medical records of patients with gynecologic cancer who died within a 2-year period from 2010 to 2012, including the date of death, the date of the last cycle of chemotherapy, and the type of gynecologic cancer. Of 115 facilities that participated in this questionnaire, 34.0 facilities (29.6%) responded to the study of death case.
Each respondent represented a facility. Respondents had an average of 20.3 years (range: 5–39 years) of clinical experience. Sixty-one facilities (53.0%) were general hospitals, 43.0 facilities (37%) were university hospitals, 10 facilities (9%) were hospitals specializing in cancer, and one facility (1%) was some other type of facility. Facilities that responded to the current study had an average of 608 beds, an average of 33 beds for patients with gynecology, and an annual average of 98.5 new patients every year who were treated for gynecologic cancer.
Results
Thirty-three facilities (29%) had a palliative care ward. One hundred and nine facilities (95%) had a palliative care team in each hospital. Eighty-two facilities (71%) charged an additional fee for palliative care. Responses to questions about the palliative care team indicated that 50% of the physicians who managed patients’ physical symptoms were dedicated members of the care team, 11% were full-time members of the team, 35% had other duties, and 4% had an unknown status. Sixty facilities (52%) had nurses specialized in cancer care, 81 facilities (70%) had nurses certified in palliative care, and 54 facilities (47%) had nurses certified in cancer pain management. Sixty-nine facilities (60%) had a palliative care specialist and 102 facilities (89%) had a physician who managed patients’ mental symptoms. Out of the 109 facilities that had a palliative care team within the hospital, 57 palliative care teams (52%) had a clinical psychologist, 78 (72%) had a social worker, and 83 (76%) had a pharmacist.
Eighteen facilities (16%) used their ties to other facilities in their region to coordinate palliative care. Twenty-one facilities (18%) had ties to other facilities in their region, but did not use them to coordinate palliative care. Sixty-three facilities (55%) had no ties to other facilities in their region. Thirteen facilities (11%) were unaware of any ties to other facilities in their region or the status of their ties to other facilities was unclear.
The definition of end-of-life care in this study is care for a patient who was not eligible for anti-cancer treatment and had a predicted survival of 6 months or less. End-of-life care was managed by physicians of the department of obstetrics and gynecology (OB and GY) in 83 facilities (72%), by physicians of the palliative care team in 10 facilities (9%), and by physicians of some other department in 22 facilities (19%). The “some other department” in the previous response most often involved care managed by both OB and GY and palliative care. When end-of-life care was needed, 20.0 facilities (17%) referred almost every patient to a facility specializing in hospice and palliative care. Eighty-three facilities (72%) referred patients individually, and 13 facilities (11%) did not refer patients or they seldom had patients to refer. End-of-life care was provided in the department of OB and GY ward (47%) or at facilities specializing in hospice (24.0%) and palliative care (14%) (Figure 1A). End-of-life care was seldom provided at a local affiliated hospital or at home. When a patient was referred to a facility with a department of palliative care or a facility specializing in hospice and palliative care, the patient had to wait a while before being transfer to another department or facility (Figure 1B). Most often, patients had to wait 1.0 to 2 weeks (35% of facilities), and patients in 43% of facilities had to wait less than 2 weeks. In contrast, in 21% of facilities, patients had to wait for over 1.0 month before transfer to another facility.
Figure 1.
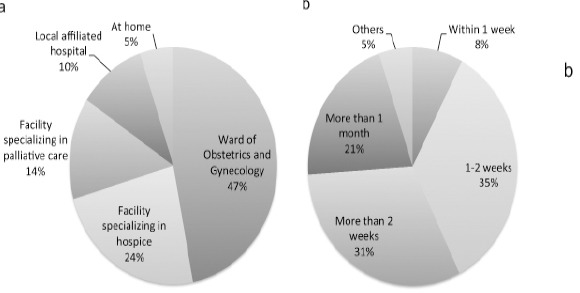
The Current State of End-of-Life Care. (A) The Places where End-of-Life Care is Provided as a First Choice. (B) The Waiting Period for Transfer to Another Department or Facility when a Patient was Referred to a Facility with a Palliative Care Department or a Facility Specializing in Hospice and Palliative Care
One hundred and thirteen facilities (98%) initiated opioid administration before the start of primary treatment for gynecologic cancer proactively. The facilities that performed opioid administration before the primary treatment proactively were as follows: OB and GY at 82.0 facilities (71%), an obstetrician/gynecologist well versed in pain control at 15 facilities (13%), the palliative care team at 13 facilities (11%), and some other department at 5 facilities (5%). The department managing pain control after the start of primary treatment was OB and GY at 68 facilities (59%), the palliative care team at 17 facilities (15%), OB and GY guided by the palliative care team at 17 facilities (15%), and some other department at 13 facilities (11%). At 110 facilities (96%), the obstetrician/gynecologist managed pain control after the start of primary treatment. The obstetrician/gynecologists prescribed an opioid, albeit in different forms such as oral agents and injections. Physicians at 54 facilities (47%) used a pain assessment scale, but those at 56 facilities (49%) did not use a pain assessment scale.
The department that managed dyspnea due to lung metastasis or lymphangitic carcinomatosis was OB and GY alone at 35 facilities (31%), OB and GY in cooperation with respiratory medicine and palliative care at 37.0 facilities (32%), the palliative care team at 20.0 facilities (17%), respiratory medicine at 10 facilities (9%) and some other department at 13 facilities (11%). The management of refractory ascites is summarized in Figure 2A. The most frequent approach was aspiration of ascites, followed by chemotherapy, cell-free and concentrated ascites reinfusion therapy (CART), use of diuretics, and then use of corticosteroids. Management of gastrointestinal obstruction due to cancer is shown in Figure 2B. The most frequent approach was to administer octreotide, followed by insertion of a nasogastric tube. In addition, a relatively large number of facilities performed surgery or inserted an intestinal tube.
Figure 2.
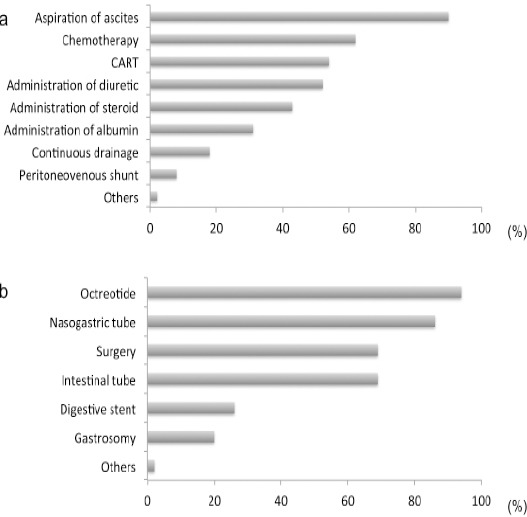
The Current State of Provision of Symptom Relief. (A) Management of Refractory Ascites. Multiple Answers Allowed. CART: Cell-Free and Concentrated Ascites Reinfusion Therapy. (B) Management of a Gastrointestinal Obstruction Due to Cancer. Multiple Answers Allowed
Palliative radiation therapy was administered by 108 facilities (93.9%). Five facilities did not administer palliative radiation therapy in principle, one facility did so only to stop bleeding, and one facility did not respond to the question about palliative radiation.
Palliative chemotherapy means chemotherapy that is not curative but intended to improve QOL or to relieve the symptoms of patients with recurrent or advanced cancer. Fifty-four facilities (47%) administered palliative chemotherapy, 52 facilities (45%) administered palliative chemotherapy depending on the patient, 8 facilities (7%) did not administer palliative chemotherapy, and one facility did not respond to the question about palliative chemotherapy. In response to the question about the timing to end palliative chemotherapy, most facilities responded that chemotherapy was ceased when the patient’s cancer becomes refractory to treatment or if the patient’s remaining life time can be definitively predicted (data not shown). For 50.0 facilities which responded that they would stop administering palliative chemotherapy when the patient’s outcome could be definitively predicted, the following question was asked: “What should a patient’s predicted survival be in order for her to undergo palliative chemotherapy?” (Figure 3). Eighty-three facilities (72%) responded that they would give a palliative chemotherapy if predicted survival was 3 months or longer.
Figure 3.
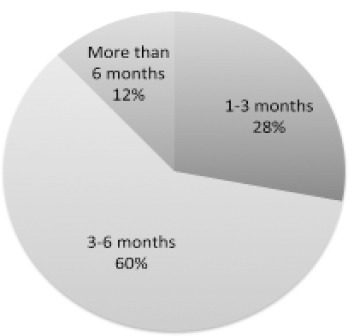
Palliative Chemotherapy. Response Rate to “What Should A Patient’s Predicted Survival be in Order for Her to Undergo Palliative Chemotherapy?”
Thirty-four facilities (8.6%) responded to questions about cases of deaths from gynecologic cancer and provided information on 1,134 patients, including 516 patients (45.5%) with ovarian cancer. The next most prevalent gynecologic cancer were cervical cancer (293 patients, 25.8%) and endometrial cancer (233 patients, 20.5%). The median number of days between the date of death and the date of the last cycle of chemotherapy and the range of those values were calculated for each primary form of gynecologic cancer and for gynecologic cancer overall (Table 1). Patients with gynecologic cancer had a median duration of survival of 81 days after the last cycle of chemotherapy. Patients with ovarian cancer, peritoneal carcinoma, endometrial cancer, or vulvar cancer had a median survival of less than 80 days. In contrast, patients with cervical cancer or vaginal cancer survived longer than patients with other gynecologic cancer (median survival of 98 days for cervical cancer and 112 days for vaginal cancer).
Table 1.
Median Number of Days Between the Date of Death and the Last Round of Chemotherapy in Cases of Gynecologic Cancers
| Gynecologic cancers | Number of cases | Median number of days between the date of death and the last cycle of chemotherapy | Range |
|---|---|---|---|
| (Days) | |||
| Ovarian cancer | 516 | 73 | 1-362 |
| Fallopian tube cancer | 15 | 82 | 5-326 |
| Peritoneal cancer | 42 | 75 | 6-318 |
| Cervical cancer | 293 | 98 | 3-362 |
| Endometrial cancer | 233 | 74 | 1.0-363.0 |
| Vulva cancer | 11 | 70 | 26-120 |
| Vaginal cancer | 10 | 122 | 58-320 |
| Total including other 14 cancers | 1,134 | 81 | 1-363 |
Eighty-one patients (7.1%) died within 14 days after their last cycle of chemotherapy, and 197 patients (17.4%) died within 30 days after their last cycle of chemotherapy (Figure 4). Six hundred and eighteen patients (54.5%) died within 90 days after their last cycle of chemotherapy.
Figure 4.
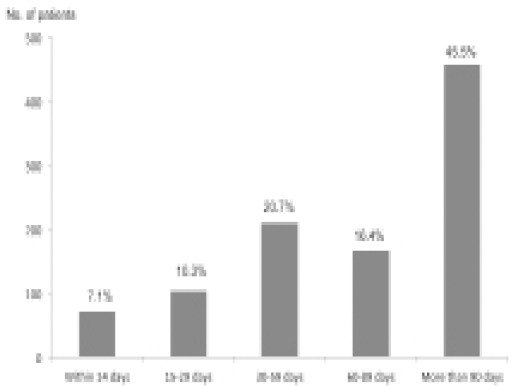
Distribution of the Number of Days From the Date of The Last Cycle of Chemotherapy to the Date of Patient Death in the Cases of Gynecological Cancers
Discussion
The white paper on hospices from palliative care teams registered with the Japanese Society for Palliative Medicine (JSPM) in 2014 reported that 20.4% of facilities have a palliative care ward and 31.8% of facilities charge an additional fee for palliative care (Japan Society for Palliative Medicine, 2014). In the present study, 95% of the facilities had a palliative care team, and the percentage of facilities with a palliative care ward and the percentage of facilities that charge an additional fee for palliative care were higher than those reported in the white paper of JSPM. As of October 2014, there were 47 prefectural/metropolitan cancer treatment centers nationwide and 304 regional cancer treatment centers in Japan. Forty-three facilities (91.5%) that participated in the current survey were prefectural/metropolitan cancer treatment centers and 57 (18.8%) were regional cancer treatment centers, suggesting that the majority of the target institutions of the present survey were institutions specializing in cancer treatment. There are many regional cancer treatment centers in Japan, but most of these hospitals usually provide general medical services including perinatal care to local population. This may be the reason why most of the prefectural/metropolitan cancer treatment centers responded to this survey although a few regional cancer treatment centers responded. In the future, medical doctors working at regional cancer treatment centers may need to be encouraged to have greater interest in palliative care.
End-of-life care was managed by physicians of OB and GY alone in 72% of facilities. In addition, physicians of OB and GY managed end-of-life care in 91% of facilities with the cooperation of the department of palliative care. When patients needed end-of-life care, only 17% of facilities referred almost every patient to a facility specializing in hospice and palliative care in the current study. The result suggested that, in certain area, there is no facility suitable for end-of-lice care for patients who desired such care near their town. Several specific natures of the gynecologic cancer, when it is terminal, might also hamper for a hospice to accept such patients. Consequently, gynecologic oncologists play a key role in establishing a medical cooperation system for the palliative care of patients with gynecologic cancer in each region.
The Japanese Ministry of Health, Labor, and Welfare reported that 79% of patients with incurable cancer died in hospital, 8.9% died at home, 1.9% died in a palliative care ward, and 9.5% died in a nursing home (Japan Society for Palliative Medicine, 2014). In Norway, Taiwan, and Canada, 60% of patients with cancer died in the hospital, whereas about 50% of patients with cancer died at home in Italy and the Netherlands (Mohsen et al., 2014). Furthermore, the patients with incurable cancer who remained at home had a longer median survival in comparison to hospitalized patients (67.0 days vs. 33.0 days, p<0.001) (Murakami et al., 2015). The patients with cancer who died in the hospital or ICU had a worse QOL at the end of life and bereaved family caregivers had a high risk of developing a mental disorder in comparison to those who cared patients that died at home (Wright et al., 2010). Most women with incurable gynecologic cancer play an important role as a mother, as a wife and sometimes as a daughter in the home. Therefore, a system that assists end-of-life care at home in each region would greatly enhance quality of life in patients suffering from in curable cancers.
The majority of patients with recurrent/advanced ovarian cancer, which accounted for half of the cases of death from gynecological cancer, had refractory ascites and malignant bowel obstruction (MBO). The management of refractory ascites is a common problem in care of ovarian cancer. In clinical practice, the easiest way to manage refractory ascites is probably needle aspiration of ascites. Anti-cancer agents may also be used depending on the circumstances. The AURELLIA study suggested that addition of bevacizumab to salvage chemotherapy may be useful for the management of refractory ascites in patients with recurrent ovarian cancer (Pujade-Lauraine et al., 2014). CART is reported to be useful in managing refractory ascites (Maeda et al., 2015; Wang et al., 2015). Physician in more than 80.0% of facilities selected administration of octreotide or insertion of a nasogastric tube for patients with MBO. Nonetheless, the best approach for management of MBO, including indications for palliative surgery, remain to be determined.
Proactive interventions such as radiotherapy, chemotherapy, and surgery are performed to improve QOL and prolong the survival of patients with incurable cancer, in addition to supportive care in some patients. Palliative radiation is given to manage symptoms associated with metastasis/recurrence of gynecologic cancer, such as pain due to bone metastasis, symptoms due to brain metastasis, and vaginal bleeding of uterine cancer. (De Meerleer G et al., 2011; Makino et al., 2016).
The term “palliative chemotherapy” was not described in the Japanese treatment guidelines for ovarian cancer. The Japanese treatment guidelines defined salvage chemotherapy as chemotherapy given to relieve symptoms associated with cancer, to maintain or improve QOL, and if possible, to prolong overall survival. The majority of Japanese gynecologic oncologists recognize palliative chemotherapy beneficial for relieving symptoms related to progressive cancer, such as refractory massive ascites with peritonitis carcinomatosa and intracranial hypertension with brain metastasis (Kobold S et al., 2009). In contrast, continuing aggressive chemotherapy for incurable patients until near death sometimes decreased QOL and resulted in unpredictable worsening after chemotherapy. Ultimately, the most important decision for gynecologic oncologists that manage patients with incurable gynecologic cancer is to decide when aggressive chemotherapy should be stopped, and to select what is the most effective chemotherapeutic regimen as a palliative care for incurable disease.
Previous studies reported that 12.6% to 23% of patients with various cancers underwent chemotherapy 1 month prior to their death, and 8% to 16% of patients underwent chemotherapy two weeks prior to their death (Hashimoto et al., 2009; Kao et al., 2009; Mack et al., 2012; Näppä et al., 2011). Few similar studies have looked specifically at gynecologic cancer (Barbera et al., 2010; Keyser et al., 2010). 57.8% of patients with gynecologic cancer underwent chemotherapy half a year prior to their death (Fauchi et al., 2012). The current study revealed the proportion of patients who died within 14 days or 30.0 days after the final cycle of chemotherapy. In this study, 72% of the facilities administered palliative chemotherapy when the patient had predicted survival of 3 months or longer. However, the median duration of survival after the final cycle of chemotherapy was 81 days, indicating that it is difficult to predict accurately the survival of patients with incurable gynecologic cancer, and some patients would not have benefitted from palliative chemotherapy. In the setting of disease progression and unstable patient condition, most gynecologic oncologists tend to inform a patient and her family that she is at the end of life relatively in a rush (Ramondetta et al., 2014; von Gruenigen et al., 2005). Half of the bereaved families of incurable patients felt that the transfer to the palliative care ward had been too late (Cheung et al., 2015; Morita et al., 2009). Studies have reported that early palliative intervention results in less hospitalization expenses for gynecologic cancer (Lowery et al., 2013; Nevadunsky et al., 2014).
Finally, there are two limitations in this study. First, low response rate to the questionnaire may reflects the low level of interest associated with palliative care in Japanese Gynecological Oncologists. Second, the evaluation concerning the median duration of survival after the final cycle of chemotherapy remains obscure. In this study, although we picked up the final date of chemotherapy in cases that died due to gynecologic cancer, some of the cases were simultaneously treated with palliative radiotherapy, surgery and best supportive care. Thus, since clinical interpretation of the present results seemed complicated, we must plan the new clinical survey concerning the detail of fatal cases treated with the palliative chemotherapy, palliative radiotherapy, palliative surgery or best supportive care.
In conclusions, the current study is the first large-scale survey of palliative care for patients with gynecologic cancer in Japan. The results of this survey indicate that regional alliance systems providing end-of-life care for patients with incurable gynecologic cancer are not sufficiently established in Japan. To provide better end-of-life care for patients with incurable gynecologic cancer, Japanese gynecologic oncologist should build the regional alliance systems to utilize existing medical resources effectively. Deciding when to halt chemotherapy is difficult. We will conduct a detailed study of deaths of patients with gynecologic cancer in the future, and those findings should help to distinguish which patients should receive interventions such as palliative therapy and which patients should not.
Acknowledgement
We would like to thank all the members who participated in this JSGPM survey.
Conflict of interest statement
The authors declare no relevant conflicts of interest.
References
- Barbera L, Elit L, Krzyzanowska M, et al. End of life care for women with gynecologic cancers. Gynecol Oncol. 2010;118:196–201. doi: 10.1016/j.ygyno.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Cheung MC, Earle CC, Rangrej J, et al. Impact of aggressive management and palliative care on cancer costs in the final month of life. Cancer. 2015;121:3307–15. doi: 10.1002/cncr.29485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meerleer G, Vandecasteele K, Ost P, et al. Whole abdominopelvicradiotherapy using intensity-modulated arc therapy in the palliative treatment of chemotherapy-resistant ovarian cancer with bulky peritoneal disease: a single-institution experience. Int J Radiat Oncol Biol Phys. 2011;79:775–81. doi: 10.1016/j.ijrobp.2009.11.039. [DOI] [PubMed] [Google Scholar]
- Donovan KA, Greene PG, Shuster JL. Treatment preferences in recurrent ovarian cancer. Gynecol Oncol. 2002;86:200–11. doi: 10.1006/gyno.2002.6748. [DOI] [PubMed] [Google Scholar]
- Fauchi J, Schneider K, Walters C, et al. The utilizeation of palliateive care in gynecologic oncology patients near the end of life. Gynecol Oncol. 2012;127:175–79. doi: 10.1016/j.ygyno.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Japanese Society for Palliative Medicine. The 2014 White Paper on Hospices from palliative care teams 2014 edition. Tokyo: Seikaisha, LTD; 2014. [Google Scholar]
- Hashimoto K, Yonemori K, Katsumata N, et al. Factors that affect the duration of the interval between the completion of palliative chemotherapy and death. Oncologist. 2009;14:752–59. doi: 10.1634/theoncologist.2008-0257. [DOI] [PubMed] [Google Scholar]
- Kao S, Shafig J, Vardy J, et al. Use of chemotherapy at end of life in oncology patients. Ann Oncol. 2009;20:1555–59. doi: 10.1093/annonc/mdp027. [DOI] [PubMed] [Google Scholar]
- Keyser EA, Reed BG, Lowery WJ, et al. Hospice enrollent for terminally ill patients with gynecologic cancer: Impact on outcomes and intervenetions. Gynecol Oncol. 2010;118:274–77. doi: 10.1016/j.ygyno.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Kobold S, Hegewisch-Becker S, Oechsle K, et al. Intraperitoneal VEGF inhibition using bevacizumab: a potential approach for the symptomatic treatment of malignant ascites? Oncologist. 2009;14:1242–51. doi: 10.1634/theoncologist.2009-0109. [DOI] [PubMed] [Google Scholar]
- Lowery WJ, Lowery AW, Barnett JC, et al. Cost-effectiveness of early palliative care intervention in recurrent platinum-resistant ovarian cancer. Gynecol Oncol. 2013;130:426–30. doi: 10.1016/j.ygyno.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Mack JW, Cronin A, Keating NL, et al. Associations between end-of-life discussion characteristics and care received near death: a prospective cohort study. J Clin Oncol. 2012;30:4387–95. doi: 10.1200/JCO.2012.43.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Yabuuchi J, Nobuta H, et al. Characteristics of Patients and Their Ascites Who Underwent Repeated Cell-Free and Concentrated Ascites Reinfusion Therapy. Ther Apher Dial. 2015;19:342–48. doi: 10.1111/1744-9987.12343. [DOI] [PubMed] [Google Scholar]
- Makino H, Nishio S, Tsubamoto H, et al. Treatment and prognosis of bone metastasis from cervical cancer. KCOG-G1202s J Obstet Gynaecol Res. 2016 doi: 10.1111/jog.12956. doi: 10.1111/jog.12956. [DOI] [PubMed] [Google Scholar]
- Mohsen H, Haddad P, Allam A, et al. Patterns in place of cancer death in the state of Quatar;A population-based Study. PLoS ONE. 2014;9:e109615. doi: 10.1371/journal.pone.0109615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Miyashita M, Tsuneto S, et al. Late referrals to palliative care units in Japan: nationwide follow-up survey and effects of palliative care team involvement after the Cancer Control. Act J Pain Symptom Manage. 2009;38:191–96. doi: 10.1016/j.jpainsymman.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Murakami N, Tanabe K, Morita T, et al. Going back to home to die: dose it make a difference to patient survival. BMC Palliative care. 2015;14:7. doi: 10.1186/s12904-015-0003-5. doi 10.1186/s12904-015-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näppä U, Lindqvist O, Rasmussen B.H, et al. Palliative chemotherapy during the last month of life. Ann Oncol. 2011;22:2375–80. doi: 10.1093/annonc/mdq778. [DOI] [PubMed] [Google Scholar]
- Nevadunsky NS, Gordon S, Spoozak L, et al. The role and timing of palliative medicine consultation for women with gynecologic cancer: association with end of life interventions and direct hospital costs. Gynecol Oncol. 2014;132:3–7. doi: 10.1016/j.ygyno.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–8. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- Ramondetta LM, Tortolero-Luna G, Bodurka DC, et al. Approaches for end-of-life care in the field of gynecologic oncology: an exploratory study. Int J Gynecol Cancer. 2014;14:580–88. doi: 10.1111/j.1048-891X.2004.14402.x. [DOI] [PubMed] [Google Scholar]
- Saito AM, Landrum MB, Neville BA, et al. The effect on survival of continuing -chemotherapy to near death. BMC Palliat Care. 2011;21:10–14. doi: 10.1186/1472-684X-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Gruenigen VE, Daly BJ. Futility: clinical decisions at the end-of-life in women with ovarian cancer. Gynecol Oncol. 2005;97:638–44. doi: 10.1016/j.ygyno.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Wang L, Okubo T, Shinsaka M, et al. Efficacy and safety of cell-free and concentrated ascites reinfusion therapy (CART) in gynecologic cancer patients with a large volume of ascites. J Obstet Gynaecol Res. 2015;41:1614–20. doi: 10.1111/jog.12763. [DOI] [PubMed] [Google Scholar]
- Wright AA, Keating NL, Balboni TA, et al. Place of death: Correlations with quality of life of patients with cancer and predictors of Bereaved caregiver’s mental health. J Clin Oncol. 2010;28:4457–64. doi: 10.1200/JCO.2009.26.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]


